Abstract
The polysaccharide capsule of Streptococcus pneumoniae inhibits phagocytic killing by innate immune mechanisms. Certain serotypes are associated with invasive disease while others with a nasopharyngeal carriage. The invasiveness of serotypes may partly be explained by ability to resist deposition of complement (C3) on the bacterial surface and consequent opsonophagocytic killing. In our previous studies, we observed that clinical isolates of serotypes 1 and 5, which are rarely detected in asymptomatic carriage, were resistant to complement deposition and opsonophagocytosis, whereas serotypes 6B and 23F, both common in carriage, were more sensitive to deposition of C3 and opsonophagocytic killing. However, presence of significant variation in C3 deposition between isolates of the same serotype indicated that factors other than the capsule also affect complement resistance. To distinguish the relative effect of the capsular serotype and other virulence factors on C3 deposition, we compared capsule-switched mutants prepared in genetic backgrounds of pneumococcal strains TIGR4, 603, and 618. Clinical isolates which had the same multilocus sequence type but expressed different serotypes were also compared. We found that the serotype had a significant impact on complement resistance and that the more resistant the strain was to complement, the higher was the concentration of polysaccharide-specific antibodies required for opsonophagocytic killing. Comparison of strains expressing the same capsular polysaccharides in the different genetic backgrounds and various capsular mutants of the same strain suggests that while the genotype affects complement resistance, the serotype is the most important determinant. Differences between serotypes were more significant than the differences between strains.
Streptococcus pneumoniae is a major global pathogen responsible for a wide range of diseases from otitis media to pneumonia, sepsis, and meningitis. Under normal circumstances, pneumococcus enjoys a commensal relationship with its host, and the frequency of invasive disease among individuals colonized by the organism is very low (15). The polysaccharide capsule is considered the major determinant of virulence, because isolates that lack the capsule hardly ever cause invasive disease. The chemical composition of the polysaccharide is also important since only a few of the more than 90 known pneumococcal serotypes are responsible for the majority of invasive infections (14).
The ability of nasopharyngeal carriage to progress into invasive disease or the risk of invasive disease after acquisition of the pathogen varies by serotype (4, 13). The relative contribution of the capsular type compared to other virulence factors in pneumococcal diseases is still unclear, but the extent of virulence cannot be predicted from the capsular type only. Clones belonging to the same serotype can have different abilities to cause invasive disease (4, 13, 43).
The complement system is an essential element of host defense against pneumococci (3, 42). Activation of the complement leads to opsonization of the bacterial surface with C3 activation products C3b and iC3b, which are recognized by complement receptors of phagocytic cells (10, 41). The pneumococcal capsule impairs clearance by preventing access of phagocytic cells to opsonins deposited on the bacterial cell wall (2). Several pneumococcal proteins have also been shown to interact with complement (19). Pneumococcal surface protein A (PspA) inhibits C3 deposition (49) by interfering with the C1q initiation step of the classical pathway (24), which is the dominant complement activation pathway in innate host defense against pneumococci (3). Pneumolysin depletes complement by activating the classical pathway at a distance from the bacterium (54). The pneumococcal surface protein C (PspC) inhibits the activation of complement by, e.g., binding factor H (7, 8), a serum protein that efficiently modulates the function of the complement (17, 18, 38). The genetic background is likely to affect the relative importance of a surface protein to the complement resistance of the strain. Loss of PspC had variable effects on C3 deposition depending largely on the strain background and less on the serotype (55). Binding of complement inhibitor C4b-binding protein is restricted to certain serotypes, which possess a particular PspC allele (9). Pneumococcal histidine triad proteins may also play a role in complement evasion (35), but the impact they have on complement deposition seems to depend on the genetic background (29).
We have previously found that pneumococcal isolates of certain serotypes, such as 1 and 5, associated with invasive disease were particularly resistant to complement deposition and opsonophagocytic killing, while serotypes such as 6B and 23F, associated with carriage, were more sensitive to deposition of C3 and opsonophagocytosis (30, 31). We found significant variation in the magnitude of complement deposition between isolates expressing the same capsular serotype, suggesting a role of serotype-independent factors in the outcome of this particular interaction. In a recent study by Hyams et al. (16) comparing C3 deposition on TIGR4 capsule-switch mutants, the resistance of pneumococcus to complement-mediated immunity was found to vary with the capsular serotype independently of capsular thickness or antibody binding. In the present study, we assessed the influence of the capsular serotype on complement deposition and opsonophagocytic killing by comparing several isogenic capsule-switched mutants and clonally related clinical isolates sharing the same sequence type but expressing different capsular polysaccharides (natural capsule switch variants of the same pneumococcal strain).
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Strains used in the present study are listed in Table 1 . Capsule-switched mutants in three different genetic backgrounds were compared to each other and to the donors of the capsule genes. In multilocus sequence typing (MLST), clones among bacterial species are identified by comparing the sequences of internal fragments of seven housekeeping genes (26). For the present study, we selected invasive pneumococcal strains, sharing the same MLST but representing different serotypes, isolated from Finnish children <2 years of age by the National Reference Laboratory for Pneumococcus (National Institute for Health and Welfare, Oulu, Finland) as a part of ongoing national infectious disease register program.
TABLE 1.
