Abstract
Tissue penetration of systemic antibiotics is an important consideration for positive outcomes in diabetic patients. Herein we describe the exposure profile and penetration of tigecycline in the interstitial fluid of wound margins versus that of uninfected thigh tissue in 8 adult diabetic patients intravenously (IV) administered 100 mg and then 50 mg of tigecycline twice daily for 3 to 5 doses. Prior to administration of the first dose, 2 microdialysis catheters were inserted into the subcutaneous tissue, the first within 10 cm of the wound margin and the second in the thigh of the same extremity. Samples for determination of plasma and tissue concentrations were simultaneously collected over 12 h under steady-state conditions. Tissue concentrations were corrected for percent in vivo recovery by the retrodialysis technique. Plasma samples were also collected for determination of protein binding at 1, 6, and 12 h postdose for each patient. Protein binding data were corrected using a fitted polynomial equation. The mean patient weight was 95.1 kg (range, 63.6 to 149.2 kg), the mean patient age was 63.5 ± 9.4 years, and 75% of the patients were males. The mean values for the plasma, thigh, and wound free area under the concentration-time curve from 0 to 24 h (fAUC0-24) were 2.65 ± 0.33, 2.52 ± 1.15, and 2.60 ± 1.02 μg·h/ml, respectively. Protein binding was nonlinear, with the percentage of free drug increasing with decreasing serum concentrations. Exposure values for thigh tissue and wound tissue were similar (P = 0.986). Mean steady-state tissue concentrations for the thigh and wound were similar at 0.12 ± 0.02 μg/ml, and clearance from the tissues appeared similar to that from plasma. Tissue penetration ratios (tissue fAUC/plasma fAUC) were 99% in the thigh and 100% in the wound (P = 0.964). Tigecycline penetrated equally well into wound and uninfected tissue of the same extremity.
Diabetic patients, due to the numerous sequelae associated with the disease, are well known to suffer from foot ulcers and their ensuing complications (15). In addition to the development of these wounds, the risk of these ulcers becoming chronically infected is well above 60% (8, 18). Some advances have been made in treatment of this complication with surgical intervention, topical antiseptics, and systemic antibiotic therapy; however, outcomes continue to remain poor and lead to limb amputation in 15% to 20% of patients within 5 years of the time of initial infection (7, 15).
The main pathogens that are identified in these wounds are Gram-positive organisms, in particular, Staphylococcus aureus (7). Concurrent with the worldwide increase in antibiotic resistance rates, the rate at which methicillin-resistant S. aureus (MRSA) is identified as one of the causative organisms in complicated skin and skin structure infections is also on the rise (20). As a result, the majority of health practitioners empirically prescribe antibiotics targeting MRSA for the treatment of chronic diabetic wound infections due to the poor outcomes seen with this pathogen (20, 21).
Tigecycline has been approved by the FDA for the treatment of complicated skin and skin structure infections caused by a variety of commonly occurring pathogens, including Staphylococcus aureus and its methicillin-resistant phenotype (22). As such, the compound has been used in the diabetic population to treat this type of infection caused by MRSA (1, 5). A pharmacoeconomic study conducted by Mallick et al. demonstrated medical cost savings of $1,469 for patients receiving tigecycline compared with vancomycin-aztreonam due to the shorter length of hospitalization for patients receiving tigecycline (10). Focusing on the MRSA-infected subgroup, tigecycline was associated with an even greater reduction in length of stay and a reduction of $2,239 in treatment costs (10).
The pharmacokinetics of tigecycline have previously been described in numerous patient populations, including investigations employing an inflammatory blister fluid model using healthy volunteers which demonstrated a tigecycline tissue penetration ratio of 74% (19). A single-intravenous-dose study by Rodvold et al. also noted that tigecycline achieved high tissue-fluid concentrations in the bile, gallbladder, colon, and lung compared with simultaneously obtained serum concentrations (16). Despite these promising results, data are lacking regarding the pharmacokinetics of tigecycline at the target site of infection. The objective of this study was to describe the pharmacokinetic profile of tigecycline in the interstitial fluid of soft tissues by using in vivo microdialysis (12, 13, 17) and to compare the degrees of penetration into uninfected and infected tissue in the diabetic population.
