Abstract
LCB01-0371 is a new oxazolidinone with cyclic amidrazone. In vitro activity of LCB01-0371 against 624 clinical isolates was evaluated and compared with those of linezolid, vancomycin, and other antibiotics. LCB01-0371 showed good activity against Gram-positive pathogens. In vivo activity of LCB01-0371 against systemic infections in mice was also evaluated. LCB01-0371 was more active than linezolid against these systemic infections. LCB01-0371 showed bacteriostatic activity against Staphylococcus aureus.
The emergence of multidrug-resistant (MDR) pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant coagulase-negative staphylococci (MRCNS), penicillin-resistant Streptococcus pneumoniae (PRSP), and vancomycin-resistant enterococci (VRE), has generated worldwide concern in the medical community (11). The requirement for effective new antimicrobial agents to treat infections caused by Gram-positive organisms is becoming urgent as resistance to existing agents arises and spreads around the world.
The oxazolidinones, a totally synthetic class of novel antibiotics, have strong activity against nearly all Gram-positive organisms, including those resistant to other agents (1, 10). They inhibit protein synthesis by binding to domain V of the 23S rRNA and thereby blocking formation of the initiation complex (6). Linezolid is the first member of the oxazolidinone class approved by the FDA in the United States. The success of linezolid and the occurrence of strains resistant to linezolid in clinical isolates of Enterococcus faecium (4, 5) and S. aureus (12) have inspired further efforts toward developing new oxazolidinones with improved safety and antibacterial activity.
LCB01-0371 (Fig. 1), a novel oxazolidinone with cyclic amidrazone, was synthesized by LegoChem BioSciences Inc. (Daejeon, Republic of Korea). In this study, in vitro activity of LCB01-0371 was compared with those of eight different antibacterial agents against 624 clinical isolates that were collected from several general hospitals in the Republic of Korea. In vivo activity of LCB01-0371 against systemic infections in mice and time-kill studies of LCB01-0371 against S. aureus giorgio (methicillin-susceptible S. aureus [MSSA]) and S. aureus p125 (MRSA) were also investigated.
FIG. 1.
Chemical structure of LCB01-0371.
(This study was presented in part at the 49th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 2009 [7].)
In vitro MICs were determined by the 2-fold agar dilution method as described by the Clinical and Laboratory Standards Institute (CLSI) (3). Mueller-Hinton agar (MHA) medium was used for testing aerobic and facultative organisms. Streptococcus pneumoniae, Streptococcus pyogenes, and Moraxella catarrhalis were grown on Mueller-Hinton agar supplemented with 5% defibrinated sheep blood (Hanil Komed Ltd., Sungnam City, Republic of Korea). Mueller-Hinton agar supplemented with 3% Fildes enrichment (Oxoid Ltd., Basingstoke, Hampshire, England) was used for Haemophilus influenzae. Bacteria (104 to 105 CFU) were spotted onto plates containing the appropriate concentration of drug. Plates were incubated at 35°C for 18 h and examined for growth. The MIC was considered to be the lowest concentration that completely inhibited growth on agar plates, disregarding a single colony or a faint haze caused by the inoculum.
Time-kill studies were performed by the M26-A method of the NCCLS (8). Test organisms incubated on tryptic soy agar (TSA) for 18 h at 37°C were diluted with fresh Mueller-Hinton broth to ∼105 CFU/ml, and the diluted cultures were preincubated for 2 h. Each drug was added to the cultures at concentrations of 0.25×, 0.5×, 1×, 2×, 4×, and 8× MIC. Aliquots (0.1 ml) of the cultures were removed at 0, 2, 4, 6, and 24 h of incubation, and serial 10-fold dilutions were prepared in saline as needed. Drug carryover effects were reduced by 100-fold dilution of the sample with agar. The numbers of viable cells on drug-free MHA plates after 24 h of incubation were determined. The compound was considered bactericidal at the concentration that reduced the original inoculum by 3 log10 CFU/ml (99.9%) at each of the time periods or considered bacteriostatic if the inoculum was reduced by ∼0 to 3 log10 CFU/ml.
