Abstract
Shunt infection is a major complication affecting approximately 10% of procedures. Propionibacterium acnes, an anaerobic skin bacterium, is increasingly recognized as a shunt pathogen, causing up to 14% of infections. Though susceptible to penicillin and cephalosporins, P. acnes shunt infections are not preventable by means of perioperative prophylaxis, due to poor cerebrospinal fluid penetration. Antimicrobial shunts with activity against staphylococci are available, but their activity against P. acnes is unknown, and the study was designed to determine this. Three methods of evaluation were used in order to determine the emergence of resistance when exposure is to high inocula for long periods, the time taken to kill 100% of the bacteria attached to the shunt, and the duration of activity under constant flow conditions with repeated bacterial challenge. Despite repeated exposure to high bacterial inocula over 70 days, no resistance was seen. The time taken to kill all attached bacteria, 96 h, was twice that taken to kill attached staphylococci. Nevertheless, under constant flow conditions with repeated challenges, the antimicrobial catheters resisted colonization by P. acnes for 56 days. Using tests that were designed to be clinically predictive when done together, the results suggest that the antimicrobial catheters will be able to prevent colonization of hydrocephalus shunts by P. acnes.
Hydrocephalus is the pathological accumulation of cerebrospinal fluid (CSF) in the cerebral ventricles and is caused by a variety of factors, such as intrauterine infection, meningitis, hemorrhage, and tumors. The standard treatment is surgical insertion of a shunt, a silicone tubular device that drains CSF from the cerebral ventricles to another body site, usually the peritoneal cavity. Infection is a serious complication of shunting, the incidence ranging from less than 1% to over 15%, with an overall incidence of about 10% (13, 18). Shunt infection in the United States has been estimated to account for more than $100 million in national health care expenditure annually (29). The causative bacteria are usually of skin origin, and coagulase-negative staphylococci (CoNS) predominate. However, a proportion of shunt infections, reported to be approximately 14%, is caused by Propionibacterium acnes, also a normal skin inhabitant (1, 4, 30). This organism is anaerobic and slow growing and often fails to be detected. Like CoNS, P. acnes adheres to the inner surfaces of the shunt catheters and develops a biofilm (4, 11), leading to antibiotic treatment failure and necessitating shunt removal.
Although antimicrobial prophylaxis is almost universally used, its role in reducing the incidence of shunt infections remains controversial. Systemic antibiotics penetrate the blood-cerebrospinal fluid barrier poorly in the absence of inflammation (2, 17, 20, 21, 22). The use of intraventricular antibiotics to overcome this has not been successful (6, 32), with the possible exception of the combination of intraventricular gentamicin and vancomycin, which has been reported to significantly reduce the incidence of perioperative shunt infection (27). However, this result remains to be confirmed.
An alternative method of preventing infection involves the use of antimicrobial shunts (8). These are impregnated with rifampin and clindamycin (Bactiseal; Codman and Shurtleff, Inc., Raynham, MA) and provide a duration of activity of approximately 50 days (9). Bacterial colonization on both inner and outer surfaces is prevented (5). Clinical trials of various quality and design have demonstrated a reduction in the rate of infection due to Gram-positive bacteria (14, 16, 24, 26), but the activity of these shunt catheters against P. acnes has not been determined. The aims of this research were to investigate the activity of antimicrobial shunt catheters against P. acnes and the duration of that activity and to determine whether exposure to the catheters caused the emergence of resistance.
MATERIALS AND METHODS
Test strains.
Three clinical isolates of P. acnes, all from proven arthroplasty infections in different patients, were characterized by established methods, including the Rapid ID 32 A (bioMérieux, Marcy-l'Etoile, France) system. All three had different identification profiles by the Rapid ID 32 A system (0403330404, 0003330604, and 0003120604). They all showed the same MICs of rifampin (0.016 mg/liter) and clindamycin (0.0125 mg/liter), measured using agar incorporation with brucella blood agar (Oxoid, Basingstoke, United Kingdom). All have been shown to develop biofilms on biomaterials (4). They were maintained under anaerobic conditions at 37°C on sheep blood agar (BA) plates (Oxoid).
Shunt catheters.
Plain silicone shunt catheters for use as controls and impregnated catheters (Bactiseal) were donated by Codman. The catheters are impregnated with 0.054% (wt/wt) rifampin and 0.15% (wt/wt) clindamycin hydrochloride.
