Abstract
Major histocompatibility complex class II (MHC-II) molecules are released by murine macrophages upon lipopolysaccharide (LPS) stimulation and ATP signaling through the P2X7 receptor. These studies show that infection of macrophages with Mycobacterium tuberculosis or M. bovis strain BCG enhances MHC-II release in synergy with ATP. Shed MHC-II was contained in two distinct organelles, exosomes and plasma membrane-derived microvesicles, which were both able to present exogenous antigenic peptide to T hybridoma cells. Furthermore, microvesicles from mycobacterium-infected macrophages were able to directly present M. tuberculosis antigen (Ag) 85B(241-256)-I-Ab complexes that were generated by the processing of M. tuberculosis Ag 85B in infected cells to both M. tuberculosis-specific T hybridoma cells and naïve P25 M. tuberculosis T-cell receptor (TCR)-transgenic T cells. In the presence of prefixed macrophages, exosomes from mycobacterium-infected macrophages provided weak stimulation to M. tuberculosis-specific T hybridoma cells but not naïve P25 T cells. Thus, infection with M. tuberculosis primes macrophages for the increased release of exosomes and microvesicles bearing M. tuberculosis peptide-MHC-II complexes that may generate antimicrobial T-cell responses.
Exosomes are 50- to 80-nm membrane vesicles that are released by many cell types, including reticulocytes, B cells, and dendritic cells (DCs) (16, 17, 33-35, 40, 42, 49, 53). Invagination of the limiting membrane of late endosomes leads to the formation of intraluminal vesicles in multivesicular endosomes. The intraluminal vesicles are secreted as exosomes upon the fusion of multivesicular endosomes with the plasma membrane.
Exosomes from B cells contain major histocompatibility complex class II (MHC-II) molecules and can stimulate CD4+ T-cell responses in vitro (40), although they may be more capable of activating primed T cells than naïve CD4+ T cells (27). The activation of naïve CD4+ T cells by DC exosomes occurs via an indirect pathway in which the exosomes and their constituent peptide-MHC-II molecules are presented in the context of intact antigen (Ag)-presenting cells (APCs) (e.g., DCs that may be MHC-II negative but must bear the costimulatory molecules CD80 and CD86 [48]). The presence of ICAM-1 on exosomes is important for naïve T-cell priming (43).
While the shedding of exosomes can be constitutive (27, 40), it can also be significantly enhanced by the stimulation of certain receptors, e.g., Toll-like receptors (TLRs) and the P2X7 purinergic receptor (P2X7R), which trigger inflammatory responses (37, 38). P2X7R can be activated by ATP, which is released into the extracellular milieu following cell death or injury (50). P2X7R signaling induces the assembly of inflammasome signaling complexes (10), which drive the proteolytic activation of caspase-1 and the maturation of interleukin 1b (IL-1b). Another P2X7R-induced response is the rapid extracellular release of MHC-II molecules (38), which was previously observed within 15 min of the addition of ATP and resulted in the release of ∼15% of the total MHC-II pool in macrophages within 90 min (38). Released MHC-II molecules were contained in two membrane fractions: larger (100- to 1,000-nm) plasma membrane-derived microvesicles and smaller (50- to 80-nm) exosomes. The ATP-stimulated release of MHC-II was markedly reduced in macrophages isolated from NLRP3 knockout or ASC knockout mice. Thus, P2X7R activation of the NLRP3 inflammasome induces the biogenesis and release of MHC-II-containing membranes. The precedent of synergy between lipopolysaccharide (LPS) and ATP suggests that MHC-II shedding might be enhanced in the context of bacterial infection, but this hypothesis has not been explored.
Mycobacterium tuberculosis is a major human pathogen that infects one-third of the world population. M. tuberculosis and the related organism Mycobacterium bovis strain BCG infect host cells and regulate host cell functions by signaling through innate immune receptors, including TLR2. Cells infected with M. tuberculosis also secrete exosomes containing mycobacterial molecules that function as PAMPs (pathogen-associated molecular patterns) (2-5, 42) and can stimulate proinflammatory responses via TLR2, TLR4, and MyD88 (4, 5). The dissemination of PAMPs by exosomes released from infected cells may induce innate immune responses by a greater number of cells than are directly infected, magnifying host responses. M. tuberculosis and other mycobacteria can activate the ASC/NLRP3/caspase-1 inflammasome in macrophages via a mechanism dependent on the mycobacterial RD1 locus (encoding components of the ESX-1 secretion system, including the ESAT-6 protein) (9, 20, 26). The ability of M. tuberculosis to stimulate inflammasome activity is dependent on increased K+ efflux and occurs in macrophages from P2X7 receptor knockout mice (20). Thus, P2X7 receptor activation and M. tuberculosis infection may elicit similar signaling pathways that converge on the NLRP3 inflammasome and, possibly, on the inflammasome-dependent release of MHC-II membranes.