In vivo activity of LCB01-0371 against systemic infections caused by S. aureus giorgio (MSSA), S. aureus p125 (MRSA), Enterococcus faecalis u810, S. pneumoniae ATCC 6305, and Haemophilus influenzae hd2 in mice was determined. Four-week-old male ICR mice weighing 18 to 22 g (Daehan Bio Link Co., Ltd., Eum-sung Gun, Republic of Korea) were used for the systemic infection model. They were maintained in animal rooms kept at 23 ± 2°C with 55% ± 20% relative humidity. Test organisms for infection were cultured in Mueller-Hinton agar medium (Difco) at 37°C for 18 h. For S. pneumoniae, Muller-Hinton agar medium was supplemented with 5% defibrinated sheep blood. For use as inocula, bacterial strains were suspended in 0.9% saline solution containing 5% gastric mucin (Sigma), except for S. pneumoniae, which was suspended in 0.9% saline solution. Mice were used in groups of six for each dose and were challenged intraperitoneally with a single 0.5-ml portion of the bacterial suspension, corresponding to an inoculum range of 10 to 100 times the minimal lethal dose of bacteria. Four dose levels were used for each antibiotic, depending on the in vitro antimicrobial activity of the compound. Antibiotics at various dose regimens were administered orally twice, at 1 and 4 h postinfection. Mortality was recorded for 7 days, and the median effective dose needed to protect 50% of the mice (ED50) was calculated by the Probit method (2). The challenge inoculum was sufficient to kill 100% of the untreated control mice, which died within 48 h postinfection.
All animal experiments were approved by the Ethics Review Committee of Handong Global University, Republic of Korea.
The comparative in vitro antibacterial activities of LCB01-0371 are shown in Table 1. The MIC90 of LCB01-0371 for MSSA and MRSA was 2 μg/ml. LCB01-0371 was as active as linezolid. Against methicillin-susceptible coagulase-negative staphylococci (MSCNS) (MIC90, 0.5 μg/ml) and MRCNS (MIC90, 0.5 μg/ml), LCB01-0371 was at least 2-fold more active than linezolid. LCB01-0371 was equally active irrespective of whether the strains were methicillin susceptible or resistant. Against S. pneumoniae (MIC90, 1 μg/ml) and S. pyogenes (MIC90, 2 μg/ml), LCB01-0371 showed antibacterial activity comparable to that of linezolid. LCB01-0371 was as active as linezolid against E. faecalis (MIC90, 2 μg/ml) and E. faecium (MIC90, 2 μg/ml). Against VRE (MIC90, 1 μg/ml), LCB01-0371 was 2-fold more active than linezolid. LCB01-0371 showed weak activity against the fastidious Gram-negative aerobes H. influenzae and M. catarrhalis. Against H. influenzae, LCB01-0371 yielded a MIC90 of 16 μg/ml, while slightly better activity against M. catarrhalis (MIC90, 8 μg/ml) was seen. The MIC90 of LCB01-0371 against H. influenzae was 2-fold lower than that of linezolid, but the MICs of LCB01-0371 against H. influenzae and M. catarrhalis were too high for clinical efficacy.
TABLE 1.
