Abstract
The mechanism of stepwise acquired multidrug resistance in Acinetobacter baumannii isolates from a hospitalized patient was investigated. Thirteen consecutive multidrug-resistant isolates were recovered from the same patient over a 2-month period. The Vitek 2 system identified the isolates as meropenem-sensitive Acinetobacter lwoffii; however, molecular identification showed that the isolates were A. baumannii. Etest revealed that the isolates were meropenem resistant. The presence of oxacillinase (OXA)-type enzymes were investigated by sequencing. The clonal relatedness of isolates was assessed by pulsed-field gel electrophoresis (PFGE). Expression of the genes encoding the efflux pumps AdeB and AdeJ was performed by semiquantitative real-time reverse transcription-PCR (qRT-PCR). The adeRS two-component system was sequenced. All isolates had identical PFGE fingerprints, suggesting clonal identity. The first six isolates were positive for the novel blaOXA-164 gene. The following seven isolates, recovered after treatment with a combination of meropenem, amikacin, ciprofloxacin, and co-trimoxazole showed an increase of >7-fold in adeB mRNA transcripts and a missense mutation in blaOXA-164, converting it to blaOXA-58. Sequencing revealed a novel mutation in adeR. These data illustrate how A. baumannii can adapt during antimicrobial therapy, leading to increased antimicrobial resistance.
Acinetobacter baumannii is a serious nosocomial pathogen. Infections are difficult to treat, as the organism is often multidrug resistant (4). The carbapenems have had good activity against A. baumannii, but resistance to this drug class is rising mainly through the action of intrinsic or acquired oxacillinases (OXAs) (18). OXAs exhibit weak activity against the carbapenems, but when they are overexpressed, they can confer resistance. This overexpression is associated with the presence of insertion elements, which are also a significant factor in A. baumannii multidrug resistance and genome plasticity (16, 19, 22). For example, the insertion element ISAba1 upregulates expression of the intrinsic blaOXA-51 gene and the efflux pump adeABC genes, but it can also mobilize antibiotic resistance genes (16, 21, 26). Efflux pumps such as AdeB and AdeJ also contribute to multidrug resistance (3, 14). Overexpression of these pumps has been selected in vivo where they also play a major role in the development of resistance to tigecycline (9, 21).
The aim of this study was to investigate the molecular epidemiology and the mechanism of increasing antimicrobial resistance observed in sequential A. baumannii isolates recovered from a patient during a prolonged hospitalization.
CASE REPORT
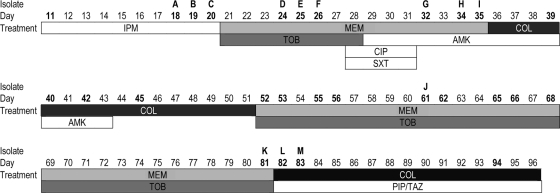
A 58-year-old patient was initially admitted to a community hospital for personality changes and confusion. After the diagnosis of an extremely large optic nerve sheath meningioma was established, the patient was transferred to the neurosurgery department of the Cologne University hospital in Cologne, Germany. The patient's condition rapidly deteriorated, and he required intubation and mechanical ventilation. The patient developed ventilator-associated pneumonia. Methicillin-resistant Staphylococcus aureus was isolated from a tracheal secretion, and the patient was started on vancomycin therapy. A follow-up sample obtained 11 days after admission to the hospital revealed a multiresistant Acinetobacter species that was identified by the Vitek 2 system (bioMérieux, Nürtingen, Germany) as Acinetobacter lwoffii, and therapy was switched to imipenem, although the isolate was tested intermediate to imipenem (MIC of 8 μg/ml). Since the patient did not improve, the antimicrobial therapy was changed several times during the patient's 96-day stay in the hospital (Fig. 1), including a short period of ciprofloxacin and co-trimoxazole for empirical coverage of Stenotrophomonas maltophilia (days 27 to 31). The Acinetobacter isolates after the ciprofloxacin-co-trimoxazole treatment course were increasingly resistant, however, and remained susceptible to colistin, which the patient received as intravenous (i.v.) therapy. Despite several attempts using combination therapy (Fig. 1), the multidrug-resistant organism could not be cleared from the respiratory tract and was later also isolated from blood cultures. Due to the poor prognosis of the underlying disease, an end-of-life decision was made, and the patient died 96 days after admission.
FIG. 1.
Timeline of antimicrobial therapy and isolation of A. baumannii. The numbers in boldface type are the dates (number of days in the hospital) after the patient was admitted to the hospital when A. baumannii was isolated. Isolates A to M were retained for further investigation. Drug abbreviations: IPM, imipenem; MEM, meropenem; TOB, tobramycin; CIP, ciprofloxacin; SXT, trimethoprim-sulfamethoxazole; AMK, amikacin; COL, colistin; PIP/TAZ, piperacillin-tazobactam.
