Abstract
Five Paracoccidioides brasiliensis isolates were grown in the presence of caspofungin (0 to 1 μg/ml). Inhibition of the yeast phase ranged from 20 to 65%, while in the mycelial form it ranged from 75% to 82%. Such variability was loosely related to the amount of cell wall β-1,3-glucan. No association with point mutations in the β-1,3-glucan synthase was detected. Caspofungin induced physical changes and cytoplasmic deterioration in both fungal phases.
The echinocandins, inhibitors of the β-1,3-d-glucan synthase, affect the assembly of the fungal cell wall, leading to cell deterioration (25). Candida and Aspergillus are caspofungin-sensitive genera (9, 15), while zygomycetes and other fungi are not (11). Dimorphic fungi, i.e., Blastomyces dermatitidis or Histoplasma capsulatum, are rather resistant (7). They and Paracoccidioides brasiliensis have β-1,3-glucan in their cell walls, mainly in their mycelial (M) phase. α-1,3-Glucan substitutes for β-1,3-glucan almost entirely when these species go into the pathogenic yeastlike (Y) phase (16-19). Herein, we report the effect of caspofungin in P. brasiliensis growth, cell wall composition, and morphology.
P. brasiliensis isolates IVIC Pb73 (ATCC 32071; patient), IVIC Pb300 (soil), IVIC Pb377 (armadillo), and IVIC Pb381 and IVIC Pb444 (recent isolates from patients) were grown for up to 4 days in RPMI 1640 (Gibco) medium buffered with 0.165 M morpholinepropanesulfonic acid (MOPS) to pH 7.0, according to CLSI M27-A2, in the presence of caspofungin (0 to 1.0 μg/ml). Y cells were incubated at 37°C; cell density was followed by turbidimetry in Klett units, every 24 h. Mycelia grew at 23°C; growth was measured daily by dry weight (20). Experiments were repeated three times.
The cell walls and fractions herein were prepared as before (20). Alkali-insoluble fraction 1 (β-1,3-glucan and chitin), alkali-soluble, acid-insoluble fraction 2 (α-1,3-glucan), and alkali- and acid-soluble fraction 3 (galactomannan, proteins, and lipids) were separated (20). Hexoses (for glucans) (21) and N-acetyl-hexosamine (for chitin) (1) were quantitatively traced in fractions 1 and 2. Fractionation was carried out twice, and chemical analyses were done in triplicate. Infrared (IR) spectroscopy with KBr pellets was performed with a Brucker FT_IR Tensor 27 infrared spectrometer (Elk Grove Village, IL). Transmission electron microscopy (TEM; Phillip CM10, Eindhoven, Netherlands) and scanning electron microscopy (SEM; Hitachi S-4500) were performed as before (2).
FKS1 gene fragments corresponding to Fks1 hot spot 1 and 2 regions were amplified. Fks protein sequences from Candida krusei (DQO17894), C. glabrata (AF229171), and C. albicans (D88815) were aligned with the protein sequence of P. brasiliensis FKS (AF148715) and the respective Fks sequences mined from the genomes of P. brasiliensis isolates Pb01, Pb18, and Pb03 (Broad Institute, MIT, Boston, MA; http://www.broadinstitute.org/annotation/genome/paracoccidioides_brasiliensis/MultiHome.html). Fks hot spots 1 and 2 were identified (5, 25); primers were designed on these regions, extending them about 100 bp upstream and downstream. The deduced amino acid sequences of Fks1 hot spot 1 and 2 regions in our P. brasiliensis isolates were compared by Clustal W (24).
Statistical analyses were done by covariance analysis (ANCOVA), with the SPSS 17.0 program, at a significance level representing P values of ≤0.05. They were run at different concentrations of caspofungin at each day, with every culture, and in both morphological phases.
The CLSI (formerly NCCLS) reference method (M38-A) (13, 14) is poorly suited to measure the in vitro activity of echinocandins against filamentous fungi (8). We adapted the method to macroculture conditions because P. brasiliensis requires continuous aeration and longer periods of time (3 to 4 days) to grow. The effects of caspofungin on P. brasiliensis growth are recorded in Tables S1 and S2 in the supplemental material. Caspofungin (1.0 μg/ml) inhibited mycelial growth in proportions that ranged between 75.4% ± 0.5% (Pb381) and 82.3% ± 1.8% (Pb73) (Table S1). Y cultures were less affected, from 20.7% ± 0.7% (Pb377) to 65.6% ± 1.1% (Pb73) (Table S2). Higher caspofungin concentrations (up to 16 μg/ml; not shown) did not improve these figures. Statistical analyses under each experimental condition indicated P values of ≤0.05 in all cases.