In vitro antibacterial activities of LCB01-0371 against clinical isolates
| Microorganism (no. of strains) and compound | MIC (μg/ml) |
||
|---|---|---|---|
| Range | 50% | 90% | |
| MSSA (69) | |||
| LCB01-0371 | ∼0.5-2 | 1 | 2 |
| Linezolid | ∼2-4 | 2 | 2 |
| Oxacillin | ∼0.06-1 | 0.25 | 0.5 |
| Erythromycin | ∼0.125->64 | 0.25 | >64 |
| Ciprofloxacin | ∼0.06->64 | 0.25 | 0.5 |
| Moxifloxacin | ∼0.015-64 | 0.06 | 0.125 |
| Gemifloxacin | ∼0.008-64 | 0.015 | 0.06 |
| Vancomycin | ∼0.25-2 | 1 | 1 |
| Quinupristin-dalfopristin | ∼0.125-0.5 | 0.25 | 0.5 |
| ∼0.125->64 | >64 | >64 | |
| MRSA (202) | |||
| LCB01-0371 | ∼0.5-4 | 1 | 2 |
| Linezolid | ∼2-2 | 2 | 2 |
| Oxacillin | ∼2->64 | >64 | >64 |
| Erythromycin | ∼0.25->64 | >64 | >64 |
| Ciprofloxacin | ∼0.125->64 | 32 | >64 |
| Moxifloxacin | ∼0.03->64 | 4 | 64 |
| Gemifloxacin | ∼0.008->64 | 2 | 64 |
| Vancomycin | ∼0.5-4 | 1 | 2 |
| Quinupristin-dalfopristin | ∼0.125-1 | 0.5 | 1 |
| ∼0.125->64 | >64 | >64 | |
| MSCNS (20) | |||
| LCB01-0371 | ∼0.5-1 | 0.5 | 0.5 |
| Linezolid | ∼1-2 | 1 | 2 |
| Oxacillin | ∼0.03-1 | 0.125 | 1 |
| Erythromycin | ∼0.06->64 | 0.25 | >64 |
| Ciprofloxacin | ∼0.06-8 | 0.125 | 8 |
| Moxifloxacin | ∼0.03-4 | 0.125 | 4 |
| Gemifloxacin | ∼0.008-0.5 | 0.015 | 0.5 |
| Vancomycin | ∼1-4 | 2 | 4 |
| Quinupristin-dalfopristin | ∼0.125-1 | 0.25 | 1 |
| ∼>64->64 | >64 | >64 | |
| MRCNS (33) | |||
| LCB01-0371 | ∼0.5-1 | 0.5 | 0.5 |
| Linezolid | ∼1-2 | 1 | 1 |
| Oxacillin | ∼2->64 | >64 | >64 |
| Erythromycin | ∼0.06->64 | >64 | >64 |
| Ciprofloxacin | ∼0.06-64 | 8 | 32 |
| Moxifloxacin | ∼0.06-16 | 2 | 8 |
| Gemifloxacin | ∼0.008-8 | 0.5 | 1 |
| Vancomycin | ∼1-4 | 2 | 2 |
| Quinupristin-dalfopristin | ∼0.125-8 | 0.25 | 1 |
| S. pneumoniae (97) | |||
| LCB01-0371 | ∼0.125-2 | 0.5 | 1 |
| Linezolid | ∼0.5-1 | 1 | 1 |
| Oxacillin | ∼0.008->64 | 8 | 16 |
| Erythromycin | ∼0.008->64 | 64 | >64 |
| Ciprofloxacin | ∼0.5-32 | 1 | 2 |
| Moxifloxacin | ∼0.06-4 | 0.25 | 0.5 |
| Gemifloxacin | ∼0.008-0.25 | 0.03 | 0.06 |
| Vancomycin | ∼0.25-1 | 0.5 | 1 |
| Quinupristin-dalfopristin | ∼0.5-4 | 1 | 2 |
| S. pyogenes (46) | |||
| LCB01-0371 | ∼0.5-2 | 1 | 2 |
| Linezolid | ∼1-2 | 2 | 2 |
| Oxacillin | ∼0.03-16 | 0.5 | 8 |
| Erythromycin | ∼0.008-8 | 0.06 | 2 |
| Ciprofloxacin | ∼0.5-2 | 1 | 2 |
