Abstract
Raltegravir's divalent metal ion chelating motif may predispose the drug to interactions with divalent cations. We determined whether a divalent cation-containing antacid interacted with raltegravir. Twelve HIV-1-seronegative subjects were enrolled in this randomized, prospective, crossover study of single-dose raltegravir (400 mg) with and without an antacid. Subjects underwent two intensive pharmacokinetic visits in the fasted state separated by a 5- to 12-day washout period. With simultaneous antacid administration, time to peak raltegravir concentration occurred 2 h sooner (P = 0.002) and there was a 67% lower raltegravir concentration at 12 h postdose (P < 0.0001) than with administration of raltegravir alone. The raltegravir area under the-concentration-time curve from 0 to 12 h and maximum concentration were unchanged with the addition of an antacid. Studies are needed to determine the clinical relevance of this interaction, whether it remains after multiple dosing to steady state, whether it is mitigated by temporal separation, and whether raltegravir interacts with divalent cation-containing vitamins, supplements, or foods.
Raltegravir ([RAL] Isentress, Merck & Co., Inc., Whitehouse Station, NJ) is used in combination with other antiretroviral drugs for the treatment of the human immunodeficiency virus (HIV). The drug has demonstrated solid efficacy with minimal toxicities (3, 8, 10, 15) and is therefore a preferred component of antiretroviral regimens for both treatment-naïve and treatment-experienced patients (16).
RAL tablets are dosed as 400 mg twice daily. The marketed poloxamer tablet formulation has highly variable plasma pharmacokinetics. The inter- and intrapatient variability in concentrations at 12 h postdose (C12) were 212% and 122%, respectively, in prior studies (11). Although RAL is approved for use without regard to meals, food does affect exposures of the drug and appears to increase the pharmacokinetic variability (11). Low-fat meals decrease RAL area under the concentration-time curve (AUC) and maximum concentration (Cmax) by approximately 50% with no change in C12, whereas high-fat meals increase AUC and Cmax 2-fold, and C12 is increased 4.1-fold. Moderate-fat meals increase AUC, Cmax, and C12 by 13%, 5%, and 66%, respectively. RAL is metabolized primarily through uridine glucuronosyl transferase (UGT) 1A1 and is not a substrate, inhibitor, or inducer of cytochrome P450 enzymes. RAL concentrations demonstrate a biphasic decline. The alpha and beta half-lives for the drug are approximately 1 and 9 h, respectively, with the alpha half-life accounting for the majority of the AUC in a dosing interval. There are limited concentration-effect data for the drug, but available evidence suggests RAL has a wide therapeutic index.
RAL is an integrase strand transfer inhibitor which prevents incorporation of viral DNA into a host's cellular DNA. RAL contains a divalent metal ion chelating motif which allows the compound to bind to divalent metal cofactors at the integrase active site (4). Though essential for its mechanism of action, RAL's metal ion chelating motif may potentially predispose the drug to interactions with drugs, foods, or supplements which contain divalent metals. Exposures of other structurally similar integrase inhibitors, elvitegravir and S/GSK1349572, were reduced 45% and 74%, respectively, with simultaneous administration of an antacid (13, 14). An additional consideration for RAL, however, is its improved solubility and intestinal absorption with higher pH (5).
A significant portion of HIV-infected patients on antiretroviral therapy takes gastric-acid modifiers including antacids (17). A survey of 200 persons with HIV in the United States found that 77% had taken antacids since initiating antiretroviral therapy, and 32% had taken antacids within the previous month (9). Thus, the primary objective of this study was to determine the effect of a magnesium and aluminum containing antacid on the pharmacokinetics of RAL.
(This study was presented as a poster at the 50th Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, 11 to 15 September 2010, poster A1-2013 [1].)
MATERIALS AND METHODS
Subjects.
This study was approved by the Colorado Multiple Institutional Review Board. All participants provided written informed consent.
