Abstract
The treatment of prosthetic joint infections caused by methicillin-resistant Staphylococcus aureus (MRSA) continues to be a challenge for the clinician. The aim of this study was to evaluate the efficacies of daptomycin at usual and high doses (equivalent to 6 and 10 mg/kg of body weight/day, respectively, in humans) and in combination with rifampin and to compare the activities to those of conventional anti-MRSA therapies. We used MRSA strain HUSA 304, with the following MICs and minimal bactericidal concentrations (MBCs), respectively: daptomycin, 1 μg/ml and 4 μg/ml; vancomycin, 2 μg/ml and 4 μg/ml; linezolid, 2 μg/ml and >32 μg/ml; and rifampin, 0.03 μg/ml and 0.5 μg/ml. In time-kill curves, only daptomycin and its combinations with rifampin achieved a bactericidal effect in log and stationary phases. For in vivo studies, we used a rat foreign-body infection model. Therapy was administered for 7 days with daptomycin at 100 mg/kg/day and 45/mg/kg/day, vancomycin at 50 mg/kg/12 h, rifampin at 25 mg/kg/12 h, and linezolid at 35 mg/kg/12 h, and each antibiotic was also combined with rifampin. Among monotherapies, daptomycin at 100 mg/kg/day and rifampin performed better than vancomycin and linezolid. In combination with rifampin, both dosages of daptomycin were significantly better than all other combinations, but daptomycin at 100 mg/kg/day plus rifampin achieved better cure rates at day 11 (P < 0.05) than daptomycin at 45 mg/kg/day plus rifampin. Resistant strains were found in monotherapies with rifampin and daptomycin at 45 mg/kg/day. In conclusion, daptomycin at high doses was the most effective monotherapy and also improved the efficacy of the combination with rifampin against foreign-body infections by MRSA. Clinical studies should confirm whether this combination may be considered the first-line treatment for foreign-body infections by MRSA in humans.
Methicillin-resistant Staphylococcus aureus (MRSA) infections are of great concern, and the limitations in activity of standard anti-MRSA antibiotics (i.e., glycopeptides) emphasize the need for the introduction of new drugs (16, 27, 29).
Daptomycin is a promising lipopeptide drug which provides concentration-dependent bactericidal activity against growing and nongrowing MRSA (25, 30). It is currently approved for use in treatment of bacteremia and right-sided endocarditis at a dosage of 6 mg/kg of body weight/day (7, 9); however, clinical failure and development of resistance during treatment have been described and are matters for concern (13, 28). Two therapeutic strategies stand out as promising alternatives for deep-seated infections: (i) the use of high doses of the drug (doses up to 12 mg/kg/day have been well tolerated in humans) and (ii) the use of the drug in combined therapies.
Foreign-body infections are difficult to treat due to the presence of bacterial biofilm and tolerance to antibiotics (4, 31). MRSA is often involved in these infections (36). In this setting, clinical experience with daptomycin is limited, and the dosage to use remains controversial and undefined (8, 23). Rifampin has proven its value in the treatment of device-related staphylococcal infections (35, 37), and so the use of a combination of daptomycin and rifampin should be considered as a promising alternative therapy.
The experimental tissue cage infection model is a reliable method for mimicking device-related infections that has been used in recent years to test several antimicrobial therapies alone and in combination (12, 18, 34). We previously reported that, by use of this model, daptomycin at high doses (equivalent to 10 mg/kg/day in humans) was as effective as rifampin alone and also prevented the emergence of daptomycin-resistant strains (19). More recently, John et al. described the good efficacy of daptomycin at usual doses (6 mg/kg/day) in combination with rifampin in a guinea pig tissue cage infection model and also noted the lack of resistance with this combination (10).
In the present study, we aimed to compare the activity of daptomycin at usual and high doses (equivalent to 6 and 10 mg/kg/day, respectively) and its activity in combination with rifampin by using a rat tissue cage infection model. We also aimed to compare the efficacies of these daptomycin-rifampin combinations with those of other available anti-MRSA drugs in association with rifampin.
MATERIALS AND METHODS
Microorganism.
A methicillin-resistant S. aureus strain (HUSA 304) was used for all in vitro and in vivo studies.
Antimicrobial agents.
For in vitro experiments, the purified powder of antibiotic was resuspended according to laboratory recommendations. For in vivo experiments, we diluted the commercial products to achieve a final volume suitable for administration to animals.
Antibiotics were kindly supplied by the manufacturers' laboratories: linezolid by Pfizer (Madrid, Spain), vancomycin by Normon (Madrid, Spain), rifampin by Sanofi-Aventis (Madrid, Spain), and daptomycin by Novartis (Barcelona, Spain).