SPTT.
The serial plate transfer test (SPTT) was performed to assess the duration of antimicrobial activity of impregnated catheters against P. acnes and to detect resistance. Three sets of BA plates were each seeded (0.2 ml of 107 CFU/ml) with one of three strains of P. acnes, and three 1-cm segments of catheter were placed on the surface of each and incubated anaerobically. The segments were removed and placed on fresh plates every 7 days. Each segment was indelibly marked to ensure that the same surface was in contact with the agar at each transfer. Upon each transfer, the zone of inhibition was measured across the short axis. This procedure was repeated until no zones of inhibition were seen. Irrespective of zone size, the presence of any bacteria immediately in contact with the catheter was noted to evaluate the development of “paradoxical” bacterial resistance, seen when bacteria attached to the antibacterial biomaterial develop a biofilm on its surface yet the biomaterial is surrounded by a zone of inhibition of the same bacterium on an agar plate. This phenomenon has been described previously (7).
tK100.
The test of time taken to kill 100% attached bacteria (tK100 test) is used to determine the time needed to kill 100% of bacteria attached to the surface of the catheters (5). Anaerobe basal broth (ABB CM0957; Oxoid) was inoculated with several colonies of P. acnes from an overnight BA plate of each of three strains, and bacteria were grown at 37°C to prepare planktonic suspensions. The optical density (OD) at 490 nm of the planktonic suspension was adjusted to 0.6 to 0.7 (∼107 CFU/ml). Catheter segments 1 cm long were cut longitudinally and placed in triplicate in Eppendorf tubes containing 1 ml of the bacterial suspension and incubated anaerobically at 37°C for 1 h. They were then removed, rinsed in 1 ml of ABB, replaced in 1 ml of 25% ABB, and incubated for 24, 48, 72, 96, or 120 h. After each 24-h incubation period, catheter segments for further incubation were removed, rinsed in 1 ml of ABB, and placed in 1 ml fresh 25% ABB. At the end of each 24-h period, three catheters were removed, rinsed, and sonicated for 20 min (Ultrawave Ltd., Cardiff, United Kingdom) at 50 Hz to remove the adhered bacteria. The sonicates were cultured anaerobically on BA for 72 to 96 h at 37°C, and any colonies were counted. Each strain was tested in triplicate on each occasion.
IVC.
An in vitro challenge (IVC) model designed to simulate in-use conditions (temperature, hydration, flow rate) (9) was used to test the ability of P. acnes to colonize the luminal surface of impregnated catheters and plain silicone catheters. Briefly, test catheters were connected in triplicate to inflow and outflow tubes and placed in a chamber filled with sodium thioglycolate (0.5 g/dl) to maintain anaerobic conditions and maintained at 37°C. The catheters were then constantly perfused with ABB using a peristaltic pump at a flow rate of 20 ml/h (the average CSF production rate) and challenged luminally every 2 weeks with 1 ml of ∼107 CFU/ml of one of the P. acnes strains. The plain control catheters were changed before each new challenge as they all became colonized. Samples of control catheters were fixed in cold acetone and viewed by scanning electron microscopy (SEM) to confirm biofilm production. Only one strain was tested by this method, as they all appeared to have similar characteristics and the method is time-consuming and labor-intensive. Effluent samples were taken from the outlet tubing daily for quantitative culture. This method determined the ability of the catheters to resist repeated bacterial challenge under flow conditions at 37°C. The protocol of repeated challenge was intended to establish the duration of protective activity and was not aimed at simulating clinical practice, where such multiple bacterial challenges would be unlikely to occur. Similarly, the ABB used to perfuse the catheters was chosen instead of artificial CSF in order to maximize bacterial survival. All manipulations were carried out under strict aseptic conditions to avoid contamination. All isolates were fully identified, and their MICs of rifampin and clindamycin were confirmed.
RESULTS
Serial plate transfer test.
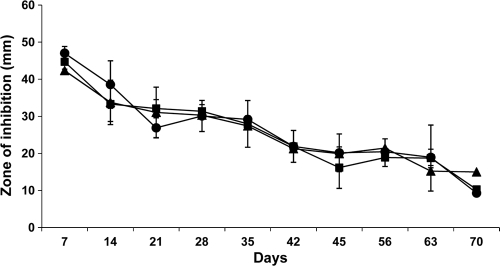
The initial mean diameter of inhibition zone of the antimicrobial catheter segments in the SPTT was 44.7 mm, and the mean final diameter after 70 days was 11.5 mm (Fig. 1). No paradoxical or other resistance was found.