In the current study, we demonstrate that infection of macrophages with mycobacteria elicits the shedding of MHC-II-containing membranes. Furthermore, M. tuberculosis increases the ATP-triggered release of exosomes and microvesicles containing MHC-II. In addition, we demonstrate that MHC-II in membranes released from mycobacterium-infected macrophages can present Ag to T cells. These findings suggest that exosomes and microvesicles from mycobacterium-infected cells may broadcast the stimulation of both innate and adaptive immune receptors beyond the directly infected host cells, contributing to the genesis of CD4 T-cell responses to mycobacterial pathogens such as M. tuberculosis.
MATERIALS AND METHODS
Cells and media.
All animal protocols were reviewed by the Institutional Animal Care and Use Committee at Case Western Reserve University. C57BL/6 and CBA/J mice were obtained from Jackson Laboratories (Bar Harbor, ME). C57BL/6 MHC-II-enhanced green fluorescent protein (EGFP) knock-in mice were obtained from Taconic (Hudson, NY). P25 T-cell receptor (TCR)-transgenic (Tg) mice (C57BL/6 background), generously provided by Kiyoshi Takatsu (University of Tokyo), express a T-cell receptor that recognizes M. tuberculosis Ag 85B(240-254) bound to I-Ab (47). Standard medium was composed of Dulbecco's modified Eagle's medium (DMEM) (Life Technologies, Grand Island, NY) supplemented with 10% decomplemented fetal calf serum (HyClone, Logan, UT), 5 × 10−5 M 2-mercaptoethanol (2-ME), l-arginine HCl (116 mg/liter), l-asparagine (36 mg/liter), NaHCO3 (2 g/liter), sodium pyruvate (1 mM), 10 mM HEPES buffer, and antibiotics (antibiotics were omitted for incubations with M. tuberculosis or M. bovis). All incubations were carried out at 37°C in 5% CO2. For the preparation of macrophages, bone marrow cell precursors were harvested from femurs of mice and cultured in 15-cm2 petri dishes (or tissue culture dishes for experiments with M. tuberculosis) in standard medium supplemented with 20% LADMAC cell-conditioned medium (containing macrophage colony-stimulating factor [M-CSF]) (46). The medium was replaced on day 5, and by day 7 the resulting cultures were confluent and contained approximately 3 × 107 to 4 × 107 cells/dish. The T-cell hybridoma line BB7 recognizes M. tuberculosis Ag 85B(241-256) bound to I-Ab (30).
Bacteria.
M. tuberculosis H37Ra, M. tuberculosis H37Rv, and M. bovis BCG (all from ATCC, Manassas, VA) were grown to an optical density (OD) of 0.2 to 0.3 in Middlebrook 7H9 broth (Difco, Detroit, MI) supplemented with 1% glycerol, 0.05% Tween (Sigma) (to prevent clumping), and 10% Middlebrook oleic albumin dextrose catalase enrichment (Difco). Bacteria were harvested and used on the same day. Prior to macrophage infection, all mycobacterial preparations were pelleted, washed in DMEM, and resuspended in DMEM. The cells were then left undisturbed for 10 min to allow clumps to settle. The supernatant was then transferred into a new tube and analyzed under a microscope to confirm the absence of clumps and to determine the concentration of bacteria. M. tuberculosis H37Rv was similarly grown in a biosafety level 3 (BSL-3) facility and used as a frozen stock.
Antibodies.
Mouse monoclonal antibody (Ab) KL295 against the MHC-II beta chain (I-Ab and I-Ad) was obtained from the ATCC. In-1, a rat IgG2b specific for the cytoplasmic region of the invariant chain, was generously provided as a hybridoma supernatant by Andrea Sant (University of Rochester). Anti-CD86, a rat anti-mouse monoclonal Ab (B7-2; clone GL1), was purchased from BD Pharmingen (San Jose, CA). Anti-ICAM-1, a rat anti-mouse monoclonal Ab (clone YN1/1.7.4) was purchased from Southern Biotech (Birmingham, AL). Anti-calnexin and anti-Rab7, both rabbit polyclonal Abs, were purchased from Abcam (Cambridge, MA). APC-conjugated rat anti-mouse CD4 (clone RM4-5) was purchased from BD Pharmingen.
