Abstract
Telavancin displays potent in vitro and in vivo activity against methicillin-resistant Staphylococcus aureus (MRSA), including strains with reduced susceptibility to vancomycin. We compared the efficacies of telavancin and vancomycin against MRSA strains with vancomycin MICs of ≥1 μg/ml in a neutropenic murine lung infection model. Thirteen clinical MRSA isolates (7 vancomycin-susceptible, 2 vancomycin-heteroresistant [hVISA], and 4 vancomycin-intermediate [VISA] isolates) were tested after 24 h, and 7 isolates (1 hVISA and 4 VISA isolates) were tested after 48 h of exposure. Mice were administered subcutaneous doses of telavancin at 40 mg/kg of body weight every 12 h (q12h) or of vancomycin at 110 mg/kg q12h; doses were designed to simulate the area under the concentration-time curve for the free, unbound fraction of drug (fAUC) observed for humans given telavancin at 10 mg/kg q24h or vancomycin at 1 g q12h. Efficacy was expressed as the 24- or 48-h change in lung bacterial density from pretreatment counts. At dose initiation, the mean bacterial load was 6.16 ± 0.26 log10 CFU/ml, which increased by averages of 1.26 ± 0.55 and 1.74 ± 0.68 log in untreated mice after 24 and 48 h, respectively. At both time points, similar CFU reductions were noted for telavancin and vancomycin against MRSA, with vancomycin MICs of ≤2 μg/ml. Both drugs were similarly efficacious after 24 and 48 h of treatment against the hVISA strains tested. Against VISA isolates, telavancin reduced bacterial burdens significantly more than vancomycin for 1 of 4 isolates after 24 h and for 3 of 4 isolates after 48 h. These data support the potential utility of telavancin for the treatment of MRSA pneumonia caused by pathogens with reduced susceptibility to vancomycin.
Over the course of the last 15 years, methicillin resistance among Staphylococcus aureus strains has increased steadily. Recent surveys report methicillin-resistant S. aureus (MRSA) rates of upwards of 50% for hospitalized patients with staphylococcal infections and upwards of 60% for patients in intensive care units (27). Considering that S. aureus accounts for 20 to 30% of hospital-acquired pneumonia cases, MRSA is a clinically important pathogen to consider in empirically choosing a regimen to treat pneumonia (22, 33).
Vancomycin has long been regarded as the drug of choice for the treatment of MRSA infections. Current practice guidelines for the treatment of health care-associated pneumonia recommend vancomycin as a first-line therapy (2). Despite its being a first-line recommendation, studies evaluating the clinical success of vancomycin treatment in patients with MRSA pneumonia have observed failure in 45% to 77% of patients (15, 24). One possible explanation could be the recently reported vancomycin MIC creep detected among S. aureus strains (32). Over the last decade, an increase in vancomycin MICs has been noted by some centers, despite values staying within the Clinical and Laboratory Standards Institute (CLSI)-defined susceptibility range of ≤2 μg/ml (6). Among selected patients with MRSA bacteremia treated with vancomycin, Sakoulas and colleagues found significantly more treatment failures for patients infected with isolates possessing vancomycin MICs of 1 to 2 μg/ml (90.5%) than for those infected with isolates with vancomycin MICs of ≤0.5 μg/ml (44.4%) (31).
In addition to MIC creep, another explanation for vancomycin failures is the increasing appearance of vancomycin-intermediate S. aureus (VISA) and heterogeneous vancomycin-intermediate S. aureus (hVISA) strains (3). The simultaneous increases in frequency of these isolates are expected, as hVISA isolates are thought to be the strains immediately preceding VISA in its evolution (12). The parent strains of hVISA have vancomycin MICs ranging from 1 to 4 μg/ml. However, when these strains are subjected to increasing concentrations of vancomycin, a subpopulation of resistant clones develops, with MICs of at least 8 μg/ml (21).
Clinically, poor outcomes have been reported for both VISA and hVISA infections treated with vancomycin (21). In one study, hVISA-infected bacteremia patients were shown to have a significantly longer length of hospital stay, to have a greater proportion of high-bacterial-load infections, and to more commonly fail vancomycin therapy than those with vancomycin-susceptible MRSA infections (5). For this reason, it is necessary to characterize other treatment options not only for MRSA but also for the increasingly prevalent hVISA and VISA isolates.