FIG. 1.
Serial plate transfer test (SPTT) results for the antimicrobial shunt catheter against three P. acnes strains are shown. There was no significant difference among the results (P = 0.47). Filled squares, filled circles, and filled triangles represent the three strains, each tested in triplicate. Prolonged antimicrobial activity of up to 70 days was demonstrated for all three strains without any development of resistance.
tK100 assay.
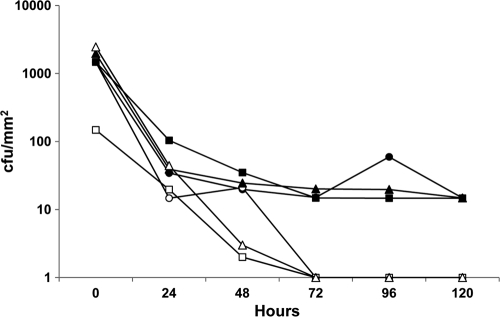
The time taken to kill all P. acnes, expressed as the duration of viability of bacteria attached to control and antimicrobial catheters, is shown in Fig. 2, which is a representative result for the three strains. For all three strains, all attached bacteria were killed by 96 h and did not regrow. Attached bacteria remained viable in the control catheters throughout the test period.
FIG. 2.
The antimicrobial shunt catheters achieved killing of 100% attached bacteria against P. acnes strains at 96 h (tK100) (P < 0.05). Squares, circles, and triangles represent the three strains, each tested in triplicate. Filled symbols, plain control shunt catheters; open symbols, antimicrobial shunt catheters.
In vitro challenge.
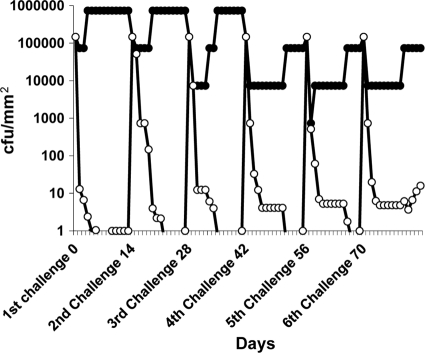
The IVC results shown in Fig. 3 demonstrate that the antimicrobial catheters resisted bacterial challenge without becoming colonized for 56 days but failed the next challenge at 70 days. All catheters were cleared of P. acnes between days 6 and 12 after each challenge except the last. All plain controls became colonized with the challenge strain, as shown by culture and SEM.
FIG. 3.
During the in vitro challenge (IVC), unlike the controls, the antimicrobial shunt catheter (open circles) protected against P. acnes colonization for 5 successive challenges up to 56 days (P < 0.05) but failed the 70-day challenge, becoming colonized. After each challenge period of 14 days, the colonized control catheter (filled circles) was removed and replaced with a fresh catheter. Catheters were tested in triplicate.
