Abstract
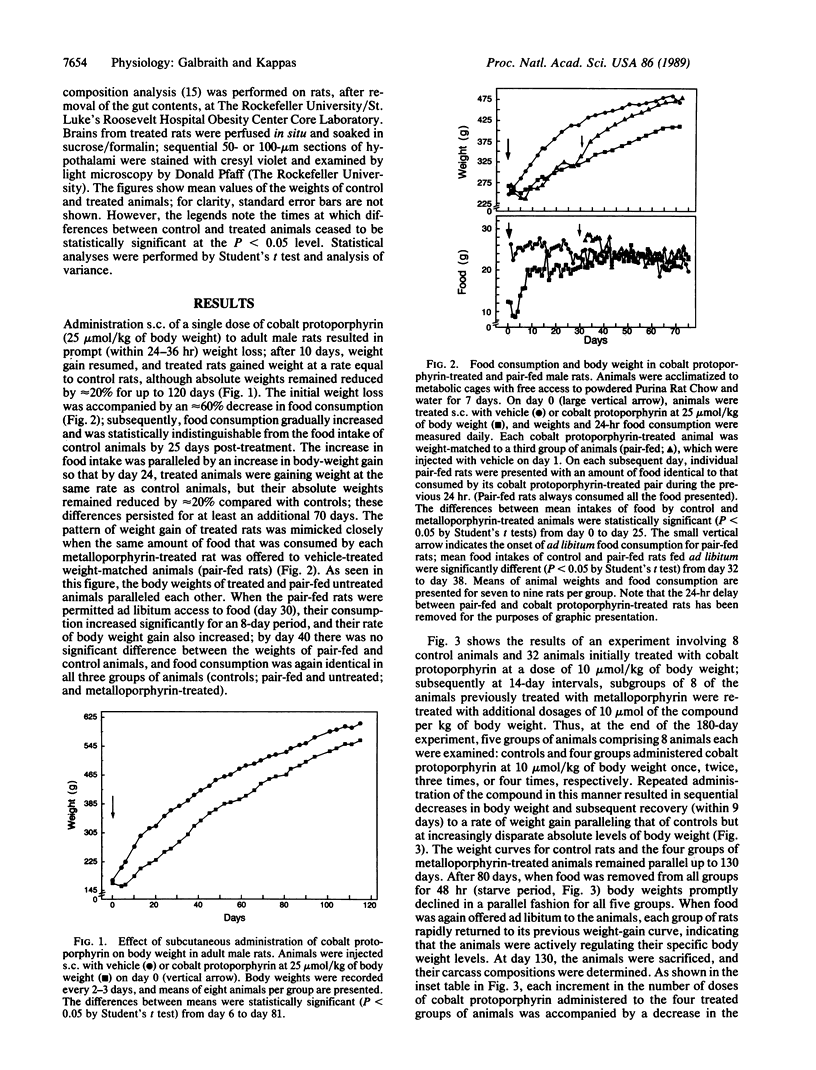
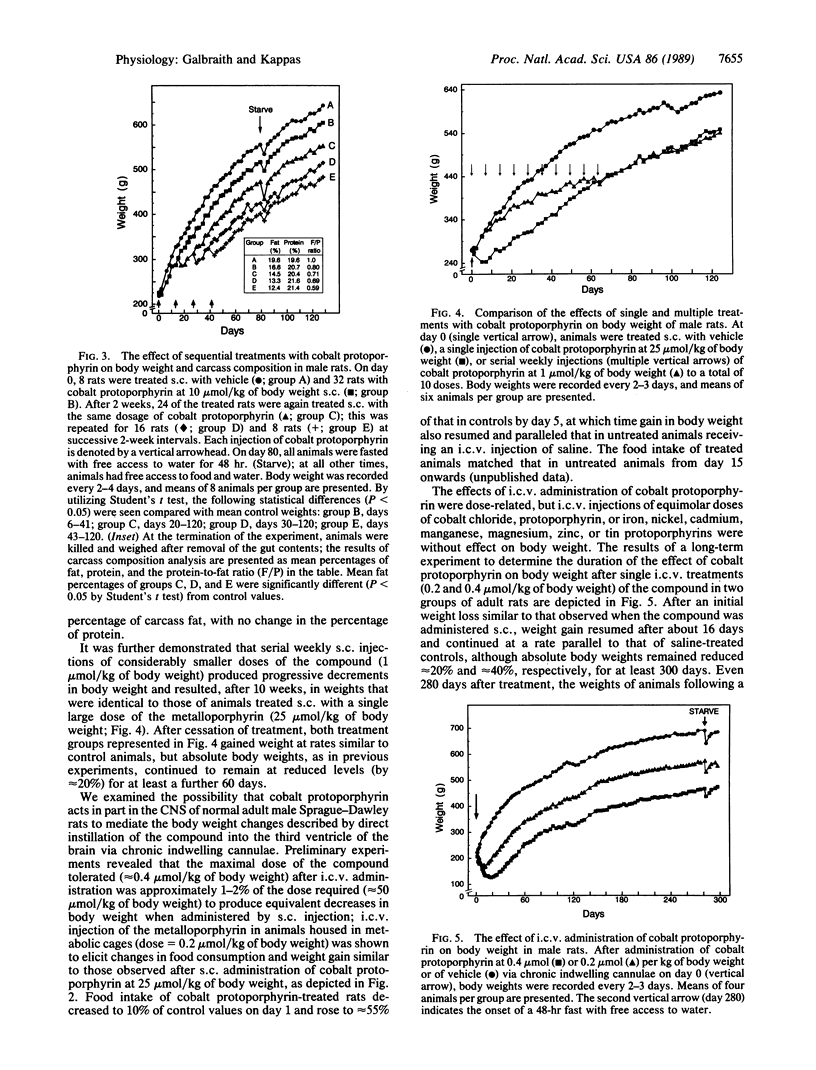
Cobalt-substituted protoporphyrin administered subcutaneously to normal adult rats elicited prompt decreases in food intake and sustained decreases in body weight. Repetitive parenteral administration of small doses of this synthetic heme analogue resulted in dose-related diminutions of carcass fat content without changes in carcass protein content. Direct injection of the compound into the third ventricle of the brain produced changes in food intake and body weight that were quantitatively similar to those observed after parenteral treatment but required only 1-2% of the parenteral dose. The effects of intracerebroventricularly administered cobalt protoporphyrin on body weight were dose-related and were not produced by inorganic cobalt, heme, and a number of other metal-substituted protoporphyrins. Differential body weights between control and treated animals persisted for at least 300 days after intracerebroventricular injections of a single dose (0.2 or 0.4 mumol/kg of body weight) of the compound. Similar effects were observed after subcutaneous administration of the metalloporphyrin to genetically obese Zucker (fa/fa) rats and normal and genetically obese (ob/ob) mice as well as chickens and dogs. Cobalt-substituted mesoporphyrin elicited comparable effects on food intake and body weight. The results of these studies define a new biological action of cobalt protoporphyrin and demonstrate that this and certain other cobalt porphyrins can act, at least in part, in the central nervous system to regulate appetite and to produce long-sustained diminutions in body weight and carcass content of fat in animals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAND B. K., BROBECK J. R. Hypothalamic control of food intake in rats and cats. Yale J Biol Med. 1951 Nov;24(2):123–140. [PMC free article] [PubMed] [Google Scholar]
- ANAND B. K., DUA S., SHOENBERG K. Hypothalamic control of food intake in cats and monkeys. J Physiol. 1955 Jan 28;127(1):143–152. doi: 10.1113/jphysiol.1955.sp005244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger L. L., Williams F. E. Aphagia and adipsia after kainic acid lesioning of the dorsomedial hypothalamic area. Am J Physiol. 1983 Mar;244(3):R389–R399. doi: 10.1152/ajpregu.1983.244.3.R389. [DOI] [PubMed] [Google Scholar]
- Berglund L., Angelin B., Blomstrand R., Drummond G., Kappas A. Sn-protoporphyrin lowers serum bilirubin levels, decreases biliary bilirubin output, enhances biliary heme excretion and potently inhibits hepatic heme oxygenase activity in normal human subjects. Hepatology. 1988 May-Jun;8(3):625–631. doi: 10.1002/hep.1840080331. [DOI] [PubMed] [Google Scholar]