| Moxifloxacin | ∼0.125-0.25 | 0.125 | 0.25 |
| Gemifloxacin | ∼0.015-0.125 | 0.03 | 0.06 |
| Vancomycin | ∼0.5-1 | 1 | 1 |
| Quinupristin-dalfopristin | ∼0.25-4 | 1 | 2 |
| E. faecalis (109) | |||
| LCB01-0371 | ∼1-2 | 2 | 2 |
| Linezolid | ∼1-2 | 2 | 2 |
| Oxacillin | ∼8->64 | 16 | >64 |
| Erythromycin | ∼0.125->64 | >64 | >64 |
| Ciprofloxacin | ∼0.06->64 | 2 | 64 |
| Moxifloxacin | ∼0.06-64 | 1 | 32 |
| Gemifloxacin | ∼0.008-16 | 0.125 | 4 |
| Vancomycin | ∼0.5-64 | 2 | 4 |
| Quinupristin-dalfopristin | ∼0.25-16 | 4 | 16 |
| E. faecium (29) | |||
| LCB01-0371 | ∼1-2 | 2 | 2 |
| Linezolid | ∼1-2 | 2 | 2 |
| Oxacillin | ∼16->64 | >64 | >64 |
| Erythromycin | ∼0.125->64 | >64 | >64 |
| Ciprofloxacin | ∼1-64 | 4 | 64 |
| Moxifloxacin | ∼0.25->64 | 4 | 32 |
| Gemifloxacin | ∼0.03-64 | 2 | 16 |
| Vancomycin | ∼0.5-8 | 1 | 2 |
| Quinupristin-dalfopristin | ∼0.25-32 | 0.5 | 4 |
| VRE (16) | |||
| LCB01-0371 | ∼1-1 | 1 | 1 |
| Linezolid | ∼2-2 | 2 | 2 |
| Oxacillin | ∼32->64 | >64 | >64 |
| Erythromycin | ∼>64->64 | >64 | >64 |
| Ciprofloxacin | ∼0.5-4 | 4 | 4 |
| Moxifloxacin | ∼0.25-4 | 2 | 4 |
| Gemifloxacin | ∼0.015-2 | 0.5 | 2 |
| Vancomycin | ∼>64->64 | >64 | >64 |
| Quinupristin-dalfopristin | ∼0.5-2 | 2 | 2 |
| M. catarrhalis (20) | |||
| LCB01-0371 | ∼2-8 | 4 | 8 |
| Linezolid | ∼4-8 | 8 | 8 |
| Oxacillin | ∼0.25-32 | 8 | 16 |
| Ciprofloxacin | ∼<0.008-0.06 | 0.03 | 0.06 |
| Moxifloxacin | ∼0.015-0.06 | 0.06 | 0.06 |
| Gemifloxacin | ∼<0.008-0.03 | <0.008 | 0.015 |
| Vancomycin | ∼64->64 | 64 | >64 |
| Quinupristin-dalfopristin | ∼0.5-2 | 1 | 1 |
| H. influenzae (13) | |||
| LCB01-0371 | ∼2-16 | 8 | 16 |
| Linezolid | ∼8-32 | 16 | 32 |
| Oxacillin | ∼>32->32 | >32 | >32 |
| Erythromycin | ∼0.5-8 | 2 | 8 |
| Ciprofloxacin | ∼<0.008-<0.008 | <0.008 | <0.008 |
| Moxifloxacin | ∼0.008-0.015 | 0.008 | 0.008 |
| Gemifloxacin | ∼<0.008-<0.008 | <0.008 | <0.008 |
| Vancomycin | ∼>64->64 | >64 | >64 |
| Quinupristin-dalfopristin | ∼2-8 | 4 | 8 |
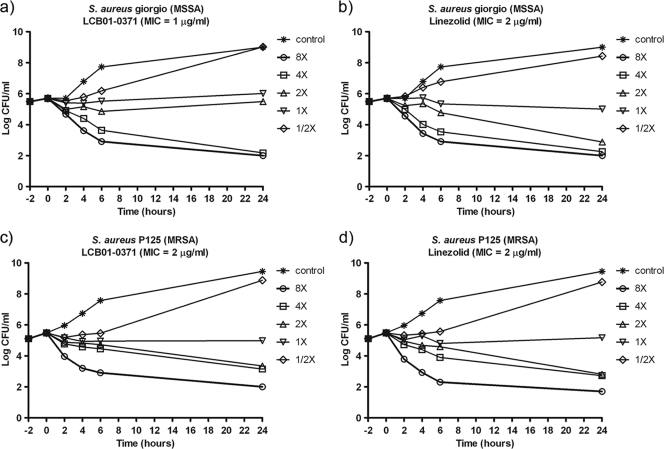