Hyphal morphology deteriorated in the presence of caspofungin (Fig. 1), not only through disappearance of the hyphal segments but with disorganization of organelles within the cytoplasmic structure and a coarser appearance of the outer cell wall. Y cells were similarly affected by caspofungin (Fig. 2).
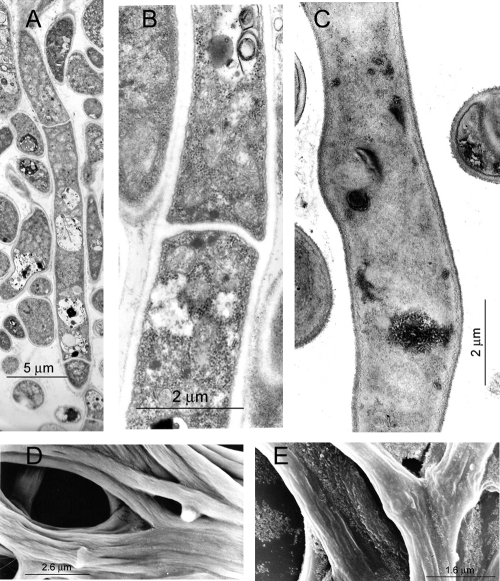
FIG. 1.
Transmission (TEM) and scanning (SEM) electron micrographs of P. brasiliensis Pb73 in its mycelial phase. (A, B, and C) TEM of cultures grown for 4 days in the absence (A and B) and presence (C) of caspofungin (1 μg/ml). (D) Corresponding SEM for panels A and B. (E) Corresponding SEM of panel C. Cell wall roughness, membrane deterioration, and disappearance of hyphal septa, with concurrent cytoplasmic disorganization, were the effects of caspofungin exposure. Similar results were obtained with strain Pb300. Measuring scales are shown in the micrographs.
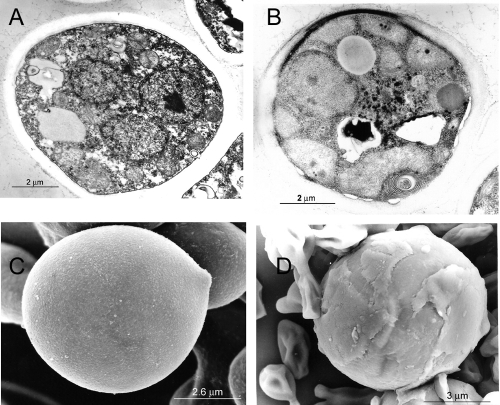
FIG. 2.
Transmission (TEM) and scanning (SEM) electron micrographs of P. brasiliensis Pb73 in its yeastlike phase. (A and B) TEM of cells grown for 4 days in the absence (A) and presence (B) of caspofungin (1 μg/ml). (C and D) Corresponding SEM for panels A and B, respectively. An abnormal cell wall layering is observed under the effect of caspofungin, together with membrane detachment and cytoplasmic disorganization. Similar results were obtained with strain Pb300. Measuring scales are shown in the micrographs.
Cell wall analyses (see Table S3 in the supplemental material) indicated the presence of β-1,3-glucan as the prevalent neutral polysaccharide in the M cell walls of all isolates, from 20.2% (Pb444) to 31.4% (Pb73) of the total wall. Instead, the Y cell wall reduced these amounts to between 3.9 and 10.6% (for Pb377 and Pb73, respectively), while replacing this polysaccharide with α-1,3-glucan (22.4 to 32.6% for Pb73 and Pb381, respectively). Chitin was three times more abundant in the Y cell walls than in the corresponding M cell walls.