MATERIALS AND METHODS
Bacterial isolates.
A total of 33 Acinetobacter isolates were recovered from the patient over 96 days (Fig. 1). The majority of isolates were recovered from tracheal secretions, but they were also recovered from tracheostoma (day 26), nose (day 32), bedsore (day 65), and bloodstream (days 81 and 82). Thirteen of these isolates were retained for further investigation (isolates A to M). Isolates were identified as A. lwoffii by the Vitek 2 system. Species identification was repeated by gyrB multiplex (7).
Susceptibility testing.
Antimicrobial susceptibility of all isolates was initially tested by the Vitek 2 system using AST-N118 and AST-N110 cards. The susceptibilities of isolates A to M to imipenem, meropenem, tigecycline, levofloxacin, and tobramycin were tested by Etest (bioMérieux) following the manufacturer's instructions and retested by the Vitek 2 system. The breakpoint values used for interpretation were those proposed by the Clinical and Laboratory Standards Institute (CLSI) (2). Tigecycline MIC breakpoints are currently not available from CLSI for A. baumannii.
Molecular typing.
Isolates were molecularly typed by repetitive-sequence-based PCR (rep-PCR) using the DiversiLab system (bioMérieux) and pulsed-field gel electrophoresis (PFGE) as previously described (5, 23).
Presence of carbapenemase genes.
On the basis of the results of carbapenem susceptibility, the isolates were screened for known blaOXA carbapenemase genes and sequenced (28). The presence of insertion elements was investigated as previously described (19, 26). Plasmids were isolated from the strains and used to transform electrocompetent A. baumannii ATCC 19606 and used as PCR template for the presence of blaOXA carbapenemases to determine whether resistance to carbapenem was encoded in genes carried on a plasmid.
Cloning blaOXA-164 and blaOXA-58.
The genes encoding oxacillinase 164 (OXA-164) and OXA-58 were amplified from isolates A and G using primers oxa-164/58_up and oxa-164/58_down (Table 1), which include 673 bp upstream of the start codon. PCR products were ligated into EcoRI/BamHI double digested pWH1266 which is an Escherichia coli-Acinetobacter shuttle plasmid (11) using the In-Fusion dry-down PCR cloning kit (Clontech, Saint-Germain-en-Laye, France) and transformed in E. coli NEB 5-alpha (New England BioLabs, Frankfurt, Germany). Plasmids were isolated from transformants and used to transform electrocompetent A. baumannii ATCC 19606 and A. baumannii ATCC 17978 selected on Mueller-Hinton agar containing 100 μg/ml ticarcillin. To confirm transfer of blaOXA-164 and blaOXA-58, plasmid inserts were sequenced. The susceptibility of the transformants to imipenem and meropenem was examined by Etest.
TABLE 1.
Primer sequences used in this study
| Primer | Primer sequence (5′-3′) | Application | Target gene(s) | Reference |
|---|---|---|---|---|
| rpob_rtF | GAGTCTAATGGCGGTGGTTC | qRT-PCR | rpoB housekeeping gene | 10 |
| rpob_rtR | ATTGCTTCATCTGCTGGTTG | qRT-PCR | rpoB housekeeping gene | 10 |
| adeB_rtF | GAATAAGGCACCGCAACAAT | qRT-PCR | adeB | This study |
| adeB_rtR | TTTCGCAATCAGTTGTTCCA | qRT-PCR | adeB | This study |
| adeJ_rtF | GCGAATGGACGTATGGTTCT | qRT-PCR | adeJ | This study |
| adeJ_rtR | CATTGCTTTCATGGCATCAC | qRT-PCR | adeJ | This study |
| adeR_rtF | TGGGTTAAAAGGCTTCACCA | qRT-PCR | adeR | This study |
| adeR_rtR | ACGCCAAAAAGCTCAGACTC | qRT-PCR | adeR | This study |
| adeS_rtF | GCATTTTTGACGGAAACCTC | qRT-PCR | adeS | This study |
| adeS_rtR | TTAGTCACGGCGACCTCTCT | qRT-PCR | adeS | This study |
| oxa51_rtF | GGAAGTGAAGCGTGTTGGTT | qRT-PCR | blaOXA-51 | This study |
| oxa51_rtR | CAAACTGTGCCTCTTGCTGA | qRT-PCR | blaOXA-51 | This study |
| oxa58_rtF | TCAAGAATTGGCACGTCGTA | qRT-PCR | blaOXA-58 | This study |
| oxa58_rtR | GCCCTTTCAACCAAAATTGA | qRT-PCR | blaOXA-58 | This study |
| oxa-164/58_up | TCGTCTTCAAGAATTCCCCTACGATCCGTGTTTTTG | Cloning blaOXA-164 and blaOXA-58 | blaOXA-164 and blaOXA-58 including the upstream promoter region | This study |
| oxa-164/58_down | CCGGCGTAGAGGATCCAATGACTTTGCTGAGGCTGAA | Cloning blaOXA-164 and blaOXA-58 | blaOXA-164 and blaOXA-58 including the upstream promoter region | This study |
| adeS_F | GAGGGAGTGCTCGAATTTGT | Sequencing | adeS and adeR including the promoter region of adeS | This study |
| adeR_R | GCTCAGCTTGAGCGACTTCT | Sequencing | adeS and adeR including the promoter region of adeS | This study |
qRT-PCR.
