Abstract
Tafenoquine (TFQ), an 8-aminoquinoline analogue of primaquine, which is currently under clinical trial (phase IIb/III) for the treatment and prevention of malaria, may represent an alternative treatment for leishmaniasis. In this work, we have studied the mechanism of action of TFQ against Leishmania parasites. TFQ impaired the overall bioenergetic metabolism of Leishmania promastigotes, causing a rapid drop in intracellular ATP levels without affecting plasma membrane permeability. TFQ induced mitochondrial dysfunction through the inhibition of cytochrome c reductase (respiratory complex III) with a decrease in the oxygen consumption rate and depolarization of mitochondrial membrane potential. This was accompanied by ROS production, elevation of intracellular Ca2+ levels and concomitant nuclear DNA fragmentation. We conclude that TFQ targets Leishmania mitochondria, leading to an apoptosis-like death process.
Leishmaniasis includes a wide variety of clinical manifestations caused by the protozoan parasite Leishmania. Visceral leishmaniasis is the most severe form of the disease and is usually fatal if not treated (http://www.who.int/leishmaniasis/burden/en/). In the absence of a reliable vaccine, leishmaniasis treatment relies exclusively on chemotherapy. Resistance to organic pentavalent antimonials (until recently considered to be the standard treatment) in northeast India (4), together with the severe side effects associated with their use, has led to the use of alternative treatments based on the incorporation of drugs such as amphotericin B, miltefosine, and paromomycin into the arsenal of antileishmanial drugs (8). Nevertheless, the limited number of active drugs has prompted the WHO to recommend a combined therapy in order to extend the life expectancy of these compounds.
Among the new drugs under development, sitamaquine (WR6026; GlaxoSmithKline), an 8-aminoquinoline, currently under phase IIb clinical trials, represents a promising drug for the oral treatment of leishmaniasis (35). In addition, another 8-aminoquinolines have been synthesized and evaluated for their leishmanicidal activity (29, 36). However, the leishmanicidal mechanism of 8-aminoquinolines is still unknown. Sitamaquine, for example, accumulates in the acidocalcisomes, but this organelle has been ruled out as its final target (17). The collapse of mitochondrial potential in digitonized Leishmania donovani promastigotes has also been reported (39). Tafenoquine (TFQ), formerly known as WR238605, is an analogue of primaquine with much lower toxicity than the parental drug. It has demonstrated significant leishmanicidal activity in the mouse experimental model (41) and may represent an alternative treatment for leishmaniasis.
In the present study, we have shown that TFQ inhibits the mitochondrial cytochrome c reductase of Leishmania promastigotes. This inhibition causes a drop in the intracellular ATP levels of the parasite and the loss of mitochondrial membrane potential. TFQ induces ROS production and deregulation of Ca2+ homeostasis, followed by nicking and fragmentation of DNA in Leishmania promastigotes leading to an apoptosis-like death. Our results provide the first insight into the mechanistic lethal pathway of an 8-aminoquinoline in Leishmania. This information may be useful for the design of more specific and less toxic compounds against leishmaniasis.
MATERIALS AND METHODS
Reagents.
Tafenoquine (TFQ) was kindly provided by GlaxoSmithKline (Greenford, United Kingdom). A stock solution of 10 mM TFQ was prepared in ethanol. DMNPE-luciferin {d-luciferin-1[-(4, 5-dimethoxy-2-nitrophenyl) ethyl ester]}, Fluo4-AM, Lysotracker Green DND-26, MitoSOX Red, Pluronic F-127, rhodamine 123 (Rh123) and SYTOX Green, were purchased from Invitrogen (Carlsbad, CA). Fatty acid-free bovine serum albumin (BSA), digitonin, ADP, antimycin A, ascorbate, cytochrome c (from equine heart), FCCP (carbonyl cyanide 4-trifluoromethoxyphenylhydrazone), α-glycerophosphate, malonate, MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide), KCN, TMPD (tetramethyl-p-phenylenediamine), succinate, propidium iodide (PI), and Triton X-100 were purchased from Sigma-Aldrich (Madrid, Spain). All other chemicals were of the highest quality available.
Strains and parasite cultures.
Leishmania donovani promastigotes (MHOM/ET/67/HU3) were grown at 28°C in RPMI 1640-modified medium (Invitrogen) supplemented with 20% heat-inactivated fetal bovine serum (HIFBS; Invitrogen). The L. donovani strain MHOM/SD/00/1S-2D and its derived line, L. donovani 3-Luc, which expresses cytoplasmic firefly luciferase (19), were grown at 25°C in RPMI 1640 medium with 10% HIFBS. L. major MHOM/JL/80/Friedlin parental and AP3δ-null mutant (ΔAP3) were kindly provided by J. Mottram (Institute of Biomedical and Life Sciences, University of Glasgow, Glasgow, United Kingdom) (2) and cultured at 28°C in RPMI 1640-modified medium supplemented with 20% HIFBS.
TFQ sensitivity assay.
Logarithmic-phase parasites (2 × 106 promastigotes/ml) were incubated with TFQ (0.5 to 20 μM) for 72 h at 28°C in culture medium. Cell proliferation was determined by the MTT colorimetric assay, as described previously (15). Parasites incubated with 0.2% ethanol were used as control.
Bioluminescence assays.
The in vivo variation in intracellular ATP levels was monitored in promastigotes expressing a cytoplasmic form of firefly luciferase, as described previously (19). Briefly, parasites from the L. donovani 3-Luc strain (2 × 107 promastigotes/ml) were resuspended in HEPES-buffered saline (HBS; 21 mM HEPES, 0.7 mM Na2HPO4, 137 mM NaCl, 5 mM KCl and 6 mM d-glucose, pH 7.1) and DMNPE-luciferin was added at a final concentration of 25 μM. Aliquots of this suspension (100 μl/well) were immediately distributed into a 96-well black polystyrene microplate. Once the luminescence reached a plateau, different TFQ concentrations were added. Changes in luminescence were recorded with an Infinite F200 microplate reader (Tecan Austria GmbH, Austria). In vitro inhibition of recombinant firefly luciferase activity by TFQ was discarded using the ATP determination kit (Invitrogen) in the presence of saturable ATP concentrations. The release of ATP from L. donovani promastigotes into the external medium was determined using the same kit.
Plasma membrane permeabilization.
Plasma membrane integrity was assessed by the entry of the vital dye SYTOX Green, as described previously (20) with some modifications. Briefly, parasites (2 × 106 promastigotes/ml) were treated with 1, 5, and 10 μM TFQ for 45 min at 28°C in HBS, washed twice with HBS, and incubated with 2 μM SYTOX Green (final concentration) for 15 min at 28°C. The parasites were then transferred into a 96-well microplate (100 μl/well) and fluorescence, due to the binding of the dye to intracellular nucleic acids, was recorded with an Infinite F200 microplate reader (Tecan Austria GmbH, Austria) equipped with 485- and 535-nm filters for excitation and emission wavelengths, respectively. Control for maximum fluorescence was obtained by addition of 0.05% Triton X-100.
Analysis of ΔΨm.