The recently approved lipoglycopeptide telavancin is the newest option for the treatment of resistant S. aureus. In vitro studies of telavancin demonstrated potent activity against a number of Gram-positive organisms, including MRSA, hVISA, and VISA (7, 8, 18). A murine lung infection model of human simulated exposures of telavancin and vancomycin showed an increased efficacy of telavancin against a single MRSA isolate (28). Similar results were noted against an hVISA and 2 VISA isolates in a murine bacteremia model (10). The present study was designed to merge these two findings by comparing the efficacies of human simulated exposures to telavancin and vancomycin against a number of MRSA isolates, including hVISA and VISA isolates, in a murine lung infection model.
(These data were presented in part at the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy.)
MATERIALS AND METHODS
Antimicrobials.
Telavancin for injection (Theravance Inc., San Francisco, CA) was used for all in vivo analyses, and analytic-grade vancomycin powder (Sigma-Aldrich, St. Louis, MO) was used for both in vitro and in vivo studies. The entire contents of the telavancin vial was reconstituted with 0.9% sodium chloride as recommended by the manufacturer and served as the stock solution for dilution to the desired dosing concentration. Based on the supplied potency, vancomycin powder was weighed in a quantity sufficient to achieve the required concentration and reconstituted with normal saline immediately prior to use. Antibiotic solutions were stored at 4°C, protected from light, and discarded after 48 h.
Bacterial isolates.
A total of 13 clinical MRSA isolates were used in this analysis. These included five hospital-associated MRSA (HA-MRSA), two community-associated MRSA (CA-MRSA), two hVISA, and four VISA isolates. The HA-MRSA isolates consisted of isolate 56 (MRSA-494) (13), isolate 152 (USA100, staphylococcal cassette chromosome mec element type II [SCCmec II], Panton-Valentine leukocidin [PVL] negative), and clinically obtained isolates 336, 360, and 412. Both CA-MRSA strains were confirmed to be USA300 and SCCmec IV strains. Isolate 145 is PVL positive, while isolate 156 is PVL negative. The hVISA isolates utilized were isolate 38 (Mu3) and the population analysis profiling-confirmed clinical isolate 443. The VISA isolates included previously published clinical isolates 435 and 440 (30), as well as isolates 453 (NRS23) and 454 (NRS404). MICs of telavancin, vancomycin, and a number of commonly used antimicrobials were determined in triplicate for each isolate by broth microdilution assay using dry-form panels (Sensititre; Trek Diagnostics, Cleveland, OH), and the modal MICs are reported. Isolates were maintained in double-strength skim milk (BD Biosciences, Sparks, MD) at −80°C. Each isolate was subcultured twice on Trypticase soy agar with 5% sheep blood (BD Biosciences) and grown at 35°C prior to use in all experiments.
Murine lung infection model.
This study was reviewed and approved by the Hartford Hospital Institutional Animal Care and Use Committee. Pathogen-free female BALB/c mice weighing approximately 20 g were acquired from Harlan Sprague-Dawley, Inc. (Indianapolis, IN), and utilized throughout these experiments. Animals were maintained and used in accordance with National Research Council recommendations and were provided food and water ad libitum. The murine lung infection model used in this analysis has been described previously (16, 19). Briefly, mice were rendered neutropenic by intraperitoneal injections with 100 and 250 mg/kg of body weight of cyclophosphamide (Cytoxan; Bristol-Myers Squibb, Princeton, NJ) given 1 and 4 days prior to inoculation, respectively. Six hours prior to the initiation of antimicrobial therapy, isoflurane-anesthetized mice were held upright and inoculated with 0.05 ml of a 107-CFU suspension of the infecting MRSA isolate in 3% mucin (Sigma-Aldrich, St. Louis, MO). Inocula were administered directly into the buccal cavity of the mice, and their nares were blocked to induce aspiration. Mice with lung infections were used for all pharmacodynamic and pharmacokinetic analyses.
Dosing regimen determination.