Healthy HIV-seronegative men and women between 18 and 60 years, weighing ≥50 kg, and within ±30% of their ideal body weight were eligible. Subjects were instructed not to consume alcohol for 48 h prior to the screening visit, for 24 h preceding the intensive pharmacokinetics study visits, and for 24 h following the completion of the study visits. The following were criteria for exclusion from study participation: allergy/sensitivity to RAL; allergy/sensitivity to antacids; active drug or alcohol abuse or dependence; participation in any investigational drug studies within 30 days prior to study entry; history of or active cardiovascular, renal, hematologic, hepatic, neurologic, gastrointestinal, psychiatric, endocrine, or immunologic disease(s) including chronic illnesses such as hypertension, coronary artery disease, arthritis, diabetes, or any chronic gastrointestinal conditions that might interfere with drug absorption or any medical condition that, in the opinion of the investigator, would interfere with the subject's ability to participate in this protocol; use of investigational, prescription, and over-the-counter medications within 14 days of study entry with the exceptions of aspirin, acetaminophen, ibuprofen, and oral contraceptives; creatine kinase of more than three times the upper limit of normal (ULN); hemoglobin of <11 g/dl; platelet count of <125,000/mm3; white blood cell count of <2,500/mm3; serum creatinine (SCr) of >1.3 mg/dl; serum transaminases (alanine aminotransferase [ALT] or aspartate transaminase [AST]) of >1.25× ULN; and any other laboratory abnormalities greater than or equal to grade 2 in the 2004 Division of AIDS toxicity tables (1). Women who were pregnant or breast feeding and women and men of reproductive potential who were actively engaging in sexual activity or assisted reproductive technology with the intent of pregnancy were also excluded.
Design.
The study was a randomized, crossover study designed to determine the effects of an antacid on the pharmacokinetics of a single dose of RAL in HIV-seronegative subjects. Subjects were admitted to the Clinical Translational Research Center following an 8-h overnight fast and administered a single, observed oral dose of 400 mg of RAL with or without 30 ml of an aluminum, magnesium, and simethicone-containing antacid (Maalox Plus Extra Strength). There are 2.7 g of magnesium hydroxide, 3 g of aluminum hydroxide dried gel, and 240 mg of simethicone in 30 ml of the antacid. Subjects continued to fast for 4 h following study drug(s) administration. Five milliliters of whole blood was obtained at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 24, and 48 h after study drug administration. After a 5- to 12-day washout period, subjects returned for the second intensive pharmacokinetic visit. A single predose blood sample was also drawn at this visit to check for residual RAL in the bloodstream.
Bioanalysis.
Whole blood for determination of RAL was processed by centrifugation, and the plasma was stored (−70°C) within 30 min of collection. RAL plasma concentrations were determined using a validated, reversed-phase liquid chromatography-tandem mass spectrometry (LC/MS/MS) method (Colorado Antiviral Pharmacology Laboratory, University of Colorado Denver, Aurora, CO). This method utilized a stable internal standard uniformly labeled with six 13C carbon atoms on the ring structure of the RAL molecule. An acetonitrile protein precipitation method was used to prepare the samples. The chromatographic separation was performed on a Mac-Mod ACE C18, 2.0- by 50-mm, reversed-phase column with a 3-μm particle size. The mobile phase consisted of an isocratic flow of 50:50 (vol/vol) of acetonitrile-water containing 0.1% formic acid at 0.2 ml/min. The detection and quantitation of RAL and the internal standard were achieved by MS/MS-selective reaction monitoring (SRM) at precursor/product transitions of 445.1/109.1 and 451.1/115.1 m/z, respectively. The assay was linear in the range of 5 ng/ml to 10,000 ng/ml. The assay has a minimum quantifiable limit of 5.00 ng/ml when 0.100 ml of human EDTA plasma is used. Inter- and intraday accuracy and precision were within ±20% at the lower limit of quantification and ±15% at all other concentrations during validation.
Pharmacokinetic analysis.
RAL pharmacokinetics were determined by noncompartmental methods (WinNonLin, version 5.2.1; Pharsight Corporation, Mountain View, CA). A RAL area under the concentration-time curve from 0 to 12 h (AUC0-12) was determined using the linear-log trapezoidal rule. Cmax, time to Cmax (Tmax), and concentration at 12, 24, and 48 h postdose were determined visually.
Safety and tolerability assessments.
Clinical adverse events were assessed during the two 12-h intensive pharmacokinetic visits and at 24 and 48 h postdose. Laboratory tests (complete blood counts, electrolytes, SCr, bilirubin, ALT, and AST) were performed at the screening visit and at 24 h postdose. Urine pregnancy tests (for women of childbearing potential) were performed prior to administering study medication. Clinical and laboratory adverse events were graded by study investigators using the 2004 Division of AIDS toxicity tables (1a), and subjects experiencing a grade 2 or greater clinical or laboratory adverse event were permanently discontinued from the study.