The variation of mitochondrial membrane potential (ΔΨm) in the promastigotes was monitored using rhodamine 123 (Rh123) accumulation, as described previously (9). To this end, the parasites (107 promastigotes/ml) were incubated with 5 μM TFQ for 1, 5, 10, and 30 min at 28°C in HBS, and then 0.8 μM Rh123 was added and incubated for 5 min. Subsequently, the parasites were washed twice, resuspended in phosphate-buffered saline (PBS) and analyzed by flow cytometry in a FACScan flow cytometer (Becton-Dickinson, San Jose, CA) equipped with an argon laser operating at 488 nm. Fluorescence emission between 515 and 545 nm was quantified using Cell Quest software. Parasites that were either untreated or fully depolarized by incubation with 10 μM FCCP for 10 min were used as controls.
Determination of oxygen consumption rates.
Oxygen consumption rates were measured with a Clark-oxygen electrode (Hansatech, KingsLynn, United Kingdom) at 25°C, using 1 ml of promastigote suspension (108 cells/ml) in respiration buffer (10 mM Tris-HCl, pH 7.2, 125 mM saccharose, 65 mM KCl, 1 mM MgCl2, 2.5 mM NaH2PO4, 0.3 mM EGTA) supplemented with 5 mM succinate and 1 mg/ml fatty acid-free BSA, as described previously (1). Cells were permeabilized with 60 μM digitonin, which allows selective permeation of the plasma membrane but not of the inner mitochondrial membrane (38). Subsequently, 100 μM ADP was added to restore state 3 respiration; once a steady rate was reached, TFQ was added. In order to map the site of inhibition by TFQ within the respiratory chain, a set of substrates and inhibitors specific to the different complexes inside the respiratory chain were used. Their final concentrations were: 6.7 mM α-glycerophosphate, 0.1 mM TMPD plus 1.7 mM ascorbate, and 2 mM malonate.
Isolation of mitochondrial fraction.
A mitochondrion-enriched fraction was obtained as described by Chen et al. (5). Leishmania promastigotes were washed twice in phosphate-buffered saline (PBS), resuspended in hypo-osmotic buffer (5 mM Tris-HCl, pH 7.4) for 10 min at 25°C, and homogenized in a Potter-Elvehjem homogenizer on ice. Cell debris was removed by centrifugation (1,000 × g, 10 min, 4°C). The supernatant was next centrifuged at 13,000 × g (20 min, 4°C). The pellet, containing the mitochondrial fraction, was resuspended in 75 mM sodium phosphate, pH 7.4, and the protein content was adjusted to 2 mg/ml, as measured using Bradford reagent (Bio-Rad).
Measurement of CcR activity.
Determination of cytochrome c reductase (CcR) activity was carried out according to Sottocasa et al. (34), based on the reduction of oxidized cytochrome c. Briefly, different TFQ concentrations were added to the incubation mixture (200 μg/ml of mitochondrial fraction, 0.02% Triton X-100 and 32 μM oxidized cytochrome c in 75 mM sodium phosphate pH 7.4). The reaction started by addition of 10 mM succinate (final concentration). The increase of absorbance at 550 nm was monitored for 5 min at 37°C in a Varioskan Flash (Thermo Scientific) microplate reader. Oxidation of reduced cytochrome c by cytochrome c oxidase (respiratory complex IV) was precluded by previous inhibition of this enzyme with 10 mM KCN. Samples without succinate or in the presence of 2 μM antimycin A, an inhibitor of cytochrome c reductase, were taken as controls.
Determination of ROS production.
To detect mitochondrial reactive oxygen species (ROS) production we used the cell-permeable fluorogenic probe MitoSOX Red, which targets mitochondria selectively, being oxidized by local superoxide (32). Parasites (2 × 107 promastigotes/ml) were preincubated with 0.5 μM MitoSOX Red for 30 min at 28°C in HBS and then treated with 5 μM TFQ for 1, 5, 10, and 30 min. Fluorescence emission at 580 nm was measured by flow cytometry, in a FACScan flow cytometer, with an excitation wavelength of 488 nm. Antimycin A (0.3 μg/ml) was used as a positive control for ROS generation.
Variation in free intracellular Ca2+.
Changes in the cytosolic Ca2+ levels were monitored using Fluo4-AM, as described previously (11). Briefly, parasites (2 × 107 promastigotes/ml) were incubated with 5 μM Fluo4-AM for 60 min at 28°C in RPMI 1640 medium devoid of phenol red and supplemented with 0.02% pluronic acid F127 to improve dispersion of the nonpolar acetyloxy-methyl ester in aqueous media. After incubation, cells were washed and incubated with 2, 5, and 10 μM TFQ in a fresh medium, with and without addition of 8 mM EGTA. The fluorescence of Ca2+-bound Fluo4 was analyzed at 28°C in an Aminco-Bowman series 2 fluorometer (490/518 nm, excitation and emission wavelengths, respectively). Parasites treated with NH4Cl (20 mM) were used as positive control for calcium release from intracellular organelles as described previously (18).
TFQ-induced alkalinization.
Parasites (4 × 106 promastigotes/ml), treated either with or without 5 μM TFQ (10 min at 28°C), were incubated with 100 nM the acidotropic dye LysoTracker Green DND-26 for 10 min in HBS at 28°C. Afterwards, the parasites were washed and then resuspended in PBS, and dye fluorescence was measured by flow cytometry in a FACScan flow cytometer (excitation at 488 nm; emission between 515 and 545 nm).
TFQ localization.
Using the intrinsic fluorescence of TFQ (excitation at 340 nm; emission at 388 nm), its intracellular distribution was ascertained by fluorescence microscopy. Parasites were treated with 5 μM TFQ for 15 min at 28°C in culture medium, washed successively with the same medium and then with PBS, and finally observed under a Zeiss Axiophot (Germany) epifluorescence microscope; images were captured with a SPOT camera (Diagnostic Instrument, Inc.). Parasites labeled with Lysotracker Green (as mentioned above) were used to visualize the distribution of acidocalcisomes and analyzed in parallel by fluorescence microscopy.
Analysis of autophagy in Leishmania induced by TFQ treatment.
Induction of autophagy by TFQ was analyzed in L. donovani lines using autophagy-related protein 8 (ATG8) fused to green fluorescent protein (GFP) as a marker of autophagosomes (3). Parasites were transfected with an episomal expression vector plasmid containing the GFP-ATG8 construct, kindly provided by J. Mottram, as described previously (31). Parasites expressing GFP-ATG8 were treated with 5 μM TFQ for 24 h in culture medium at 28°C. The presence of autophagic bodies was observed under a Zeiss Axiophot epifluorescence microscope. Images were captured with a SPOT camera. The percentage of autophagosome-bearing cells and the number of these structures per cell were analyzed. At least four series of 200 cells were counted, and this was repeated three times.
DNA fragmentation analysis.
DNA fragmentation was analyzed by terminal deoxynucleotidyltransferase (TdT)-mediated dUTP end labeling (TUNEL) using the Roche in situ cell death detection kit. Parasites (107 promastigotes/ml) were incubated with 5, 10, and 20 μM TFQ for 4 h at 28°C in culture medium, fixed with 4% formaldehyde in PBS for 15 min at room temperature, and permeabilized with 0.1% Triton X-100 for 2 min at 4°C. The cells were labeled with TUNEL reaction mixture following the manufacturer's instructions. Fluorescence was measured by flow cytometry, in a FACScan flow cytometer, with excitation and emission wavelengths of 488/520 nm. Unfixed parasites were labeled with 0.4 μg/ml PI for 5 min at 4°C and measured at 488/617 nm to detect necrotic cells.