In vitro studies. (i) Determination of MICs and MBCs.
The MICs and minimal bactericidal concentrations (MBCs) in log phase were determined according to standard recommendations (3). The MIC was defined as the minimum concentration of antibiotic that was able to inhibit visible bacterial growth, and the MBC was defined as the lowest concentration that killed 99.9% of the original inoculum.
We also determined MBCs during the stationary phase of growth. We used a methodology previously reported in detail (18, 35), which has proven to be a reliable approach for correlating the in vivo efficacies of antibiotics in the tissue cage infection model. The MBCs were defined as described above.
The MICs of vancomycin, rifampin, linezolid, and daptomycin in log phase were 2, 0.03, 2, and 1 μg/ml, respectively, and the MBCs were 4, 0.5, >32, and 4 μg/ml, respectively. The MBCs for these antibiotics in stationary phase were >32, >8, >32, and 24 μg/ml, respectively.
(ii) Twenty-four-hour kill curves in log and stationary phases.
For kill curves in log phase, we used standard methodology (21), and for kill curves in stationary phase, we used a methodology reported elsewhere (18).
The concentrations of antibiotics selected for kill curves in log phase were pre-fixed (ranging from 0.5× to 128× MIC) to represent subinhibitory and clinically achievable levels greater than the MIC. Due to the bacterial tolerance to antibiotics expressed in stationary phase, drug concentrations tested were higher than those in log phase; these concentrations were equivalent to the total peak and trough levels achieved in the tissue cage fluid (TCF).
For all experiments, bactericidal activity was defined as a ≥3-log10 decrease in CFU/ml of the initial inoculum at 24 h. The results of the combination were compared with those of the most active single drug; synergy, indifference, and antagonism were then defined as a ≥2-log increase in killing, a <2-log change (increase or decrease) in killing, and a ≥2-log decrease in killing, respectively.
To avoid carryover antimicrobial-agent interference, the sample was placed on the plate in a single streak down the center and allowed to be absorbed into the agar until the plate surface appeared dry; the inoculum was then spread over the plate.
In all in vitro experiments with daptomycin, the medium was supplemented with 50 mg/liter of calcium (Sigma).
Animal studies.
The animal model was approved by the Ethical Committee for Animal Experiments at the University of Barcelona.
The rat model was previously standardized by our group, and all methodology used has been reported in our earlier study (19). Briefly, two Teflon tissue cages with one coverslip (CV) each were subcutaneously implanted in male Wistar rats. After 3 weeks, the TCF was checked for sterility and infected with 0.1 ml of a MRSA preparation (106 CFU/ml). At 72 h postinoculation (day 1), TCF was obtained to quantify bacterial counts; therapy was then started and administered intraperitoneally for 7 days. One and 4 days after the end of treatment (days 8 and 11, respectively), TCF was again recovered to quantify bacterial counts. On day 11, animals were sacrificed and coverslips were removed to quantify adherent bacteria.
All procedures for processing TCF and coverslips have been reported as being harmless for bacteria and have been described in detail elsewhere (2, 12, 18). Briefly, TCF obtained was sonicated to disrupt bacterial clumps; samples of 100 μl of the sonicated fluids and their 10-fold dilutions were plated on a Trypticase soy agar plate with 5% sheep blood for 48 h at 37°C, and then bacterial counts were recorded as log CFU per ml. Once animals were sacrificed, coverslips from tissue cages were removed and rinsed three times in 1 ml of phosphate-buffered saline (PBS); they were then incubated in 1 ml of PBS with trypsin (6 U/ml; Sigma, Madrid, Spain) for 20 min at 37°C. Finally, the remaining PBS was sonicated to recover adherent bacteria, with the final fluid being used to perform bacterial counts and to screen resistant bacteria (see below).
The criterion of efficacy was defined as the decrease in bacterial count from TCF between the beginning and the end of treatment; it was evaluated twice, on days 8 and 11. The antibiotic efficacy against adherent bacteria from coverslips removed on day 11 was also evaluated by determining the bacterial counts. Finally, the cure rate of infection was calculated on day 11 with bacteria from both the TCF and the coverslips; it was defined as the percentage of samples with bacterial counts under the limit of detection with respect to the total samples.
For all cases, the lower limit of detection of bacterial counts was 10 CFU/ml.
Therapeutic groups.
Animals were divided into therapeutic groups and received drugs at the following dosages: linezolid at 35 mg/kg/12 h, vancomycin at 50 mg/kg/12 h, rifampin at 25 mg/kg/12 h, daptomycin at 100 mg/kg/day, daptomycin at 45 mg/kg/day, linezolid plus rifampin, vancomycin plus rifampin, daptomycin at 100 mg/kg/day plus rifampin, and daptomycin at 45 mg/kg/day plus rifampin. Controls received no drugs.