DISCUSSION
P. acnes is now a recognized pathogen in neurosurgery (23, 28), often giving rise to infections with delayed presentation (3, 12). Shunt infections due to P. acnes bacteria, also often with delayed presentation, have been reported (1, 4, 30, 31). Despite their late clinical presentation, it is reasonable to assume that, like Staphylococcus epidermidis, P. acnes bacteria gain access to the shunt at insertion or at a subsequent revision, and such infections are therefore preventable. P. acnes is almost always fully susceptible to penicillin, but as with many other antimicrobials, cerebrospinal fluid concentrations of penicillin after a single intravenous injection are too low to be of prophylactic value (15). The alternative approach of using an antimicrobial shunt catheter accepts that skin bacteria will inevitably gain access to the shunt lumen in most patients but proposes that they can be prevented from colonizing the shunt by the antimicrobial activity of the catheter. Such a catheter was first described in 1989 (8) and has since been developed for clinical use as the Bactiseal catheter used here. The time taken for these catheters to kill all staphylococci that become attached to their surfaces is now known to be approximately 48 h (5). The low rate of kill is considered to be due to the change in bacterial phenotype that is known to occur upon the attachment of the bacteria to a surface (25), and it is therefore essential that any preclinical evaluation of such catheters includes testing for activity against attached rather than planktonic bacteria. Here we have shown that the time taken for the catheters to kill all attached P. acnes bacteria is twice as long as that for S. epidermidis bacteria, and this might be related to the lower growth rate of the former. However, all bacteria were eventually killed. Similarly, the in vitro challenge (IVC) test showed that the antimicrobial catheters resisted P. acnes colonization for 56 days, similar to other Gram-positive bacteria (9), but that P. acnes cleared more slowly from the challenged catheters than staphylococci (10 to 11 days versus 2 to 3 days). The emergence of resistance in the serial plate transfer test usually takes the form of bacterial growth on the surface of the catheter segment, despite there being a large zone of inhibition. This “paradoxical” resistance is sometimes seen after exposure to antimicrobial biomaterials containing single agents and is thought to be due to inhibited but viable bacteria attaching to the catheter material at the time of transfer and developing a biofilm, thus becoming phenotypically nonsusceptible (7). In clinical use, this would not only lead to failure of the antimicrobial catheter and to infection but would also increase the risk of emergence of mutational resistance. No resistance, paradoxical or otherwise, was seen on continuous exposure to high inocula over 70 days in the SPTT. Neither was any increase in MIC seen after exposure to the catheters, as measured by agar incorporation.
Only one other antimicrobial shunt catheter is commercially available (Silverline; Spiegelberg GmbH and Co., Hamburg, Germany), and this shunt contains near-nanoparticulate silver. Investigations using techniques similar to those described here have shown no antimicrobial activity of this shunt against P. acnes (10). Several antimicrobial catheters are available for temporary external ventricular drains (19, 33), but P. acnes is not a problem for these. To our knowledge, no other antimicrobial catheter materials have been tested for activity against P. acnes.
The results presented here show that the antimicrobial catheters were able to eradicate all P. acnes bacteria that had become attached to the catheters, even under flow conditions. Based on the clinical predictivity of the tests used here in the case of other shunt pathogens (5, 8, 9, 14, 16, 26, 29), antimicrobial catheters promise to give protection against P. acnes shunt infection, extending from the perioperative to the immediate postoperative period, and are likely also to prevent late-presenting cases, as these are also contracted at initial surgery.
Acknowledgments
Research support for consumables and a salary contribution for W.A. were received from Codman & Shurtleff, Inc., Raynham, MA. The company had no role in the design or execution of the research nor any input to the publication.