MATERIALS AND METHODS
Study protocol.
This study was an open-label pharmacokinetic study of 8 diabetic patients admitted to Hartford Hospital in Hartford, CT. The study protocol was reviewed and approved by the Institutional Review Board at Hartford Hospital. Written informed consent was obtained from all patients before inclusion in the study.
Patients.
Patients were enrolled and included if they had a documented medical history of type 1 or type 2 diabetes for which they were actively receiving insulin or oral antihyperglycemic agents. The patient enrollment was limited to those with an ongoing chronic diabetic foot infection deemed mild to moderate by the Infectious Diseases Society of America (8) or classified as grade 2 or 3 according to the guidelines of the International Consensus on the Diabetic Foot (9). Patients were permitted to receive other anti-infective agents during the study.
Clinical laboratory data, including the results obtained with serum electrolyte panels, serum creatinine, liver function panels, complete blood counts, glycosylated hemoglobin (HbA1C), and urine analysis, were collected. Prior to enrollment, patients were excluded who were <18 years of age, had a history of hypersensitivity to the study agent (tigecycline or tetracycline agents) or anesthetics (lidocaine or lidocaine derivatives), were pregnant or breast-feeding, had no palpable pedal pulses, or exhibited a level of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, total bilirubin, or amylase more than twice the upper limit of the normal range.
Microdialysis procedure.
After the patients were taken to surgery but before they received any study drug, two microdialysis probes (CMA 60 microdialysis catheter; CMA Microdialysis AB, Solna, Sweden) with membrane lengths of 30 mm and molecular mass cutoffs of 20 kDa were inserted under sterile conditions into the subcutaneous tissue, one within 10 cm of the wound margin and the second in the uninfected thigh of the same extremity, by the use of a guidance canula and following the local injection of a 0.5% lidocaine solution. Once the catheters were implanted, the microdialysis systems were connected, flushed (15 μg/ml) for 15 min, and then continuously perfused using Lactated Ringer's solution and microinfusion pumps (CMA 107 microdialysis pump; CMA Microdialysis AB, Solna Sweden) at a rate of 2 μl/min.
Study medication.
Tigecycline was supplied by Pfizer Inc. (New York, NY). Each 50-mg vial of tigecycline for injection was prepared according to the manufacturer's instructions and diluted with 0.9% normal saline to a total volume of 100 ml (22).
Each subject received an intravenous loading dose of 100 mg of tigecycline over 1 h through a peripheral catheter placed in the cephalic or antecubital vein. Beginning 12 h following the loading dose, each patient received tigecycline (50 mg) administered over 1 h every 12 h for an additional 2 to 3 doses.
Sample collection.
Venous blood was collected from a peripheral intravenous catheter placed in the arm contralateral to the dosing arm at the following time points: baseline (before the administration of drug), 0 (start of the infusion), 1 (end of the infusion), 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12 h. Blood samples were collected in a 10-ml BD Vacutainer (Becton, Dickinson and Company, Franklin Lakes, NJ) containing sodium heparin. Immediately after collection, blood samples were centrifuged (2,000 × g for 10 min) to obtain the separated plasma and were then stored in polypropylene tubes at −80°C until analysis.
Simultaneously with the blood sampling, dialysate samples of approximately 120 μl were obtained from both microdialysis catheters at baseline and at each of the corresponding blood sample time points. Dialysate samples were collected in 200-μl microvials (CMA Microdialysis AB, Solna, Sweden), which were then stored within polypropylene tubes at −80°C until analysis.
Protein binding studies.