Bacterial strains used in this study
| Strain | Serotype | MLST | Capsule genes (source/target) | Reference and/or sourcea |
|---|---|---|---|---|
| Isogenic capsule switch mutants | ||||
| TIGR4-R | Rough | Janus cassette | 48 | |
| TIGR4-1 | 1 | GA07694 | 51 | |
| TIGR4-2 | 2 | GA03901 | This study | |
| TIGR4-3 | 3 | GA07650 | This study | |
| TIGR4 | 4 | Host strain | 47; BAA-334, ATCC | |
| TIGR4-4 | 4 | TIGR4 | 51 | |
| TIGR4-5 | 5 | 501 | 51 | |
| TIGR4-6B | 6B | NY00216 | 48 | |
| TIGR4-14 | 14 | GA02190 | 48 | |
| TIGR4-19F | 19F | GA71 | 48 | |
| TIGR4-23F | 23F | TN82328 | 51 | |
| 603-1 | 1 | GA02290 | 51 | |
| 603-5 | 5 | 501 | 51 | |
| 603-6B | 6B | Host strain | 27 | |
| 603-14 | 14 | GA02190 | 51 | |
| 603-19F | 19F | GA71 | 51 | |
| 618-6B | 6B | Host strain | This study | |
| 618-14 | 14 | GA02190 | This study | |
| 618-19F | 19F | GA71 | This study | |
| Donors of the capsule genes | ||||
| GA07694 | 1 | TIGR4 | 44; clinical isolate, ABC | |
| GA02290 | 1 | 603 | 44; clinical isolate, ABC | |
| GA03901 | 2 | TIGR4 | 44; clinical isolate, ABC | |
| GA07650 | 3 | TIGR4 | 44; clinical isolate, ABC | |
| 501 | 5 | TIGR4, 603 | 51 | |
| NY00216 | 6B | TIGR4 | 44; clinical isolate, ABC | |
| GA02190 | 14 | TIGR4, 603, 618 | 44; clinical isolate, ABC | |
| GA71 | 19F | TIGR4, 603, 618 | 6 | |
| Clinical isolates sharing the same MLST but different serotypes | ||||
| 199-19F | 19F | 199 | 13; invasive clinical isolate | |
| 199-6B | 6B | 199 | 13; invasive clinical isolate | |
| 156-14 | 14 | 156 | 13; invasive clinical isolate | |
| 156-9V | 9V | 156 | 13; invasive clinical isolate | |
| 156-19F | 19F | 156 | 13; invasive clinical isolate | |
| 162-14 | 14 | 162 | 13; invasive clinical isolate | |
| 162-9V | 9V | 162 | 13; invasive clinical isolate | |
| 162-19F | 19F | 162 | 13; invasive clinical isolate | |
| 66-23F | 9V | 66 | 13; invasive clinical isolate | |
| 66-9V | 23F | 66 | 13; invasive clinical isolate |
ABC, Active Bacterial Core Surveillance of Centers for Disease Control and Prevention, Atlanta, GA. ATCC, American Type Culture Collection.
Fresh pneumococcal cultures were used in flow cytometric assays measuring C3 deposition on the pneumococcal surface. Bacteria were cultured in the Todd-Hewitt broth supplemented with 0.5% yeast extract (THYE) fortified with 5% (wt/vol) fetal bovine serum (FBS) to reach the early logarithmic growth phase. Culturing the bacteria in liquid medium has been shown to enhance expression of genes associated with invasive disease (34), and the culture conditions favored opaque colony morphology.
Frozen stocks of bacterial cultures were prepared for the opsonophagocytic assay as described previously (30, 40), with minor modifications. Bacteria were cultured in THYE with 5% FBS until the early logarithmic growth phase, and culture stocks were frozen slowly in 15% glycerol at −70°C.
Construction of the isogenic capsular variants.
The capsular variants of otherwise isogenic strains were constructed in three different genetic backgrounds—TIGR4 (originally of serotype 4) and 603 and 618 (both originally of serotype 6B)—by using a previously described method (48). In short, the parent strain was rendered unencapsulated by insertion of a cassette into the capsule locus using the transformation protocol published by Pozzi et al. (37) and the bicistronic Janus cassette constructed by Sung et al. (46). Encapsulated mutants were generated by replacing the cassettes with a cps locus from a donor strain. The procedure was repeated three times using genomic DNA of previously constructed capsular variants in order to eliminate the effect of simultaneous random recombinational replacements outside the cps locus. An exception was made with TIGR4 serotypes 1 and 5 and 603 serotype 5 mutant, in which only one backcross could be accomplished. Capsular serotypes were confirmed in all new capsule variants by the Quellung reaction.
Serum samples used in the complement assays.
Complement deposition on pneumococci was measured using sera from 10 healthy adults (normal human sera [NHS]) as the source of complement. Sera were drawn from voluntary donors, and written informed consents were obtained. None of the subjects had been immunized with a pneumococcal vaccine. The concentrations of existing IgG to the corresponding capsular and protein antigens PspA (family 1 and 2), PspC (CbpA), and PhtD were measured by using enzyme immunoassay (EIA) as previously described (45). Each strain was also analyzed once with an agammaglobulinemic human serum (AGS) with nondetectable antibody concentrations to the relevant capsular antigens. Sera were divided into small volumes and stored at −70°C to preserve intact complement activity. Once thawed the serum was used immediately as a source of complement for the C3 deposition assay.
Serum samples used in the opsonophagocytic assay.
The ability of anticapsular antibodies to enhance opsonophagocytosis was assessed by analyzing the pneumococcal isolates with pooled sera from infants immunized in a previous study with an 11-valent pneumococcal conjugate vaccine (53). Each serum pool was collected from post-booster sera of 5 to 11 different children and contained low, medium, or high concentrations of serotype-specific antibodies. The mean capsule type-specific antibody concentrations of the serum pools were calculated based on concentrations of the individual sera previously measured with EIA (52).
Complement C3 deposition assay.
The deposition of C3 on pneumococci was measured with a flow cytometric assay as described previously (30). In short, pneumococcal strains were incubated in 20% human serum with active complement components for 5 min, and bound C3 molecules (C3b and iC3b) were detected by incubating the bacteria with fluorescein isothiocyanate-conjugated rabbit polyclonal anti-human complement C3c (Dako Immunoglobulins, Denmark). The data from 20,000 gated events was collected by flow cytometer (FACSCalibur; Becton Dickinson). Geometric mean intensity of fluorescence (GMF) was analyzed for each sample.
Opsonophagocytic assay.
The functional activity of serum antibodies was measured by standard opsonophagocytic killing assay (40) using differentiated HL-60 cells (promyelotic leukemia cells, CCL240; American Type Culture Collection, Rockville, MD), as described previously (30). Shortly thereafter, polymorphonuclear cells were allowed to phagocytose bacteria in the presence of serum pools containing anticapsular antibodies and baby rabbit complement (Peel-Freez Biologicals/Dynal) or, as a control, complement only. Each bacterial strain was analyzed with three different serum pools containing different concentrations of serotype-specific antibodies. The results were interpreted as the serum dilution that resulted in 50% of bacteria being killed compared to the bacteria present in the control well in which only complement but no antibodies was present.
Statistical methods.