Statistical analysis.
The primary endpoint for this study was the RAL AUC0-12 with versus without an antacid. Secondary endpoints included changes in RAL Cmax, Tmax, and C12 values with versus without an antacid. Twelve subjects provided 82% power to detect a 50% difference in the geometric mean RAL AUC0-12 with versus without an antacid at a significance level of 0.05 using a paired two-sided one-sample t test. AUC0-12, Cmax, and C12 were log transformed to reduce skew, and values were compared (with versus without an antacid) using paired t tests. Geometric mean ratios and the corresponding 90% confidence intervals were determined for interpretation. Tmax values were compared with versus without an antacid using a Wilcoxon signed rank test. No adjustments were made for multiple comparisons. SAS, version 9.2, was used for data analyses.
RESULTS
Subjects.
Seventeen subjects were consented, and 12 completed the study. Two of the subjects consented were ineligible: one had syncope during the screening blood draw, and the other had exclusionary medical conditions. One subject was discontinued due to a grade 2 adverse event (chills). After providing consent, two subjects were discontinued from the study due to conflicting personal schedules. Among the 12 subjects who completed the study, 8 were female and 4 were on oral contraceptives. Ten were Caucasian (seven females and three males), and two were Hispanic (one female and one male). The median (range) age, weight, and height of the subjects were 29 years (19 to 59 years), 61.8 kg (55 to 82.95 kg), and 66.75 in (63.3 to 73.25 in), respectively.
Pharmacokinetics.
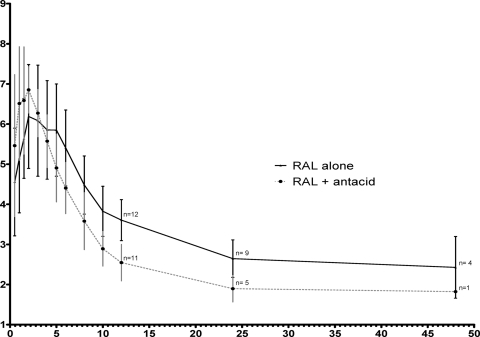
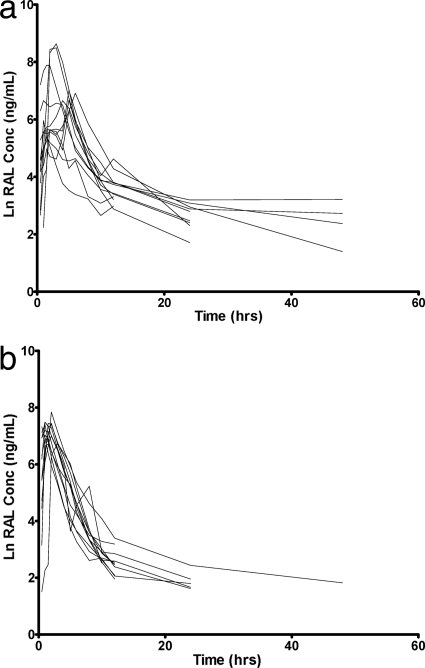
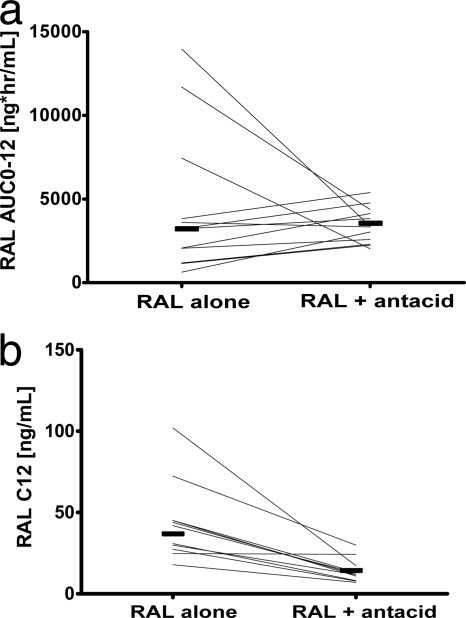
Values of RAL AUC0-12 and Cmax were not significantly different with versus without an antacid, but the presence of the antacid caused an earlier RAL Tmax (1 versus 3 h; P = 0.002) and lower RAL C12 (13 versus 37 ng/ml; P < 0.0001), as shown in Table 1 and Fig. 1. There was a high degree of interindividual variability in concentrations, particularly in the phase without the antacid (Fig. 2), as well as variability in the magnitude of the interaction (Fig. 3a). Eleven of the 12 subjects had a decrease in C12 with addition of an antacid (Fig. 3b). Nine had a C12 of <15 ng/ml, the protein-adjusted 95% inhibitory concentration (IC95) for RAL in the presence of an antacid. Due to the variability in when the concentrations began the second phase of decline and the frequent occurrences of undetectable concentrations after 12 h (Fig. 2), the half-lives were not calculated and compared. Because of this, a partial AUC0-12 is provided in addition to the AUC0-∞. Apparent oral clearance and volume of distribution were not calculated without the half-life estimates.