For each patient, protein binding studies were conducted in triplicate at 1, 6, and 12 h after the administration of the last dose of tigecycline. These studies were conducted using Centrifree ultrafiltration devices with 30-kDa molecular mass cutoff filters per the manufacturer's package insert (Milipore Corporation, Billerica, MA). At the aforementioned time points, an extra blood sample was collected into a 10-ml BD Vacutainer containing sodium heparin and immediately centrifuged (2,000 × g for 10 min) to obtain separated plasma. Exactly 0.9 ml of plasma was transferred into each ultrafiltration device and centrifuged at 2,000 × g for 40 min at 25°C to generate an ultrafiltrate (Cultrafiltrate). Aliquots of plasma were also retained at each time point for determination of the total drug concentration in the plasma (Cplasma). The level of protein binding was calculated using the following equation: % protein binding = 100 − (100 × Cultrafiltrate/Cplasma).
Microdialysis probe recovery: in vivo retrodialysis.
Calibration of the microdialysis probes was conducted for each patient and for each catheter; retrodialysis was performed over an interval of 1 h at 2 μl/min, after the final blood and dialysate sampling was obtained. Tigecycline solutions for calibration were freshly prepared by diluting tigecycline with Lactated Ringer's solution and refrigerated until use. Tigecycline was added to the perfusate (Cperfusate) at a concentration of 500 μg/ml, and its rate of disappearance through the membrane (representing the level of recovery) was determined. The level of in vivo recovery was calculated using the following equation: % recovery in vivo = 100 − (100 × Cdialysate/Cperfusate), where Cdialysate is defined as the concentration obtained with the microdialysis probe during calibration.
Analytical procedures.
Concentrations of tigecycline in plasma and dialysate were determined by a validated high-performance liquid chromatography (HPLC) assay at the Center for Anti-Infective Research and Development, Hartford, CT, by methods previously described (6). The plasma assay results were linear over a range of 0.05 to 5 μg/ml (r2 = 0.998). Intra- and interday coefficients of variation (CV) for the low concentration (0.1 μg/ml) were 5.75% and 5.68%, respectively. Intra- and interday CV for the high concentration (3 μg/ml) were 3.41% and 3.79%, respectively. The dialysate assay results were linear over a range of 0.05 to 5 μg/ml (r2 = 0.998). Intra- and interday CV for the low concentration (0.1 μg/ml) were 4.47% and 5.17%, respectively. Intra- and interday CV for the high concentration (3 μg/ml) were 2.00% and 6.52%, respectively.
Pharmacokinetic analysis. (i) Plasma.
The free and total tigecycline concentrations in plasma from each patient were analyzed by noncompartmental methods using the WinNonLin software program (version 5.2.1; Pharsight Corporation, Mountain View, CA). The maximum concentration of tigecycline (Cmax) was determined by visual inspection of the concentration-time profile. The area under the concentration-time curve from 0 to 24 h (AUC0-24) was calculated using the log-linear trapezoidal rule. The half-life (t1/2) was calculated as 0.693/λz, where λz is the terminal elimination rate constant. The terminal rate constant was estimated by linear regression analysis of the terminal portion of the concentration-time curve by using no fewer than three data points. The clearance was calculated as Vss × λz, where Vss is the volume of distribution. Regression analyses were then employed to fit the free fraction of drug (Fu) over the concentration range by using the SigmaPlot software program (version 7.101; Systat Software Inc., San Jose, CA.).
(ii) Wound and thigh.
The wound and thigh interstitial fluid concentrations from each patient were analyzed by noncompartmental methods using the WinNonLin software program (version 5.2.1; Pharsight Corporation, Mountain View, CA). The maximum concentration of tigecycline (Cmax) in tissues was determined by visual inspection of the concentration-time profile. The area under the concentration-time curve from 0 h to 24 h (AUC0-24) was calculated using the log-linear trapezoidal rule. The half-life (t1/2) was calculated as 0.693/λz, where λz is the terminal elimination rate constant. The terminal rate constant was estimated by linear regression analysis of the terminal portion of the concentration-time curve by using no fewer than three data points. The percentage of penetration into tissue was calculated with AUC0-24 results determined for wound and thigh (AUCwound; AUCthigh) and for plasma (using the free drug concentration value [fAUCplasma]) as follows: AUCwound/fAUCplasma × 100 and AUCthigh/fAUCplasma × 100.