- Corbett S. W., Keesey R. E. Energy balance of rats with lateral hypothalamic lesions. Am J Physiol. 1982 Apr;242(4):E273–E279. doi: 10.1152/ajpendo.1982.242.4.E273. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Molliver M. E., Kuhar M. J. In situ injection of kainic acid: a new method for selectively lesioning neural cell bodies while sparing axons of passage. J Comp Neurol. 1978 Jul 15;180(2):301–323. doi: 10.1002/cne.901800208. [DOI] [PubMed] [Google Scholar]
- Drummond G. S., Galbraith R. A., Sardana M. K., Kappas A. Reduction of the C2 and C4 vinyl groups of Sn-protoporphyrin to form Sn-mesoporphyrin markedly enhances the ability of the metalloporphyrin to inhibit in vivo heme catabolism. Arch Biochem Biophys. 1987 May 15;255(1):64–74. doi: 10.1016/0003-9861(87)90294-3. [DOI] [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. Chemoprevention of neonatal jaundice: potency of tin-protoporphyrin in an animal model. Science. 1982 Sep 24;217(4566):1250–1252. doi: 10.1126/science.6896768. [DOI] [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. Prevention of neonatal hyperbilirubinemia by tin protoporphyrin IX, a potent competitive inhibitor of heme oxidation. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6466–6470. doi: 10.1073/pnas.78.10.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. The cytochrome P-450-depleted animal: an experimental model for in vivo studies in chemical biology. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2384–2388. doi: 10.1073/pnas.79.7.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith R. A., Drummond G. S., Krey L., Kappas A. Relationship of suppression of the androgenic axis by cobalt-protoporphyrin to its effects on weight loss and hepatic heme oxygenase induction. Pharmacology. 1987;34(5):241–249. doi: 10.1159/000138275. [DOI] [PubMed] [Google Scholar]
- Galbraith R. A., Jellinck P. H. Cobalt-protoporphyrin causes prolonged inhibition of catechol estrogen synthesis by rat liver microsomes. Biochem Biophys Res Commun. 1987 May 29;145(1):376–383. doi: 10.1016/0006-291x(87)91332-5. [DOI] [PubMed] [Google Scholar]
- Galbraith R. A., Jellinck P. H. Cobalt-protoporphyrin, a synthetic heme analogue, feminizes hepatic androgen metabolism in the rat. J Steroid Biochem. 1989 Mar;32(3):421–426. doi: 10.1016/0022-4731(89)90216-1. [DOI] [PubMed] [Google Scholar]
- Galbraith R. A., Kappas A. Pharmacokinetics of tin-mesoporphyrin in man and the effects of tin-chelated porphyrins on hyperexcretion of heme pathway precursors in patients with acute inducible porphyria. Hepatology. 1989 Jun;9(6):882–888. doi: 10.1002/hep.1840090616. [DOI] [PubMed] [Google Scholar]