Additionally, DNA fragmentation was detected as described by Vergnes et al. (40). After treatment with TFQ, total DNA was extracted from the parasites using the “salting-out DNA extraction” method. Briefly, parasites were pelleted and treated with lysis buffer (10 mM Tris-HCl, 5 mM EDTA, pH 8.0, 0.5% SDS, 200 mM NaCl, 100 μg/ml proteinase K) for 1 h at 65°C. Two volumes of ice-cold absolute ethanol were added, and the DNA was centrifuged for 15 min at 13,000 × g. The supernatant was discarded, and the dried pellet was resuspended in 100 μl of a mixture of 10 mM Tris-HCl, pH 7.4, and 0.1 mM EDTA and treated with RNase A (0.3 μg/ml) for 1 h at 37°C. The DNA was analyzed on 2% agarose gel.
RESULTS
TFQ inhibits Leishmania proliferation in vitro.
After 72 h of TFQ exposure, proliferation of L. donovani and Leishmania major promastigotes was inhibited at the micromolar range in a dose-dependent manner, with 50% effective concentrations (EC50s) of 5.6 ± 1.0 μM and 5.3 ± 2.1 μM for L. donovani MHOM/ET/67/HU3 and MHOM/SD/00/1S-2D, respectively, and 2.2 ± 0.2 μM and 2.2 ± 0.1 μM for L. major MHOM/JL/80/Friedlin parental strain and ΔAP3 mutant, respectively.
TFQ reduces the intracellular ATP level of L. donovani promastigotes.
In order to ascertain the general metabolic processes underlying the leishmanicidal effect of this drug, the bioenergetic state of L. donovani promastigotes was first studied. Parasites of the 3-Luc strain, expressing a cytoplasmic form of luciferase, were employed together with the membrane-permeable luciferase substrate DMNPE-luciferin. Under these conditions, the luminescence of these promastigotes is directly related to the concentration of free cytoplasmic ATP, the limiting substrate of the reaction (21). TFQ decreased the luminescence in a concentration-dependent manner, reaching the respective endpoint level about 15 min after addition of the drug (Fig. 1A), leading to the bioenergetic collapse of the promastigotes.
FIG. 1.
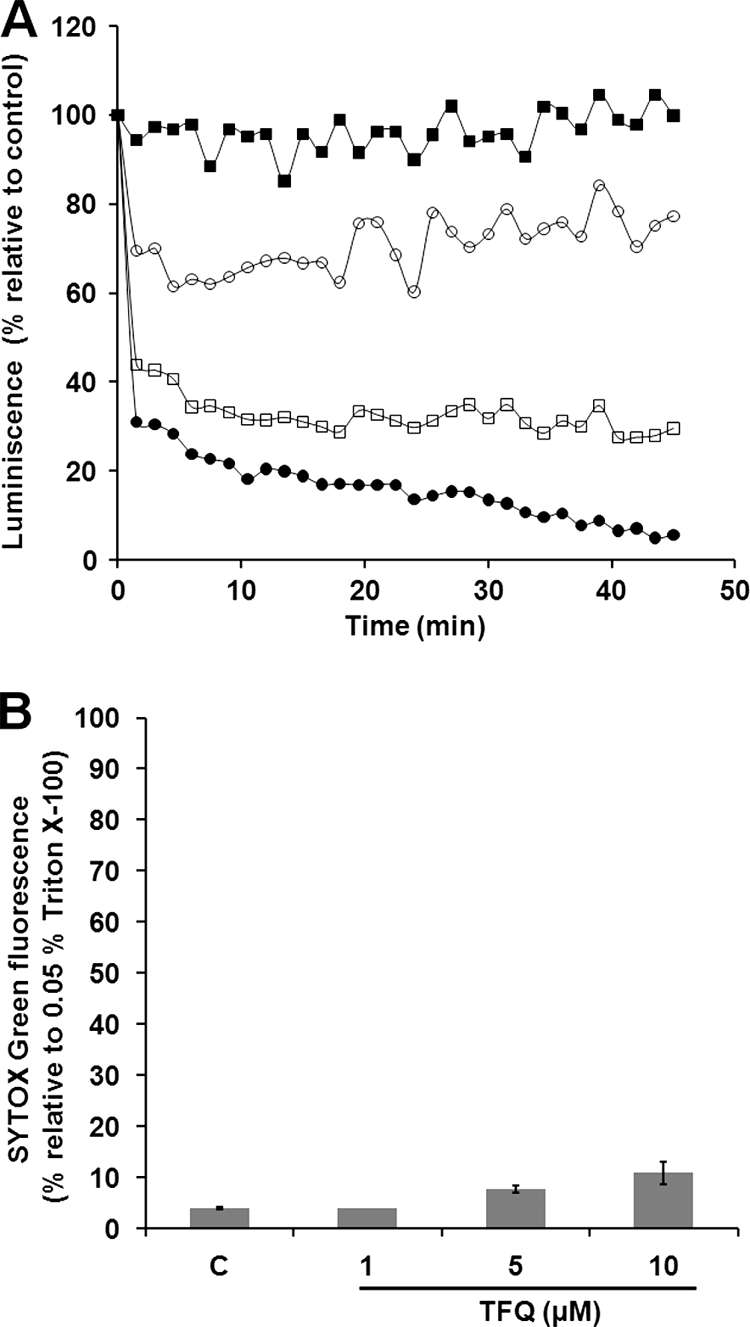
TFQ reduces intracellular ATP level without plasma membrane permeabilization. (A) Changes in the intracellular ATP levels were determined by variation of luminescence in L. donovani 3-Luc promastigotes treated with different TFQ concentrations: 2 (▪), 5 (○), 8 (□), and 10 μM (•). Promastigotes were preloaded with 25 μM DMNPE-luciferin, and when luminescence reached a plateau, TFQ was added (t = 0) and luminescence was monitored as described in Materials and Methods. Variation of luminiscence was normalized relative to the level in the control untreated parasites. Similar results were obtained in three independent experiments. At the concentrations tested, TFQ did not inhibit the activity of recombinant firefly luciferase. (B) The effect of TFQ on the plasma membrane permeability was determined by incubating promastigotes without (control [c]) and with 1, 5, and 10 μM TFQ for 45 min in HBS at 28°C and then treating them with 2 μM SYTOX Green for 15 min at 28°C. SYTOX Green fluorescence is represented relative to parasites treated with 0.05% Triton X-100 used as 100% permeabilization. Results are means ± standard deviations (SD) from three independent experiments.
TFQ does not affect the plasma membrane integrity.
One of the more likely reasons to account for the observed decrease in the intracellular ATP content may be that TFQ compromises plasma membrane integrity. To test this hypothesis, the entrance of the vital dye SYTOX Green (molecular weight [MW], 600) into the cytoplasm of L. donovani promastigotes, induced by TFQ, was monitored. At the highest TFQ concentration assayed (10 μM, 45 min), the increase in SYTOX Green fluorescence accounted for only 11% of the maximal permeabilization, defined as that obtained with 0.05% Triton X-100 (Fig. 1B), ruling out the formation of large lesions on the parasite plasma membrane as a key event in the mechanism of action of TFQ. Furthermore, we measured leakage of ATP into the supernatant; TFQ does not induce an enhanced ATP liberation to the medium when compared to the control parasites (<50 pmol ATP × 10−6 cells).
TFQ induces ΔΨm depolarization.