Activation and ATP stimulation of macrophages.
Macrophages were seeded into six-well plates at a density of 2 × 106 cells/well or grown in large 155-mm petri dishes (for LPS priming) or 155-mm tissue culture plates (for M. tuberculosis incubations) and were stimulated with 2 ng/ml gamma interferon (IFN-γ) (Genzyme) for 24 h to induce MHC-II expression. Cells were primed with various concentrations of LPS or infected with bacteria (M. tuberculosis H37Ra, M. tuberculosis H37Rv, or M. bovis BCG) at a multiplicity of infection (MOI) of 3 for 4 to 16 h at 37°C. Cells were washed once in phosphate-buffered saline (PBS) and then incubated with 1 ml (six-well plate) or 12 ml (155-mm plates) of prewarmed basal saline solution (BSS) medium containing 130 mM sodium gluconate, 5 mM KCl, 20 mM HEPES, 1.5 mM CaCl2, and 1.0 mM MgCl2 (pH 7.5) supplemented with 5 mM glucose, 5 mM glycine, and 0.01% bovine serum albumin (BSA) for 5 min. Cells were then stimulated with 5 mM ATP (Sigma) for 15 to 20 min.
Western blotting.
For Western blot analysis of extracellular medium (six-well plates), the medium was centrifuged at 10,000 × g for 30 s to pellet detached cells and bacteria, and the supernatant was transferred into a new tube. Proteins were precipitated by the addition of 72 μl of 100% trichloroacetic acid (TCA) and 15 μl of 10% cholic acid per 1 ml of extracellular medium and incubation for 30 min on ice. The precipitate was pelleted and washed three times with 1 ml of cold acetone, dissolved in 10 μl of 0.2 M NaOH, and then diluted to a final volume of 60 μl in SDS-PAGE sample buffer. For Western blot analysis of cell lysates, cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (1% NP-40, 0.1% SDS, and 0.5% sodium deoxycholate in PBS [pH 7.4]) supplemented with protease inhibitor cocktail 1 (Sigma) for 30 min on ice. The lysate was spun at 8,000 × g for 10 min at 4°C, and the supernatant was collected and supplemented with SDS-PAGE sample buffer. Purified microvesicles and exosomes were suspended in SDS-PAGE sample buffer. All samples were boiled and electrophoresed on 12% SDS-PAGE gels, blotted, and probed using standard procedures.
Purification of microvesicles and exosomes.
Extracellular medium from large 155-mm dishes was collected into 50-ml tubes on ice, spun at 300 × g followed by 2,000 × g at 4°C (to get rid of detached cells and debris), and then spun at 10,000 × g at 4°C to collect microvesicles. The supernatant was then concentrated to a volume of 25 ml using Centriprep (Ultracel YM-3 membrane; Millipore) that had been sterilized using 70% ethanol. The concentrated supernatant was centrifuged at 100,000 × g for 15 h (50.2 Ti rotor; Beckman Coulter, Fullerton, CA) to collect exosomes. The purified microvesicles and exosomes were subsequently analyzed by electron microcopy, Western blotting, or T hybridoma assay for the presentation of peptide-MHC-II complexes.
Electron microscopy analysis of exosomes and microvesicles.
Purified microvesicles and exosomes were fixed in 0.05 M phosphate buffer (pH 7.4) containing 2% glutaraldehyde and 4% sucrose for 2 h and then postfixed in 1% osmium tetroxide for 1 h at room temperature. Samples were then block stained in 0.5% aqueous uranyl acetate, dehydrated in an ascending concentration of ethanol, and embedded in Epon 812 resin. Ultrathin sections (60-nm thickness) were cut on an RMC MT6000-XL microtome, stained with 2% uranyl acetate in 50% methanol and with lead citrate, and then examined with a Jeol 1200EX electron microscope at 80 kV.
Microvesicle and exosome antigen presentation assays.