R.B. is a consultant to, and a member of the speakers' bureau for, Codman & Shurtleff, Inc., a manufacturer of an antimicrobial catheter. No other author has a conflict of interest.
Footnotes
Published ahead of print on 13 September 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Arnell, K., K. Cesarini, A. Lagerqvist-Widh, T. Wester, and J. Sjölin. 2008. Cerebrospinal fluid shunt infections in children over a 13-year period: anaerobic cultures and comparison of clinical signs of infection with Propionibacterium acnes and with other bacteria. J. Neurosurg. Pediatr. 1:366-372. [DOI] [PubMed] [Google Scholar]
- 2.Bafeltowska, J. J., E. Buszman, K. M. Mandat, and J. K. Hawranek. 2004. Therapeutic vancomycin monitoring in children with hydrocephalus during treatment of shunt infections. Surg. Neurol. 62:142-150. [DOI] [PubMed] [Google Scholar]
- 3.Barazi, S., K. K. Gnanalingham, I. Chopra, and J. R. van Dellen. 2003. Delayed postoperative intracerebral abscess caused by Propionibacterium acnes: case report and review of the literature. Br. J. Neurosurg. 17:336-339. [DOI] [PubMed] [Google Scholar]
- 4.Bayston, R., W. Ashraf, R. Barker-Davies, E. Tucker, R. Clement, J. Clayton, B. J. C. Freeman, and B. Nuradeen. 2007. Biofilm formation by Propionibacterium acnes on biomaterials in vitro and in vivo: impact on diagnosis and treatment. J. Biomed. Mater. Res. 81A:705-709. [DOI] [PubMed] [Google Scholar]
- 5.Bayston, R., W. Ashraf, and C. Bhundia. 2004. Mode of action of an antimicrobial biomaterial for use in hydrocephalus shunts. J. Antimicrob. Chemother. 53:778-782. [DOI] [PubMed] [Google Scholar]
- 6.Bayston, R., C. Bannister, V. Boston, R. Burman, B. Burns, F. Cooke, R. Cooke, R. Cudmore, R. Fitzgerald, C. Goldberg, H. T. Green, E. Guiney, C. Hardwidge, C. A. Hart, A. E. Holmes, J. Meigh, J. Miles, A. Rampling, J. Walker, H. Webb, and K. Whale. 1990. A prospective randomised controlled trial of antimicrobial prophylaxis in hydrocephalus shunt surgery. Z. Kinderchirurg. 45:5-7. [DOI] [PubMed] [Google Scholar]
- 7.Bayston, R., L. Fisher, and K. Weber. 2009. An antimicrobial modified silicone peritoneal catheter with activity against both Gram positive and Gram negative bacteria. Biomaterials 30:3167-3173. [DOI] [PubMed] [Google Scholar]
- 8.Bayston, R., N. Grove, J. Siegel, D. Lawellin, and S. Barsham. 1989. Prevention of hydrocephalus shunt catheter colonisation in vitro by impregnation with antimicrobials. J. Neurol. Neurosurg. Psychiatr. 52:605-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayston, R., and E. Lambert. 1997. Duration of protective activity of cerebrospinal fluid shunt catheters impregnated with antimicrobial agents to prevent bacterial catheter-related infection. J. Neurosurg. 87:247-251. [DOI] [PubMed] [Google Scholar]
- 10.Bayston, R., L. Vera, A. Mills, W. Ashraf, O. Stevenson, and S. M. Howdle. 2010. In vitro antimicrobial activity of silver-processed catheters for neurosurgery. J. Antimicrob. Chemother. 65:258-265. [DOI] [PubMed] [Google Scholar]
- 11.Coenye, T., E. Peeters, and H. J. Nelis. 2007. Biofilm formation by Propionibacterium acnes is associated with increased resistance to antimicrobial agents and increased production of putative virulence factors. Res. Microbiol. 158:386-392. [DOI] [PubMed] [Google Scholar]
- 12.Critchley, G., and R. Strachan. 1996. Postoperative subdural empyema caused by Propionibacterium acnes—a report of two cases. Br. J. Neurosurg. 10:321-323. [DOI] [PubMed] [Google Scholar]
- 13.Duhaime, A. C. 2006. Evaluation and management of shunt infections in children with hydrocephalus. Clin. Pediatr. 45:705-713. [DOI] [PubMed] [Google Scholar]
- 14.Eymann, R., S. Chehab, M. Strowitzki, W.-I. Steudel, and M. Kiefer. 2008. Clinical and economic consequences of antibiotic impregnated cerebrospinal fluid shunt catheters. J. Neurosurg. Pediatr. 1:444-450. [DOI] [PubMed] [Google Scholar]
- 15.Frenz, G., P. B. Nielsen, F. Espersen, A. Czartoryski, and H. Aastrup. 1984. Penicillin concentrations in blood and spinal fluid after a single intramuscular injection of penicillin G benzathine. Eur. J. Clin. Microbiol. 3:147-149. [DOI] [PubMed] [Google Scholar]