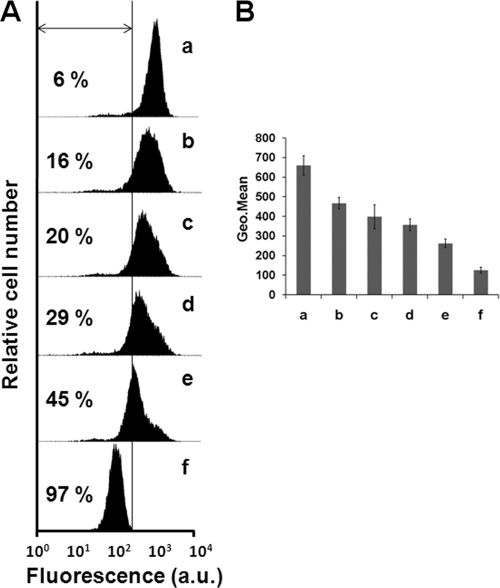
The decrease in intracellular ATP levels caused by TFQ is consistent with impairment of the processes involved in ATP synthesis. In Leishmania, mitochondrial oxidative phosphorylation accounts for most of the ATP expenditure of the parasite, with glycolysis being of minor importance in this process (37). As ΔΨm is essential to drive mitochondrial ATP synthesis, its variation with TFQ was monitored in Leishmania promastigotes by cytofluorometry using Rh123 accumulation. After the addition of 5 μM TFQ to promastigotes, in the first 30 min, dye accumulation falls to levels approximately 7 times lower than that for the untreated parasites, similar to those obtained by incubation of parasites with the uncoupling reagent FCCP (10 μM, 30 min), used as control for full depolarized promastigotes (Fig. 2).
FIG. 2.
ΔΨm depolarization induced by TFQ. L. donovani promastigotes were treated with 5 μM TFQ for 1, 5, 10, and 30 min (b to e, respectively) and analyzed for fluorescence by flow cytometry, after being stained with 0.8 μM Rh123. TFQ-untreated parasites were used as a control (a), and treatment with 10 μM FCCP for 10 min was used as a depolarization control (f). (A) Histogram of a representative experiment. Percentages of depolarized cells are shown. (B) Geometrical (Geo.) mean channel fluorescence values ± SD from three experiments versus the control were significantly different by Student's t test (P < 0.05).
Inhibition of CcR activity by TFQ.
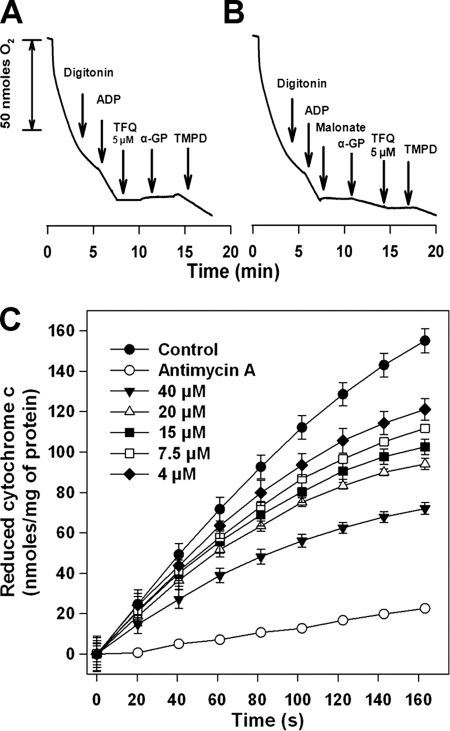
Mitochondrial respiratory chain complexes I, III, and IV drive the maintenance of ΔΨm through the proton pumping generated during electron transfer. In trypanosomatids, the contribution of complex I to this process was questioned as it lacks subunits essential for H+ extrusion present in other eukaryotes (30). We hypothesized whether the observed TFQ-induced ΔΨm depolarization may correspond to a specific inhibition within the respiratory chain. To pinpoint the TFQ target in the respiratory chain of Leishmania, parasites were permeabilized with digitonin to allow unrestricted access of specific substrates and inhibitors to the different complexes of the respiratory chain. Preservation of the integrity of the inner mitochondrial membrane was demonstrated by the increase of respiration after the addition of ADP (state 3), as it is no longer the limiting substrate for oxidative phosphorylation. At this state, using succinate as the sole substrate that feeds complex II (Fig. 3A and B), the oxygen consumption rate of digitonin-permeabilized parasites was 8.9 ± 0.6 nmol × min−1 × 10−8 cells (Fig. 3A). The addition of 5 μM TFQ led to a full inhibition of this process (Fig. 3A), which was restored to 50% after addition of TMPD-ascorbate, an electron donor to cytochrome c (Fig. 3A). This excluded cytochrome c oxidase (complex IV) as the site for TFQ inhibition, and pinpoints the potential target as complex II or III (Fig. 4).
FIG. 3.
Identification of the inhibition site of the respiratory chain of Leishmania by TFQ. (A and B) Inhibition of the oxygen consumption rates of permeabilized L. donovani promastigotes in the presence of 5 mM succinate as the substrate. The arrows indicate the addition of the indicated substrates and inhibitors at their respective final concentrations as stated: 60 μM digitonin, 100 μM ADP, 0.1 mM TMPD plus 1.7 mM ascorbate, 2 mM malonate, 6.7 mM α-glycerophosphate (α-GP), and 5 μM TFQ. (C) Inhibition of CcR activity by TFQ. CcR activity ± SD was monitored by the increase of absorbance at 550 nm due to the reduction of initially oxidized cytochrome c solution (32 μM) after addition of different TFQ concentrations to mitochondrial fraction as described in Materials and Methods. Samples without succinate or in the presence of antimycin A, as the inhibitor of CcR, were considered as controls. Activity was measured at 37°C.
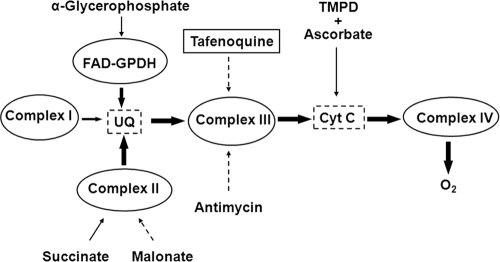
FIG. 4.
Scheme of the respiratory chain in Leishmania with the specific substrates and inhibitors used in this study. Sites of electron feeding or inhibition are indicated by solid or dotted lines, respectively. UQ, ubiquinone; Cyt C, cytochrome c. Feeding of the respiratory chain by α-glycerophosphate through flavin adenine dinucleotide-dependent glycerophosphate dehydrogenase (FAD-GPDH) is according to references 13 and 30.
In Leishmania, the pool of reduced ubiquinone used by complex III as substrate to reduce cytochrome c comes from two sources: the mitochondrial FAD-dependent glycerol-3-phosphate dehydrogenase, channeling the α-glycerophosphate produced by glycolysis (13); and complex II, which is of much higher relevance in Leishmania, using succinate as substrate. The addition of 5 μM TFQ led to a full inhibition of the oxygen consumption rate using succinate as sole substrate (Fig. 3A), and a further addition of 6.7 mM α-glycerophosphate did not reverse at all the inhibition caused by TFQ (Fig. 3A). The modest rate increase obtained by α-glycerophosphate after full inhibition of complex II by malonate was also inhibited after the addition of TFQ (Fig. 3B). Altogether, these results pinpointed complex III (cytochrome c reductase [CcR]) as the main target for TFQ inside the respiratory chain. To corroborate this result, the inhibition of CcR by increasing TFQ concentrations was assayed in mitochondrial fractions. Figure 3C shows the specific dose-dependent inhibition of CcR activity by TFQ. Total inhibition of this enzyme was achieved with 2 μM antimycin A.