The time-kill analyses of LCB01-0371 against S. aureus giorgio (MSSA) and S. aureus p125 (MRSA) are presented in Fig. 2. LCB01-0371 and linezolid showed similar patterns of the time-kill effect irrespective of whether the strain was methicillin susceptible or resistant. LCB01-0371, at concentrations of 1× MIC and 2× MIC, had bacteriostatic activity against MSSA and MRSA after 24 h. At concentrations of 4× MIC and 8× MIC, LCB01-0371 showed bacteriostatic activity, but there was no regrowth at concentrations of 4× MIC and 8× MIC after 24 h of incubation.
FIG. 2.
Time-kill curves of LCB01-0371 and linezolid against S. aureus giorgio (MSSA) and S. aureus p125 (MRSA).
The protective efficacy of LCB01-0371 against systemic infections in mice was compared with that of linezolid (Table 2). When administered orally, LCB01-0371 showed more-potent protective effects than linezolid against systemic infections caused by Gram-positive and Gram-negative bacteria. Against infection caused by S. aureus giorgio (MSSA), the ED50s of LCB01-0371 and linezolid were 4.53 and 8.05 mg/kg of body weight, respectively. Against S. aureus p125 (MRSA), LCB01-0371 (ED50, 2.96 mg/kg) was more active than linezolid (ED50, 4.84 mg/kg). Against E. faecalis u810, the ED50s of LCB01-0371 and linezolid were 4.53 and 5.97 mg/kg, respectively. LCB01-0371 (ED50, 2.28 mg/kg) was also more active than linezolid (ED50, 9.10 mg/kg) against S. pneumoniae ATCC 6305. Against H. influenzae hd2, the ED50s of LCB01-0371 and linezolid were 9.96 and 21.43 mg/kg, respectively. In general, the ED50s of LCB01-0371 were well correlated with in vitro MICs.
TABLE 2.
In vivo activities of LCB01-0371 against systemic infection in mice
| Microorganism (inoculum, CFU/mousea) | Antimicrobial agentb | MIC (μg/ml) | ED50, mg/kg (95% confidence limit) |
|---|---|---|---|
| S. aureus giorgio, MSSA | LCB01-0371 | 1 | 4.53 (∼2.26-7.87) |
| (1 × 107) | Linezolid | 2 | 8.05 (∼4.70-13.85) |
| S. aureus p125, MRSA | LCB01-0371 | 1 | 2.96 (∼0.00-5.81) |
| (1 × 108) | Linezolid | 2 | 4.84 (∼0.01-12.66) |
| E. faecalis u810 | LCB01-0371 | 2 | 4.53 (∼2.26-7.87) |
| (2 × 108) | Linezolid | 2 | 5.97 (∼2.23-7.87) |
| S. pneumoniae ATCC 6305 | LCB01-0371 | 0.5 | 2.28 (∼0.00-4.49) |
| (1 × 104) | Linezolid | 1 | 9.10 (∼4.92-23.72) |
| H. influenzae hd2 | LCB01-0371 | 8 | 9.96 (∼4.26-16.75) |
| (7.5 × 108) | Linezolid | 16 | 21.43 (∼9.99-450.60) |
Bacterial strains were suspended in 0.9% saline solution containing 5% mucin, except for S. pneumoniae ATCC 6305, which was suspended in 0.9% saline solution.