TABLE 1.
RAL pharmacokinetic parametersa
| Parameter | Value for the parameter |
|||
|---|---|---|---|---|
| RAL alone | RAL with antacid | GMR | P value | |
| Geometric mean AUC0-12 (ng·h/ml [90% CI]) | 3,042 (2,152-4,298) | 3,296 (2,928-3,707) | 1.08 (0.68-1.73) | 0.762 |
| Geometric mean AUC0-∞ (ng·h/ml [90% CI]) | 3,544 (2,298-5,464) | 3,415 (2,922-3,996) | 0.96 (0.62-1.5) | 0.884 |
| Geometric mean Cmax (ng/ml [90% CI]) | 945 (643-1,386) | 1,445 (1,295-1,626) | 1.53 (0.9-2.6) | 0.175 |
| Geometric mean C12 (ng/ml [90% CI]) | 37 (31-44) | 13 (11-15) | 0.33 (0.26-0.42) | <0.0001 |
| Median Tmax (h [range]) | 3 (1-6) | 1 (0.5-3) | −1.75 (−4.0-0.5)b | 0.004 |
CI, confidence interval; GMR, geometric mean ratio of RAL with antacid/RAL alone; AUC0-12, area under the concentration-time curve from 0 to 12 h after the observed dose; AUC0-∞, AUC from 0 to infinity; C12, concentration at 12 h after the observed dose; Tmax, time to maximum concentration.
Not a geometric mean ratio; the median(range) difference between the value for RAL with antacid and RAL alone.
FIG. 1.
Mean (± standard deviations) RAL concentration-time curves with versus without an antacid. The numbers at 12, 24, and 48 h postdose correspond to the number of patients with detectable RAL concentrations at these time points. Values on the y axis represent the natural log of RAL concentrations. Values on the x axis represent times (hours) postdose.
FIG. 2.
Individual RAL concentration-time curves for subjects taking RAL alone (a) and the combination of RAL and antacid (b).
FIG. 3.
(a) Individual change in RAL AUC0-12 values with versus without an antacid. Geometric means are shown as shaded lines. (b) Individual Change in RAL C12 values with versus without an antacid. Geometric means are shown as shaded lines.
Safety and tolerability.
The most commonly reported adverse event was mild headache, reported by three subjects (25%) during treatment with RAL alone and five (42%) during treatment with RAL plus antacid. One subject reported mild dizziness, fever, nausea, and moderate (grade 2) chills 24 h after the first dose of study medication in which a causal relationship to the study or study drug could not be ruled out, and this subject was thus discontinued from the study. None of the enrolled subjects experienced any laboratory events greater than grade 1.
DISCUSSION
This study found that when HIV-seronegative volunteers took a single dose of RAL simultaneously with an antacid, RAL AUC0-12 and Cmax were unchanged, but the time to peak RAL concentration occurred about 2 h sooner, and there was a 67% lower RAL C12 relative to the concentration when RAL was administered alone. For reference, 75% of the subjects had a RAL C12 of less than 15 ng/ml (the 95% inhibitory concentration for the drug in 50% human serum). The mechanism and clinical relevance of this interaction are unclear.