Expression of the efflux pump genes adeB and adeJ, the adeABC two-component system sensor (adeS) and regulator (adeR), the intrinsic oxacillinase blaOXA-51 gene, and the acquired blaOXA-58 and blaOXA-164 genes (gene with a missense mutation in blaOXA-164, converting it to blaOXA-58) were investigated in five selected isolates by semiquantitative reverse transcription-PCR (qRT-PCR) as previously described with minor modifications (9). Bacterial isolates were grown in Luria-Bertani broth until mid-log phase. Aliquots were treated with RNA protect (Qiagen, Hilden, Germany), and RNA was isolated using the RNeasy minikit (Qiagen) following the manufacturer's instructions. cDNA was synthesized from 1 μg of total RNA using Quantitect reverse transcription kit (Qiagen), and real-time PCR was performed in a LightCycler with Quantifast SYBR green (Qiagen) following the manufacturer's instructions. Primer sequences are shown in Table 1. All qRT-PCR was done in triplicate using freshly prepared cDNA. qRT-PCR was repeated at least three times. rpoB was used as a control housekeeping gene and was quantified in tandem with genes under investigation (10).
Characterization of adeB upregulation.
The genes encoding the two-component regulatory system AdeRS lie upstream of the adeABC operon. This area was investigated by sequencing. Primers adeS_F and adeR_R (Table 1) were designed using the published sequence of A. baumannii AB307-0294. The PCR product includes the upstream regions of both regulatory genes. PCR products from isolates A, F, G, and J were amplified using these primers and sequenced.
Nucleotide sequence accession numbers.
The nucleotide sequences reported in this paper have been submitted to the EMBL/GenBank nucleotide sequence database under accession numbers GU831575, HM440347, and HM440348.
RESULTS
Susceptibility testing.
The results of susceptibility testing are summarized in Table 2. The Vitek 2 system recorded all isolates as resistant to cefepime, cefotaxime, cefoxitin, ceftazidime, ciprofloxacin, moxifloxacin, fosfomycin, gentamicin, piperacillin, piperacillin-tazobactam, and ampicillin-sulbactam and susceptible only to colistin (≤1 μg/ml) and tobramycin (≤1 μg/ml) (except for isolates J and K). Amikacin MICs were ≥32 μg/ml for all isolates except for isolates D and E, which had MICs of ≤2 μg/ml. Tigecycline MICs were 2 μg/ml for isolates A to F and ≥8 μg/ml for isolates G to M. According to Vitek 2, all isolates except isolate A were resistant to imipenem, and only isolates G and M were resistant to meropenem (MIC of ≥16 μg/ml). Etest recorded higher MICs than Vitek 2 did (Table 2). In particular, 12 isolates were resistant to meropenem (MIC range, 16 to ≥64 μg/ml), and one was intermediate. Isolates A to F exhibited heteroresistance to imipenem and meropenem. We define heteroresistance as a resistant subpopulation appearing within the Etest inhibition zone ellipse (Fig. 2 and 3). The patient received combination antimicrobial therapy on days 28 to 31, consisting of meropenem, ciprofloxacin, amikacin, and co-trimoxazole (Fig. 1). Posttherapy isolates G to M recorded higher MICs against co-trimoxazole, tigecycline, meropenem, and levofloxacin than those isolated before therapy (Table 2). This simultaneous rise in MIC against four different drug classes was interpreted as enhanced efflux.
TABLE 2.