The pharmacokinetics of telavancin in mice have been described previously (28). In the previous study, a dose of 40 mg/kg given subcutaneously every 12 h (q12h) resulted in a similar free drug exposure profile in mice to that for a daily 10-mg/kg dose in humans. Assuming respective protein binding values of 93% and 96% for mice and humans, respectively, the areas under the concentration-time curve for the free, unbound fraction of drug (fAUC) reported for these doses were 52.3 μg·h/liter and 44.3 μg·h/liter, respectively (28). As such, a 40-mg/kg dose of telavancin given every 12 h was used throughout this analysis to simulate human exposures.
A number of recently published studies have conducted pharmacokinetic analyses of vancomycin in mice to determine doses that approximate the exposure profile for humans given 1 g every 12 h. The final doses purported by the various studies have ranged from 110 to 180 mg/kg given subcutaneously every 12 h (11, 20, 28). Given this variability, we conducted pharmacokinetic studies of infected mice to confirm the proper dose selection. The fAUC we targeted for these studies was 208 μg·h/liter, which corresponds with the reported total AUC (454 μg·h/liter) for humans with normal renal function administered 1 g every 12 h (1, 9). Calculations of free drug profiles for mice and humans were done by assuming 30% and 54% protein binding, respectively (1, 14, 20). For confirmatory pharmacokinetic studies, mice were dosed with a single dose of 110 mg/kg, and groups of 6 mice were euthanized at 3 time points throughout the dosing interval. Blood samples were taken via cardiac puncture, and sera were stored at −80°C until analysis. Vancomycin concentrations were analyzed with a colorimetric enzymatic assay (Roche Diagnostic Corporation, Indianapolis, IN) by a spectrophotometric detection method (Cobas c501; Roche Diagnostics Corporation, Indianapolis, IN). The assay was linear for vancomycin concentrations ranging from 1.7 to 80 μg/ml. The AUC for the observed regimen was calculated using the trapezoidal rule.
Efficacy determination.
For each of the 13 S. aureus isolates, groups of 12 mice were administered human simulated exposures of telavancin or vancomycin beginning 6 h after the initiation of infection; all doses were administered as 0.2-ml subcutaneous injections. To serve as control animals, an additional group of 12 mice was administered normal saline at the same volume, route, and frequency as the treatment regimens. One set of experiments ended after 24 h (all 13 isolates), and another ended after 48 h (7 of 13 isolates); 6 mice were harvested from each group at both time points (i.e., telavancin treatment, vancomycin treatment, and control groups). The harvesting procedure began with euthanasia by CO2 exposure followed by cervical dislocation. After sacrifice, all of the lobes of the lungs were removed and homogenized in 5 ml of normal saline. Serial dilutions of the lung homogenate were plated on Trypticase soy agar with 5% sheep blood for CFU determination. In addition to the above-mentioned treatment and control groups, another group of 6 infected, untreated mice were harvested at the initiation of dosing (i.e., 0-h control group). Efficacy, designated the change in bacterial density, was calculated as the change in log10 bacterial CFU/ml obtained for treated mice after 24 and 48 h compared to that obtained for the 0-h control animals. A comparison of the efficacies of telavancin and vancomycin against each isolate was made by using the Student t test or the Mann-Whitney U test if data were not normally distributed. A P value of <0.05 was defined a priori as statistically significant.
RESULTS
In vitro potency.
The phenotypic profile for each of the S. aureus isolates utilized is shown in Table 1. MICs of telavancin were 1 to 2 dilutions lower than those of vancomycin across the range of vancomycin MICs.
TABLE 1.