Pharmacokinetic studies.
The methodology used for pharmacokinetic studies was described in detail in our earlier studies (18, 20). On the basis of previously reported data, for all drugs we selected the dose at which the area under the concentration-time curve from 0 to 24 h (AUC0-24) values in TCF and serum in animals were close to those in human serum (5, 32).
Peak and trough levels in TCF for each drug were also determined on day 4 to check the equilibrium test concentrations during treatment.
All drug concentrations, except those of vancomycin, were determined using a bioassay method (1). The vancomycin concentrations were determined by fluorescent polarization immunoassays using a TDX analyzer (Abbott, Madrid, Spain) (18). The pharmacokinetic and pharmacodynamic parameters achieved by the selected dosage of each antibiotic were reported in our earlier works (18, 19).
Resistance studies.
The screening of resistant strains from TCF at day 8 and day 11 and from coverslips at day 11 was performed using agar plates containing 4 μg/ml linezolid, 2 μg/ml vancomycin, or 1 μg/ml daptomycin or rifampin. The plates containing daptomycin were supplemented with calcium (50 mg/liter).
In all cases, a sample of 100 μl from TCF or fluid from processed coverslips was inoculated onto the agar plates, and the plates were incubated at 37°C for 48 h. Results were interpreted as positive (some macroscopic growth) or negative (no growth).
For the particular case of daptomycin, resistant strains obtained from screening agar plates were recovered following standard methodology (3) to determine the MIC.
Statistical analysis.
All bacterial counts are presented as log CFU/ml (means ± standard deviations [SD]). Differences in bacterial counts between treated and untreated animals were evaluated for statistical significance by using analysis of variance. An unpaired Student t test with the Bonferroni correction was used to determine statistical significance. For all tests, differences were considered to be statistically significant when P values were <0.05.
RESULTS
In vitro time-kill studies.
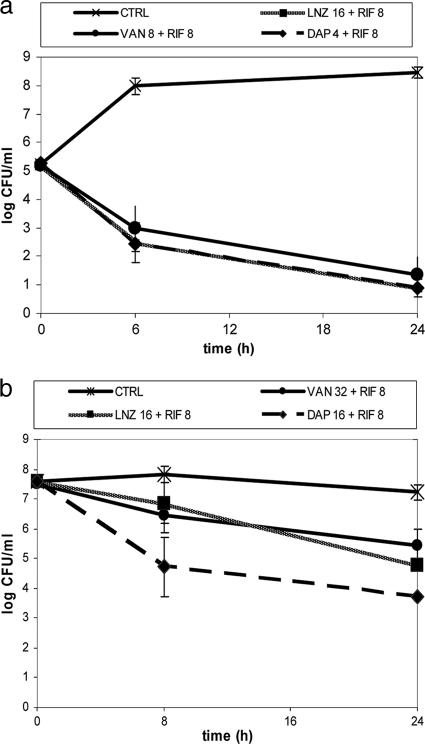
In log phase, the addition of rifampin to bactericidal concentrations of daptomycin delayed bacterial killing at 6 h but not at 24 h with respect to killing achieved with daptomycin alone. When subinhibitory or nonbactericidal concentrations of daptomycin were combined with rifampin, this delay was not observed. The combinations of rifampin with vancomycin and linezolid showed bactericidal activity, and in both cases this efficacy was similar to that of rifampin alone.
In stationary phase, daptomycin-rifampin was bactericidal at a greater range of daptomycin concentrations (≥16× MIC), thus improving the efficacy of each antibiotic alone and exerting an additive effect. The vancomycin-rifampin and linezolid-rifampin combinations were not bactericidal, although both showed an additive effect.
The most representative results of kill curves in log and stationary phases with antibiotics in combination with rifampin are shown in Fig. 1.
FIG. 1.
Time-kill curves in log (a) and stationary (b) phases with various antibiotics in combination with rifampin. Data for antibiotics alone are not shown. Concentrations are given in μg/ml. Error bars indicate standard deviations. LNZ, linezolid; VAN, vancomycin; RIF, rifampin; DAP, daptomycin.
Animal studies.