ROS production in Leishmania by TFQ.
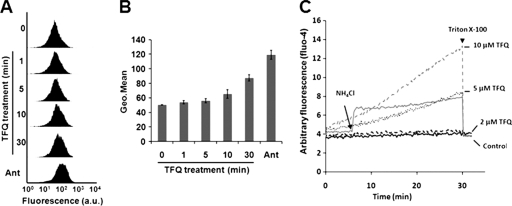
Due to the inherent relationship between ROS generation and respiratory chain inhibition, and as respiratory complex III was described as the main source of superoxide anion radicals in both mammals and Leishmania (12, 24), we explored the potential of TFQ to induce ROS production. Leishmania promastigotes were incubated with the ROS-sensitive probe MitoSOX Red and treated with 5 μM TFQ for 30 min. Flow cytometry analysis showed a time-dependent increase in ROS generation after treatment with TFQ (Fig. 5A and B). As a positive control for ROS generation, parasites were treated with antimycin A (0.3 μg/ml) as reported previously (24).
FIG. 5.
TFQ induces ROS generation and changes in cytosolic Ca2+ levels. ROS levels were measured using the specific fluorescent dye MitoSOX Red. L. donovani promastigotes preloaded with 0.5 μM MitoSOX Red were incubated without (0) and with 5 μM TFQ at the time points indicated. Antimycin A (Ant) (0.3 μg/ml, 30 min) was used as the control of ROS generation. Fluorescence intensity (arbitrary units [a.u.]) was determined by flow cytometry analysis, as described in Materials and Methods. (A) Histogram of a representative experiment. (B) Geometrical (Geo.) mean channel fluorescence values ± SD of three experiments were significantly different versus control by Student's t test (P < 0.05), except for parasites treated for 1 min. (C) Variation in cytosolic Ca2+ levels of L. donovani promastigotes. Fluo4-preloaded parasites were treated with different concentrations of TFQ as indicated and then analyzed for increasing fluorescence over a period of 30 min at 28°C using an Aminco-Bowman series 2 spectrometer. The experiments were assessed in the presence of Ca2+ chelator EGTA. The arrow indicates the addition of NH4Cl, taken as positive control. The arrowhead (t = 30 min) indicates the addition of 0.05% Triton X-100 to assess complete permeabilization of parasites. The trace “Control” represents TFQ-untreated parasites. Similar results were obtained in three independent experiments.
TFQ increases free cytosolic Ca2+ level.
Mitochondrial damage, exemplified by ΔΨm depolarization, is frequently associated not only with ROS production but also with an increase of intracellular Ca2+ concentration (28). The relevance of Ca2+ mobilization from intracellular stores during the progression of apoptosis in Leishmania has been reported previously (33). With this premise in mind, we studied the variation of cytosolic Ca2+ levels in both control and TFQ-treated Leishmania promastigotes, using Fluo4 as a calcium fluorescent probe. In the presence of EGTA, 5 and 10 μM TFQ induced Ca2+ mobilization from its intracellular stores, resulting in a significant increase of cytosolic Ca2+ concentration (Fig. 5C). In the absence of EGTA, parasites treated for 30 min with 5 μM TFQ showed a Ca2+-bound Fluo4 fluorescence 30% higher than that obtained with EGTA (data not shown), suggesting that mobilization from intracellular stores is the main source of the observed cytosolic Ca+2 increase.
TFQ is partly localized in acidocalcisomes.
Acidocalcisomes are important Ca2+ reservoirs in Leishmania (10); this, together with our previous report on the privileged accumulation of sitamaquine, a close analogue of TFQ (17), in these organelles, prompted us to investigate whether TFQ may accumulate in acidocalcisomes, inducing the release of Ca2+.
First, we studied the decrease in the accumulation of the acidotropic probe LysoTracker Green, mediated by TFQ through the alkalinization of acidic organelles. The results showed that TFQ reduced the cellular accumulation of LysoTracker Green measured by flow cytometry (Fig. 6A), due, at least in part, to an increase in the pH of acidocalcisomes induced by TFQ. The feasibility of quenching of the LysoTracker Green fluorescence signal by TFQ was discarded by spectrofluorometry (data not shown).
FIG. 6.
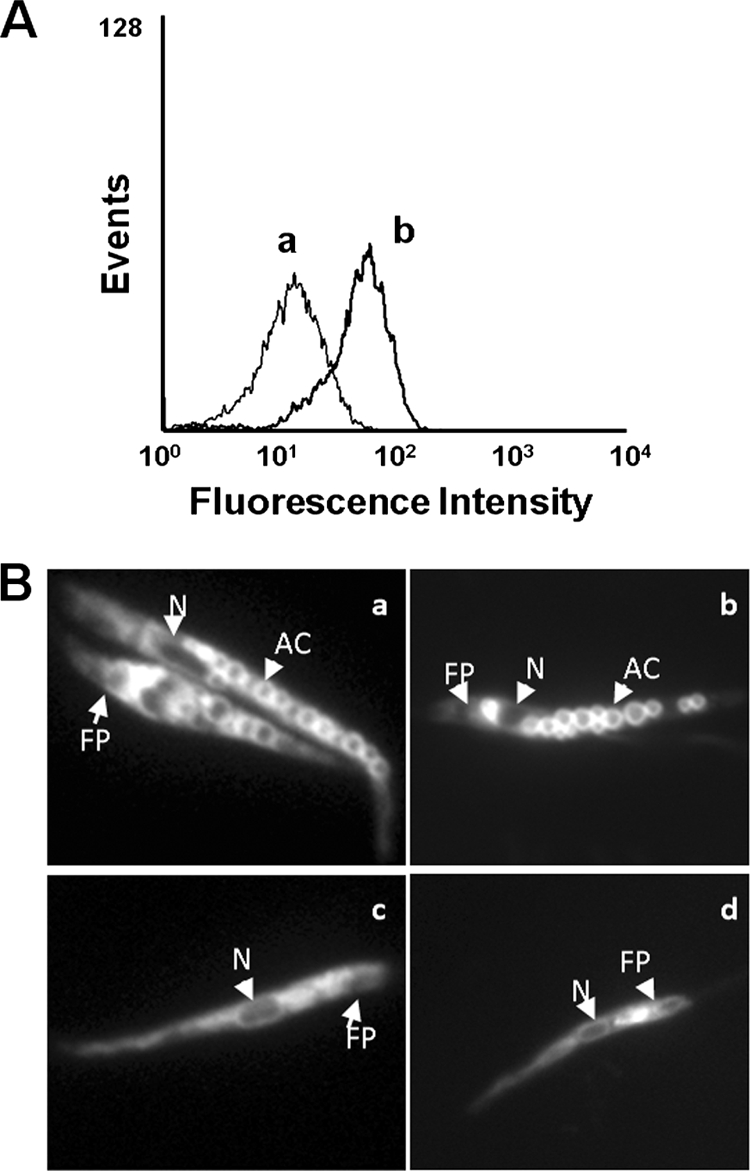
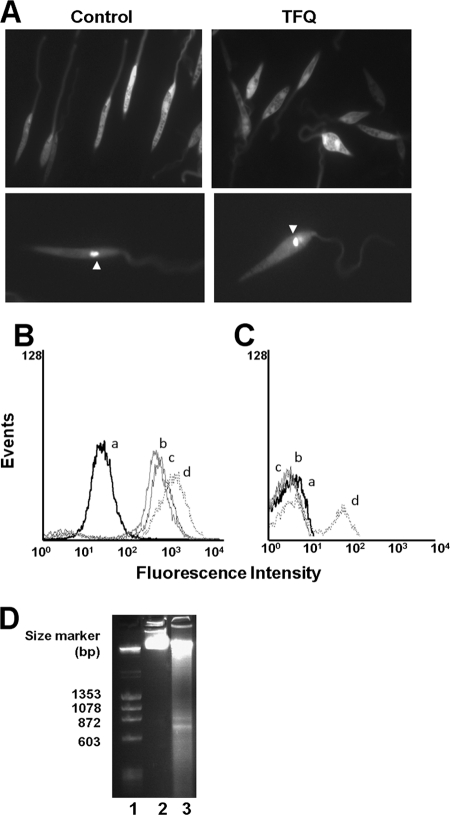
Intracellular localization of TFQ in Leishmania. (A) L. donovani promastigotes were pretreated with (a) and without (b) 5 μM TFQ for 10 min at 28°C and then labeled with 100 nM Lysotracker Green DND-26 for 10 min. Fluorescence was analyzed by flow cytometry as described in Materials and Methods. Representative histograms of three independent experiments are shown. (B) Intracellular localization of TFQ and Lysotracker Green in the control (a and b, respectively) and ΔAP3 (c and d, respectively) L. major promastigote lines were visualized by fluorescence microscopy after incubation with 5 μM TFQ or 100 nM Lysotracker Green DND-26 for 10 min at 28°C, as described in Materials and Methods. AC, acidocalcisome; N, nucleus; FP, flagellar pocket.