For Ag presentation assays using T hybridoma cells, microvesicles and exosomes were isolated from macrophages grown on 16 (for LPS) or 24 (for M. tuberculosis) large 150-mm petri dishes (tissue culture dishes for M. tuberculosis). Macrophages were activated with LPS for 4 h or infected with M. tuberculosis H37Ra at an MOI of 1 to 3 for 12 h and then stimulated with ATP for 20 min. Microvesicles and exosomes were isolated as described above and resuspended in 200 μl of medium. For direct T-cell presentation assays, microvesicles (50 μl) and exosomes (50 μl) were incubated with 105 BB7 T hybridoma cells for 24 h at 37°C in U-bottom 96-well plates with or without 10 μM exogenous synthetic peptide [M. tuberculosis Ag 85B(241-256)] in a total volume of 200 μl. For indirect T-cell presentation assays, 105 bone marrow-derived macrophages from CBA/J mice (expressing I-Ak) were plated into flat-bottom 96-well plates and fixed by using 1% paraformaldehyde. Exosomes and BB7 T hybridoma cells were added and incubated as described above. Supernatants (100 μl) were harvested and assessed for IL-2 by using a CTLL-2 proliferation assay, monitored by the addition of Alamar blue (Alamar Biosciences, Sacramento, CA) as an indicator dye, and measured as the difference between absorbances at 550 nm and 595 nm after 24 h. Blanks for spectrophotometry were provided by wells containing medium alone (added at the initiation of the CTLL-2 assay) and Alamar blue (added at the same time as that for the other wells). All analyses were performed in triplicate.
For Ag presentation assays using naïve P25 TCR-Tg T cells, microvesicles and exosomes were isolated from macrophages grown on 24 large 150-mm tissue culture dishes. Macrophages were infected with M. tuberculosis H37Ra at an MOI of 1 to 3 for 12 h and then stimulated with ATP for 15 min. Microvesicles and exosomes were isolated as described above and resuspended in 200 μl of standard medium. Untouched CD4+ T cells were isolated from spleens of P25 TCR-Tg mice using microbead kits from Miltenyi Biotec (Germany). Naïve T cells (106 cells/ml) were labeled with carboxyfluorescein succinimidyl ester (CFSE) (2 μM; Invitrogen) in PBS for 15 min at room temperature and washed in cold quench buffer (2% fetal bovine serum in PBS) before use. M. tuberculosis-induced microvesicles (10.8 μg, 5.4 μg, or 2.7 μg) or exosomes (20.8 μg or 10.4 μg) were incubated with 1 × 105 CFSE-labeled naïve T cells in a total volume of 200 μl in round-bottom plates for 6 days. Cells were labeled with an anti-CD4 Ab, and the percentage of CD4+ T cells undergoing CFSE dilution was assessed by flow cytometry.
Confocal microscopy.
Bone marrow-derived macrophages (105 cells) from C57BL/6 MHC-II-EGFP knock-in mice were plated into eight-chamber slides (Lab-Tek II chamber 1.5 German coverglass system; Nalge Nunc International) in standard medium (without antibiotics for M. tuberculosis). Macrophages were stimulated with 2 ng/ml IFN-γ for 24 h and then primed with 10 ng/ml LPS or incubated for 4 h with Bact-Light Red (Molecular Probes)-labeled M. tuberculosis H37Ra at an MOI of 3. Cells were washed in PBS, and the chamber slide was mounted onto a temperature- and CO2-controlled high-speed Leica (Wetzlar, Germany) SP5 broadband confocal microscope fitted with an HCX Plan Apo CS 63× oil immersion objective (numerical aperture [NA], 1.4). The cells were incubated in stimulation medium and monitored in real-time for vesicular release using Leica Application Suite software. Postcollection image analysis was performed by using the Imaris software suite (Bitplane Software, St. Paul, MN).
Statistical analysis.
T-cell assays were performed with duplicate samples of exosomes or microvesicles, and mean values were computed for each condition and normalized to the maximum CTLL-2 assay response. Normalized values for each condition from three independent experiments were assessed for statistical significance by an unpaired Student's t test using GraphPad software 2002-2005 (GraphPad Software, La Jolla, CA).
RESULTS
Mycobacterial infection promotes ATP-dependent shedding of MHC-II.