MICs of various antimicrobials for the 13 S. aureus isolates utilized in the lung infection model
| Isolate | MIC (μg/ml)a |
||||||
|---|---|---|---|---|---|---|---|
| VAN | TLV | CIP | CLI | DAP | LZD | TGC | |
| HA-MRSA 56 (494) | 1 | 0.5 | 0.5 | 0.25 | 0.5 | 2 | 0.5 |
| HA-MRSA 152 | 1 | 0.25 | 0.5 | 0.125 | 0.5 | 2 | 0.25 |
| CA-MRSA 156 | 1 | 0.5 | ≥16 | ≤0.5 | 0.5 | 2 | 0.125 |
| CA-MRSA 145 | 2 | 0.5 | 8 | ≥8 | 0.5 | 2 | 0.25 |
| HA-MRSA 336 | 2 | 0.5 | ≥16 | ≥8 | 0.5 | 2 | 0.5 |
| HA-MRSA 360 | 2 | 0.5 | ≥16 | ≥8 | 0.5 | 2 | 0.25 |
| HA-MRSA 412 | 2 | 0.5 | ≥16 | ≤0.5 | 0.5 | 4 | 0.25 |
| hVISA 38 (Mu3) | 2 | 0.5 | ≥16 | ≥8 | 0.5 | 2 | 0.5 |
| hVISA 443 | 2 | 0.5 | ≥16 | ≥8 | 0.5 | 2 | 0.25 |
| VISA 435 | 4 | 1 | ≥16 | ≤0.5 | 0.5 | 2 | 0.25 |
| VISA 440 (A6298) | 4 | 1 | ≥16 | ≥8 | 1 | 2 | 0.125 |
| VISA 453 (NRS23) | 4 | 1 | ≥16 | ≥8 | 1 | 2 | 0.125 |
| VISA 454 (NRS404) | 4 | 1 | ≥16 | ≤0.5 | 1 | 2 | 0.125 |
VAN, vancomycin; TLV, telavancin; CIP, ciprofloxacin; CLI, clindamycin; DAP, daptomycin; LZD, linezolid; TGC, tigecycline.
Pharmacokinetics.
Confirmatory pharmacokinetic studies of a single dose of vancomycin (110 mg/kg) revealed an area under the concentration-time curve from 0 to 12 h (AUC0-12) of 139.8 μg·h/liter. When single-dose data were extrapolated to a 24-h dosing interval (i.e., 110 mg/kg every 12 h) and protein binding was accounted for, this dosing regimen resulted in a free drug exposure level (195.7 μg·h/liter) very similar to that for humans given 1 g every 12 h (208 μg·h/liter). Accordingly, all pharmacodynamic analyses of vancomycin were conducting using a regimen of 110 mg/kg every 12 h.
In vivo efficacy.
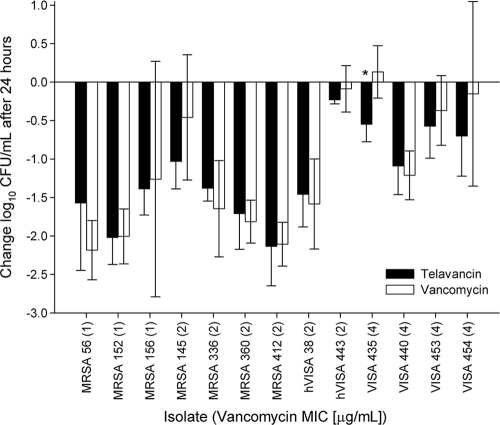
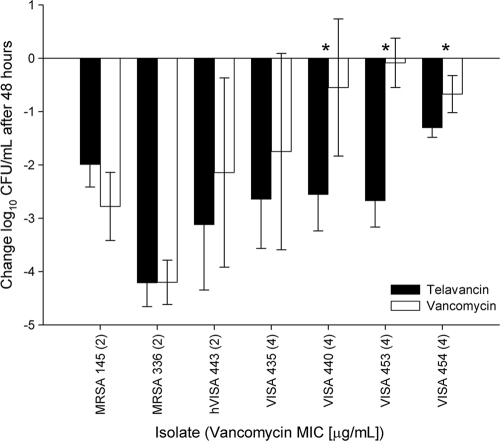
At dose initiation, the mean bacterial density was 6.16 ± 0.26 log10 CFU/ml, which increased by an average of 1.26 ± 0.55 log in untreated mice after 24 h and an average of 1.74 ± 0.68 log in untreated mice after 48 h. The results for the 24-h efficacy studies of telavancin and vancomycin against all 13 isolates are shown in Fig. 1. After 24 h, the efficacies of human simulated telavancin and vancomycin exposures were similar against all isolates except for VISA 435, for which telavancin treatment resulted in statistically greater reductions in CFU than did vancomycin (P = 0.002). The efficacy results for the 7 isolates tested after 48 h of treatment are shown in Fig. 2. At this time point, CFU reductions by telavancin were statistically greater against 3 of the 4 VISA isolates (for isolate 440, P = 0.007; for isolate 453, P < 0.001; and for isolate 454, P = 0.003) than those with vancomycin, while efficacies were similar for all other isolates.
FIG. 1.