Statistical analysis.
All pharmacokinetic parameters and indices for wound and thigh exposures were compared using the t test or Mann-Whitney rank sum test for non-normally distributed data and SigmaStat version 2.03 (SPSS Inc., Chicago, IL). A P value of <0.05 was considered significant.
RESULTS
Patients.
Baseline characteristics for the 8 enrolled patients are provided in Table 1. Seven adverse events occurred during the study. Three patients complained of nausea and vomiting, one patient experienced hypoglycemia (not attributable to tigecycline), two patients experienced loose stools, and, finally, one patient experienced an increase in lactate dehydrogenase (LDH) to 512 U/liter while on therapy, a level that then returned to baseline. All other laboratory values were either within normal limits or clinically insignificant (as determined by the study physician) over the course of the study.
TABLE 1.
Characteristics of patients
| Patient characteristic (n = 8) | Valuea |
|---|---|
| Age (yr) | 63 ± 9 |
| Male (%) | 75 (6/8) |
| Race (%) | |
| African-American | 38 (3/8) |
| Caucasian | 62 (5/8) |
| Height (in) | 69.6 ± 4.4 |
| Weight (kg) | 95.1 ± 26.9 |
| Body mass index (kg of wt/m2 of ht) | 30.4 ± 6.2 |
| Wound location (%) | |
| Right foot | 37.5 (3/8) |
| Left foot | 62.5 (5/8) |
| Pedal pulse (%) | |
| +1 | 50 (4/8) |
| +2 | 50 (4/8) |
| Aspartate aminotransferaseb (U/liter) | 20 ± 3 |
| Alanine transaminaseb (U/liter) | 16 ± 3 |
| HbA1Cb (%) | 7.8 ± 1.4 |
Data are reported as means ± standard deviations, unless otherwise specified.
Values reflect laboratory results obtained at enrollment prior to the administration of the study drug.
Pharmacokinetic analyses. (i) Plasma.
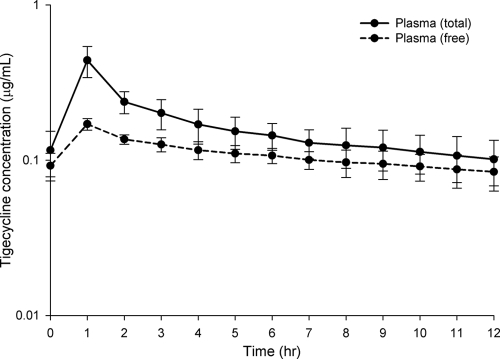
Tigecycline protein binding, calculated using Centrifree ultrafiltration devices and the equation % protein binding = 100 − (100 × Cultrafiltrate/Cplasma), was found to decrease with decreasing plasma concentrations for every patient such that the percentages of free drug at times 1, 6, and 12 h were 35.6 ± 8.22%, 80.3 ± 23.05%, and 100 ± 34.5%, respectively. These percentages of free drug corresponded to mean plasma concentrations of 0.61, 0.14, and 0.10 μg/ml, respectively. Using these data, the percent free drug-concentration profile (Fig. 1) best fit the following polynomial, with r2 = 0.783: y = y0 + a/x + b/x2 + c/x3, where y = percent unbound, y0 = 9.0896, a = 15.339, b = −0.999, c = 0.0232, and x = plasma concentration. Through the application of this polynomial equation, all total plasma concentrations were corrected with respect to free drug levels. The time courses of free and total tigecycline plasma concentrations paralleled each other (Fig. 2). The free plasma pharmacokinetic parameters are provided in Table 2.
FIG. 1.
Tigecycline percent free drug-plasma concentration profile fit (determined using regression analysis) to the following polynomial equation, with r2 = 0.783: y = y0 + a/x + b/x2 + c/x3, where y = percent unbound, y0 = 9.0896, a = 15.339, b = −0.999, c = 0.0232, and x = the plasma concentration.
FIG. 2.