MIC values of clinical isolates as determined by Vitek 2 and Etest
| Isolate | blaOXA gene | Day of isolationa | Source of isolateb | MIC (μg/ml) of the indicated drugc as determined by: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitek 2 |
Etest |
|||||||||||||
| IPM | MEM | TGC | TOB | AMK | SXT | IPM | MEM | TGC | TOB | LEV | ||||
| A | blaOXA-164 | 18 | TS | 8 | 2 | 2 | ≤1 | ≥64 | 40 | 32* | 32* | 4 | 2 | 8 |
| B | blaOXA-164 | 19 | TS | ≥16 | 4 | 2 | ≤1 | ≥64 | 40 | 32* | 32* | 4 | 4 | 8 |
| C | blaOXA-164 | 20 | TS | ≥16 | 8 | 2 | ≤1 | 32 | 40 | 32* | 8 | 4 | 2 | 8 |
| D | blaOXA-164 | 24 | TS | ≥16 | 8 | 2 | ≤1 | ≤2 | 40 | 32* | 32* | 4 | 2 | 8 |
| E | blaOXA-164 | 25 | TS | ≥16 | 8 | 2 | ≤1 | ≤2 | 40 | 32* | 32* | 4 | 2 | 16 |
| F | blaOXA-164 | 26 | TST | ≥16 | 4 | 2 | ≤1 | ≥64 | 40 | 32* | 16* | 4 | 2 | 8 |
| G | blaOXA-58 | 32 | TS | ≥16 | ≥16 | ≥8 | ≤1 | ≥64 | ≥320 | ≥64 | ≥64 | 16 | 4 | ≥64 |
| H | blaOXA-58 | 34 | TS | ≥16 | 8 | ≥8 | ≤1 | ≥64 | ≥320 | ≥64 | ≥64 | 16 | 4 | ≥64 |
| I | blaOXA-58 | 35 | TS | ≥16 | 8 | ≥8 | ≤1 | ≥64 | ≥320 | ≥64 | ≥64 | 16 | 4 | ≥64 |
| J | blaOXA-58 | 61 | BS | ≥16 | 8 | ≥8 | 8 | ≥64 | ≥320 | ≥64 | ≥64 | 16 | 16 | ≥64 |
| K | blaOXA-58 | 81 | BC | ≥16 | 8 | ≥8 | 8 | ≥64 | ≥320 | ≥64 | ≥64 | 16 | 16 | ≥64 |
| L | blaOXA-58 | 82 | TS | ≥16 | ≥16 | ≥8 | ≤1 | ≥64 | ≥320 | ≥64 | ≥64 | 16 | 4 | ≥64 |
| M | blaOXA-58 | 83 | TS | ≥16 | 8 | ≥8 | ≤1 | ≥64 | ≥320 | ≥64 | ≥64 | 16 | 4 | ≥64 |
The day of isolation is the date (number of days after the patient was admitted to the hospital) that the particular isolate was recovered from the patient.
TS, tracheal secretion; TST, tracheostoma; BS, bedsore; BC, blood culture.
Drug abbreviations: IPM, imipenem; MEM, meropenem; TGC, tigecycline; TOB, tobramycin; AMK, amikacin; SXT, trimethoprim-sulfamethoxazole; LEV, levofloxacin. The asterisks for IMP and MEM values indicate that the isolate exhibited heteroresistance.
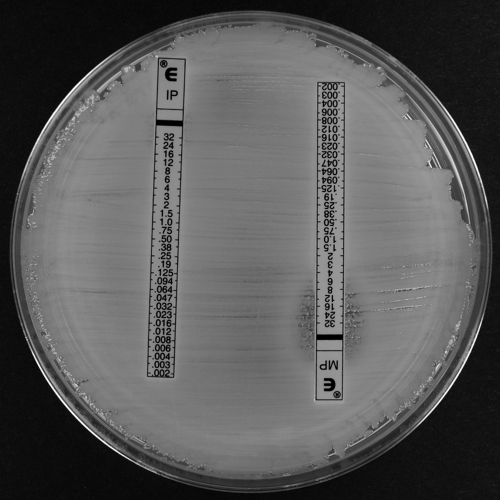
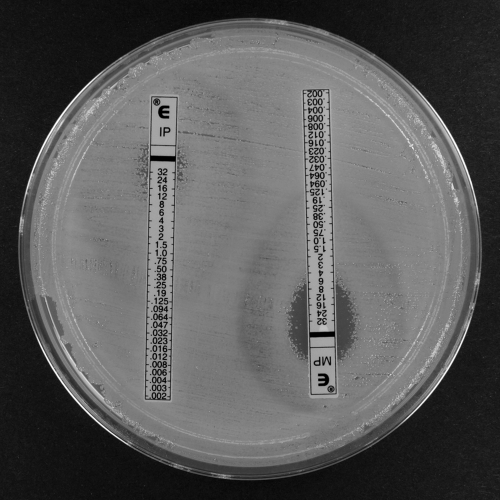
FIG. 2.
Imipenem (IP) and meropenem (MP) Etests for A. baumannii clinical isolate A (blaOXA-164) showing colonies within the imipenem and meropenem ellipse (heteroresistance).