Comparative efficacies of human simulated exposures of telavancin and vancomycin against S. aureus in a murine pneumonia model after 24 h. Data are expressed as means ± standard deviations (SD) for 6 mice per group. The asterisk indicates statistical significance in comparing telavancin and vancomycin activities (P < 0.05).
FIG. 2.
Comparative efficacies of human simulated exposures of telavancin and vancomycin against S. aureus in a murine pneumonia model after 48 h. Data are expressed as means ± SD for 6 mice per group. Asterisks indicate statistical significance in comparing telavancin and vancomycin activities (P < 0.05).
DISCUSSION
Telavancin is the most recent addition to the antimicrobial armamentarium for Gram-positive infections. As a result of its dual mechanism of action, telavancin exhibits increased in vitro potency relative to that of vancomycin against MRSA. This enhanced potency may prove increasingly more significant as vancomycin-nonsusceptible MRSA strains continue to emerge. In the present analysis, we compared the efficacies of human simulated exposures of telavancin and vancomycin against a number of these emergent strains in a mouse lung infection model. We found telavancin to be more efficacious against 1 of 13 isolates after 24 h of treatment and against 3 of 7 isolates after 48 h of treatment, while telavancin and vancomycin efficacies were similar for the remaining isolates.
In considering the results of the 24-h studies, human simulated exposures of telavancin resulted in at least a 1-log reduction in bacterial density against MRSA isolates with telavancin MICs of ≤0.5 μg/ml and a ≥0.5-log reduction against hVISA and VISA isolates with a MIC of 1 μg/ml. While an exposure-response target has yet to be identified for telavancin in lung infection, by assuming an fAUC of 52.3 μg·h/liter for the human simulated regimen, it can be extrapolated from our study that, in general, fAUC/MIC ratios of 208, 104, and 52 resulted in approximately 2-, 1-, and 0.5-log reductions in bacterial density, respectively. Interestingly, for the two hVISA isolates with a telavancin MIC of 0.5 μg/ml, telavancin efficacy against one resulted in a ≥1-log decrease, while the other resulted in only a 0.25-log decrease after 24 h. In the current analysis, we did not retest the MICs of the various isolates after in vivo exposure to telavancin, so it cannot be confirmed that resistance did not develop. However, given the results against that isolate (hVISA 443) after 48 h (3-log decrease), the development of resistance seems unlikely. Notably, a recent in vitro resistance selection study did show an increase in telavancin MICs, from 0.5 μg/ml to 2 μg/ml, for two hVISA strains, albeit after 50 serial passages (17).
The currently recognized exposure-response target for vancomycin derived from clinical studies of humans is a total drug AUC/MIC ratio of 400 or a free drug target of approximately 184, assuming 54% protein binding (24, 25). With this target, one would expect vancomycin given at 1 g q12h in a patient with normal renal function to produce a predictable antimicrobial effect against isolates with MICs of ≤1 μg/ml (29). However, using this human simulated regimen of vancomycin in mice with infected lungs, we noted predictable efficacy against isolates with vancomycin MICs of ≤2 μg/ml, with only one isolate (MIC = 2 μg/ml) resulting in a <1-log decrease in bacterial density after 24 h. While the AUC/MIC target of 400 is a total drug target, in this study we humanized exposures based on free drug concentrations, assuming 54% protein binding in humans and 30% binding in mice (1, 14, 20). This approach was chosen given the observation that only unbound drug is microbiologically active (23, 26). It should be noted that the relationship between proteins, antimicrobials, and bacteria is a very complex interaction that is not entirely understood, especially for vancomycin. Specifically, a recent study evaluating in vivo protein binding in 15 patients treated with vancomycin for Gram-positive infections found the percentage of free drug to range from 12 to 100% (4). This degree of variability could certainly impact actual free drug exposures. Unfortunately, no study simultaneously evaluating both free drug vancomycin concentrations and outcomes in the same patients has been completed to date.
Despite potential confounders, the efficacy of vancomycin at 24 h against the entire VISA population and a majority of the hVISA isolates was reduced relative to that against isolates with lower MICs, as predicted. Given the heterogeneity of the hVISA strains, it is possible that a 24-h dosing interval was not long enough to induce a sufficient rise in the resistant subpopulation and thus show notable effects on CFU reductions for these isolates. For this reason, studies were extended to evaluate the bacterial density after 48 h of treatment.