Concentration-time profiles for total and free plasma samples from diabetic patients following doses of tigecycline administered intravenously (IV) at 100 mg and then at 50 mg IV twice daily. Data are reported as means ± standard deviations of the results for 8 patients in total. Free drug concentrations were calculated using the following equation and an r2 = 0.783: y = y0 + a/x + b/x2 + c/x3, where y = percent unbound, y0 = 9.0896, a = 15.339, b = −0.999, c = 0.0232, and x = the plasma concentration.
TABLE 2.
Steady-state pharmacokinetic parameters representing tigecycline concentrations in plasma, wound interstitial fluid, and uninfected thigh interstitial fluid samplesa
| Sample category | Parameterb |
||||||
|---|---|---|---|---|---|---|---|
| Cmax (μg/ml) | Tmax (h) | AUC0-24 (μg·h/ml) | t1/2 (h) | CLss (liters/h/kg) | Vss (liters/kg) | Penetrationc (%) | |
| Plasma (total) | 0.42 ± 0.11 | 1.13 ± 0.35 | 3.99 ± 0.75 | 9.73 ± 4.62 | 0.28 ± 0.09 | 3.95 ± 2.31 | |
| Plasma (free) | 0.16 ± 0.01 | 1.13 ± 0.35 | 2.65 ± 0.33 | ||||
| Wound | 0.16 ± 0.06 | 4.38 ± 3.38 | 2.60 ± 1.02 | 24.88 ± 28.67 | 100.00 ± 44.78 | ||
| Thigh | 0.18 ± 0.13 | 3.38 ± 3.54 | 2.52 ± 1.15 | 15.96 ± 13.2 | 98.94 ± 52.75 | ||
Steady-state conditions consisted of a 100-mg loading dose and then 3 to 4 doses of 50 mg twice daily.
Cmax, peak concentration; Tmax, time to reach peak concentration; CLss, clearance at steady state; Vss, volume of distribution at steady state. Data are reported as means ± standard deviations. P values (representing statistical analysis of wound and thigh concentrations): for Cmax, 0.699; for Tmax, 0.573; for AUC0-24, 0.885; for t1/2, 0.437; for percent penetration, 0.966.
Percent penetration calculated as follows: AUCthigh/fAUCplasma × 100 and AUCwound/fAUCplasma × 100.
(ii) Thigh and wound tissue exposures.
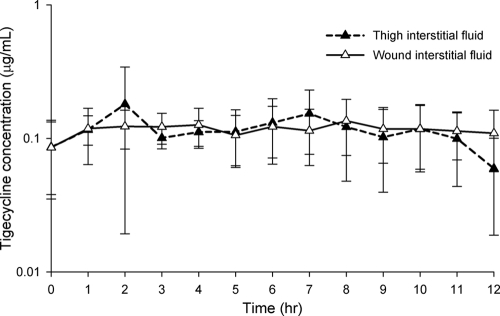
The tigecycline concentration-time profiles for wound and thigh interstitial fluid are displayed in Fig. 3. The mean recovery rates for the thigh and wound catheters were 53.48 ± 9.32% and 57.03 ± 10.66%, respectively. Tigecycline concentrations within the interstitium of both the thigh and wound were generally ≥0.1 μg/ml for the entire 12-h dosing interval. No statistical difference was noted between wound and thigh tissue results for any of the parameters (Table 2). The tigecycline concentrations in uninfected thigh and wound tissues closely paralleled those of the free drug concentrations in the plasma. This lack of difference is best illustrated by the similarity in the degrees of tissue penetration, defined as AUCtissue/fAUCplasma ratios, for the thigh and wound (98.94% [range, 38.78 to 100%] and 100.00% [range, 42.86 to 100%], respectively) (P = 0.966) (Table 2).
FIG. 3.
Concentration-time profiles for thigh and wound interstitial fluid samples from diabetic patients following doses of tigecycline administered intravenously (IV) at 100 mg IV and then at 50 mg IV twice daily. Data are reported as means ± standard deviations of the results for 8 patients in total.