Subsequently, the intrinsic fluorescence of TFQ was used to assess its intracellular distribution. Fluorescence microscopy images showed a vesiculated pattern throughout the whole cell body (Fig. 6Ba), resembling that for acidocalcisomes stained with LysoTracker Green (Fig. 6Bb), pointing toward a privileged accumulation of TFQ in these organelles. Apparently, TFQ did not localize in the acidocalcisomes of L. major ΔAP3 (Fig. 6Bc); a mutant line that is devoid of several acidocalcisome membrane proteins, which is therefore defective for polyphosphate accumulation and internal acidic milieu, and has an alkaline pH inside these organelles (2). As no differences for TFQ sensitivity were observed between the wild type and the ΔAP3 mutant, resembling the results with sitamaquine, the acidocalcisome could be rule out as final target for TFQ (17). However, an increase in pH reduced the TFQ fluorescence intensity (data not shown), and consequently, we cannot exclude the possibility that TFQ accumulates in the acidocalcisomes of the ΔAP3 mutant.
TFQ induces an apoptosis-like death in Leishmania.
Since elevation of cytosolic Ca2+, mitochondrial dysfunction, ROS generation, and reduction of intracellular ATP levels are all distinctive events during the progression of an apoptosis-like death process, we examined this possibility for TFQ. In trypanosomatids, there are several classes of programmed cell death (25). The induction of autophagy by TFQ was determined by using an L. donovani line overexpressing the autophagosome marker GFP-ATG8 (3). No significant increase in the number of autophagosomes was observed after 24 h of TFQ treatment versus untreated parasites (Fig. 7A). Accordingly, in a further step, TFQ-induced DNA nicking, typical of apoptotic-like processes, was tested using the TUNEL assay. Parasites treated with different concentrations of TFQ for 4 h were significantly TUNEL positive (Fig. 7B). Necrotic cell death was rejected due to the lack of parasite staining with PI, as it requires a permeable plasma membrane in order to reach the intracellular nucleic acid. Only at 20 μM TFQ, a concentration 4-fold higher than the EC50, were parasites PI positive (Fig. 7C). These conclusions were confirmed by the fragmentation of genomic DNA in parasites treated with 5 μM TFQ for 4 h (Fig. 7D).
FIG. 7.
TFQ induces programmed cell death in Leishmania. (A) Autophagosome formation in L. donovani promastigotes. (Upper panel) Distribution of GFP-ATG8 in L. donovani promastigotes untreated or treated with 5 μM TFQ. (Lower panel) Autophagosomes could be identified as punctate structures clearly observable in the cytoplasm (arrowhead). (B and C) Representative histogram of TUNEL analysis and PI labeling, respectively, of L. donovani promastigotes treated with TFQ. Parasites were treated with different concentrations of TFQ: 5 (b), 10 (c) and 20 (d) μM for 4 h at 28°C, using untreated parasites (a) as controls. Fluorescein-dUTP and PI nucleic acid labeling were analyzed by flow cytometry as described in Materials and Methods. PI was used as the control of necrosis. Histograms are representative of three independent experiments with 10,000 parasites analyzed per group. (D) DNA fragmentation in L. donovani promastigotes. Genomic DNAs were isolated from parasites either untreated (lane 2) or treated for 4 h with 5 μM TFQ in HBS (lane 3), run through a 2% agarose gel, and visualized by ethidium bromide as described in Materials and Methods. DNA size markers (lane 1) are shown in base pairs (bp).
DISCUSSION
The 8-aminoquinoline scaffold has been extensively used in the development of antiprotozoal drugs (36), typically as antiplasmodial compounds. The most recent application for these drugs is the use of sitamaquine and TFQ as alternative leishmanicidal compounds, currently at different stages of implementation (35, 41). Unfortunately their mechanism of action is scarcely known, jeopardizing the rational optimization of new analogues. In order to address this problem, we decided to outline the leishmanicidal mechanism of TFQ.
At the concentrations at which TFQ inhibits promastigote proliferation, the most conspicuous effect observed is the rapid drop in the intracellular ATP levels. A similar effect has been described for the mechanism of action of other potential and established leishmanicidal drugs (19-23). Arguably, the two most likely hypotheses to account for this result are (i) inhibition of ATP synthesis and (ii) permeabilization of the plasma membrane with release of intracellular ATP into the external medium. The last hypothesis is typical for the membrane active reagents, including antimicrobial peptides and polyenes such as amphotericin B (21, 22). Nevertheless, even at concentrations producing above 95% growth inhibition, entry of the vital dye SYTOX Green only reached a small percentage (11%) of that corresponding to full permeabilization. Thus, the observed drop in ATP levels is more likely to be due to inhibition of ATP synthesis. In Leishmania, approximately 70% of the total bioenergetic requirements are fulfilled by oxidative phosphorylation, tightly coupled with ΔΨm, which appeared to be decreased in TFQ-treated parasites. This prompted us to carry out a systematic study to identify the TFQ target among the different complexes involved in oxidative phosphorylation. As a result, complex III was defined as its main target; this statement was further validated by the inhibition of CcR in vitro by TFQ. It is noteworthy that, complex III is the crossroads where the reduced ubiquinone pools from complex II and the oxidation of α-glycerophosphate converge (13).