Antimicrobial agents were administered orally at 1 and 4 h postinfection.
Although linezolid has been recognized as an effective antibiotic against infections with Gram-positive bacteria, such as MRSA and VRE, it produced side effects, such as myelosuppression and peripheral neuropathy, in long-term applications (9). Therefore, it is important to develop new oxazolidinones with good safety profiles, broad antibacterial spectrum, improved pharmacokinetic (PK) parameters, and good water solubility for parenteral administration. LCB01-0371 showed good in vitro and in vivo activities against Gram-positive bacteria and had high aqueous solubility and good absorption, distribution, metabolism, excretion, and toxicity (ADMET) and PK profiles (7). In view of its improved antibacterial activities against Gram-positive bacteria and good pharmacokinetic profiles in animals, the clinical usefulness of LCB01-0371 should be established by further studies.
Acknowledgments
This study was supported by a grant from the Ministry of Health and Welfare, Republic of Korea (A080660).
Footnotes
Published ahead of print on 20 September 2010.
REFERENCES
- 1.Anonymous. 2000. The complete guide to anti-infectives. Scrip 2526:18. [Google Scholar]
- 2.Bliss, C. I. 1985. Statistics in bioassay. Academic Press, Inc., New York, NY.
- 3.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Gonzales, R. D., P. C. Schreckenberger, M. B. Graham, S. Kelkar, K. DenBesten, and J. P. Quinn. 2001. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 357:1179. [DOI] [PubMed] [Google Scholar]
- 5.Herrero, I. A., N. C. Issa, and R. Patel. 2002. Nosocomial spread of linezolid-resistant, vancomycin-resistant Enterococcus faecium. N. Engl. J. Med. 346:867-869. [DOI] [PubMed] [Google Scholar]
- 6.Leach, K. L., S. M. Swaney, J. R. Colca, W. G. McDonald, J. R. Blinn, L. M. Thomasco, R. C. Gadwood, D. Shinabarger, L. Siong, and A. S. Mankin. 2007. The site of action of oxazolidinone antibiotics in living bacteria and in human mitochondria. Mol. Cell 26:393-402. [DOI] [PubMed] [Google Scholar]
- 7.Lee, H. S., D. S. Cha, D. H. Kang, K. M. Oh, Y. L. Cho, J. H. Kwak, T. K. Park, Y. Z. Kim, and S. H. Woo. 2009. New oxazolidinones with cyclic amidrazone(III): pharmacokinetics and repeated dose toxicity studies of LCB01-0183 and LCB01-0371, abstr. F1-1510. Abstr. 49th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (ISDA) 47th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 8.National Committee for Clinical Laboratory Standards. 1999. Methods for determining bactericidal activity of antimicrobial agents; approved standard M26-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 9.Park, I. N., S. B. Hong, Y. M. Oh, M. N. Kim, C. M. Lim, S. D. Lee, Y. Koh, W. S. Kim, D. S. Kim, W. D. Kim, and T. S. Shim. 2006. Efficacy and tolerability of daily-half dose linezolid in patients with intractable multidrug-resistant tuberculosis. J. Antimicrob. Chemother. 58:701-704. [DOI] [PubMed] [Google Scholar]
- 10.Perry, C. M., and B. Jarvis. 2001. Linezolid: a review of its use in the management of serious Gram-positive infections. Drugs 61:525-551. [DOI] [PubMed] [Google Scholar]
- 11.Schwalbe, R. S., J. T. Stapleton, and P. H. Giligan. 1987. Emergence of vancomycin resistance in coagulase-negative staphylococci. N. Engl. J. Med. 316:927-931. [DOI] [PubMed] [Google Scholar]
- 12.Tsiodras, S., H. S. Gold, G. Sakoulas, G. M. Eliopoulos, C. Wennersten, L. Venkataraman, R. C. Moellering, and M. J. Ferraro. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207-208. [DOI] [PubMed] [Google Scholar]