DISCUSSION
Herein we describe the pharmacokinetic profile of tigecycline for both the uninfected (thigh) and infected (lower extremity wound) tissues of diabetic patients by means of in vivo microdialysis. In this study, we found that tigecycline pharmacokinetics were similar for the plasma, wound, and uninfected thigh tissues (with plasma values corrected for protein binding). The plasma protein binding was found to be nonlinear, with the percentage of free drug increasing with decreasing serum concentrations. Tigecycline penetrated equally well into wound and uninfected tissues of the same extremity, with penetration at almost 100% of free plasma concentrations.
The total plasma pharmacokinetic data for our patients are consistent with what has been reported previously for multiple-dose studies of tigecycline administered to similar patients with skin and skin structure infections when using noncompartmental analysis (3, 14). In a study by Darling et al., values for Cmax and AUC0-24 under steady-state conditions were found to be 0.40 μg/ml and 4.48 mg·h/liter, respectively (3). Our findings were very similar, with a total Cmax of 0.42 ± 0.11 μg/ml and an AUC0-24 (under steady-state conditions) of 3.99 ± 0.75 mg·h/liter. As was also noted in the previous studies, our data demonstrated large intersubject variations, which may be explained in part by the presence of multiple comorbidities (3). There is currently no literature available with which to compare our free plasma pharmacokinetic data, presumably due to issues involved in determining tigecycline protein binding.
In vitro studies have shown that tigecycline exhibits nonlinear concentration-dependent plasma protein binding; however, in contrast to other agents that also display nonlinear protein binding, the percentage of tigecycline protein binding decreases as concentrations decrease (14). In the present study, we corroborated this observation in vivo by showing that the percentage of protein binding by tigecycline decreased as the concentration of the drug decreased. This phenomenon has also been demonstrated in vivo in a immunocompromised mouse thigh infection model (2). Multiple theories have been suggested to explain the mechanism for this atypical pattern of protein binding, with the most common pointing to the ability of tigecycline to form metal ion complexes (14). These formations affect diffusion rates across semipermeable membranes and also affect binding to cellular proteins (14). Despite the aforementioned studies, ours is the first (to our knowledge) that shows this alteration in protein binding over a concentration range in one patient. This unusual protein binding was fit over the concentration range using regression analysis; by using the derived polynomial equation, we were able to correct for the free fraction of tigecycline over the entire concentration range.
Tigecycline has been reported to have a vast ability to penetrate all tissues within the body, but there have not been any data previously published regarding this penetration in the target tissues of a diabetic foot wound (16). Our results demonstrate that tigecycline penetration into both the wound and the uninfected thigh occurred relatively quickly, within 4 h of the dose administration. Tigecycline accumulated within the interstitium of the wound and thigh, with concentrations at both sites remaining at constant levels for the entire dosing interval. Concentrations of tigecycline in the thigh and wound were almost 100% of the free plasma concentrations, suggesting that the drug penetrates equally and fully into both uninfected and diseased tissue. These penetration ratios are larger than the 74% value reported in an inflammatory blister study done by Sun et al. (19). This discordance was not unexpected, because the blister study interstitial concentrations were compared with total plasma concentrations. When the thigh and wound penetration ratios in our study are recalculated using the total plasma AUC0-24 value, the resulting penetration ratios, at around 65% for both the wound and thigh, were similar to those reported by Sun et al. (19).