Geometric mean fluorescence intensities of C3 deposition and geometric means of serum antibody concentrations required for 50% opsonophagocytic killing with 95% confidence intervals (CI) were calculated. Pearson coefficient of correlation was calculated for complement deposition and opsonophagocytic killing using geometric means of C3 deposition and antibody concentration. One-way analysis of variance (ANOVA) was applied for comparisons of isolates of the same genetic background expressing different capsular serotypes or isolates from different genetic backgrounds isolates expressing the same capsular serotype. One-way ANOVA was followed by Tukey's post-hoc test when appropriate. A Student t test was used in comparison of capsule donor strains with recipients. All statistical analyses were performed on log-transformed data, and P values of <0.05 were considered to indicate a statistically significant difference.
RESULTS
Deposition of C3 on pneumococci.
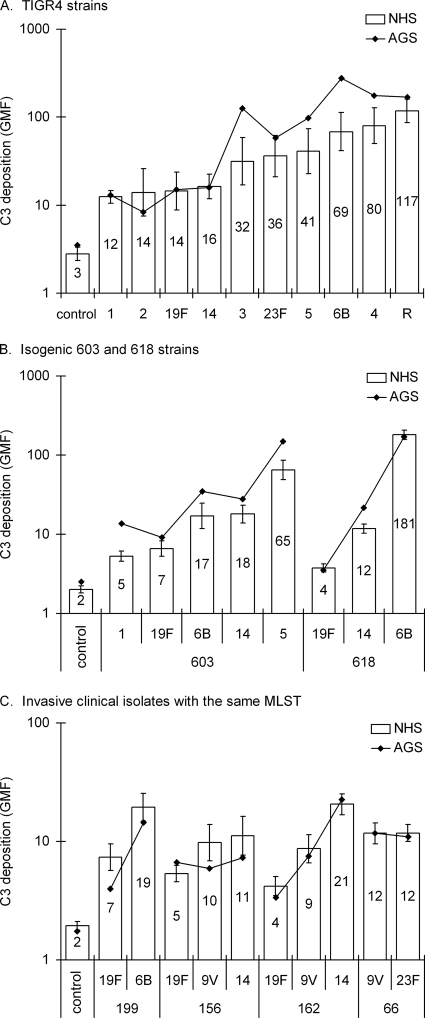
Serotypes were ranked from most resistant to most susceptible according to C3 deposition, expressed as the geometric mean intensity of fluorescence (GMF) (Fig. 1). C3 deposition varied significantly between capsular variants of the same S. pneumoniae strain. Of the in vitro capsule-switch mutants, serotypes 1 (TIGR4-1 and 603-1) and 19F (all three strains) were among the most resistant to complement (Fig. 1A and B). C3 deposition varied significantly also between the clinical isolates with the same MLST but of different serotype (Fig. 1C). For all of those, serotype 19F strains were more resistant to C3 deposition than the same MLST type isolates of any other capsule type (P < 0.001, one-way ANOVA, followed by Tukey's post-hoc test). Because antibody to the capsular polysaccharides in NHS could potentially enhance complement deposition differently on strains expressing different capsular serotypes, each strain was analyzed once with an agammaglobulinemic serum (AGS). In the absence of antibodies the order of resistance remained essentially the same (Fig. 1).
FIG. 1.
Complement deposition on pneumococci expressing different capsules in the genetic background of TIGR4 (A) and 603 and 618 (B) and clinical isolates, which share the same MLST but have different capsular serotypes (C). For a control, a random isolate was analyzed with serum diluted in EDTA, which inhibits both alternative and classical pathways of complement activation. NHS, normal human serum; AGS, agammaglobulinemic serum. Geometric mean intensities of fluorescence (GMF) are shown with the 95% CI.
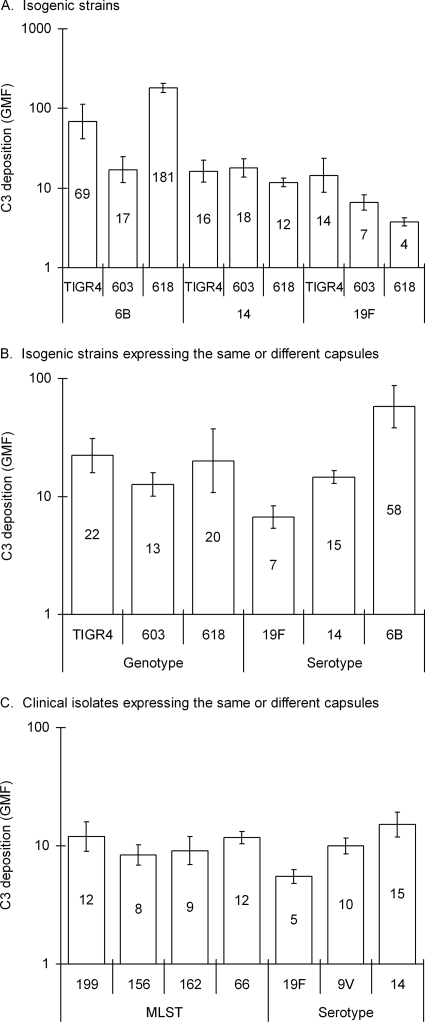
Isogenic strains TIGR4, 603, and 618 expressing the same capsule (Fig. 2A) had different susceptibilities to complement C3 deposition (P < 0.001, one-way ANOVA), but no significant differences were found between the genotypes when the results obtained for the same genotype expressing the different capsules were combined (Fig. 2B). Instead, when the results obtained for the different strains expressing the same capsule type were combined, the differences between the serotypes were significant (P < 0.001). The smallest amount of complement C3 was deposited on 19F, intermediate on 14, and the most on 6B (Fig. 2B). When the results obtained for clinical isolates sharing the same MLST but expressing different capsules were combined, the differences between MLSTs were not significant. In contrast, among isolates with different MLSTs expressing the same capsule, significant differences were observed in C3 deposition on serotypes 14, 9V, and 19F (P < 0.001, Fig. 2C). When the isogenic capsule-switched strains were compared to the clinical isolates used as donors of the capsule genes, resistance to complement differed significantly between the donor and the recipient strains (data not shown).
FIG. 2.
Complement C3 deposition on pneumococcal strains expressing serotypes 6B, 14, and 19F in the genetic backgrounds of TIGR4, 603, and 618 (A) and geometric means of C3 deposition on the different isogenic genotypes or serotypes 19F, 14, and 6B, respectively (B). Geometric mean C3 deposition on clinical isolates representing different MLSTs or expressing different capsular serotypes, which included at least two isolates: 19F, 9V, and 14 (C). Geometric mean intensities of fluorescence (GMF) are shown with the 95% CI.