Previous studies with structurally similar integrase inhibitors found decreases in the exposures of these drugs when antacids were simultaneously administered, presumably due to the binding of the integrase inhibitors to divalent cations in the antacid. S/GSK1349572 AUC0-∞, Cmax, and concentration at 24 h postdose (C24) were reduced 74% with an antacid versus treatment with drug alone (14). Similarly, the elvitegravir AUC0-24, Cmax, and C24 were decreased approximately 45% when the drug was administered simultaneously with an antacid (13). In contrast, we found no difference in RAL exposures or Cmax values with versus without an antacid but a drop in RAL C12. We also observed a faster RAL Tmax and apparently less variability in AUC with the addition of an antacid, which was not observed in studies with S/GSK1349572 or elvitegravir and supports a previous report that RAL is preferentially absorbed at a higher gastric pH. (5) Administration of RAL 2 h after a 20-mg dose of the proton pump inhibitor omeprazole, increased RAL AUC0-∞, Cmax, and C12 by 3-, 4-, and 1.5-fold, respectively (5). Based on the findings in the present study and the observations above, antacids may have two effects on RAL. First, they might cause a faster and more uniform dissolution and intestinal absorption which may, in effect, shift the RAL concentration-time curve to the left (Fig. 1). However, instead of causing a higher AUC with the higher pH, the antacid may also bind RAL, offsetting the potential for an increase in extent of absorption (shown by the similar AUC). Indeed, we did observe a faster first-order RAL absorption constant (Ka) in the presence of antacid using a two-compartment model with WinNonLin (data not shown).
It is unknown if the reduction in RAL C12 due to antacids would compromise the antiviral efficacy of the drug. There are limited concentration-effect data with RAL, but an in vitro (18) and an in vivo (19) study suggest that overall exposure may correlate better with efficacy than troughs. Treatment response at 16 weeks was not reduced in 16 patients, with RAL C12 values less than 15 ng/ml in phase II and III trials (20), but patients with C12 values below the limits of assay detection (2 ng/ml for the Merck assay [12]) were excluded from this analysis. At 48 weeks, RAL minimum concentration, but not RAL C12, was associated with treatment response, but a geometric mean of several concentrations per patient (n = 457 patients) seemed to correlate better with efficacy. Investigators found that the odds of having an HIV-1 RNA of less than 50 copies/ml after 48 weeks of treatment were significantly (P = 0.002) higher in subjects with an average RAL concentration of 213 ng/ml than in those with an average concentration of 152 ng/ml (19). However, baseline viral load and use of darunavir/ritonavir were more strongly associated with treatment response than RAL drug concentrations. Though it has been suggested that intracellular RAL concentrations may better predict therapeutic outcomes, a pilot study of 10 patients suggested that plasma and intracellular concentrations are highly correlated (2).
There are limitations to this study which restrict the ability to generalize with regard to HIV-infected patients receiving RAL as part of their antiretroviral regimens. First, this interaction was evaluated following a single dose of RAL. It is unknown whether the same findings would be observed if the RAL were dosed to steady state. Studies with the lactose formulation of RAL in HIV-seronegative male volunteers suggest that the C12 is 2.5 times higher at steady state than with a single dose (97 versus 39 ng/ml, respectively) (6). Similarly, the geometric mean RAL C12 at steady state in phase II/III studies (HIV-infected volunteers) following unobserved dosing was 128 ng/ml (19). Second, this interaction was evaluated in a small number of HIV-seronegative volunteers. RAL pharmacokinetics is highly variable, so results may differ if a larger number of subjects were studied. Third, the magnitude of this interaction may also differ in persons with HIV if gastric pH is affected by the disease itself, comorbidities, or other medications. Lastly, the clinical implications of the interaction may also change, depending upon other antiretroviral medications in a patient's regimen. Specifically, the lower RAL C12 with coadministration of antacids may have more clinical significance when RAL is used with etravirine, tipranavir/ritonavir, or efavirenz, which lower RAL levels, than with tenofovir, atazanavir, and atazanavir/ritonavir, which raise RAL levels (11).