Our finding of almost 100% penetration into both diabetic and healthy tissue has clinically significant implications. While diabetic patients are known to suffer from peripheral vascular disease, this compromised state, as evidenced in our patients by nonhealing chronic wounds, did not appear to hinder the penetration of tigecycline into the target site. In fact, the concentrations maintained at both tissue sites were >0.1 μg/ml, allowing AUC0-24 values of 2.6 and 2.5 for the wound and thigh, respectively. In order to relate these AUC0-24 findings to tigecycline's potential for clinical and/or microbiological success, the pharmacodynamic driver of efficacy, represented by AUC0-24/MIC, requires consideration (11). A previous animal study conducted by Crandon et al. demonstrated that the fAUC0-24/MIC ratio needed to achieve a 2-log decrease in bacterial density in a murine thigh infection model was 5.7 (2). A study by Meagher et al. done in humans used classification and regression tree (CART) analysis to define an AUC0-24/MIC ratio of 17.9 as the breakpoint for clinical and microbiological success in complicated skin and skin structure infections with S. aureus and streptococcal spp. as the main pathogens (11). In contrast to the study by Crandon et al., this breakpoint was determined using total plasma concentrations to calculate the AUC0-24/MIC ratio. Applying this method to our data and using a MIC90 of 0.12 μg/ml for methicillin-resistant S. aureus, our total plasma AUC0-24/MIC ratio was 33.25, vastly exceeding the target of 17.9 (4, 11). In fact, at 22.1, 21.7, and 21, respectively, the AUC0-24/MIC ratios for free plasma, wound, and thigh tissues all exceeded this target as well. This degree of penetration, together with the resulting fAUC0-24/MIC, support the reported efficacy of tigecycline in treating patients with complicated skin and skin structure infections, including those with diabetes (1, 5).
In conclusion, tigecycline penetrates equally well into healthy and infected tissue, with concentrations that approximate the free drug concentrations in plasma. Our target tissue data, combined with the in vivo microdialysis technique, support the clinical utility of tigecycline in diabetics with lower limb infections.
Acknowledgments
We thank members of the staff at the Center for Anti-Infective Research and Development and Hartford Hospital for their assistance in the preparation and performance of this study.
The work was supported by an investigator-initiated research grant from Pfizer Inc., New York, NY. D. P. Nicolau and J. L. Kuti have received other research grants from Pfizer Inc.
Footnotes
Published ahead of print on 4 October 2010.
REFERENCES
- 1.Breedt, J., J. Teras, J. Gardovskis, F. J. Maritz, T. Vaasna, D. P. Ross, M. Gioud-Paquet, N. Dartois, E. J. Ellis-Grosse, and E. Loh. 2005. Safety and efficacy of tigecycline in treatment of skin and skin structure infections: results of a double-blind phase 3 comparison study with vancomycin-aztreonam. Antimicrob. Agents Chemother. 49:4658-4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crandon, J. L., M. A. Banevicius, and D. P. Nicolau. 2009. Pharmacodynamics of tigecycline against phenotypically diverse Staphylococcus aureus isolates in a murine thigh model. Antimicrob. Agents Chemother. 53:1165-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darling, I. M., B. B. Cirincione, and J. S. Owen. 2005. Non-compartmental pharmacokinetics of tigecycline in phase 3 studies of patients with complicated skin and skin structure and intra abdominal infections. Abstr. 45th Annu. Meet. Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-20.
- 4.Dowzicky, M. J., and C. H. Park. 2008. Update on antimicrobial susceptibility rates among Gram-negative organisms in the United States: results from the tigecycline evaluation and surveillance trial (TEST) 2005-2007. Clin. Ther. 30:2040-2050. [DOI] [PubMed] [Google Scholar]
- 5.Florescu, I., M. Beuran, R. Dimov, A. Razbadauskas, M. Bochan, G. Fichev, G. Dukart, T. Babinchak, C. A. Cooper, E. J. Ellis-Grosse, N. Dartois, and H. Gandjini. 2008. Efficacy and safety of tigecycline compared with vancomycin or linezolid for treatment of serious infections with methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococci: a Phase 3, multicentre, double-blind, randomized study. J. Antimicrob. Chemother. 62:i17-i28. [DOI] [PubMed] [Google Scholar]
- 6.Li, C., C. A. Sutherland, C. H. Nightingale, and D. P. Nicolau. 2004. Quantitation of tigecycline, a novel glycylcycline, by liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 811:225-229. [DOI] [PubMed] [Google Scholar]
- 7.Lipsky, B. A. 2004. Medical treatment of diabetic foot infections. Clin. Infect. Dis. 39:S104-S114. [DOI] [PubMed] [Google Scholar]
- 8.Lipsky, B. A., A. R. Berendt, H. G. Deery, J. M. Embil, W. S. Joseph, A. W. Karchmer, J. L. LeFrock, D. P. Lew, J. T. Mader, C. Norden, and J. S. Tan. 2004. Diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. 39:885-910. [DOI] [PubMed] [Google Scholar]
- 9.Lipsky, B. A., A. R. Berendt, J. Embil, and F. De Lalla. 2004. Diagnosing and treating diabetic foot infections. Diabetes Metab. Res. Rev. 20:S56-S64. [DOI] [PubMed] [Google Scholar]
- 10.Mallick, R., A. Kuznik, and D. Weber. 2006. Treatment of complicated skin and skin structure infections in the U.S.: expected cost differences between tigecycline and vancomycin/aztreonam. Abstr. 16th Eur. Congr. Clin. Microbiol. Infect. Dis. (ECCMID); 1-4 April 2006, Nice, France, abstr. P1494.