One hundred animals were used (200 tissue cages); as some tissue cages were lost due to spontaneous shedding, at the beginning of experiments we used 188 valid tissue cages. There were no significant differences between the groups on day 1 (beginning of the treatment). The counts (mean log CFU/ml ± SD) were as follows: 5.70 ± 0.96 for animals treated with linezolid (n = 17 tissue cages), 5.97 ± 1.25 for those treated with vancomycin (n = 17), 5.50 ± 1.06 for those treated with rifampin (n = 20), 6.15 ± 1.1 for those treated with daptomycin at 100 mg/kg/day (n = 15), 5.73 ± 1.05 for those treated with daptomycin at 45 mg/kg/day (n = 25), 5.67 ± 0.99 for those treated with linezolid plus rifampin (n = 20), 5.61 ± 1.18 for those treated with vancomycin plus rifampin (n = 20), 5.40 ± 0.80 for those treated with daptomycin at 100 mg/kg/day plus rifampin (n = 17), 5.60 ± 1.07 for those treated with daptomycin at 45 mg/kg/day plus rifampin (n = 18), and 6.12 ± 1.05 for controls (n = 19).
Efficacy of antibiotics at day 8.
All therapeutic groups performed significantly better than controls (P < 0.05). The addition of rifampin improved the efficacy of each antibiotic alone (P < 0.05). The daptomycin-rifampin groups were the most effective treatments (P < 0.05 versus all other combinations). Linezolid-rifampin and vancomycin-rifampin were as effective as rifampin alone.
The decreases in bacterial counts in TCF at day 8 are shown in Table 1.
TABLE 1.
Decreases in bacterial counts from TCF at day 8 and day 11 and bacterial counts recovered from CVs at day 11
| Therapy regimen or groupa | Bacterial count, mean log CFU/ml ± SD (no. of samples)b |
||
|---|---|---|---|
| TCF |
CVs | ||
| Day 8 | Day 11 | ||
| Monotherapies | |||
| RIF | −2.59 ± 0.91 (20)** | −2.75 ± 1.35 (19)* | 1.69 ± 1.26 (19)* |
| DAP100 | −3.14 ± 0.74 (15)** | −3.59 ± 0.49 (15)*** | 1.88 ± 0.92 (15) |
| DAP45 | −2.54 ± 1.21 (25)* | −2.71 ± 1.56 (22)* | 2.11 ± 1.41 (22) |
| Combination therapies | |||
| LNZ+RIF | −2.38 ± 1.17 (20) | −3.23 ± 1.45 (19) | 1.76 ± 1.27 (19) |
| VAN+RIF | −2.62 ± 1.19 (20) | −3.73 ± 1.48 (20) | 1.23 ± 0.52 (20) |
| DAP100+RIF | −4.57 ± 0.69 (17)†† | −4.58 ± 0.68 (17)†† | 0.95 ± 0.13 (17)†† |
| DAP45+RIF | −4.21 ± 0.99 (18)†† | −4.38 ± 0.92 (18)† | 0.91 ± 0.32 (18)†† |
| Control | 0.66 ± 1.24 (19) | 1.14 ± 1.16 (11) | 5.58 ± 0.97 (11) |
RIF, rifampin; DAP100, daptomycin at 100 mg/kg/day; DAP45, daptomycin at 45 mg/kg/day; LNZ, linezolid; VAN, vancomycin.
Data for vancomycin and linezolid alone are not shown. All therapeutic groups performed significantly better than controls (P < 0.05). Among monotherapies, *, P < 0.05 versus linezolid; **, P < 0.05 versus linezolid and vancomycin; and ***, P < 0.05 versus linezolid, vancomycin, rifampin, and daptomycin at 45 mg/kg/day. Among combination therapies, †, P < 0.05 versus linezolid-rifampin, and ††, P < 0.05 versus linezolid-rifampin and vancomycin-rifampin.
Efficacy of antibiotics at day 11.
At day 11, the efficacies for all therapeutic groups were greater than those at day 8 and better than those for controls (P < 0.05). Daptomycin at 100 mg/kg/day showed a greater reduction in bacterial counts than daptomycin at 45 mg/kg/day and other monotherapies (P < 0.05). The efficacy of linezolid-rifampin was similar to that of rifampin alone, whereas the remaining combinations achieved greater killing than rifampin alone (P < 0.05). Daptomycin at 100 mg/kg/day plus rifampin had greater efficacy than vancomycin-rifampin and linezolid-rifampin (P < 0.05), whereas daptomycin at 45 mg/kg/day plus rifampin achieved a greater reduction in bacterial counts than only linezolid-rifampin (P < 0.05). All drug combinations prevented the emergence of resistance to rifampin.
In the CV assessment, all therapeutic groups performed significantly better than controls (P < 0.05). Both dosages of daptomycin plus rifampin showed the lowest bacterial counts and were significantly better than linezolid-rifampin and vancomycin-rifampin (P < 0.05).