The unencapsulated TIGR4 isolate was predictably the most sensitive to complement deposition. The serotype 4 variant of TIGR4, which has gone through the same capsule-switching as the other capsular variants did not differ from the original TIGR4 host strain (Student's t test), indicating that the capsular switch itself had not affected the complement resistance of the serotype 4 strain (data not shown). In order to assess the possible attenuation of the capsular variants due to successive laboratory cultures, TIGR4 serotype 6B, 14, and 19F variants, inoculated, and recovered a week later by nasal wash from the nasopharynxes of mice, were analyzed for comparison. We did not find any significant differences between the serotype 6B and 14 mouse-passaged and laboratory cultures, whereas slightly, but significantly less C3 was deposited on the mouse-passaged serotype 19F culture (Student t test, data not shown).
Opsonophagocytic killing of pneumococci.
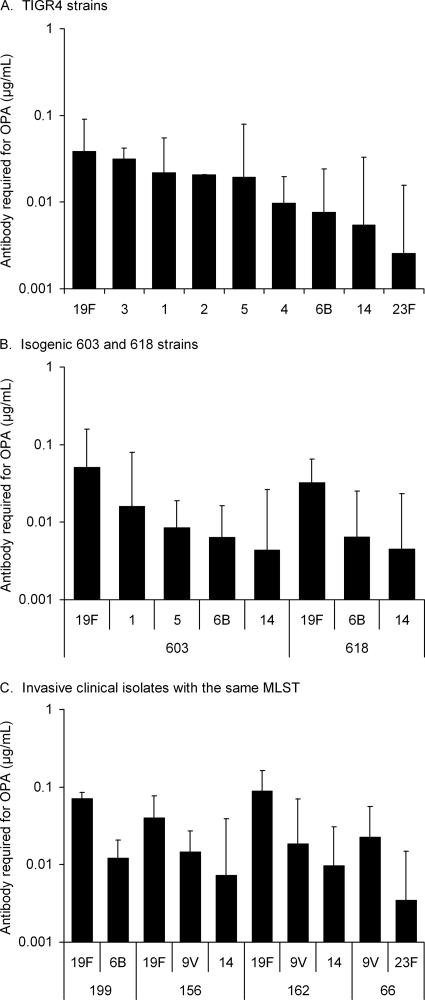
The polysaccharide-specific antibody concentration required for 50% killing of TIGR4 strains depended on the capsular serotype and significant differences existed between serotypes (one-way ANOVA, Fig. 3A). TIGR4 serotypes 19F, 3, and 1 required the highest concentration of capsule antibodies for killing in OPA. Serotype 19F was more resistant to opsonophagocytic killing than serotypes 6B and 14 in the genetic background of all three in vitro capsule-switched mutants (P < 0.05, one-way ANOVA, followed by Tukey's post-hoc test Fig. 3A and B). Mouse-passaged TIGR4 serotypes 6B, 14, and 19F did not differ from strains, which were not passaged (Student paired t test, data not shown). The clinical isolates, which have the same MLST but different capsular serotypes, also differed significantly in terms of sensitivity to opsonophagocytic killing: serotype 19F was the most resistant in three MLST backgrounds (Fig. 3C).
FIG. 3.
Anti-capsular antibody concentration required for 50% opsonophagocytic killing of capsule-switched mutants of TIGR4 (A) and strains 603 and 618 (B) and clinical isolates which share the same MLST but express different capsule types (C). Serotype 2 was analyzed with pneumococcal reference serum lot 89-SF; all of the other strains were analyzed with three serum pools with low, medium, and high concentrations of antibodies to the polysaccharides. Geometric mean concentrations with the 95% CI are shown.
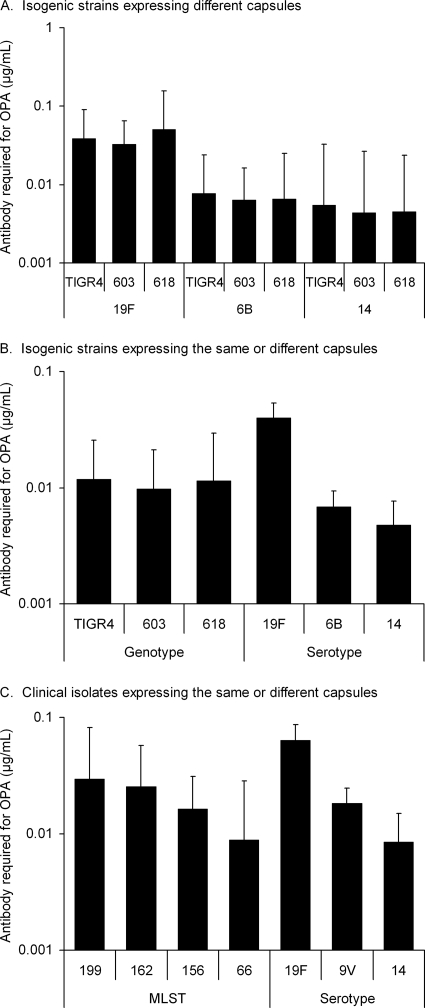
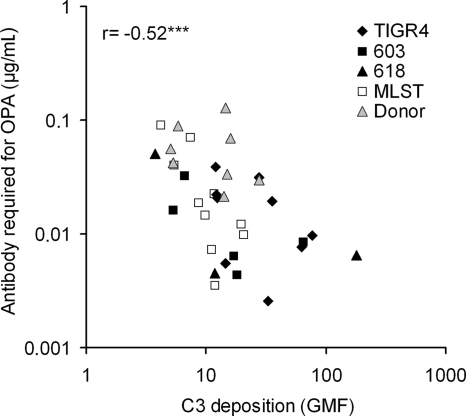
Similar concentrations of anticapsular antibodies were required for killing of isogenic strains TIGR4, 603, and 618, which expressed the same capsular type (6B, 14, or 19F) (Fig. 4A). The concentration of antibodies required for killing the three genotypes was also similar when the strains expressing different capsules were combined (Fig. 4B). In contrast, when the three genotypes were combined, significantly more antibodies were required for killing the serotype 19F strains compared to serotype 6B and 14 strains (P < 0.001, one-way ANOVA, followed by Tukey's post-hoc test, Fig. 4B). When the results of clinical isolates with the same MLST were combined, differences between MLSTs were not significant, but when clinical isolates with different MLSTs expressing the same serotype were combined significant differences between the serotypes were seen. Serotype 19F required more antibody for opsonophagocytic killing than either 9V or 14, whereas the concentration required for killing serotype 14 was lower than for either 9V or 19F (P < 0.01, Fig. 4C). Compared to the capsule donors, some of the capsule-switched mutants were significantly more sensitive to opsonophagocytic killing (data not shown). The concentration of anticapsular antibodies required for 50% opsonophagocytic killing correlated negatively with C3 deposition on the pneumococcal strains (Fig. 5).