- 11.Meagher, A. K., J. A. Passarell, B. B. Cirincione, S. A. Van Wart, K. Liolios, T. Babinchak, E. J. Ellis-Grosse, and P. G. Ambrose. 2007. Exposure-response analyses of tigecycline efficacy in patients with complicated skin and skin-structure infections. Antimicrob. Agents Chemother. 51:1939-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller, M. 2002. Science, medicine, and the future: microdialysis. BMJ 324:588-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Müller, M., O. Haag, T. Burgdorff, A. Georgopoulos, W. Weninger, B. Jansen, G. Stanek, H. Pehamberger, E. Agneter, and H. G. Eichker. 1996. Characterizations of peripheral-compartment kinetics of antibiotics by in vivo microdialysis. Antimicrob. Agents Chemother. 40:2703-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muralidharan, G., M. Micalizzi, J. Speth, D. Raible, and S. Troy. 2005. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob. Agents Chemother. 49:220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramsey, S. D., K. Newton, D. Blough, D. K. McCulloch, N. Sandhu, G. E. Reiber, and E. H. Wagner. 1999. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 22:382-387. [DOI] [PubMed] [Google Scholar]
- 16.Rodvold, K. A., M. H. Gotfried, M. Cwik, J. M. Korth-Bradley, G. Dukart, and E. J. Ellis-Grosse. 2006. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J. Antimicrob. Chemother. 58:1221-1229. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt, S., R. Banks, V. Kumar, K. H. Rand, and H. Derendorf. 2008. Clinical microdialysis in skin and soft tissues: an update. J. Clin. Pharmacol. 48:351-364. [DOI] [PubMed] [Google Scholar]
- 18.Sotto, A., N. Bouziges, N. Jourdan, J.-L. Richard, and J.-P. Lavigne. 2007. In vitro activity of tigecycline against strains isolated from diabetic foot ulcers. Pathol. Biol. 55:398-406. [DOI] [PubMed] [Google Scholar]
- 19.Sun, H. K., C. T. Ong, A. Umer, D. Harper, S. Troy, C. H. Nightingale, and D. P. Nicolau. 2005. Pharmacokinetic profile of tigecycline in serum and skin blister fluid of healthy subjects after multiple intravenous administrations. Antimicrob. Agents Chemother. 49:1629-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tentolouris, N., G. Petrikkos, N. Vallianou, C. Zachos, G. L. Daikos, P. Tsapogas, G. Markou, and N. Katsilambros. 2006. Prevalence of methicillin-resistant Staphylococcus aureus in infected and uninfected diabetic foot ulcers. Clin. Microbiol. Infect. 12:186-189. [DOI] [PubMed] [Google Scholar]
- 21.Vardakas, K. Z., M. Horianopoulou, and M. E. Falagas. 2008. Factors associated with treatment failure in patients with diabetic foot infections: an analysis of data from randomized controlled trials. Diabetes Res. Clin. Pract. 80:344-351. [DOI] [PubMed] [Google Scholar]
- 22.Wyeth Pharmaceuticals Inc. 2010. Tygacil (tigecycline) package insert. Wyeth Pharmaceuticals Inc., Philadelphia, PA.