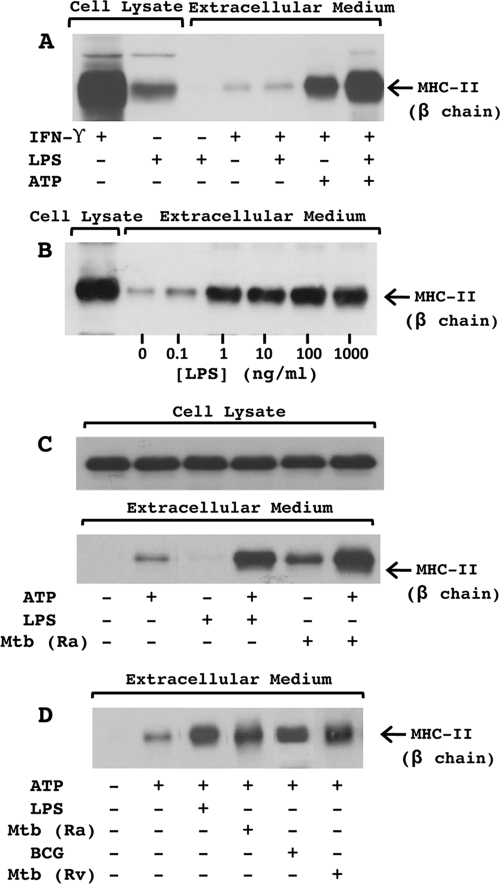
We assessed whether mycobacterial infection enhances the ATP-induced release of MHC-II from macrophages. Consistent with prior results (38), the activation of macrophages with LPS for 4 h enhanced the subsequent rapid release of MHC-II molecules upon stimulation with 5 mM ATP for 20 min (Fig. 1A). Although similar observations can be seen with lower concentrations of ATP (1 or 3 mM), 5 mM ATP produces optimum responses (37). The ATP-induced release of MHC-II was enhanced by concentrations of LPS as low as 1 ng/ml (Fig. 1B). Overnight infection with virulent M. tuberculosis H37Rv, avirulent M. tuberculosis H37Ra, or M. bovis BCG clearly enhanced the ATP-induced release of MHC-II (Fig. 1C and D) but had no effect on MHC-II levels in macrophages (Fig. 1C). Since all three strains of mycobacteria were able to enhance the release of MHC-II in response to ATP (Fig. 1D), subsequent experiments were done by using M. tuberculosis H37Ra. Interestingly, M. tuberculosis by itself enhanced the release of MHC-II independent of ATP, an effect that was absent or minimal with LPS alone, but the combination of M. tuberculosis and ATP induced the greatest level of MHC-II release (Fig. 1C).
FIG. 1.
Mycobacterial infection promotes ATP-dependent shedding of MHC-II. C57BL/6 macrophages (2 × 106 cells/well in six-well plates) were activated with IFN-γ (2 ng/ml) for 24 h. Cells were then primed for 4 h with LPS or infected overnight with mycobacteria (MOI of 3). Cells were transferred into BSS and stimulated with or without 5 mM ATP for 20 min. Extracellular medium was collected, and released proteins were precipitated with TCA. TCA-precipitated proteins and cell lysate were analyzed for MHC-II by Western blotting. (A) Analysis of MHC-II in TCA-precipitated proteins and cell lysates from macrophages activated with or without IFN-γ, primed with or without LPS (1 μg/ml), and stimulated with or without ATP. (B) Analysis of MHC-II in TCA-precipitated proteins and cell lysates from macrophages activated with IFN-γ, primed with various concentrations of LPS, and stimulated with ATP. (C) Analysis of MHC-II in TCA-precipitated proteins and cell lysates from macrophages activated with IFN-γ, primed with or without LPS (10 ng/ml) or M. tuberculosis H37Ra (Mtb Ra), and stimulated with or without ATP. (D) Analysis of MHC-II in TCA-precipitated proteins from macrophages activated with IFN-γ; primed with or without LPS (10 ng/ml), M. tuberculosis H37Ra, M. bovis BCG, and M. tuberculosis H37Rv (Mtb Rv); and stimulated with or without ATP. Results are representative of data from four independent experiments.