FIG. 4.
Opsonophagocytic killing of isogenic pneumococcal strains expressing serotype 6B, 14, and 19F capsules in the genetic backgrounds of TIGR4, 603, and 618 (A) and killing of the different genotypes or serotypes 19F, 14, and 6B, respectively (B). Opsonophagocytic killing of clinical isolates representing different MLSTs or different serotypes, which included at least two isolates: 19F, 9V, and 14 (C). Geometric mean concentrations are shown with the 95% CI.
FIG. 5.
The concentration of antibodies required for 50% opsonophagocytic killing was associated with complement deposition on pneumococci. ***, P < 0.001 (Pearson correlation).
DISCUSSION
Pneumococcal serotypes and strains differ significantly in their virulence, which results from differences in the capsular structure, as well as in the genetic background. In our previous study we found that the invasiveness of certain serotypes could be associated with increased resistance to complement deposition and opsonophagocytic killing (31). Analysis of several capsule-switched mutants in the present study indicates that exchange of the capsule can directly alter the resistance of the strain to complement and opsonophagocytosis. However, other virulence factors besides the capsule also contribute to complement resistance since differences were found among the strains expressing the same capsular serotype. Nevertheless, particular capsules seem to increase resistance irrespective of the genetic background.
The ability of pneumococcal isolates to kill mice has been found to be strongly associated with capsular type (1). The fact that strains of the same capsular type varied in virulence indicates that the genetic background also influences the virulence of the strains. To overcome the genetic variation confounding the assessment of the role of capsular serotype in virulence, capsule-switched mutants expressing different serotypes in the same genetic background have been compared in mouse models of infection (20, 21, 32). Expression of serotype 3 capsule in the genetic background of clinical isolates of capsular types 2, 5, and 6B resulted in profound changes in virulence of the strains originally of serotype 5 and 6B in an invasive disease model (21). In intranasal infection of mice the disease outcome was more similar for a serotype 14 clinical isolate and its capsule-switched serotype 9V variant than for two clinical serotype 14 isolates, which had different genetic backgrounds (32). The combination of the capsule and the genetic background of the pneumococcal strain was shown to be important but vary with the site of infection in respiratory tract infections of mice (20). Two strains expressing serotype 3 capsule survived equally well in the nasopharynx but in the lungs the mutant of serotype 2 strain became avirulent compared to either wild-type serotype 3 strain (20). In invasive pneumococcal disease the capsular serotype is assumed to be more important than the genotype, because individual serotypes vary in their invasive disease potential (4, 13). Serogroups 1, 4, 5, 7, and 14 are strongly associated with invasive disease, whereas serotype 23F is associated with carriage (5). In mucosal infections the serotype may be less important. Differences between serotypes in the risk of progression from carriage to acute otitis media were not as significant (12). Furthermore, carriage isolates tend to be genotypically more diverse than invasive isolates, whereas particular, clonal characteristics are likely to be advantageous for invasiveness (4, 39).
Despite the importance of capsular serotype as a determinant of invasiveness, clinical pneumococcal isolates belonging to different MLSTs have been found to have different invasive disease potentials (13). MLST 156 is the penicillin-resistant Spain9V-3 clone, which can also express serotypes 14, 9A, or other serogroup 19 types (28). It was derived from the penicillin-susceptible strain 162, and strains 162 and 156 are believed to have essentially the same genotype (13). The MLST 156 included in the present study had a particularly high invasive disease potential, whereas MLSTs 66, 199, and 162 were not statistically associated with invasiveness (13). We found that the isolates expressing different capsular serotypes differed significantly in their resistance to complement deposition and opsonophagocytic killing but differences between MLSTs were much smaller than the differences between serotypes. Comparison of isogenic strains expressing different capsules led to the same conclusion: a switch of the capsule to a particular serotype changed the complement resistance to the same direction in the three genetic backgrounds. The concentration of anticapsular antibodies required for opsonophagocytic killing was strongly dependent on the serotype but not the genotype. The results suggest that the capsule is more important than the genetic background for the ability of pneumococci to resist complement deposition and opsonophagocytic killing. It is likely that other factors, such as resistance to antimicrobial peptides or enzymes, affect the success of certain clones to cause invasive disease.
At the early stages of colonization the capsule inhibits trapping of the pathogen to the luminal mucus and clearance by mucociliary flow (33). Unencapsulated mutants colonized nasal spaces at a density of 10- to 100-fold less than the encapsulated parent strains (33). Increased encapsulation ensures that a portion of the bacterial population overcomes the initial clearance mechanism and progresses to the epithelial surface where stable colonization occurs (33). Timely regulation of capsule expression could be more important than the thickness of the capsule for the ability of pneumococci to colonize and cause invasive disease. A reduced amount of capsule at the later stage promotes colonization by exposure of adhesins and strengthening of the intimate contact with the epithelial cells and subsequent uptake (11). Once invasive disease is established, heavily encapsulated bacteria are better protected from phagocytes (22). If clearance of pneumococci from the nasopharynx is mediated by T cells (25, 50), the ability of pneumococcal serotypes to persist in the nasopharynx would depend on their ability to resist killing by neutrophils (51). Heavily encapsulated serotypes were found to be more resistant to nonopsonic phagocytosis by neutrophils, and it was suggested that the structure of the capsular polysaccharide would determine the success of a serotype during nasopharyngeal carriage (51). Serotypes 19F and 23F were among the most resistant to nonopsonic phagocytosis, which correlates with their high prevalence in carriage, whereas serotypes 4 and 5 were more efficiently killed (51).