The decreases in the bacterial counts from TCF at day 11 and the bacterial counts recovered from CVs are shown in Table 1.
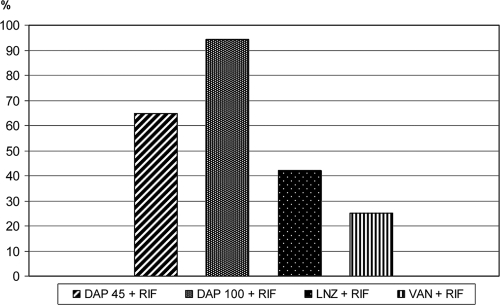
The overall cure rates from the TCF and the coverslips were evaluated at day 11. The cure rate from TCF showed that all antibiotics in combination with rifampin showed significantly better cure rates than controls (P < 0.05). Daptomycin at 100 mg/kg/day plus rifampin achieved the best cure rate (94%), and it was also significantly better than that for daptomycin at 45 mg/kg/day plus rifampin (64%) and that for linezolid-rifampin (42%). Both daptomycin-rifampin combinations were significantly better than vancomycin-rifampin (cure rate of 25%) (Fig. 2). The cure rates from coverslips showed similar results, but there were no significant differences between the two dosages of daptomycin in combination with rifampin.
FIG. 2.
Cure rates of infection for antibiotic combinations with rifampin at day 11. Data for antibiotics alone are not shown. LNZ, linezolid; VAN, vancomycin; RIF, rifampin; DAP45, daptomycin at 45 mg/kg/day; DAP100, daptomycin at 100 mg/kg/day.
Resistance studies.
Among monotherapies, we did not find resistant strains with linezolid, vancomycin, or daptomycin at 100 mg/kg/day, but resistance emerged with rifampin and daptomycin at 45 mg/kg/day.
All cases of daptomycin-resistant strains in the group treated with daptomycin at 45 mg/kg/day showed a MIC of 2 μg/ml (a 2-fold increase with respect to that for the wild-type strain). Results for the presence of resistant strains in TCF and on coverslips at the end of the therapy (days 8 and 11) are shown in Table 2.
TABLE 2.
Results from screening bacteria from TCF and coverslips for the presence of resistant strains at the end of the therapy (days 8 and 11)
| Therapy regimena | No. of resistant strains/total no. of strains screened |
||
|---|---|---|---|
| TCF |
CVs | ||
| Day 8 | Day 11 | ||
| Monotherapies | |||
| LNZ | 0/17 | 0/16 | 0/16 |
| VAN | 0/17 | 0/17 | 0/17 |
| RIF | 5/20 | 3/19 | 2/19 |
| DAP100 | 0/15 | 0/15 | 0/15 |
| DAP45 | 0/25 | 2/22 | 1/22 |
| Combination therapies | |||
| LNZ+RIF | 0/20 | 0/19 | 0/19 |
| VAN+RIF | 0/20 | 0/20 | 0/20 |
| DAP100+RIF | 0/17 | 0/17 | 0/17 |
| DAP45+RIF | 0/18 | 0/18 | 0/18 |
LNZ, linezolid; VAN, vancomycin; RIF, rifampin; DAP100, daptomycin at 100 mg/kg/day; DAP45, daptomycin at 45 mg/kg/day.
All rifampin combinations protected against the appearance of resistance to each drug included in the combined therapy.
DISCUSSION
The present study focused on the efficacies of usual and high doses of daptomycin administered alone and in combination with rifampin against foreign-body infection by MRSA.
Daptomycin was the only drug with in vitro bactericidal activity in log and stationary phases; these results corroborate previous reports (14, 19). The differences that we found in the daptomycin-rifampin combination according to the phase of growth require discussion. In log phase, the addition of rifampin delayed bactericidal activity in the first hours with respect to results for daptomycin alone, although the 24-h final efficacies were similar. In contrast, daptomycin-rifampin was the best combination against stationary-phase bacteria and improved the efficacy of either drug alone, showing bactericidal activity over a greater range of daptomycin concentrations.
Previous studies testing the in vitro activity of the daptomycin-rifampin combination have reported controversial results. Most were performed with kill curves and in vitro pharmacodynamic models of endocardial vegetations that used high inocula of bacteria in the log phase of growth. To our knowledge, the efficacy of daptomycin plus rifampin against stationary-phase bacteria has not previously been reported. While a delay in bactericidal killing due to the addition of rifampin has been described, the final efficacy of this combination has been reported variously as indifferent, antagonistic, or synergistic (6, 11, 24). The existing probability of moderate or high bacterial inocula altering the activity of daptomycin and rifampin alone could also be responsible for these differences (17, 22, 33). Our results from the in vitro log phase were performed using a standard low bacterial inoculum (105 CFU/ml), and the effect of the daptomycin-rifampin combination was indifferent. In contrast, this combination was effective in the stationary-phase studies, where high inocula and nutrient restriction were both required, and these in vitro studies have been shown to correlate better with in vivo efficacy against device-related infection (18, 35).