In comparing the efficacies of telavancin and vancomycin at 48 h, it was observed that telavancin was statistically more efficacious against 3 of the 5 hVISA and VISA isolates. Furthermore, telavancin efficacy increased from 24 to 48 h against all 7 isolates (range, −0.6 to −2.9 log10 CFU/ml), while an additional 24 h of vancomycin treatment resulted in a decrease in efficacy (i.e., increased bacterial density) for 2 of the 7 isolates (range, 0.3 to −2.6 log10 CFU/ml). As mentioned above, it is possible that the extension in the amount of time that isolates were exposed to vancomycin may have allowed amplification of the subpopulation of resistant isolates, at least for the hVISA strains. Future studies extending the endpoint further may help to elucidate these findings.
Only one other study has compared the efficacies of telavancin and vancomycin for the treatment of MRSA lung infection in mice (28). Against the single MRSA isolate tested in that study (with telavancin and vancomycin MICs of 0.5 and 1 μg/ml, respectively), human simulated doses of telavancin (40 mg/kg every 12 h) resulted in statistically greater efficacy than did those of vancomycin (110 mg/kg every 12 h) at the end of the dosing interval. Two different infection models were used in that analysis. One utilized a 12-h incubation period between inoculation and dosing and resulted in CFU reductions of 3.9 log and 1.8 log for telavancin and vancomycin, respectively, after 36 h of dosing. The second infection model utilized a 24-h incubation period and resulted in a 2.3-log reduction for telavancin and in 0.1-log growth for vancomycin after 24 h of dosing. Both methods represented findings quite different from those observed for isolates with similar MICs in our study. The mean bacterial densities at the initiation of dosing were similar between our model and the 12-h incubation model of Reyes et al. (i.e., 6.2 versus 6.3 log) and subsequently served as the best comparator. The increased efficacy noted for telavancin in that study can potentially be explained by the extra 12 h of dosing prior to harvesting (i.e., 36 versus 24 h) and is supported by the increase in efficacy noted with MRSA 336 in our 48-h model (i.e., 4-log decrease). However, an explanation for the disproportionate lack of vancomycin efficacy in that model is not abundantly clear. One notable difference is that while we dosed vancomycin subcutaneously, doses in the previous analysis were given intravenously. Nevertheless, in light of the fact that the efficacy of vancomycin is AUC driven (29), this difference should have no bearing on the observed effect. Given that the study by Reyes et al. consisted of a single observation, it is possible that the results noted are consistent with the specific MRSA isolate tested in that analysis, as a certain degree of interisolate variability exists for isolates studied in vivo.
In this report, we describe the efficacy of telavancin for the treatment of pneumonia caused by a collection of variably resistant MRSA strains. We found that human simulated dosing regimens of telavancin and vancomycin resulted in similar efficacies against MRSA strains with vancomycin MICs of ≤2 μg/ml. Against hVISA strains, similar efficacies were noted for telavancin and vancomycin after 24 h, while telavancin was more efficacious after 48 h against one of the two strains tested. Lastly, mean reductions in bacterial density were greater for telavancin against all VISA strains at both 24 and 48 h and were statistically greater against one of three strains after 24 h and three of four strains after 48 h. As telavancin undergoes further review by the FDA for the treatment of nosocomial pneumonia, these data support the potential utility of telavancin for the treatment of pneumonia caused by MRSA strains with reduced susceptibility to vancomycin.
Acknowledgments
We thank Mary Anne Banevicius, Henry Christensen, Jennifer Hull, Pornpan Koomanachai, Debora Santini, and Lindsay Tuttle for their assistance with the conduct of animal experimentation and George Sakoulas, Gary Stein, and Tom Lodise for providing the hVISA and VISA isolates.
This work was supported by Theravance Inc., San Francisco, CA, and Astellas Pharma Inc., Deerfield, IL.
Footnotes
Published ahead of print on 13 September 2010.