Although nonopsonic phagocytic receptors can mediate phagocytosis directly (51), neutrophil phagocytosis is markedly increased by opsonization of pneumococcus with complement (56). Complement deposition on TIGR4 mutants expressing different serotypes correlated with neutrophil phagocytosis in the presence of complement, but differences in phagocytosis between strains were abolished when the bacteria were not opsonized with complement (16). We found that complement deposition on pneumococci correlates with the anticapsular antibody concentration required for opsonophagocytic killing: the serotypes that were least resistant to C3 deposition required the lowest concentration of capsule antibodies. TIGR4 serotypes 4 and 7F have previously been reported to be more resistant to C3 deposition than serotypes 6A and 23F (16), whereas in the present study TIGR4 serotype 4 was less resistant than 23F. Since the mutants of these two studies were constructed independently, the source of the TIGR4 parent strain (serotype 4) could affect the results. Laboratory strains purchased from different sources could have accumulated different mutations as has been observed for D39 and its derivatives (23). It is also possible that the methodologies applied and sera used as a source of complement could result in differences between the studies.
The clinical isolates analyzed here were more resistant to complement deposition and opsonophagocytosis than the capsule-switched mutants, which could result from attenuation and reduced expression of the capsule or other virulence factors in the laboratory strains compared to the clinical isolates. Passage in the mouse nasopharynx increased the resistance of the serotype 19F but not serotype 6B or 14 variants of TIGR4. Another reason why the clinical isolates are more resistant could be that the genetic background of the isolates is adapted to a particular serotype. In our previous study we found that serotype 5 clinical isolates were relatively resistant to complement and required a high concentration of polysaccharide-specific antibodies for opsonophagocytic killing (31). The serotype 5 capsule-switched mutants of the present study, prepared in the genetic backgrounds of TIGR4 and 603, were less resistant to complement in comparison with many other serotypes expressed in the same genetic background. The invasive disease potential of serotype 4 has been reported to be high compared to other serotypes (4, 13). However, the exceedingly high estimation of the invasive disease potential of serotype 4 was based on a total of six cases, four of which had the same MLST, 205 (4). Serotype 4 invasive isolates in the previous study (three isolates, two of which MLST 205) were more resistant to complement deposition than serotypes 14 and 23F, whereas the serotype 4 reference strain used in the standard opsonophagocytic assay was significantly more sensitive to complement than the clinical isolates (31). In the present study the serotype 4 TIGR4 strain was inferior to all other capsule-switched mutants. The invasiveness of serotype 4 clinical isolates could depend on a particular genotype, which is beneficial for invasiveness, rather than the capsular serotype per se. TIGR4 was originally isolated from meningitis, and although it is able to cause meningitis in mice, its yield in blood was low in contrast to serotype 2 D39, which attains high titers in blood after challenge (36). This could indicate that the invasiveness of the TIGR4 strain is not as high as it is for serotype 4 clinical isolates from invasive disease. Successful combinations of the capsule and virulence proteins may have coevolved to optimal sets of genes in virulent strains. The same set of proteins may work differently with various capsular types, which could explain why the capsule switch did not always result in the same outcome as expected from clinical isolates. In populations immunized with a pneumococcal conjugate vaccine the severity of replacement disease could depend on the preexisting serotypes and the genotypes of capsule-switched clones.
Acknowledgments
We thank Marc Lipsitch from the Harvard School of Public Health, Boston, MA, for scientific advice and for sharing the mutant strains used in this study. We also thank Tarja Kaijalainen from the National Institute for Health and Welfare, Oulu, Finland, for supplying the clinical pneumococcal isolates collected from Finnish patients and William P. Hanage from the Imperial College, London, United Kingdom, for sharing the MLST data of the isolates. The clinical isolates that were used as donors of the capsule genes came from Active Bacterial Core Surveillance of Centers for Disease Control and Prevention, Atlanta, GA, from Chris van Beneden. Leena Tikkanen and Sanna Piipponen are acknowledged for skillful assistance in the laboratory analyses and statistician Mika Lahdenkari for advice in statistical analysis of the data at The National Institute for Health and Welfare, Helsinki, Finland.
Editor: A. Camilli
Footnotes
Published ahead of print on 20 September 2010.
REFERENCES
- 1.Briles, D. E., M. J. Crain, B. M. Gray, C. Forman, and J. Yother. 1992. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect. Immun. 60:111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown, E. J., S. W. Hosea, C. H. Hammer, C. G. Burch, and M. M. Frank. 1982. A quantitative analysis of the interactions of antipneumococcal antibody and complement in experimental pneumococcal bacteremia. J. Clin. Invest. 69:85-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, J. S., T. Hussell, S. M. Gilliland, D. W. Holden, J. C. Paton, M. R. Ehrenstein, M. J. Walport, and M. Botto. 2002. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc. Natl. Acad. Sci. U. S. A. 99:16969-16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brueggemann, A. B., D. T. Griffiths, E. Meats, T. Peto, D. W. Crook, and B. G. Spratt. 2003. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J. Infect. Dis. 187:1424-1432. [DOI] [PubMed] [Google Scholar]
- 5.Brueggemann, A. B., T. E. Peto, D. W. Crook, J. C. Butler, K. G. Kristinsson, and B. G. Spratt. 2004. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J. Infect. Dis. 190:1203-1211. [DOI] [PubMed] [Google Scholar]
- 6.Coffey, T. J., M. C. Enright, M. Daniels, J. K. Morona, R. Morona, W. Hryniewicz, J. C. Paton, and B. G. Spratt. 1998. Recombinational exchanges at the capsular polysaccharide biosynthetic locus lead to frequent serotype changes among natural isolates of Streptococcus pneumoniae. Mol. Microbiol. 27:73-83. [DOI] [PubMed] [Google Scholar]