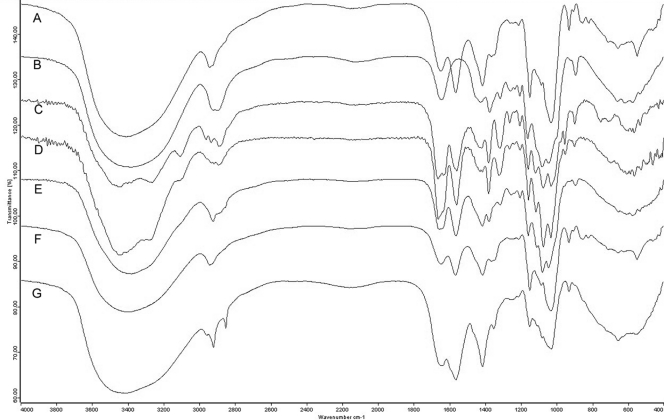
IR spectra were characteristic of polysaccharides (Fig. 3), with a strong and wide band around 3,400 cm−1 (OH stretching) and additional bands at 2,921 cm−1 (C-H stretching), 1,641 cm−1 (OH twisting), 1,414 cm−1 (CH twisting), 1,211/1,242 cm−1 (C-O-C twisting), and 1,147 to 1,023 cm−1 (C-C stretching). Fraction 2 of all strains in their Y phase showed signals at 929, 851, and 823 cm−1, suggestive of α-1,3-linkages (3). The corresponding fraction 2 region in the M phases presented the band at 929 cm−1 as the only one in this region. Bands at 897 and 1,378 cm−1 were characteristic of β-glucan in fraction 1 of both phases (3). Since this fraction was composed of a mixture of chitin and β-1,3-glucan, spectra were the result of bands associated with both polysaccharides, with additional bands characteristic of chitin (1,557, 1,662, and 3,442 cm−1).
FIG. 3.
Infrared spectra of α-1,3-glucan (A), laminarin (β-1,3-glucan) (B), chitin (C), fraction 1 from P. brasiliensis Pb73 yeastlike phase (D), fraction 1 from P. brasiliensis Pb73 mycelial phase (E), fraction 2 from P. brasiliensis Pb73 yeastlike phase (F), and fraction 2 from P. brasiliensis Pb300 yeastlike phase (G).
Sequence analysis of the deduced PbFKS1p failed to show any point mutation in “hot spot” regions 1 and 2 (aa 641 to 649 and aa 1351 to 1358), respectively.
The inhibitory effect of echinocandins has been associated with a blockage in the synthesis of cell wall β-1,3-glucan through inhibition of the corresponding synthase (25). Resistance to echinocandin may be due to a lack of this polysaccharide (12), the presence of outer capsules (10), or melanin (4, 10, 23). Melanization reduced P. brasiliensis susceptibility to amphotericin B and also protected against azoles (4). In Candida isolates, resistance may be related, although not exclusively, to point mutations in the deduced amino acid sequence of the β-1,3-glucan synthase, specifically the so-called hot spot 1 and 2 regions (5, 25). This does not seem to apply to the β-1,3-glucan synthase in our P. brasiliensis isolates, inasmuch as their sequences on their hot spot 1 and 2 regions and those downloaded from the publicly released P. brasiliensis genomes (Pb01, Pb03, and Pb18; Broad Institute, MIT) are 100% identical. Whether some other point mutations are at work is an open question (25).
Recently (22), we confirmed the presence of α-1,3-glucan in the Y cell wall of P. brasiliensis, albeit with a different structural arrangement than that proposed before (18), a glucan that is also present in our 5 isolates (Fig. 3). Because of the small β-1,3-glucan amounts and high-level α-1,3-glucan contents in the Y cell wall, we hypothesized a minimal effect of caspofungin on this phase. Caspofungin (1 μg/ml) partially inhibited Y growth at different proportions: Pb73 (65%) > Pb381 (52%) > Pb300 (35%) ≈ Pb444 (34%) > Pb377 (20%) (see Table S2 in the supplemental material). This order kept no direct relationship with the amounts of α-1,3-glucan (Pb381 [32%] > Pb444 [24%] = Pb377 [24%] = Pb300 [24%] ≈ Pb73 [22%]), although a loose relationship to the amounts of β-1,3-glucan was noted (Pb73 [11%] ≈ Pb444 [8%] ≈ Pb300 [7%] ≈ Pb381 [6%] > Pb377 [4%]) (Table S3).