Regarding the in vitro activities of the linezolid-rifampin and vancomycin-rifampin combinations against high bacterial inocula in stationary phase, neither combination achieved a bactericidal effect, although both ensured the efficacy of rifampin and prevented the emergence of rifampin resistance.
Currently, the use of rifampin combinations against device-related staphylococcal infection is recommended (36, 37). However, MRSA antimicrobial susceptibilities limit the number of drugs that can be used. Daptomycin has emerged as a promising anti-MRSA antibiotic with bactericidal efficacy against growing and nongrowing bacteria (14, 30). In the setting of foreign-body infection, the appropriate dosage and the clinical efficacy of daptomycin (alone and in combination) remain poorly defined (8, 23).
In our previous work using the rat tissue cage model, we noted the good efficacy of high doses of daptomycin alone in comparison with the efficacies of other anti-MRSA drugs (19). In the present study, we showed that daptomycin was significantly more effective at high doses (equivalent to 10 mg/kg/day) than at usual doses (6 mg/kg/day) and found that it was even better than rifampin alone. In order to evaluate antimicrobial efficacy in our rat tissue cage model, we used several criteria, including efficacy at day 8 and day 11 in TCF, efficacy against adherent bacteria from coverslips, and cure rate of infection. While bacterial decreases in TCF were always slightly more pronounced at day 11 than at day 8, it is difficult to assume which analysis might be the most suitable to compare antimicrobial efficacies. We think that any difference among therapeutic groups should be taken into account and that the evaluation of all criteria together could offer more-accurate information about efficacy.
Corroborating previous studies, we also noted the appearance of resistance to daptomycin at usual doses but not at higher doses (24). In fact, when the use of antibiotic monotherapy against foreign-body infections by rifampin-resistant MRSA strains is imperative, our results support the use of high doses of daptomycin and also suggest the need for determining the appropriate high dosage of daptomycin in terms of efficacy and safety, since doses up to 12 mg/kg/day have been well tolerated in humans.
Among the combined therapies tested, we first noted that any rifampin combination had greater efficacy than either antibiotic alone. Therefore, our results stress the benefits of adding rifampin in the setting of device-related staphylococcal infections (37). Specifically, with the daptomycin-rifampin combination, this benefit was recently reported by John et al. (10); testing the efficacy of daptomycin at doses equivalent to or less than 6 mg/kg/day in humans, those authors reported the improved efficacy of these daptomycin-rifampin combinations, which proved to be the best treatment for eradicating foreign-body infection. To our knowledge, the present study is the first to compare the efficacies of daptomycin plus rifampin at usual (6 mg/kg/day) and higher doses of daptomycin against foreign-body infection. We noted that the higher dosage of daptomycin improved the final efficacy of this combination and that these combined therapies each ensured protection against drug resistance; overall, once again these results were in accordance with in vitro findings in stationary phase (19, 35).
In contrast to the benefits of daptomycin-rifampin reported in the setting of foreign-body infection, the results obtained with this combination using animal models of endocarditis are more contradictory (15, 26). We think that the use of high inocula and the presence of bacteria mainly in the log phase of growth in these endocarditis models may both contribute to explaining differences with respect to activity against foreign-body infections involving a moderate inoculum (106 CFU/ml) and stationary-phase bacteria. Likewise, concerns regarding the emergence of resistance to daptomycin based on in vitro (“false mutants”) or in vivo studies (22, 28) also seem to be related to high inocula, and resistance has not been detected in these experimental foreign-body infections.
Finally, the daptomycin-rifampin combination had greater efficacy than the vancomycin-rifampin comparator, which can be considered standard therapy against device-related rifampin-susceptible MRSA infections. The linezolid-rifampin combination protected against rifampin resistance and was as effective as the vancomycin-rifampin combination.
In conclusion, the in vitro bactericidal activity of the daptomycin-rifampin combination showed benefits compared to the efficacy of each drug alone against nongrowing bacteria in stationary phase. Daptomycin at high doses (equivalent to 10 mg/kg/day in humans) achieved the best efficacy among monotherapies, and this dosage of daptomycin also improved the efficacy of the combination with rifampin against foreign-body infections by MRSA. Overall, our results support the use of high doses of daptomycin alone or in combination with rifampin against device-related infection caused by rifampin-resistant or -susceptible MRSA strains, respectively, in humans.