- 7.Dave, S., A. Brooks-Walter, M. K. Pangburn, and L. S. McDaniel. 2001. PspC, a pneumococcal surface protein, binds human factor H. Infect. Immun. 69:3435-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dave, S., M. K. Pangburn, C. Pruitt, and L. S. McDaniel. 2004. Interaction of human factor H with PspC of Streptococcus pneumoniae. Indian J. Med. Res. 119(Suppl.):66-73. [PubMed] [Google Scholar]
- 9.Dieudonne-Vatran, A., S. Krentz, A. M. Blom, S. Meri, B. Henriques-Normark, K. Riesbeck, and B. Albiger. 2009. Clinical isolates of Streptococcus pneumoniae bind the complement inhibitor C4b-binding protein in a PspC allele-dependent fashion. J. Immunol. 182:7865-7877. [DOI] [PubMed] [Google Scholar]
- 10.Fearon, D. T., and W. W. Wong. 1983. Complement ligand-receptor interactions that mediate biological responses. Annu. Rev. Immunol. 1:243-271. [DOI] [PubMed] [Google Scholar]
- 11.Hammerschmidt, S., S. Wolff, A. Hocke, S. Rosseau, E. Muller, and M. Rohde. 2005. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect. Immun. 73:4653-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanage, W. P., K. Auranen, R. Syrjanen, E. Herva, P. H. Makela, T. Kilpi, and B. G. Spratt. 2004. Ability of pneumococcal serotypes and clones to cause acute otitis media: implications for the prevention of otitis media by conjugate vaccines. Infect. Immun. 72:76-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanage, W. P., T. H. Kaijalainen, R. K. Syrjanen, K. Auranen, M. Leinonen, P. H. Makela, and B. G. Spratt. 2005. Invasiveness of serotypes and clones of Streptococcus pneumoniae among children in Finland. Infect. Immun. 73:431-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hausdorff, W. P., J. Bryant, P. R. Paradiso, and G. R. Siber. 2000. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin. Infect. Dis. 30:100-121. [DOI] [PubMed] [Google Scholar]
- 15.Hava, D. L., J. LeMieux, and A. Camilli. 2003. From nose to lung: the regulation behind Streptococcus pneumoniae virulence factors. Mol. Microbiol. 50:1103-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyams, C., J. Yuste, K. Bax, E. Camberlein, J. N. Weiser, and J. S. Brown. 2010. Streptococcus pneumoniae resistance to complement-mediated immunity is dependent on the capsular serotype. Infect. Immun. 78:716-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janulczyk, R., F. Iannelli, A. G. Sjoholm, G. Pozzi, and L. Bjorck. 2000. Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J. Biol. Chem. 275:37257-37263. [DOI] [PubMed] [Google Scholar]
- 18.Jarva, H., R. Janulczyk, J. Hellwage, P. F. Zipfel, L. Bjorck, and S. Meri. 2002. Streptococcus pneumoniae evades complement attack and opsonophagocytosis by expressing the pspC locus-encoded Hic protein that binds to short consensus repeats 8-11 of factor H. J. Immunol. 168:1886-1894. [DOI] [PubMed] [Google Scholar]
- 19.Jarva, H., T. S. Jokiranta, R. Wurzner, and S. Meri. 2003. Complement resistance mechanisms of streptococci. Mol. Immunol. 40:95-107. [DOI] [PubMed] [Google Scholar]
- 20.Kadioglu, A., S. Taylor, F. Iannelli, G. Pozzi, T. J. Mitchell, and P. W. Andrew. 2002. Upper and lower respiratory tract infection by Streptococcus pneumoniae is affected by pneumolysin deficiency and differences in capsule type. Infect. Immun. 70:2886-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly, T., J. P. Dillard, and J. Yother. 1994. Effect of genetic switching of capsular type on virulence of Streptococcus pneumoniae. Infect. Immun. 62:1813-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, J. O., S. Romero-Steiner, U. B. Sorensen, J. Blom, M. Carvalho, S. Barnard, G. Carlone, and J. N. Weiser. 1999. Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect. Immun. 67:2327-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanie, J. A., W. L. Ng, K. M. Kazmierczak, T. M. Andrzejewski, T. M. Davidsen, K. J. Wayne, H. Tettelin, J. I. Glass, and M. E. Winkler. 2007. Genome sequence of Avery's virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J. Bacteriol. 189:38-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, J., D. T. Glover, A. J. Szalai, S. K. Hollingshead, and D. E. Briles. 2007. PspA and PspC minimize immune adherence and transfer of pneumococci from erythrocytes to macrophages through their effects on complement activation. Infect. Immun. 75:5877-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu, Y. J., J. Gross, D. Bogaert, A. Finn, L. Bagrade, Q. Zhang, J. K. Kolls, A. Srivastava, A. Lundgren, S. Forte, C. M. Thompson, K. F. Harney, P. W. Anderson, M. Lipsitch, and R. Malley. 2008. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 4:e1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maiden, M. C., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. U. S. A. 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malley, R., M. Lipsitch, A. Stack, R. Saladino, G. Fleisher, S. Pelton, C. Thompson, D. Briles, and P. Anderson. 2001. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect. Immun. 69:4870-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGee, L., L. McDougal, J. Zhou, B. G. Spratt, F. C. Tenover, R. George, R. Hakenbeck, W. Hryniewicz, J. C. Lefevre, A. Tomasz, and K. P. Klugman. 2001. Nomenclature of major antimicrobial-resistant clones of Streptococcus pneumoniae defined by the pneumococcal molecular epidemiology network. J. Clin. Microbiol. 39:2565-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melin, M., E. Di Paolo, L. Tikkanen, H. Jarva, C. Neyt, H. Kayhty, S. Meri, J. Poolman, and M. Vakevainen. 2010. Interaction of pneumococcal histidine triad proteins with human complement. Infect. Immun. 78:2089-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melin, M., H. Jarva, L. Siira, S. Meri, H. Kayhty, and M. Vakevainen. 2009. Streptococcus pneumoniae capsular serotype 19F is more resistant to C3 deposition and less sensitive to opsonophagocytosis than serotype 6B. Infect. Immun. 77:676-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melin, M., K. Trzciński, M. Antonio, S. Meri, R. Adegbola, T. Kaijalainen, H. Käyhty, and M. Väkeväinen. 2010. Serotype-related variation in susceptibility to complement deposition and opsonophagocytosis among clinical isolates of Streptococcus pneumoniae. Infect. Immun. 78:5252-5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizrachi-Nebenzahl, Y., N. Porat, S. Lifshitz, S. Novick, A. Levi, E. Ling, O. Liron, S. Mordechai, R. K. Sahu, and R. Dagan. 2004. Virulence of Streptococcus pneumoniae may be determined independently of capsular polysaccharide. FEMS Microbiol. Lett. 233:147-152. [DOI] [PubMed] [Google Scholar]