There is a wealth of knowledge linking 8-aminoquinolines to mitochondrial dysfunction in their respective target cells, such as primaquine in Plasmodium falciparum gametocytes (16) or sitamaquine in Leishmania mitochondria (39). The Leishmania mitochondrion is the target for a wide variety of leishmanicidal drugs; including some in clinical use such as pentamidine (27) and miltefosine (20), and others at different stages of development, such as chalcones or histatin 5, (5, 23). The inhibition of complex III by TFQ led to the production of superoxide radicals at levels similar to those produced by antimycin A (24). ROS, either produced inside the cell by drugs (7) or added exogenously (6), trigger an apoptosis-like death process.
Programmed cell death (PCD) was classified by biochemical and morphological criteria under three major classes, which are not mutually exclusive (25). Autophagy was discarded as being the predominant PCD induced by TFQ, as no significant differences were found between treated and control parasites for distribution and expression levels of the autophagosome marker GFP-ATG8. Necrosis (another PCD class) was also ruled out as plasma membrane disruption (one of its hallmarks) only reached significant values at concentrations far higher than those that are lethal. In contrast, TFQ-treated parasites showed typical features for apoptosis-like PCD, including a decrease in ΔΨm (28), a rise in intracellular Ca2+ levels, and late-stage apoptosis markers (such as DNA nucleosomal fragmentation), which is similar in many aspects to that described in metazoan organisms (6).
The increased intracellular Ca2+ levels induced by TFQ came from both an external medium and intracellular stores, as the addition of EGTA led to only a 30% abrogation. The entry of external Ca2+ was described in Leishmania apoptosis induced by external H2O2 (28), camptothecin (33), and curcumin (7) and mediated by specific channels activated by ROS or its derived metabolites inside the cells (14). Accordingly, the remaining 70% came from intracellular stores, mainly the endoplasmic reticulum, glycosomes, acidocalcisomes, and/or mitochondria (26).
All these molecular events activate PCD with DNA nicking and fragmentation as the final outcome, both of which are characteristic of an apoptosis-like death in Leishmania. Studies are currently in progress to determine whether other potential leishmanicidal 8-aminoquinolines act through a similar mechanism. In conclusion, our studies suggest that TFQ inhibits the functionality of the mitochondrial respiratory chain through CcR (complex III) inhibition, inducing a rapid drop in the intracellular ATP levels in Leishmania. At the same time, the increase in mitochondrial ROS production and elevation of intracellular Ca2+ leads to a depolarization of ΔΨm. Taken together, these biological events induced by TFQ, trigger an apoptosis-like death in Leishmania.
Acknowledgments
This work was supported by the Spanish grants SAF2006-02093 (to F.G.), ISCIII-Red de Investigación Cooperativa en Enfermedades Tropicales (RICET)-FEDER RD06/0021/0002 (F.G.) and RD 06/0021/0006 (L.R.) European Union (HEALTH—2007-223414) (L.R.) and FIS PS09-01928 (L.R.) and by the Plan Andaluz de Investigación (Cod. BIO130).
We acknowledge the support of GlaxoSmithKline (Greenford, United Kingdom) for the tafenoquine used throughout this research work.
Footnotes
Published ahead of print on 13 September 2010.
REFERENCES
- 1.Alvarez-Fortes, E., L. M. Ruiz-Perez, F. Bouillaud, E. Rial, and L. Rivas. 1998. Expression and regulation of mitochondrial uncoupling protein 1 from brown adipose tissue in Leishmania major promastigotes. Mol. Biochem. Parasitol. 93:191-202. [DOI] [PubMed] [Google Scholar]
- 2.Besteiro, S., D. Tonn, L. Tetley, G. H. Coombs, and J. C. Mottram. 2008. The AP3 adaptor is involved in the transport of membrane proteins to acidocalcisomes of Leishmania. J. Cell Sci. 121:561-570. [DOI] [PubMed] [Google Scholar]
- 3.Besteiro, S., R. A. Williams, L. S. Morrison, G. H. Coombs, and J. C. Mottram. 2006. Endosome sorting and autophagy are essential for differentiation and virulence of Leishmania major. J. Biol. Chem. 281:11384-11396. [DOI] [PubMed] [Google Scholar]
- 4.Chappuis, F., S. Sundar, A. Hailu, H. Ghalib, S. Rijal, R. W. Peeling, J. Alvar, and M. Boelaert. 2007. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat. Rev. Microbiol. 5:873-882. [DOI] [PubMed] [Google Scholar]
- 5.Chen, M., L. Zhai, S. B. Christensen, T. G. Theander, and A. Kharazmi. 2001. Inhibition of fumarate reductase in Leishmania major and L. donovani by chalcones. Antimicrob. Agents Chemother. 45:2023-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das, M., S. B. Mukherjee, and C. Shaha. 2001. Hydrogen peroxide induces apoptosis-like death in Leishmania donovani promastigotes. J. Cell Sci. 114:2461-2469. [DOI] [PubMed] [Google Scholar]
- 7.Das, R., A. Roy, N. Dutta, and H. K. Majumder. 2008. Reactive oxygen species and imbalance of calcium homeostasis contributes to curcumin induced programmed cell death in Leishmania donovani. Apoptosis 13:867-882. [DOI] [PubMed] [Google Scholar]
- 8.den Boer, M. L., J. Alvar, R. N. Davidson, K. Ritmeijer, and M. Balasegaram. 2009. Developments in the treatment of visceral leishmaniasis. Expert Opin. Emerg. Drugs 14:395-410. [DOI] [PubMed] [Google Scholar]
- 9.Diaz-Achirica, P., J. Ubach, A. Guinea, D. Andreu, and L. Rivas. 1998. The plasma membrane of Leishmania donovani promastigotes is the main target for CA(1-8)M(1-18), a synthetic cecropin A-melittin hybrid peptide. Biochem. J. 330:453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Docampo, R., W. de Souza, K. Miranda, P. Rohloff, and S. N. Moreno. 2005. Acidocalcisomes—conserved from bacteria to man. Nat. Rev. Microbiol. 3:251-261. [DOI] [PubMed] [Google Scholar]
- 11.Dolai, S., R. K. Yadav, S. Pal, and S. Adak. 2009. Overexpression of mitochondrial Leishmania major ascorbate peroxidase enhances tolerance to oxidative stress-induced programmed cell death and protein damage. Eukaryot. Cell 8:1721-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drose, S., and U. Brandt. 2008. The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J. Biol. Chem. 283:21649-21654. [DOI] [PubMed] [Google Scholar]
- 13.Guerra, D. G., A. Decottignies, B. M. Bakker, and P. A. Michels. 2006. The mitochondrial FAD-dependent glycerol-3-phosphate dehydrogenase of Trypanosomatidae and the glycosomal redox balance of insect stages of Trypanosoma brucei and Leishmania spp. Mol. Biochem. Parasitol. 149:155-169. [DOI] [PubMed] [Google Scholar]
- 14.Halliwell, B., and J. M. Gutteridge. 1990. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 186:1-85. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy, M. L., F. Cortes-Selva, J. M. Perez-Victoria, I. A. Jimenez, A. G. Gonzalez, O. M. Munoz, F. Gamarro, S. Castanys, and A. G. Ravelo. 2001. Chemosensitization of a multidrug-resistant Leishmania tropica line by new sesquiterpenes from Maytenus magellanica and Maytenus chubutensis. J. Med. Chem. 44:4668-4676. [DOI] [PubMed] [Google Scholar]