Acknowledgments
This work was supported by a research grant from the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III (FIS 08/014); grants from the Spanish Network for the Research in Infectious Diseases (REIPI C03/14 and REIPI RD06/0008); and grants from Pfizer (Spain) and Novartis (Spain). C.G. was supported by a grant from the REIPI.
Footnotes
Published ahead of print on 4 October 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Chapin-Robertson, K., and S. C. Edberg. 1991. Measurements of antibiotics in human body fluids: techniques and significance, p. 295-366. In V. Lorian (ed.), Antibiotics in laboratory medicine. Williams & Wilkins Co., New York, NY.
- 2.Chuard, C., J. C. Lucet, P. Rohner, M. Herrmann, R. Auckenthaler, F. A. Waldvogel, and D. P. Lew. 1991. Resistance of Staphylococcus aureus recovered from infected foreign body in vivo to killing by antimicrobials. J. Infect. Dis. 163:1369-1373. [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2009. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. Approved standard, 8th ed., M7-A8. CLSI, Wayne, PA.
- 4.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 5.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 6.Credito, K., G. Lin, and P. C. Appelbaum. 2007. Activity of daptomycin alone and in combination with rifampin and gentamicin against Staphylococcus aureus assessed by time-kill methodology. Antimicrob. Agents Chemother. 51:1504-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drees, M., and H. Boucher. 2006. New agents for Staphylococcus aureus endocarditis. Curr. Opin. Infect. Dis. 19:544-550. [DOI] [PubMed] [Google Scholar]
- 8.Falagas, M. E., K. P. Giannopoulou, F. Ntziora, and P. J. Papagelopoulos. 2007. Daptomycin for treatment of patients with bone and joint infections: a systematic review of the clinical evidence. Int. J. Antimicrob. Agents 30:202-209. [DOI] [PubMed] [Google Scholar]
- 9.Fowler, V. G., Jr., H. W. Boucher, G. R. Corey, E. Abrutyn, A. W. Karchmer, M. E. Rupp, D. P. Levine, H. F. Chambers, F. P. Tally, G. A. Vigliani, C. H. Cabell, A. S. Link, I. DeMeyer, S. G. Filler, M. Zervos, P. Cook, J. Parsonnet, J. M. Bernstein, C. S. Price, G. N. Forrest, G. Fatkenheuer, M. Gareca, S. J. Rehm, H. R. Brodt, A. Tice, and S. E. Cosgrove. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 355:653-665. [DOI] [PubMed] [Google Scholar]
- 10.John, A. K., D. Baldoni, M. Haschke, K. Rentsch, P. Schaerli, W. Zimmerli, and A. Trampuz. 2009. Efficacy of daptomycin in implant-associated infection due to methicillin-resistant Staphylococcus aureus: importance of combination with rifampin. Antimicrob. Agents Chemother. 53:2719-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaPlante, K. L., and S. Woodmansee. 2009. Activities of daptomycin and vancomycin alone and in combination with rifampin and gentamicin against biofilm-forming methicillin-resistant Staphylococcus aureus isolates in an experimental model of endocarditis. Antimicrob. Agents Chemother. 53:3880-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucet, J. C., M. Herrmann, P. Rohner, R. Auckenthaler, F. A. Waldvogel, and D. P. Lew. 1990. Treatment of experimental foreign body infection caused by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 34:2312-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangili, A., I. Bica, D. R. Snydman, and D. H. Hamer. 2005. Daptomycin-resistant, methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 40:1058-1060. [DOI] [PubMed] [Google Scholar]
- 14.Mascio, C. T., J. D. Alder, and J. A. Silverman. 2007. Bactericidal action of daptomycin against stationary-phase and nondividing Staphylococcus aureus cells. Antimicrob. Agents Chemother. 51:4255-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miro, J. M., C. Garcia-de-la-Maria, Y. Armero, D. Soy, A. Moreno, A. del Rio, M. Almela, M. Sarasa, C. A. Mestres, J. M. Gatell, M. T. Jimenez de Anta, and F. Marco. 2009. Addition of gentamicin or rifampin does not enhance the effectiveness of daptomycin in treatment of experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:4172-4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moellering, R. C., Jr. 2008. Current treatment options for community-acquired methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 46:1032-1037. [DOI] [PubMed] [Google Scholar]
- 17.Moise, P. A., D. North, J. N. Steenbergen, and G. Sakoulas. 2009. Susceptibility relationship between vancomycin and daptomycin in Staphylococcus aureus: facts and assumptions. Lancet Infect. Dis. 9:617-624. [DOI] [PubMed] [Google Scholar]
- 18.Murillo, O., A. Domenech, A. Garcia, F. Tubau, C. Cabellos, F. Gudiol, and J. Ariza. 2006. Efficacy of high doses of levofloxacin in experimental foreign-body infection by methicillin-susceptible Staphylococcus aureus. Antimicrob. Agents Chemother. 50:4011-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murillo, O., C. Garrigos, M. E. Pachon, G. Euba, R. Verdaguer, C. Cabellos, J. Cabo, F. Gudiol, and J. Ariza. 2009. Efficacy of high doses of daptomycin versus alternative therapies against experimental foreign-body infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:4252-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murillo, O., M. E. Pachon, G. Euba, R. Verdaguer, F. Tubau, C. Cabellos, J. Cabo, F. Gudiol, and J. Ariza. 2008. Antagonistic effect of rifampin on the efficacy of high-dose levofloxacin in staphylococcal experimental foreign-body infection. Antimicrob. Agents Chemother. 52:3681-3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. 1999. Methods for determining bactericidal activity of antimicrobial agents. M26-A. NCCLS, Wayne, PA.