- Galbraith R. A., Krey L. C. Cobalt-protoporphyrin suppresses testosterone secretion by multiple interactions within the brain-pituitary-testicular axis. Neuroendocrinology. 1989 Jun;49(6):641–648. doi: 10.1159/000125181. [DOI] [PubMed] [Google Scholar]
- Hirsch J. The regulation of food intake. Discussion. Adv Psychosom Med. 1972;7:229–242. [PubMed] [Google Scholar]
- Kappas A., Drummond G. S. Control of heme metabolism with synthetic metalloporphyrins. J Clin Invest. 1986 Feb;77(2):335–339. doi: 10.1172/JCI112309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappas A., Drummond G. S., Manola T., Petmezaki S., Valaes T. Sn-protoporphyrin use in the management of hyperbilirubinemia in term newborns with direct Coombs-positive ABO incompatibility. Pediatrics. 1988 Apr;81(4):485–497. [PubMed] [Google Scholar]
- Keesey R. E., Boyle P. C., Storlien L. H. Food intake and utilization in lateral hypothalamically lesioned rats. Physiol Behav. 1978 Aug;21(2):265–268. doi: 10.1016/0031-9384(78)90051-3. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N., Powley T. L. Set points for body weight and fat. Behav Biol. 1977 Jun;20(2):205–223. doi: 10.1016/s0091-6773(77)90773-8. [DOI] [PubMed] [Google Scholar]
- Powley T. L., Keesey R. E. Relationship of body weight to the lateral hypothalamic feeding syndrome. J Comp Physiol Psychol. 1970 Jan;70(1):25–36. doi: 10.1037/h0028390. [DOI] [PubMed] [Google Scholar]
- Presta E., Yang M. U., Segal K. R., Bjorntorp P. Energy depot replenishment in rats during refeeding after fasting: effect of exercise. Am J Clin Nutr. 1984 Nov;40(5):1011–1016. doi: 10.1093/ajcn/40.5.1011. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Drummond G. S., Kappas A. Cobalt-protoporphyrin suppresses thyroid and testicular hormone concentrations in rat serum: a novel action of this synthetic heme analogue. Pharmacology. 1987;34(1):9–16. doi: 10.1159/000138242. [DOI] [PubMed] [Google Scholar]
- Stricker E. M., Swerdloff A. F., Zigmond M. J. Intrahypothalamic injections of kainic acid produce feeding and drinking deficits in rats. Brain Res. 1978 Dec 15;158(2):470–473. doi: 10.1016/0006-8993(78)90692-3. [DOI] [PubMed] [Google Scholar]
- TEITELBAUM P., EPSTEIN A. N. The lateral hypothalamic syndrome: recovery of feeding and drinking after lateral hypothalamic lesions. Psychol Rev. 1962 Mar;69:74–90. doi: 10.1037/h0039285. [DOI] [PubMed] [Google Scholar]
- Van den Pol A. N. Lateral hypothalamic damage and body weight regulation: role of gender, diet, and lesion placement. Am J Physiol. 1982 Mar;242(3):R265–R274. doi: 10.1152/ajpregu.1982.242.3.R265. [DOI] [PubMed] [Google Scholar]