The M phases of all P. brasiliensis isolates were, as expected, highly susceptible to caspofungin, with inhibition varying from 74% (Pb444) to 81% (Pb73) in the following sequence: Pb73 > Pb300 > Pb377 ≈ Pb444 ≈ Pb381 (see Table S1 in the supplemental material). Results for the inhibitory capacity of micafungin in P. brasiliensis have been reported (12); although higher concentrations of the echinocandin were used, those results cannot be directly compared with ours, as different protocols were used (15). The sequence of inhibition was loosely related to the amount of β-1,3-glucan in the mycelial cell walls of the strains (Pb73 [31%] > Pb377 [27%] > Pb300 [25%] > Pb381 [22%] > Pb444 [20%]) (Table S3). In no case was a 100% inhibition achieved, even at concentrations as high as 16 μM caspofungin (not shown), for which 91% inhibition (the highest in our tests) was the observed value for isolate Pb73 in M phase. Similar observations were commented upon by Espinel-Ingroff et al. (8), who indicated that echinocandins do not generally yield 100% inhibition susceptibility endpoints, because of trailing growth; similar outcomes have been reported for Candida kefyr (6) and Aspergillus species (25), an effect explained as the result of a fungistatic, rather than fungicidal, activity (6) or lysis of hyphal tips but not subapical hyphal compartments (25).
The possibility of using combinations of antifungals to improve the inhibitory capacity of caspofungin against P. brasiliensis remains to be studied.
Supplementary Material
Acknowledgments
Acknowledgements are due to Merck, Sharp & Dohme (Caracas, Venezuela) for partial financial support, to Mirtha Romano (IVIC, Caracas, Venezuela) and Antonio Bretaña (Universidad Nacional Experimental Simón Rodríguez, Caracas, Venezuela) for transmission and scanning electron micrographs, respectively, to Pedro Moncada (Universidad Simón Bolívar, Caracas, Venezuela) for infrared spectra and Maria de Lourdes Corradi da Silva (Universidade Estadual Paulista, São Paulo, Brazil) for help in their interpretation, and to María de Lourdes González for statistical analyses.
Footnotes
Published ahead of print on 11 October 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Belcher, R., A. J. Mutlen, and C. M. Sambrook. 1954. The determination of glucosamine. Analyst 79:201-208. [Google Scholar]
- 2.Bretaña, A., B. Nañez, M. Contreras-Bretaña, and S. Giardina. 2002. Multiple infection in bovines from the tropics: observation of blood parasites by scanning and transmission electron microscope. Parasitologia 44:173-178. [PubMed] [Google Scholar]
- 3.Corradi da Silva, M. L., E. K. Fukuda, A. F. D. Vasconcelos, R. F. H. Dekker, A. C. Matias, N. K. Monteiro, M. S. Cardoso, A. M. Barbosa, J. L. M. Silveira, G. L. Sassaki, and E. R. Carbonero. 2008. Structural characterization of the cell wall d-glucans isolated from the mycelium of Botryosphaeria rhodina MAMB-05. Carbohydr. Res. 343:793-798. [DOI] [PubMed] [Google Scholar]
- 4.da Silva, M. B., A. F. Marques, J. D. Nosanchuk, A. Casadevall, L. R. Travassos, and C. P. Taborda. 2006. Melanin in the dimorphic fungal pathogen Paracoccidioides brasiliensis: effects on phagocytosis, intracellular resistance and drug susceptibility. Microbes Infect. 8:197-205. [DOI] [PubMed] [Google Scholar]
- 5.Desnos-Ollivier, M., S. Bretagne, D. Raoux, D. Hoinard, F. Dromer, E. Dannaoui, and the European Committee on Antibiotic Susceptibility Testing. 2008. Mutations in the fks1 gene in Candida albicans, C. tropicalis, and C. krusei correlate with elevated caspofungin MICs uncovered in AM3 medium using the method of the European Committee on Antibiotic Susceptibility Testing. Antimicrob. Agents Chemother. 52:3092-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Bonaventura, G., I. Spedicato, C. Picciani, D. D'Antonio, and R. Piccolomini. 2004. In vitro pharmacodynamic characteristics of amphotericin B, caspofungin, fluconazole, and voriconazole against bloodstream isolates of infrequent Candida species from patients with hematologic malignancies. Antimicrob. Agents Chemother. 48:4453-4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinel-Ingroff, A. 1998. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff, A., A. Fothergill, M. Ghannoum, E. Manavathu, L. Ostrosky-Zeichner, M. A. Pfaller, M. G. Rinaldi, W. Schell, and T. J. Walsh. 2007. Quality control and reference guidelines for CLSI broth microdilution method (M38-A document) for susceptibility testing of anidulafungin against molds. J. Clin. Microbiol. 45:2180-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lass-Flörl, C., A. Mayr, S. Perkhofer, G. Hinterberger, J. Hausdorfer, C. Speth, and M. Fille. 2008. Activities of antifungal agents against yeasts and filamentous fungi: assessment according to the methodology of the European Committee on Antimicrobial Susceptibility Testing. Antimicrob. Agents Chemother. 52:3637-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McFadden, D. C., and A. Casadevall. 2001. Capsule and melanin synthesis in Cryptococcus neoformans. Med. Mycol. 39(Suppl. 1):19-30. [PubMed] [Google Scholar]
- 11.Martos, A. I., A. Romero, M. T. González, A. González, C. Serrano, C. Castro, J. Pemán, E. Cantón, and E. Martín-Mazuelos. 2010. Evaluation of the Etest method for susceptibility testing of Aspergillus spp. and Fusarium spp. to three echinocandins. Med. Mycol. 48:858-861. [DOI] [PubMed] [Google Scholar]
- 12.Nakai, T., J. Uno, F. Ikeda, S. Tawara, K. Nishimura, and M. Miyaji. 2003. In vitro antifungal activity of micafungin (FK463) against dimorphic fungi: comparison of yeast-like and mycelial forms. Antimicrob. Agents Chemother. 47:1376-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard. NCCLS document M38-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 14.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. NCCLS document M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 15.Odds, F. C., M. Motyl, R. Andrade, J. Bille, E. Cantón, M. Cuenca-Estrella, A. Davidson, C. Durussel, D. Ellis, E. Foraker, A. W. Fothergill, M. A. Ghannoum, R. A. Giacobbe, M. Gobernado, R. Handke, M. Laverdière, W. Lee-Yang, W. G. Merz, L. Ostrosky-Zeichner, J. Pemán, S. Perea, J. R. Perfect, M. A. Pfaller, L. Proia, J. H. Rex, M. G. Rinaldi, J. L. Rodriguez-Tudela, W. A. Schell, C. Shields, D. A. Sutton, P. E. Verweij, and D. W. Warnock. 2004. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J. Clin. Microbiol. 42:3475-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rappleye, C. A., J. T. Engle, and W. E. Goldman. 2004. RNA interference in Histoplasma capsulatum demonstrates a role for alpha-(1,3)-glucan in virulence. Mol. Microbiol. 53:153-165. [DOI] [PubMed] [Google Scholar]
- 17.San-Blas, G., and G. Niño-Vega. 2001. Paracoccidioides brasiliensis: virulence and host response, p. 205-226. In R. L. Cihlar and R. A. Calderone (ed.), Fungal pathogenesis: principles and clinical applications. Marcel Dekker, Inc., New York, NY.
- 18.San-Blas, G., and G. Niño-Vega. 2004. Morphogenesis in other agents of systemic mycoses, p. 167-220. In G. San-Blas and R. Calderone (ed.), Pathogenic fungi: structural biology and taxonomy. Caister Academic Press, Norfolk, England.
- 19.San-Blas, G., G. Niño-Vega, and T. Iturriaga. 2002. Paracoccidioides brasiliensis and paracoccidioidomycosis: molecular approaches to morphogenesis, diagnosis, epidemiology, taxonomy and genetics. Med. Mycol. 40:225-242. [DOI] [PubMed] [Google Scholar]
- 20.San-Blas, F., and G. San-Blas. 1992. Mutants of Paracoccidioides brasiliensis strain IVIC Pb9 affected in dimorphism. J. Med. Vet. Mycol. 30:51-60. [PubMed] [Google Scholar]
- 21.Scott, T. A., and E. H. Melvin. 1953. Determination of dextran with anthrone. Anal. Chem. 25:1656-1661. [Google Scholar]
- 22.Sorais, F., L. Barreto, J. A. Leal, M. Bernabe, G. San-Blas, and G. A. Niño-Vega. 2010. Cell wall glucan synthases and GTPases in Paracoccidioides brasiliensis. Med. Mycol. 48:35-47. [DOI] [PubMed] [Google Scholar]
- 23.Taborda, C. P., M. B. da Silva, J. D. Nosanchuk, and L. R. Travassos. 2008. Melanin as a virulence factor of Paracoccidioides brasiliensis and other dimorphic pathogenic fungi: a minireview. Mycopathologia 165:331-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker, L. A., N. A. Gow, and C. A. Munro. 2010. Fungal echinocandin resistance. Fungal Genet. Biol. 47:117-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.