RAL offers many therapeutic advantages as a component of antiretroviral regimens because it is efficacious, well tolerated, and has fewer drug interactions than many other agents used in the treatment of HIV. It is not completely devoid of interactions, however, as demonstrated in this study with an antacid. The RAL prescribing information states that “coadministration of medicinal products that increase gastric pH (e.g., omeprazole) may increase RAL levels based on increased RAL solubility at higher pH” (11). While this may be the case for H2 blockers and proton pump inhibitors, it does not appear to be the case for antacids. Additional studies are needed to determine the clinical relevance of this interaction, whether it remains after multiple dosing to steady state, whether it can be mitigated by temporal separation, and whether similar interactions occur with divalent cation-containing vitamins, supplements, or foods. In the absence of such studies, providers should caution patients on the use of antacids with RAL.
Acknowledgments
This work was supported by the Colorado Clinical Translational Sciences Institute (1UL1 RR025780).
We acknowledge the study participants, the nurses and staff of the University of Colorado Hospital Clinical Translational Research Center, and Merck Laboratories for supplying raltegravir.
Footnotes
Published ahead of print on 4 October 2010.
REFERENCES
- 1.Bumpass, J. B., R. Brundage, A. Meditz, P. L. Anderson, L. Bushman, M. Ray, J. Predhomme, S. MaWhinney, and J. J. Kiser. 2010. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., Boston, MA, poster A1-2013. [DOI] [PMC free article] [PubMed]
- 1a.Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. 2004. Division of AIDS table for grading the severity of adult and pediatric adverse events. National Institutes of Health, Bethesda, MD.
- 2.Fayet-Mello, A., T. Buclin, C. Franc, S. Colombo, S. Cruchon, N. Guignard, J. Biollaz, A. Telenti, L. A. Decosterd, and M. Cavassini. 2010. Intracellular and plasma pharmacokinetics of raltegravir in HIV-infected patients, abstr. 614. Abstr. 17th Conf. Retrovir. Oppor. Infect., San Francisco, CA, 16 to 19 February 2010.
- 3.Grinsztejn, B., B. Y. Nguyen, C. Katlama, J. M. Gatell, A. Lazzarin, D. Vittecoq, C. J. Gonzalez, J. Chen, C. M. Harvey, and R. D. Isaacs. 2007. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet 369:1261-1269. [DOI] [PubMed] [Google Scholar]
- 4.Hazuda, D., M. Iwamoto, and L. Wenning. 2009. Emerging pharmacology: inhibitors of human immunodeficiency virus integration. Annu. Rev. Pharmacol. Toxicol. 49:377-394. [DOI] [PubMed] [Google Scholar]
- 5.Iwamoto, M., L. A. Wenning, B. Y. Nguyen, H. Teppler, A. R. Moreau, R. R. Rhodes, W. D. Hanley, B. Jin, C. M. Harvey, S. A. Breidinger, N. Azrolan, H. F. Farmer, Jr., R. D. Isaacs, J. A. Chodakewitz, J. A. Stone, and J. A. Wagner. 2009. Effects of omeprazole on plasma levels of raltegravir. Clin. Infect. Dis. 48:489-492. [DOI] [PubMed] [Google Scholar]
- 6.Iwamoto, M., L. A. Wenning, A. S. Petry, M. Laethem, M. De Smet, J. T. Kost, S. A. Merschman, K. M. Strohmaier, S. Ramael, K. C. Lasseter, J. A. Stone, K. M. Gottesdiener, and J. A. Wagner. 2008. Safety, tolerability, and pharmacokinetics of raltegravir after single and multiple doses in healthy subjects. Clin. Pharmacol. Ther. 83:293-299. [DOI] [PubMed] [Google Scholar]
- 7.Reference deleted.
- 8.Lennox, J. L., E. DeJesus, A. Lazzarin, R. B. Pollard, J. V. Madruga, D. S. Berger, J. Zhao, X. Xu, A. Williams-Diaz, A. J. Rodgers, R. J. Barnard, M. D. Miller, M. J. DiNubile, B. Y. Nguyen, R. Leavitt, and P. Sklar. 2009. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 374:796-806. [DOI] [PubMed] [Google Scholar]
- 9.Luber, A., V. Garg, S. Gharakhanian, and the Vertex HIV Program Team. 2004. Survey of medication used by HIV-infected patients that affect gastrointestinal (GI) acidity and potential for negative drug interactions with HAART, abstr. 206. Abstr. 7th Int. Conf. Drug Ther. HIV Infect., Glasgow, United Kingdom, 14 to 18 November 2008.