- 16.Govender, S. T., N. Nathoo, and J. R. van Dellen. 2003. Evaluation of an antibiotic-impregnated shunt system for the treatment of hydrocephalus. J. Neurosurg. 99:831-839. [DOI] [PubMed] [Google Scholar]
- 17.Jorgensen, L., P. D. Reiter, J. E. Freeman, K. R. Winston, D. Fish, L. A. McBride, and M. H. Handler. 2007. Vancomycin disposition and penetration into ventricular fluid of the central nervous system following intravenous therapy in patients with cerebrospinal devices. Pediatr. Neurosurg. 43:449-455. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni, A. V., J. M. Drake, and M. Lamberti-Pasculli. 2001. Cerebrospinal fluid shunt infection: a prospective study of risk factors. J. Neurosurg. 94:195-201. [DOI] [PubMed] [Google Scholar]
- 19.Lackner, P., R. Beer, G. Broessner, R. Helbok, K. Galiano, C. Pleifer, B. Pfausler, C. Brennais, C. Huck, K. Engelhardt, A. A. Obwegeser, and E. Schmutzhard. 2008. Efficacy of silver nanoparticles-impregnated external ventricular drain catheters in patients with acute occlusive hydrocephalus. Neurocrit. Care. 8:360-365. [DOI] [PubMed] [Google Scholar]
- 20.LeRoux, P., M. A. Howard, and H. R. Winn. 1990. Vancomycin pharmacokinetics in hydrocephalus shunt prophylaxis and relationship to ventricular volume. Surg. Neurol. 34:366-372. [DOI] [PubMed] [Google Scholar]
- 21.Müller, C., A. Netland, A. F. Dawson, and E. Andrew. 1980. The penetration of cefuroxime into the cerebrospinal fluid through inflamed and non-inflamed meninges. J. Antimicrob. Chemother. 6:279-283. [DOI] [PubMed] [Google Scholar]
- 22.Nau, R., H. W. Prange, P. Muth, G. Mahr, S. Menck, H. Kolenda, and F. Sörgel. 1993. Passage of cefotaxime and ceftriaxone into cerebrospinal fluid of patients with uninflamed meninges. Antimicrob. Agents Chemother. 37:1518-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nisbet, M., S. Briggs, R. Ellis-Pegler, M. Thomas, and D. Holland. 2007. Propionibacterium acnes: an under-appreciated cause of post-neurosurgical infection. J. Antimicrob. Chemother. 60:1097-1103. [DOI] [PubMed] [Google Scholar]
- 24.Parker, S. L., F. J. Attenello, D. M. Sciubba, G. L. Garces-Ambrossi, E. Ahn, J. Weingart, B. Carson, and G. Jallo. 2009. Comparison of shunt infection incidence in high-risk subgroups receiving antibiotic-impregnated versus standard shunts. Childs Nerv. Syst. 25:77-83. [DOI] [PubMed] [Google Scholar]
- 25.Patel, R. 2005. Biofilms and antimicrobial resistance. Clin. Orthop. Relat. Res. 437:41-47. [DOI] [PubMed] [Google Scholar]
- 26.Pattavilakom, A., C. Xenos, O. Bradfield, and R. A. Danks. 2007. Reduction in shunt infection using antibiotic impregnated CSF shunt catheters: an Australian prospective study. J. Clin. Neurosci. 14:526-531. [DOI] [PubMed] [Google Scholar]
- 27.Ragel, B. T., S. R. Brown, and R. H. Schmidt. 2006. Surgical shunt infection: significant reduction when using intraventricular and systemic agents. J. Neurosurg. 105:242-247. [DOI] [PubMed] [Google Scholar]
- 28.Richards, S. R., and K. M. Emara. 2001. Delayed infections after posterior TSRH spinal instrumentation for idiopathic scoliosis. Spine 26:1990-1996. [DOI] [PubMed] [Google Scholar]
- 29.Sciubba, D. M., L.-M. Lin, G. F. Woodworth, M. J. McGirt, B. Carson, and G. I. Jallo. 2007. Factors contributing to the medical costs of cerebrospinal fluid shunt infection treatment in pediatric patients with standard shunt components compared with those in patients with antibiotic-impregnated components. Neurosurg. Focus. 22:1-4. [DOI] [PubMed] [Google Scholar]
- 30.Thompson, T. P., and A. L. Albright. 1998. Propionibacterium acnes infections of cerebrospinal fluid shunts. Childs Nerv. Syst. 14:378-380. [DOI] [PubMed] [Google Scholar]
- 31.Viraraghavan, R., B. Jantausch, and J. Campos. 2004. Late-onset central nervous system shunt infections with Propionibacterium acnes: diagnosis and management. Clin. Pediatr. 43:393-397. [DOI] [PubMed] [Google Scholar]
- 32.Younger, J. J., J. C. H. Simmons, and F. F. Barrett. 1987. Failure of single dose intraventricular vancomycin for cerebrospinal fluid shunt surgery prophylaxis. Pediatr. Infect. Dis. J. 6:212-213. [DOI] [PubMed] [Google Scholar]
- 33.Zabramski, J. M., D. Whiting, R. O. Darouiche, T. G. Horner, J. Olson, C. Robertson, and A. J. Hamilton. 2003. Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. J. Neurosurg. 98:725-730. [DOI] [PubMed] [Google Scholar]