- 22.Quinn, B., S. Hussain, M. Malik, K. Drlica, and X. Zhao. 2007. Daptomycin inoculum effects and mutant prevention concentration with Staphylococcus aureus. J. Antimicrob. Chemother. 60:1380-1383. [DOI] [PubMed] [Google Scholar]
- 23.Rice, D. A., and L. Mendez-Vigo. 2009. Daptomycin in bone and joint infections: a review of the literature. Arch. Orthop. Trauma Surg. 129:1495-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rose, W. E., S. N. Leonard, and M. J. Rybak. 2008. Evaluation of daptomycin pharmacodynamics and resistance at various dosage regimens against Staphylococcus aureus isolates with reduced susceptibilities to daptomycin in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 52:3061-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Safdar, N., D. Andes, and W. A. Craig. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob. Agents Chemother. 48:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakoulas, G., G. M. Eliopoulos, J. Alder, and C. T. Eliopoulos. 2003. Efficacy of daptomycin in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:1714-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakoulas, G., and R. C. Moellering, Jr. 2008. Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin. Infect. Dis. 46(Suppl. 5):S360-S367. [DOI] [PubMed] [Google Scholar]
- 28.Skiest, D. J. 2006. Treatment failure resulting from resistance of Staphylococcus aureus to daptomycin. J. Clin. Microbiol. 44:655-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soriano, A., F. Marco, J. A. Martinez, E. Pisos, M. Almela, V. P. Dimova, D. Alamo, M. Ortega, J. Lopez, and J. Mensa. 2008. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46:193-200. [DOI] [PubMed] [Google Scholar]
- 30.Steenbergen, J. N., J. Alder, G. M. Thorne, and F. P. Tally. 2005. Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive infections. J. Antimicrob. Chemother. 55:283-288. [DOI] [PubMed] [Google Scholar]
- 31.Stewart, P. S., and J. W. Costerton. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135-138. [DOI] [PubMed] [Google Scholar]
- 32.Trampuz, A., M. Wenk, Z. Rajacic, and W. Zimmerli. 2000. Pharmacokinetics and pharmacodynamics of levofloxacin against Streptococcus pneumoniae and Staphylococcus aureus in human skin blister fluid. Antimicrob. Agents Chemother. 44:1352-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Udekwu, K. I., N. Parrish, P. Ankomah, F. Baquero, and B. R. Levin. 2009. Functional relationship between bacterial cell density and the efficacy of antibiotics. J. Antimicrob. Chemother. 63:745-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaudaux, P., P. Francois, C. Bisognano, J. Schrenzel, and D. P. Lew. 2002. Comparison of levofloxacin, alatrofloxacin, and vancomycin for prophylaxis and treatment of experimental foreign-body-associated infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 46:1503-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimmerli, W., R. Frei, A. F. Widmer, and Z. Rajacic. 1994. Microbiological tests to predict treatment outcome in experimental device-related infections due to Staphylococcus aureus. J. Antimicrob. Chemother. 33:959-967. [DOI] [PubMed] [Google Scholar]
- 36.Zimmerli, W., A. Trampuz, and P. E. Ochsner. 2004. Prosthetic-joint infections. N. Engl. J. Med. 351:1645-1654. [DOI] [PubMed] [Google Scholar]
- 37.Zimmerli, W., A. F. Widmer, M. Blatter, R. Frei, and P. E. Ochsner. 1998. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA 279:1537-1541. [DOI] [PubMed] [Google Scholar]