- 33.Nelson, A. L., A. M. Roche, J. M. Gould, K. Chim, A. J. Ratner, and J. N. Weiser. 2007. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect. Immun. 75:83-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oggioni, M. R., C. Trappetti, A. Kadioglu, M. Cassone, F. Iannelli, S. Ricci, P. W. Andrew, and G. Pozzi. 2006. Switch from planktonic to sessile life: a major event in pneumococcal pathogenesis. Mol. Microbiol. 61:1196-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogunniyi, A. D., M. Grabowicz, L. K. Mahdi, J. Cook, D. L. Gordon, T. A. Sadlon, and J. C. Paton. 2009. Pneumococcal histidine triad proteins are regulated by the Zn2+-dependent repressor AdcR and inhibit complement deposition through the recruitment of complement factor H. FASEB J. 23:731-738. [DOI] [PubMed] [Google Scholar]
- 36.Orihuela, C. J., J. N. Radin, J. E. Sublett, G. Gao, D. Kaushal, and E. I. Tuomanen. 2004. Microarray analysis of pneumococcal gene expression during invasive disease. Infect. Immun. 72:5582-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pozzi, G., L. Masala, F. Iannelli, R. Manganelli, L. S. Havarstein, L. Piccoli, D. Simon, and D. A. Morrison. 1996. Competence for genetic transformation in encapsulated strains of Streptococcus pneumoniae: two allelic variants of the peptide pheromone. J. Bacteriol. 178:6087-6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quin, L. R., S. Carmicle, S. Dave, M. K. Pangburn, J. P. Evenhuis, and L. S. McDaniel. 2005. In vivo binding of complement regulator factor H by Streptococcus pneumoniae. J. Infect. Dis. 192:1996-2003. [DOI] [PubMed] [Google Scholar]
- 39.Robinson, D. A., K. M. Edwards, K. B. Waites, D. E. Briles, M. J. Crain, and S. K. Hollingshead. 2001. Clones of Streptococcus pneumoniae isolated from nasopharyngeal carriage and invasive disease in young children in central Tennessee. J. Infect. Dis. 183:1501-1507. [DOI] [PubMed] [Google Scholar]
- 40.Romero-Steiner, S., D. Libutti, L. B. Pais, J. Dykes, P. Anderson, J. C. Whitin, H. L. Keyserling, and G. M. Carlone. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin. Diagn. Lab. Immunol. 4:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rothlein, R., and T. A. Springer. 1985. Complement receptor type three-dependent degradation of opsonized erythrocytes by mouse macrophages. J. Immunol. 135:2668-2672. [PubMed] [Google Scholar]
- 42.Saeland, E., G. Vidarsson, J. H. Leusen, E. Van Garderen, M. H. Nahm, H. Vile-Weekhout, V. Walraven, A. M. Stemerding, J. S. Verbeek, G. T. Rijkers, W. Kuis, E. A. Sanders, and J. G. Van De Winkel. 2003. Central role of complement in passive protection by human IgG1 and IgG2 anti-pneumococcal antibodies in mice. J. Immunol. 170:6158-6164. [DOI] [PubMed] [Google Scholar]
- 43.Sandgren, A., K. Sjostrom, B. Olsson-Liljequist, B. Christensson, A. Samuelsson, G. Kronvall, and B. Henriques Normark. 2004. Effect of clonal and serotype-specific properties on the invasive capacity of Streptococcus pneumoniae. J. Infect. Dis. 189:785-796. [DOI] [PubMed] [Google Scholar]
- 44.Schuchat, A., T. Hilger, E. Zell, M. M. Farley, A. Reingold, L. Harrison, L. Lefkowitz, R. Danila, K. Stefonek, N. Barrett, D. Morse, and R. Pinner. 2001. Active bacterial core surveillance of the emerging infections program network. Emerg. Infect. Dis. 7:92-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simell, B., M. Lahdenkari, A. Reunanen, H. Kayhty, and M. Vakevainen. 2008. Effects of ageing and gender on naturally acquired antibodies to pneumococcal capsular polysaccharides and virulence-associated proteins. Clin. Vaccine Immunol. 15:1391-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sung, C. K., H. Li, J. P. Claverys, and D. A. Morrison. 2001. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 67:5190-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 48.Trzcinski, K., C. M. Thompson, and M. Lipsitch. 2003. Construction of otherwise isogenic serotype 6B, 7F, 14, and 19F capsular variants of Streptococcus pneumoniae strain TIGR4. Appl. Environ. Microbiol. 69:7364-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tu, A. H., R. L. Fulgham, M. A. McCrory, D. E. Briles, and A. J. Szalai. 1999. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect. Immun. 67:4720-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Rossum, A. M., E. S. Lysenko, and J. N. Weiser. 2005. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect. Immun. 73:7718-7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weinberger, D. M., K. Trzcinski, Y. J. Lu, D. Bogaert, A. Brandes, J. Galagan, P. W. Anderson, R. Malley, and M. Lipsitch. 2009. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathog. 5:e1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wuorimaa, T., R. Dagan, M. Vakevainen, F. Bailleux, R. Haikala, M. Yaich, J. Eskola, and H. Kayhty. 2001. Avidity and subclasses of IgG after immunization of infants with an 11-valent pneumococcal conjugate vaccine with or without aluminum adjuvant. J. Infect. Dis. 184:1211-1215. [DOI] [PubMed] [Google Scholar]
- 53.Wuorimaa, T. K., R. Dagan, F. Bailleux, R. Haikala, N. Ekstrom, J. Eskola, M. Yaich, and H. Kayhty. 2005. Functional activity of antibodies after immunization of Finnish and Israeli infants with an 11-valent pneumococcal conjugate vaccine. Vaccine 23:5328-5332. [DOI] [PubMed] [Google Scholar]
- 54.Yuste, J., M. Botto, J. C. Paton, D. W. Holden, and J. S. Brown. 2005. Additive inhibition of complement deposition by pneumolysin and PspA facilitates Streptococcus pneumoniae septicemia. J. Immunol. 175:1813-1819. [DOI] [PubMed] [Google Scholar]
- 55.Yuste, J., S. Khandavilli, N. Ansari, K. Muttardi, L. Ismail, C. Hyams, J. Weiser, T. Mitchell, and J. S. Brown. 2010. The effects of PspC on complement-mediated immunity to Streptococcus pneumoniae vary with strain background and capsular serotype. Infect. Immun. 78:283-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuste, J., A. Sen, L. Truedsson, G. Jonsson, L. S. Tay, C. Hyams, H. E. Baxendale, F. Goldblatt, M. Botto, and J. S. Brown. 2008. Impaired opsonization with C3b and phagocytosis of Streptococcus pneumoniae in sera from subjects with defects in the classical complement pathway. Infect. Immun. 76:3761-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]