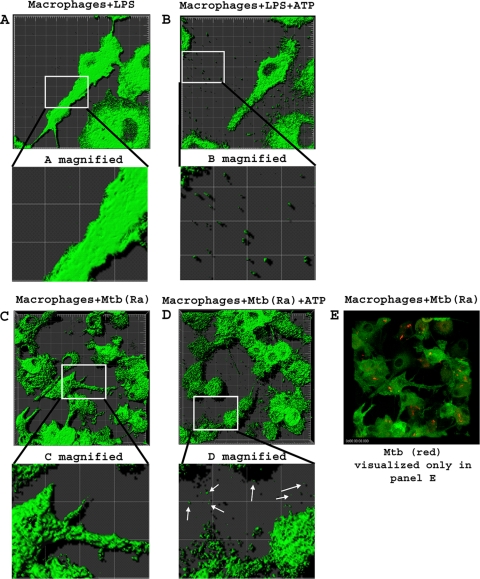
Macrophages from MHC-II-EGFP knock-in mice were used to analyze the release of MHC-II-containing membranes by time-lapse confocal fluorescence microscopy (Fig. 2). Macrophages were treated for 4 h with LPS or Bact-Light Red-labeled M. tuberculosis H37Ra, and the cells were assessed by microscopy (see Movies S1A and S1C in the supplemental material). ATP was then added, and the cells were analyzed over the following 10 min for the release of MHC-II-EGFP-containing membranes (Movies S1B and S1D). Approximately 3 min following the addition of ATP, vesicles with green fluorescence (indicating the presence of MHC-II-EGFP) were released from LPS- or M. tuberculosis H37Ra-stimulated macrophages (Fig. 2B and D), and the number of released vesicles continued to increase for the length of time that the images were taken (total of 10 min). In the absence of ATP, little or no release of EGFP-positive vesicles was observed (Fig. 2A and C). The size of the released vesicles was approximately 1,000 nm, larger than exosomes, which are approximately 50 to 80 nm in diameter (too small to be observed by this imaging technique). We also observed blebs or irregular bulges appearing on the plasma membrane following ATP stimulation, consistent with prior observations of plasma membrane blebbing and microvesicle formation following the activation of P2X7R (1, 6, 24, 36, 51). However, no lysis of cells was observed for up to 30 min following the addition of ATP. In conclusion, LPS and mycobacteria promote the ATP-dependent shedding of microvesicles containing MHC-II.
FIG. 2.
Microscopy reveals ATP-dependent shedding of membrane vesicles. Macrophages from C57BL/6 MHC-II-EGFP mice (green is MHC-II-EGFP) were plated into eight-chamber slides and activated with IFN-γ (2 ng/ml) for 24 h. Cells were incubated with 10 ng/ml LPS for 4 h (A and B) (magnification, ×189) or infected for 4 h with Bact-Light Red-labeled M. tuberculosis H37Ra at an MOI of 3 (C and D) (magnification, ×126). Cells were then transferred into BSS and monitored every 30 s for 10 min before and after the addition of 5 mM ATP using a high-speed Leica SP5 broadband confocal microscope. Images were acquired by using Leica Application Suite software and analyzed by using the Imaris software suite. Results are representative of data from independent experiments. Movies S1A to S1D in the supplemental material depict time-lapse microscopy under these conditions. Since Bact-Light Red-labeled M. tuberculosis H37Ra was highly susceptible to bleaching, the red laser was activated initially to assess M. tuberculosis infection but was not activated when analyzing the release of vesicles over the 10-min period. For each LPS experiment, five separate chambers of cells were analyzed with or without ATP. Within each chamber, a field containing approximately 10 cells was analyzed. Since two independent experiments were performed, the analysis included a total of approximately 100 cells, including those shown in C to E. For each M. tuberculosis experiment, three separate chambers containing cells were analyzed with or without ATP. Within each chamber, a field containing approximately 20 cells was analyzed. Since two independent experiments were performed, the analysis included a total of approximately 120 cells, including those shown in C to E.
Shed MHC-II is included in two distinct organelles, microvesicles and exosomes.
Constitutively shed MHC-II from dendritic cells and B cells is associated predominantly with 50- to 80-nm exosomes derived by the exocytosis of multivesicular endosomes, but our confocal microscopy results suggested the release of MHC-II in larger microvesicles. To investigate the relative contributions of exosomes and microvesicles in the shedding of MHC-II, shed membrane fractions were purified by differential centrifugation and analyzed by electron microscopy and Western blotting.
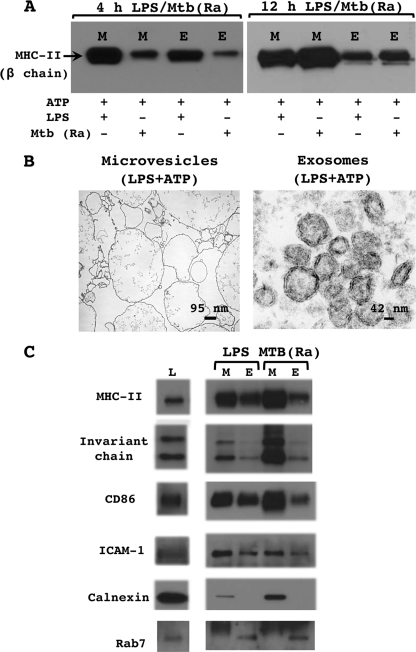
To define the period of M. tuberculosis infection that was optimum for the production of microvesicles and exosomes, macrophages were incubated with M. tuberculosis H37Ra or LPS for 4, 12, or 16 h and then stimulated with ATP for 15 min. The extracellular medium was collected and centrifuged at 300 × g and then at 1,200 × g to pellet detached cells, cell debris, and bacteria. The supernatant was centrifuged at 10,000 × g to pellet microvesicles and then at 100,000 × g to pellet exosomes. MHC-II levels (and pellet sizes) in the 4-h M. tuberculosis microvesicles and exosomes were lower (and pellets were smaller) than in the 4-h LPS microvesicles and exosomes, but by 12 h the levels of MHC-II (and pellet sizes) in M. tuberculosis microvesicles and exosomes were as high or higher than those of the 12-h LPS microvesicles and exosomes (Fig. 3A). Levels of MHC-II (and pellet sizes) did not increase in LPS microvesicles and exosomes following a longer incubation of macrophages with LPS. We also observed that incubation with M. tuberculosis for more than 12 h reduced the yield of microvesicles and MHC-II in microvesicles (data not shown). Thus, a 12-h incubation with M. tuberculosis was determined to be optimum for the production of exosomes and microvesicles containing MHC-II. This time point also avoided other long-term effects of mycobacterial infection, e.g., the loss of MHC-II expression after 18 h or more of mycobacterial infection (19, 30-32).