- 10.Markowitz, M., J. O. Morales-Ramirez, B. Y. Nguyen, C. M. Kovacs, R. T. Steigbigel, D. A. Cooper, R. Liporace, R. Schwartz, R. Isaacs, L. R. Gilde, L. Wenning, J. Zhao, and H. Teppler. 2006. Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment-naive HIV-1-infected individuals. J. Acquir. Immune Defic. Syndr. 43:509-515. [DOI] [PubMed] [Google Scholar]
- 11.Merck. 2010. Isentress product information. Merck and Co., Whitehouse Station, NJ.
- 12.Merschman, S. A., P. T. Vallano, L. A. Wenning, B. K. Matuszewski, and E. J. Woolf. 2007. Determination of the HIV integrase inhibitor, MK-0518 (raltegravir), in human plasma using 96-well liquid-liquid extraction and HPLC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 857:15-24. [DOI] [PubMed] [Google Scholar]
- 13.Ramanathan, S., G. Shen, J. Hinkle, J. Enejosa, and B. P. Kearney. 2007. Pharmacokinetic evaluation of drug interactions with ritonavir-boosted HIV integrase inhibitor GS-9137 (elvitegravir) and acid-reducing agents, abstr. 69. Eighth Int. Workshop Clin. Pharmacol. HIV Ther., Budapest, Hungary, 16 to 18 April 2007.
- 14.Song, I., A. Patel, S. Min, Y. Lou, S. Chen, P. Patel, T. Wajima, and S. Piscitelli. 2009. Evaluation of antacid and multivitamin (MVI) effects on S/GSK1349572 pharmacokinetics in healthy subjects, abstr. A1-1305. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother.
- 15.Steigbigel, R. T., D. A. Cooper, P. N. Kumar, J. E. Eron, M. Schechter, M. Markowitz, M. R. Loutfy, J. L. Lennox, J. M. Gatell, J. K. Rockstroh, C. Katlama, P. Yeni, A. Lazzarin, B. Clotet, J. Zhao, J. Chen, D. M. Ryan, R. R. Rhodes, J. A. Killar, L. R. Gilde, K. M. Strohmaier, A. R. Meibohm, M. D. Miller, D. J. Hazuda, M. L. Nessly, M. J. DiNubile, R. D. Isaacs, B. Y. Nguyen, and H. Teppler. 2008. Raltegravir with optimized background therapy for resistant HIV-1 infection. N. Engl. J. Med. 359:339-354. [DOI] [PubMed] [Google Scholar]
- 16.U. S. Department of Health and Human Services. 2009. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Department of Health and Human Services, Washington, DC. http://www.aidsinfo.nih.gov/Contentfiles/AdultandAdolescentGL.pdf.
- 17.van Lunzen, J., H. Liess, K. Arasteh, R. Walli, B. Daut, and D. Schurmann. 2007. Concomitant use of gastric acid-reducing agents is frequent among HIV-1-infected patients receiving protease inhibitor-based highly active antiretroviral therapy. HIV Med. 8:220-225. [DOI] [PubMed] [Google Scholar]
- 18.Weng, Q., R. Kulaway, J. J. McSharry, and G. L. Drusano. 2009. Dose range and dose fractionation studies for raltegravir pharmacodynamics in an in vitro hollow fiber model infection system, abstr. O-09. Tenth Int. Workshop Clin. Pharmacol. HIV Ther., Amsterdam, Netherlands, 15 to 17 April 2009.
- 19.Wenning, L., E. Hwang, B.-Y. Nguyen, H. Teppler, R. Danovich, M. Iwamoto, J. A. Wagner, D. Panebianco, and J. Stone. 2008. Pharmacokinetic/pharmacodynamic (PK/PD) analyses for raltegravir (RAL) in phase III studies in treatment-experienced HIV-infected patients following 48 weeks of treatment, abstr. H-4054. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 20.Wenning, L. A., B. Y. Nguyen, X. Sun, E. Hwang, Y. Chen, H. Teppler, C. Harvey, R. Rhodes, D. Ryan, N. Azrolan, and J. Stone. 2008. Pharmacokinetic/pharmacodynamic (PK/PD) analyses for raltegravir in phase II and III studies in treatment experienced HIV-infected patients, abstr. 021. Ninth Int. Workshop Clin. Pharmacol. HIV Ther., New Orleans, LA, 8 to 10 April 2008.