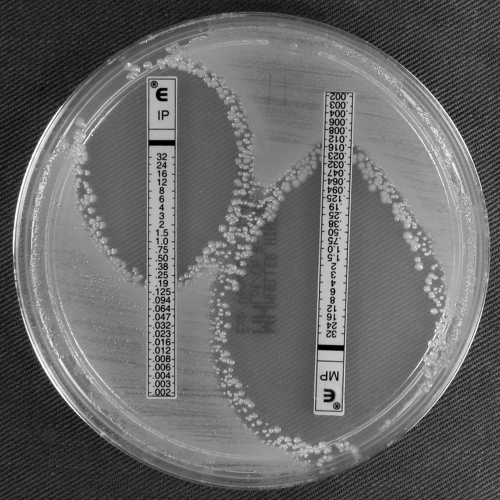
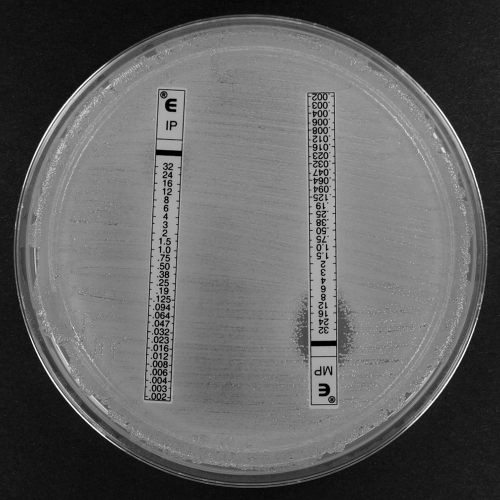
FIG. 3.
Imipenem and meropenem Etests for A. baumannii clinical isolate G (blaOXA-58) showing confluent growth up to the imipenem Etest strip and a reduced meropenem ellipse compared to that of the blaOXA-164 isolate (Fig. 2).
Species identification and presence of carbapenemase genes.
The isolates failed to utilize d-glucose, d-mannose, and malonic acid, essential substrates for identification of A. baumannii in the Vitek 2 system, thus leading to their misidentification as A. lwoffii. Species identification using gyrB multiplex identified all isolates as A. baumannii. Sequencing of the intrinsic chromosomal blaOXA-51-like gene revealed blaOXA-69, which was not associated with ISAba1. In addition, isolates A to F possessed the novel blaOXA-164 gene, which is a blaOXA-58-like variant. Isolates G to M all possessed blaOXA-58, which differs from the latter by a single A-to-T mutation at position 342, resulting in the Leu114→Phe amino acid replacement. blaOXA-164 and blaOXA-58 were flanked by IS18 and ISAba3 as previously described (29). The blaOXA-164 and blaOXA-58 genes were not transferrable; however, plasmid preparations were positive by PCR for blaOXA-164 or blaOXA-58 but not blaOXA-69, suggesting a plasmid location.
Cloning blaOXA-164 and blaOXA-58.
The results of cloning blaOXA-164 and blaOXA-58 are summarized in Table 3. Clinical resistance to the carbapenems was achieved in A. baumannii transformants, but not E. coli. blaOXA-164 and blaOXA-58 transformants recorded similar MICs. However, in both E. coli and A. baumannii, blaOXA-164 transformants exhibited imipenem heteroresistance. The meropenem ellipse was reduced in A. baumannii blaOXA-58 transformants compared to that in blaOXA-164 transformants (Fig. 4 to 7).
TABLE 3.
MICs determined by Etest for OXA-164- and OXA-58-producing A. baumannii ATCC 19606 and ATCC 17978 and E. coli NEB 5-alpha
| Isolate | MIC (μg/ml) |
|
|---|---|---|
| Imipenem | Meropenem | |
| E. coli NEB 5-alpha(pWH1266) | 0.19 | 0.012 |
| E. coli NEB 5-alpha(pWH1266::blaOXA-164) | 0.25 | 0.032 |
| E. coli NEB 5-alpha(pWH1266::blaOXA-58) | 0.5 | 0.032 |
| A. baumannii ATCC 19606(pWH1266) | 0.125 | 0.19 |
| A. baumannii ATCC 19606(pWH1266::blaOXA-164) | >32 | 32 |
| A. baumannii ATCC 19606(pWH1266::blaOXA-58) | >32 | >32 |
| A. baumannii ATCC 17978(pWH1266) | 0.25 | 0.25 |
| A. baumannii ATCC 17978(pWH1266::blaOXA-164) | >32 | >32 |
| A. baumannii ATCC 17978(pWH1266::blaOXA-58) | >32 | >32 |
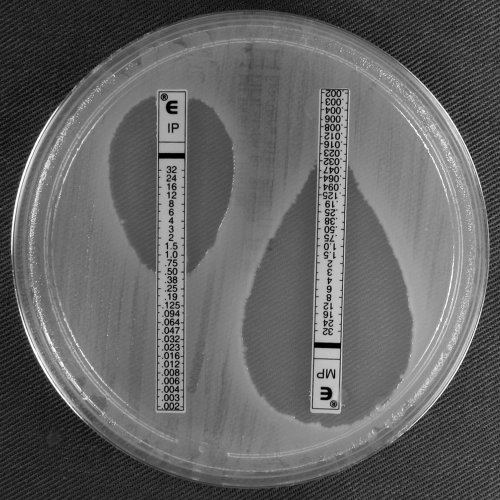
FIG. 4.
Imipenem and meropenem Etests for E. coli NEB 5-alpha carrying pWH1266::blaOXA-164 and showing imipenem and meropenem heteroresistance.