REFERENCES
- 1.Albrecht, L. M., M. J. Rybak, L. H. Warbasse, and D. J. Edwards. 1991. Vancomycin protein binding in patients with infections caused by Staphylococcus aureus. DICP 25:713-715. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171:388-416. [DOI] [PubMed] [Google Scholar]
- 3.Appelbaum, P. C. 2006. The emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 12(Suppl. 1):16-23. [DOI] [PubMed] [Google Scholar]
- 4.Berthoin, K., E. Ampe, P. M. Tulkens, and S. Carryn. 2009. Correlation between free and total vancomycin serum concentrations in patients treated for Gram-positive infections. Int. J. Antimicrob. Agents 34:555-560. [DOI] [PubMed] [Google Scholar]
- 5.Charles, P. G., P. B. Ward, P. D. Johnson, B. P. Howden, and M. L. Grayson. 2004. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin. Infect. Dis. 38:448-451. [DOI] [PubMed] [Google Scholar]
- 6.Clinical Laboratory Standards Institute. 2008. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 8th ed. CLSI publication M07-A8. CLSI, Wayne, PA.
- 7.Draghi, D. C., B. M. Benton, K. M. Krause, C. Thornsberry, C. Pillar, and D. F. Sahm. 2008. Comparative surveillance study of telavancin activity against recently collected Gram-positive clinical isolates from across the United States. Antimicrob. Agents Chemother. 52:2383-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draghi, D. C., B. M. Benton, K. M. Krause, C. Thornsberry, C. Pillar, and D. F. Sahm. 2008. In vitro activity of telavancin against recent Gram-positive clinical isolates: results of the 2004-05 Prospective European Surveillance Initiative. J. Antimicrob. Chemother. 62:116-121. [DOI] [PubMed] [Google Scholar]
- 9.Healy, D. P., R. E. Polk, M. L. Garson, D. T. Rock, and T. J. Comstock. 1987. Comparison of steady-state pharmacokinetics of two dosage regimens of vancomycin in normal volunteers. Antimicrob. Agents Chemother. 31:393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hegde, S. S., S. Difuntorum, R. Skinner, J. Trumbull, and K. M. Krause. 2009. Efficacy of telavancin against glycopeptide-intermediate Staphylococcus aureus in the neutropenic mouse bacteraemia model. J. Antimicrob. Chemother. 63:763-766. [DOI] [PubMed] [Google Scholar]
- 11.Hegde, S. S., N. Reyes, R. Skinner, and S. Difuntorum. 2008. Efficacy of telavancin in a murine model of pneumonia induced by methicillin-susceptible Staphylococcus aureus. J. Antimicrob. Chemother. 61:169-172. [DOI] [PubMed] [Google Scholar]
- 12.Hiramatsu, K. 2001. Vancomycin-resistant Staphylococcus aureus: a new model of antibiotic resistance. Lancet Infect. Dis. 1:147-155. [DOI] [PubMed] [Google Scholar]
- 13.Kaatz, G. W., S. L. Barriere, D. R. Schaberg, and R. Fekety. 1987. Ciprofloxacin versus vancomycin in the therapy of experimental methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob. Agents Chemother. 31:527-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knudsen, J. D., K. Fuursted, F. Espersen, and N. Frimodt-Moller. 1997. Activities of vancomycin and teicoplanin against penicillin-resistant pneumococci in vitro and in vivo and correlation to pharmacokinetic parameters in the mouse peritonitis model. Antimicrob. Agents Chemother. 41:1910-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kollef, M. H., J. Rello, S. K. Cammarata, R. V. Croos-Dabrera, and R. G. Wunderink. 2004. Clinical cure and survival in Gram-positive ventilator-associated pneumonia: retrospective analysis of two double-blind studies comparing linezolid with vancomycin. Intensive Care Med. 30:388-394. [DOI] [PubMed] [Google Scholar]