- 16.Lanners, H. N. 1991. Effect of the 8-aminoquinoline primaquine on culture-derived gametocytes of the malaria parasite Plasmodium falciparum. Parasitol. Res. 77:478-481. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Martin, C., J. M. Perez-Victoria, L. Carvalho, S. Castanys, and F. Gamarro. 2008. Sitamaquine sensitivity in Leishmania species is not mediated by drug accumulation in acidocalcisomes. Antimicrob. Agents Chemother. 52:4030-4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu, H. G., L. Zhong, K. P. Chang, and R. Docampo. 1997. Intracellular Ca2+ pool content and signaling and expression of a calcium pump are linked to virulence in Leishmania mexicana amazonesis amastigotes. J. Biol. Chem. 272:9464-9473. [DOI] [PubMed] [Google Scholar]
- 19.Luque-Ortega, J. R., S. Martinez, J. M. Saugar, L. R. Izquierdo, T. Abad, J. G. Luis, J. Pinero, B. Valladares, and L. Rivas. 2004. Fungus-elicited metabolites from plants as an enriched source for new leishmanicidal agents: antifungal phenyl-phenalenone phytoalexins from the banana plant (Musa acuminata) target mitochondria of Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 48:1534-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luque-Ortega, J. R., and L. Rivas. 2007. Miltefosine (hexadecylphosphocholine) inhibits cytochrome c oxidase in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 51:1327-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luque-Ortega, J. R., O. M. Rivero-Lezcano, S. L. Croft, and L. Rivas. 2001. In vivo monitoring of intracellular ATP levels in Leishmania donovani promastigotes as a rapid method to screen drugs targeting bioenergetic metabolism. Antimicrob. Agents Chemother. 45:1121-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luque-Ortega, J. R., J. M. Saugar, C. Chiva, D. Andreu, and L. Rivas. 2003. Identification of new leishmanicidal peptide lead structures by automated real-time monitoring of changes in intracellular ATP. Biochem. J. 375:221-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luque-Ortega, J. R., W. van't Hof, E. C. Veerman, J. M. Saugar, and L. Rivas. 2008. Human antimicrobial peptide histatin 5 is a cell-penetrating peptide targeting mitochondrial ATP synthesis in Leishmania. FASEB J. 22:1817-1828. [DOI] [PubMed] [Google Scholar]
- 24.Mehta, A., and C. Shaha. 2004. Apoptotic death in Leishmania donovani promastigotes in response to respiratory chain inhibition: complex II inhibition results in increased pentamidine cytotoxicity. J. Biol. Chem. 279:11798-11813. [DOI] [PubMed] [Google Scholar]
- 25.Menna-Barreto, R. F., K. Salomao, A. P. Dantas, R. M. Santa-Rita, M. J. Soares, H. S. Barbosa, and S. L. de Castro. 2009. Different cell death pathways induced by drugs in Trypanosoma cruzi: an ultrastructural study. Micron 40:157-168. [DOI] [PubMed] [Google Scholar]
- 26.Moreno, S. N., and R. Docampo. 2003. Calcium regulation in protozoan parasites. Curr. Opin. Microbiol. 6:359-364. [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee, A., P. K. Padmanabhan, M. H. Sahani, M. P. Barrett, and R. Madhubala. 2006. Roles for mitochondria in pentamidine susceptibility and resistance in Leishmania donovani. Mol. Biochem. Parasitol. 145:1-10. [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee, S. B., M. Das, G. Sudhandiran, and C. Shaha. 2002. Increase in cytosolic Ca2+ levels through the activation of non-selective cation channels induced by oxidative stress causes mitochondrial depolarization leading to apoptosis-like death in Leishmania donovani promastigotes. J. Biol. Chem. 277:24717-24727. [DOI] [PubMed] [Google Scholar]
- 29.Nanayakkara, N. P., A. L. Ager, Jr., M. S. Bartlett, V. Yardley, S. L. Croft, I. A. Khan, J. D. McChesney, and L. A. Walker. 2008. Antiparasitic activities and toxicities of individual enantiomers of the 8-aminoquinoline 8-[(4-amino-1-methylbutyl)amino]-6-methoxy-4-methyl-5-[3,4-dichlorophenoxy] quinoline succinate. Antimicrob. Agents Chemother. 52:2130-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Opperdoes, F. R., and P. A. Michels. 2008. Complex I of Trypanosomatidae: does it exist? Trends Parasitol. 24:310-317. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Victoria, F. J., F. Gamarro, M. Ouellette, and S. Castanys. 2003. Functional cloning of the miltefosine transporter. A novel P-type phospholipid translocase from Leishmania involved in drug resistance. J. Biol. Chem. 278:49965-49971. [DOI] [PubMed] [Google Scholar]
- 32.Piacenza, L., F. Irigoin, M. N. Alvarez, G. Peluffo, M. C. Taylor, J. M. Kelly, S. R. Wilkinson, and R. Radi. 2007. Mitochondrial superoxide radicals mediate programmed cell death in Trypanosoma cruzi: cytoprotective action of mitochondrial iron superoxide dismutase overexpression. Biochem. J. 403:323-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sen, N., B. B. Das, A. Ganguly, T. Mukherjee, S. Bandyopadhyay, and H. K. Majumder. 2004. Camptothecin-induced imbalance in intracellular cation homeostasis regulates programmed cell death in unicellular hemoflagellate Leishmania donovani. J. Biol. Chem. 279:52366-52375. [DOI] [PubMed] [Google Scholar]
- 34.Sottocasa, G. L., B. Kuylenstierna, L. Ernster, and A. Bergstrand. 1967. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J. Cell Biol. 32:415-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundar, S., and M. Chatterjee. 2006. Visceral leishmaniasis—current therapeutic modalities. Indian J. Med. Res. 123:345-352. [PubMed] [Google Scholar]
- 36.Tekwani, B. L., and L. A. Walker. 2006. 8-Aminoquinolines: future role as antiprotozoal drugs. Curr. Opin. Infect. Dis. 19:623-631. [DOI] [PubMed] [Google Scholar]
- 37.Van Hellemond, J. J., and A. G. Tielens. 1997. Inhibition of the respiratory chain results in a reversible metabolic arrest in Leishmania promastigotes. Mol. Biochem. Parasitol. 85:135-138. [DOI] [PubMed] [Google Scholar]
- 38.Vercesi, A. E., C. F. Bernardes, M. E. Hoffmann, F. R. Gadelha, and R. Docampo. 1991. Digitonin permeabilization does not affect mitochondrial function and allows the determination of the mitochondrial membrane potential of Trypanosoma cruzi in situ. J. Biol. Chem. 266:14431-14434. [PubMed] [Google Scholar]
- 39.Vercesi, A. E., and R. Docampo. 1992. Ca2+ transport by digitonin-permeabilized Leishmania donovani. Effects of Ca2+, pentamidine and WR-6026 on mitochondrial membrane potential in situ. Biochem. J. 284:463-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vergnes, B., B. Gourbal, I. Girard, S. Sundar, J. Drummelsmith, and M. Ouellette. 2007. A proteomics screen implicates HSP83 and a small kinetoplastid calpain-related protein in drug resistance in Leishmania donovani clinical field isolates by modulating drug-induced programmed cell death. Mol. Cell Proteomics 6:88-101. [DOI] [PubMed] [Google Scholar]
- 41.Yardley, V., F. Gamarro, and S. L. Croft. 2010. Antileishmanial and antitrypanosomal activities of the 8-aminoquinoline tafenoquine. Antimicrob. Agents Chemother. 54:XXX-XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]