FIG. 5.
Imipenem and meropenem Etests for E. coli NEB 5-alpha(pWH1266::blaOXA-58).
FIG. 6.
Imipenem and meropenem Etests for A. baumannii 19606(pWH1266::blaOXA-164) showing heteroresistance to imipenem and meropenem.
FIG. 7.
Imipenem and meropenem Etests for A. baumannii 19606(pWH1266::blaOXA-58) showing confluent growth up to the imipenem Etest strip and a reduction in meropenem heteroresistance compared to A. baumannii 19606(pWH1266::blaOXA-164) (Fig. 6).
Molecular typing.
All isolates had similar rep-PCR fingerprint and clustered with control isolates from European clonal complex I (WW1), which is in agreement with the presence of blaOXA-69. All 13 isolates had an identical PFGE pattern, confirming their clonality (data not shown).
qRT-PCR and characterization of adeB upregulation.
Expression of the adeB and adeJ efflux pump genes was investigated by qRT-PCR with isolates A, E, F, G, and J. We did not detect any difference in adeJ transcripts in isolates A, E, and J (Table 4). Isolates G and J expressed similar amounts of adeB mRNA, which were >7-fold greater than the amounts expressed by isolates A, E, and F obtained prior to hospital day 32. There was no difference in expression of blaOXA-69 transcripts. The blaOXA-58-like gene was overexpressed in isolates J and G with levels 2- to 4-fold higher than those expressed by isolates A and F. We did not detect the presence of IS elements in the vicinity of the adeABC operon. The adeR and adeS genes of isolates A, F, G, and J were sequenced. Isolates G and J had a single missense mutation in adeR at nucleotide 58 (G→A) leading to an Asp20→Asn amino acid replacement. No other nucleotide changes were found. qRT-PCR with adeR and adeS primers showed no significant difference in expression of these genes in the four isolates.
TABLE 4.
Relative expression of adeB, adeJ, adeR, adeS, blaOXA-69, and blaOXA-164 and blaOXA-58 as determined by semiquantitative RT-PCR
| Isolate | Relative expressiona |
|||||
|---|---|---|---|---|---|---|
| adeB | adeJ | adeR | adeS | blaOXA-69 | blaOXA-164/58 | |
| A | 1 | 1 | 1 | 1 | 1 | 1 |
| E | 0.6 ± 0.2 | 1.3 ± 0.3 | NT | NT | NT | NT |
| F | 0.7 ± 0.1 | NT | 0.7 ± 0.03 | 0.9 ± 0.1 | 0.8 ± 0.1 | 1.1 ± 0.04 |
| G | 7.7 ± 1.5 | NT | 1.4 ± 0.2 | 1.8 ± 0.4 | 1.7 ± 0.2 | 4.2 ± 0.1 |
| J | 9.0 ± 1 | 1.3 ± 0.4 | 1 ± 0.1 | 1.5 ± 0.2 | 0.6 ± 0.1 | 2.8 ± 0.1 |
The expression of the genes is normalized to rpoB expression and relative to the number of transcripts from isolate A. NT, not tested.
DISCUSSION
The failure of the Vitek 2 system to correctly identify A. baumannii and to predict carbapenem susceptibility has been described previously (12, 13). More recently, almost half of the carbapenem-resistant A. baumannii isolates identified using routine microbiological methods were sensitive to carbapenems upon retesting by Etest (25). What makes the present study unusual is that Vitek 2 identified these isolates uniformly as A. lwoffii, a commensal species that is rarely involved or not at all involved in nosocomial infections (4). The species misidentification and false antimicrobial susceptibility subsequently may have compromised the clinical outcome. As these isolates cluster with WW1, one of the most frequently encountered carbapenem-resistant A. baumannii lineages (8), it opens the possibility that species misidentification may be more common than previously thought and warrants greater vigilance. It is of interest that isolates A to F were heteroresistant to the carbapenems. This may be due in part to slight differences between the biochemical properties of OXA-164 and OXA-58 as observed with the E. coli and A. baumannii transformants. However, we cannot discount the small increase in blaOXA-58 and adeB transcripts which may contribute to overall resistance levels (6, 9). Carbapenem-heteroresistant A. baumannii has been reported in the absence of an acquired carbapenemase (20); however, to our knowledge, this is the first description of heteroresistance with an acquired oxacillinase.
Overexpression of adeB has previously been attributed to mutations in the adeS and adeR genes and to insertion of ISAba1 in adeS (15, 21). We found a novel adeR mutation leading to an Asp20→Asn replacement in adeB overexpressors. AdeR is composed of two major domains, a signal receiver domain and an effector domain which is a DNA binding site (14). The signal receiver domain in AdeR is composed of a phosphorylation and dimerization site and receives the signal from its sensor partner (AdeS) (15, 17). The A. baumannii AdeR residue Asp20 corresponds to Asp10 in the E. coli PhoB response regulator and is part of an acidic triad making up the active site for phosphorylation (1, 24). Thus, sustaining a mutation near this phosphorylation site may alter the interactions between the AdeS and AdeR subunit which likely results in the overexpression of the adeABC genes (27). However, the exact mechanistic details may need to be elucidated by site-directed mutagenesis.