- 16.Koomanachai, P., J. L. Crandon, M. A. Banevicius, L. Peng, and D. P. Nicolau. 2009. Pharmacodynamic profile of tigecycline against methicillin-resistant Staphylococcus aureus in an experimental pneumonia model. Antimicrob. Agents Chemother. 53:5060-5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosowska-Shick, K., C. Clark, G. A. Pankuch, P. McGhee, B. Dewasse, L. Beachel, and P. C. Appelbaum. 2009. Activity of telavancin against staphylococci and enterococci determined by MIC and resistance selection studies. Antimicrob. Agents Chemother. 53:4217-4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krause, K. M., M. Renelli, S. Difuntorum, T. X. Wu, D. V. Debabov, and B. M. Benton. 2008. In vitro activity of telavancin against resistant Gram-positive bacteria. Antimicrob. Agents Chemother. 52:2647-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laohavaleeson, S., P. R. Tessier, and D. P. Nicolau. 2008. Pharmacodynamic characterization of ceftobiprole in experimental pneumonia caused by phenotypically diverse Staphylococcus aureus strains. Antimicrob. Agents Chemother. 52:2389-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaPlante, K. L., S. N. Leonard, D. R. Andes, W. A. Craig, and M. J. Rybak. 2008. Activities of clindamycin, daptomycin, doxycycline, linezolid, trimethoprim-sulfamethoxazole, and vancomycin against community-associated methicillin-resistant Staphylococcus aureus with inducible clindamycin resistance in murine thigh infection and in vitro pharmacodynamic models. Antimicrob. Agents Chemother. 52:2156-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, C., and H. F. Chambers. 2003. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob. Agents Chemother. 47:3040-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch, J. P., III. 2001. Hospital-acquired pneumonia: risk factors, microbiology, and treatment. Chest 119:373S-384S. [DOI] [PubMed] [Google Scholar]
- 23.Merrikin, D. J., J. Briant, and G. N. Rolinson. 1983. Effect of protein binding on antibiotic activity in vivo. J. Antimicrob. Chemother. 11:233-238. [DOI] [PubMed] [Google Scholar]
- 24.Moise, P. A., A. Forrest, S. M. Bhavnani, M. C. Birmingham, and J. J. Schentag. 2000. Area under the inhibitory curve and a pneumonia scoring system for predicting outcomes of vancomycin therapy for respiratory infections by Staphylococcus aureus. Am. J. Health Syst. Pharm. 57(Suppl. 2):S4-S9. [DOI] [PubMed] [Google Scholar]
- 25.Moise, P. A., and J. J. Schentag. 2000. Vancomycin treatment failures in Staphylococcus aureus lower respiratory tract infections. Int. J. Antimicrob. Agents 16(Suppl. 1):S31-S34. [DOI] [PubMed] [Google Scholar]
- 26.Nicolau, D. P., H. M. Mattoes, M. Banevicius, D. Xuan, and C. H. Nightingale. 2003. Pharmacodynamics of a novel des-F(6)-quinolone, BMS-284756, against Streptococcus pneumoniae in the thigh infection model. Antimicrob. Agents Chemother. 47:1630-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NNIS System. 2004. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992 through June 2004, issued October 2004. Am. J. Infect. Control 32:470-485. [DOI] [PubMed] [Google Scholar]
- 28.Reyes, N., R. Skinner, K. Kaniga, K. M. Krause, J. Shelton, G. P. Obedencio, A. Gough, M. Conner, and S. S. Hegde. 2005. Efficacy of telavancin (TD-6424), a rapidly bactericidal lipoglycopeptide with multiple mechanisms of action, in a murine model of pneumonia induced by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:4344-4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rybak, M. J., B. M. Lomaestro, J. C. Rotschafer, R. C. Moellering, Jr., W. A. Craig, M. Billeter, J. R. Dalovisio, and D. P. Levine. 2009. Therapeutic monitoring of vancomycin in adults. Summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy 29:1275-1279. [DOI] [PubMed] [Google Scholar]
- 30.Sakoulas, G., G. M. Eliopoulos, R. C. Moellering, Jr., C. Wennersten, L. Venkataraman, R. P. Novick, and H. S. Gold. 2002. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob. Agents Chemother. 46:1492-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakoulas, G., P. A. Moise-Broder, J. Schentag, A. Forrest, R. C. Moellering, Jr., and G. M. Eliopoulos. 2004. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 42:2398-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinkraus, G., R. White, and L. Friedrich. 2007. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001-05. J. Antimicrob. Chemother. 60:788-794. [DOI] [PubMed] [Google Scholar]
- 33.Vincent, J. L., J. Rello, J. Marshall, E. Silva, A. Anzueto, C. D. Martin, R. Moreno, J. Lipman, C. Gomersall, Y. Sakr, and K. Reinhart. 2009. International study of the prevalence and outcomes of infection in intensive care units. JAMA 302:2323-2329. [DOI] [PubMed] [Google Scholar]