FIG. 3.
Shed MHC-II is included in two distinct released membrane types, microvesicles and exosomes. C57BL/6 macrophages were activated with IFN-γ (2 ng/ml) for 24 h. Cells were then primed with LPS (10 ng/ml) for 4 h (A, left, B, and C) or 12 h (A, right) or infected with M. tuberculosis H37Ra (MOI of 3) for 4 h (A, left) or 12 h (A, right, and C). Cells were transferred into BSS and stimulated with 5 mM ATP for 20 min. The extracellular medium was collected and sequentially centrifuged at 300 × g, 2,000 × g, 10,000 ×g, and 100,000 × g. The 10,000 × g pellet (microvesicles) and the 100,000 × g pellet (exosomes) were analyzed by Western blotting (A and C) or electron microscopy (B). M, microvesicles; E, exosomes. Results are representative of data from two or more independent experiments.
Electron microscopy of the LPS pellet centrifuged at 10,000 × g showed membrane vesicles of 100 to 1,000 nm in diameter with electron-lucent internal staining (Fig. 3B), consistent with the morphological properties of microvesicles (38). The 100,000 × g pellet consisted of membrane vesicles of 50 to 80 nm in diameter with electron-dense internal staining (Fig. 3A), consistent with the morphological properties of exosomes (16, 17, 35, 40, 53). Additionally, the lack of mitochondria, lysosomes, nuclei, and apoptotic bodies in the exosome and microvesicle preparations demonstrates the purity of the fractions and indicates that ATP is not inducing the apoptosis of macrophages within the 15-min stimulation period.
Microvesicles and exosomes were further analyzed by Western blotting (Fig. 3D) for molecules involved in MHC-II antigen processing (invariant chain) or antigen presentation (CD86 and ICAM-1) as well as molecular markers for organelles, e.g., calnexin (endoplasmic reticulum [ER] marker) and rab7 (late endosomal marker). Assessment of protein content of microvesicle and exosome preparations revealed that macrophages treated with LPS released 4.2 μg of microvesicles and 3.7 μg of exosomes per plate (40 million macrophages/plate), and macrophages infected with M. tuberculosis released 3.6 μg of microvesicles and 3.5 μg of exosomes per plate. For Western blot analysis equivalent amounts (4.5 μg) of microvesicles and exosomes were analyzed. Western blotting revealed that levels of MHC-II, invariant chain, CD86, and ICAM-1 were higher in microvesicles than in exosomes. The higher levels of CD86 and ICAM-1 are consistent with the derivation of microvesicles from the plasma membrane, where CD86 and ICAM-1 are localized. The presence of calnexin (an ER marker) and the absence of rab7 in the microvesicles suggest that microvesicles may contain membranes derived from the ER (reflecting either biological trafficking or some degree of contamination) but not from the late endosomes. The presence of rab7 and the absence of calnexin in exosomes are consistent with observations that exosomes are derived from late endosomes and not the plasma membrane. In conclusion, both LPS and M. tuberculosis microvesicles and exosomes have molecules involved in antigen processing and presentation, although these molecules are expressed at higher levels in microvesicles. This observation suggests that both organelles may be competent to present antigenic peptide to T cells although perhaps to various degrees.
Exosomes and microvesicles can present peptide-MHC-II complexes to T cells.