The mechanism of tobramycin resistance in isolates J and K is not known. Gene disruption predicts tobramycin and amikacin to be substrates of AdeB (14). However, we found no significant differences in adeB or adeJ levels between tobramycin-sensitive and -resistant isolates. In addition, isolate E, which showed reduced amikacin susceptibility, expressed the same levels of adeB and adeJ mRNA as isolates A and F, suggesting that in this case, reduced amikacin susceptibly was not related to these efflux pumps.
We did not determine the agent(s) that drove the conversion of OXA-164 to OXA-58 and led to the adeR mutation. However, given that OXA-58 appears to confer greater resistance to both imipenem and meropenem, and meropenem was administered over the conversion period, it is not unreasonable to suggest that meropenem was the driving force. In addition, elevated meropenem MICs have also been associated with efflux overexpression (6, 9).
In summary, we have described the failure of the semiautomated system Vitek 2 to correctly identify carbapenem-resistant A. baumannii. The consequence was that meropenem was inappropriately administered for a prolonged period of time and may have contributed to the mutation in blaOXA-164, leading to the selection and overexpression of blaOXA-58. In addition, adeB overexpression was selected, leading to elevated tigecycline MICs without prior exposure to this drug. Our study demonstrates that A. baumannii has the propensity to rapidly acquire resistance by mutation during antimicrobial therapy. Of particular concern is the implication of the conversion of blaOXA-164 to blaOXA-58. These data serve to highlight the need for caution when interpreting the identification of a multidrug-resistant Acinetobacter isolate that is not Acinetobacter baumannii by Vitek 2.
Acknowledgments
The work of P.G.H. and H.S. was supported by a grant from the Bundesministerium für Bildung und Forschung (BMBF), Germany, Klinische Forschergruppe Infektiologie (grant 01KI0771).
We thank Gottfried Wilharm and Yvonne Pfeifer for providing us with pWH1266 and A. baumannii ATCC 17978.
Footnotes
Published ahead of print on 4 October 2010.
REFERENCES
- 1.Allen, M. P., K. B. Zumbrennen, and W. R. McCleary. 2001. Genetic evidence that the α5 helix of the receiver domain of PhoB is involved in interdomain interactions. J. Bacteriol. 183:2204-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2007. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed. Approved standard M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 3.Damier-Piolle, L., S. Magnet, S. Bremont, T. Lambert, and P. Courvalin. 2008. AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob. Agents Chemother. 52:557-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dijkshoorn, L., A. Nemec, and H. Seifert. 2007. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 5:939-951. [DOI] [PubMed] [Google Scholar]
- 5.Healy, M., J. Huong, T. Bittner, M. Lising, S. Frye, S. Raza, R. Schrock, J. Manry, A. Renwick, R. Nieto, C. Woods, J. Versalovic, and J. R. Lupski. 2005. Microbial DNA typing by automated repetitive-sequence-based PCR. J. Clin. Microbiol. 43:199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heritier, C., L. Poirel, T. Lambert, and P. Nordmann. 2005. Contribution of acquired carbapenem-hydrolyzing oxacillinases to carbapenem resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 49:3198-3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins, P. G., H. Wisplinghoff, O. Krut, and H. Seifert. 2007. A PCR-based method to differentiate between Acinetobacter baumannii and Acinetobacter genomic species 13TU. Clin. Microbiol. Infect. 13:1199-1201. [DOI] [PubMed] [Google Scholar]
- 8.Higgins, P. G., C. Dammhayn, M. Hackel, and H. Seifert. 2010. Global spread of carbapenem-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 65:233-238. [DOI] [PubMed] [Google Scholar]
- 9.Higgins, P. G., H. Wisplinghoff, D. Stefanik, and H. Seifert. 2004. Selection of topoisomerase mutations and overexpression of adeB mRNA transcripts during an outbreak of Acinetobacter baumannii. J. Antimicrob. Chemother. 54:821-823. [DOI] [PubMed] [Google Scholar]
- 10.Hornsey, M., M. J. Ellington, M. Doumith, C. P. Thomas, N. C. Gordon, D. W. Wareham, J. Quinn, K. Lolans, D. M. Livermore, and N. Woodford. 2010. AdeABC-mediated efflux and tigecycline MICs for epidemic clones of Acinetobacter baumannii. J. Antimicrob. Chemother. 65:1589-1593. [DOI] [PubMed] [Google Scholar]
- 11.Hunger, M., R. Schmucker, V. Kishan, and W. Hillen. 1990. Analysis and nucleotide sequence of an origin of DNA replication in Acinetobacter calcoaceticus and its use for Escherichia coli shuttle plasmids. Gene 87:45-51. [DOI] [PubMed] [Google Scholar]
- 12.Joyanes, P., M. D. Conejo, L. Martinez-Martinez, and E. J. Perea. 2001. Evaluation of the VITEK 2 system for the identification and susceptibility testing of three species of nonfermenting Gram-negative rods frequently isolated from clinical samples. J. Clin. Microbiol. 39:3247-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulah, C., E. Aktas, F. Comert, N. Ozlu, I. Akyar, and H. Ankarali. 2009. Detecting imipenem resistance in Acinetobacter baumannii by automated systems (BD Phoenix, Microscan WalkAway, Vitek 2); high error rates with Microscan WalkAway. BMC Infect. Dis. 9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magnet, S., P. Courvalin, and T. Lambert. 2001. Resistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrob. Agents Chemother. 45:3375-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchand, I., L. Damier-Piolle, P. Courvalin, and T. Lambert. 2004. Expression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob. Agents Chemother. 48:3298-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mugnier, P. D., L. Poirel, and P. Nordmann. 2009. Functional analysis of insertion sequence ISAba1, responsible for genomic plasticity of Acinetobacter baumannii. J. Bacteriol. 191:2414-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagasawa, S., S. Tokishita, H. Aiba, and T. Mizuno. 1992. A novel sensor-regulator protein that belongs to the homologous family of signal-transduction proteins involved in adaptive responses in Escherichia coli. Mol. Microbiol. 6:799-807. [DOI] [PubMed] [Google Scholar]
- 18.Poirel, L., and P. Nordmann. 2006. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin. Microbiol. Infect. 12:826-836. [DOI] [PubMed] [Google Scholar]
- 19.Poirel, L., and P. Nordmann. 2006. Genetic structures at the origin of acquisition and expression of the carbapenem-hydrolyzing oxacillinase gene blaOXA-58 in Acinetobacter baumannii. Antimicrob. Agents Chemother. 50:1442-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pournaras, S., A. Ikonomidis, A. Markogiannakis, A. N. Maniatis, and A. Tsakris. 2005. Heteroresistance to carbapenems in Acinetobacter baumannii. J. Antimicrob. Chemother. 55:1055-1056. [DOI] [PubMed] [Google Scholar]
- 21.Ruzin, A., D. Keeney, and P. A. Bradford. 2007. AdeABC multidrug efflux pump is associated with decreased susceptibility to tigecycline in Acinetobacter calcoaceticus-Acinetobacter baumannii complex. J. Antimicrob. Chemother. 59:1001-1004. [DOI] [PubMed] [Google Scholar]
- 22.Segal, H., S. Garny, and B. G. Elisha. 2005. Is ISABA-1 customized for Acinetobacter? FEMS Microbiol. Lett. 243:425-429. [DOI] [PubMed] [Google Scholar]
- 23.Seifert, H., L. Dolzani, R. Bressan, T. van der Reijden, B. van Strijen, D. Stefanik, H. Heersma, and L. Dijkshoorn. 2005. Standardization and interlaboratory reproducibility assessment of pulsed-field gel electrophoresis-generated fingerprints of Acinetobacter baumannii. J. Clin. Microbiol. 43:4328-4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sola, M., F. X. Gomis-Ruth, L. Serrano, A. Gonzalez, and M. Coll. 1999. Three-dimensional crystal structure of the transcription factor PhoB receiver domain. J. Mol. Biol. 285:675-687. [DOI] [PubMed] [Google Scholar]
- 25.Towner, K. J., K. Levi, and M. Vlassiadi. 2008. Genetic diversity of carbapenem-resistant isolates of Acinetobacter baumannii in Europe. Clin. Microbiol. Infect. 14:161-167. [DOI] [PubMed] [Google Scholar]
- 26.Turton, J. F., M. E. Ward, N. Woodford, M. E. Kaufmann, R. Pike, D. M. Livermore, and T. L. Pitt. 2006. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol. Lett. 258:72-77. [DOI] [PubMed] [Google Scholar]
- 27.Walthers, D., V. K. Tran, and L. J. Kenney. 2003. Interdomain linkers of homologous response regulators determine their mechanism of action. J. Bacteriol. 185:317-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodford, N., M. J. Ellington, J. M. Coelho, J. F. Turton, M. E. Ward, S. Brown, S. G. B. Amyes, and D. M. Livermore. 2006. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents 27:351-353. [DOI] [PubMed] [Google Scholar]
- 29.Zarrilli, R., D. Vitale, A. Di Popolo, M. Bagattini, Z. Daoud, A. U. Khan, C. Afif, and M. Triassi. 2008. A plasmid-borne blaOXA-58 gene confers imipenem resistance to Acinetobacter baumannii isolates from a Lebanese hospital. Antimicrob. Agents Chemother. 52:4115-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]