While constitutively shed exosomes have been demonstrated to present peptide-MHC-II complexes to T cells, the antigen-presenting capacity of ATP-induced exosomes has not been studied. Furthermore, the antigen-presenting capacity of exosomes from M. tuberculosis-infected cells has not been determined, and it is unknown whether exosomes from infected cells contain peptide-MHC-II complexes formed by the intracellular processing of M. tuberculosis antigens.
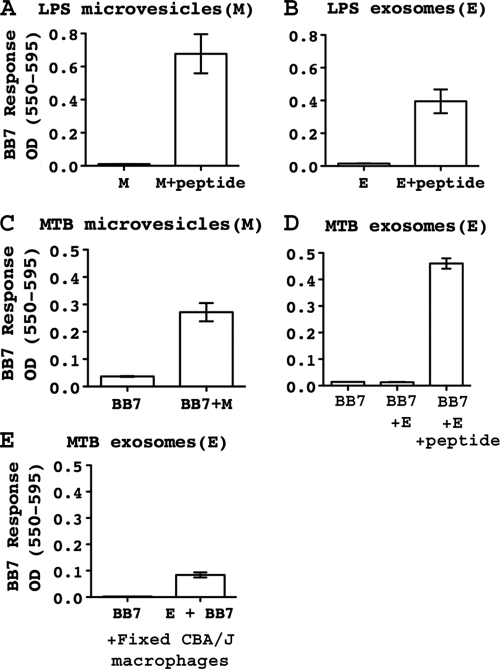
To analyze the antigen-presenting capacity of ATP-induced microvesicles and exosomes, macrophages were incubated with LPS (10 ng/ml, for 4 h) or M. tuberculosis H37Ra (MOI of 1 to 3, for 12 h) and then stimulated with ATP for 15 min. Exosomes and microvesicles were isolated by differential centrifugation under sterile conditions. Similar concentrations of LPS- or M. tuberculosis-induced exosome and microvesicle preparations were incubated directly with BB7 T hybridoma cells, which recognize M. tuberculosis Ag 85B(241-256)-I-Ab complexes. LPS-induced microvesicles and exosomes alone did not stimulate BB7 T cells, but both were able to present exogenous Ag 85B(241-256) peptide (Fig. 4A and B). Infection with M. tuberculosis provided the possibility of the endogenous processing of Ag 85B to produce M. tuberculosis Ag 85B(241-256)-I-Ab complexes, and this was confirmed by the incubation of BB7 T hybridoma cells with intact M. tuberculosis-infected macrophages (data not shown). ATP-stimulated microvesicles from M. tuberculosis-infected macrophages were able to directly present Ag 85B(241-256)-I-Ab complexes to BB7 T hybridoma cells without the addition of exogenous peptide (Fig. 4C), indicating that the microvesicles acquired endogenously processed Ag 85B(241-256)-I-Ab complexes. ATP-stimulated exosomes from M. tuberculosis-infected macrophages did not directly activate BB7 T hybridoma cells but could do so in the presence of exogenous Ag 85B(241-256) peptide (Fig. 4D).
FIG. 4.
Exosomes and microvesicles can present peptide-MHC-II complexes to T cells. C57BL/6 macrophages were activated with IFN-γ (2 ng/ml) for 24 h. Cells were then primed with LPS (10 ng/ml) for 4 h or infected for 12 h with M. tuberculosis H37Ra (MOI of 1 to 3). Cells were transferred into BSS and stimulated with 5 mM ATP for 20 min. Microvesicles and exosomes were isolated as described in the legend of Fig. 3, resuspended in standard medium, and incubated as follows. (A) LPS-induced microvesicles plus BB7 T hybridoma cells (105) with or without 10 μM peptide [M. tuberculosis Ag 85B(241-256)]. (B) LPS-induced exosomes plus BB7 T hybridoma cells (105) with or without 10 μM peptide [M. tuberculosis Ag 85B(241-256)]. (C) BB7 T hybridoma cells (105) alone or with M. tuberculosis-induced microvesicles. (D) BB7 T hybridoma cells (105) alone, with M. tuberculosis-induced exosomes, or with M. tuberculosis-induced exosomes plus 10 μM peptide [M. tuberculosis Ag 85B(241-256)]. (E) Fixed CBA/J macrophages (105) and BB7 T hybridoma cells (105) with or without M. tuberculosis-induced exosomes. M, microvesicles; E, exosomes. Results are displayed as means ± standard deviations (SD) from T-cell assays that were performed with duplicate samples of exosomes or microvesicles. Statistical analysis was performed by including data from three independent experiments. For each experiment, the mean value for each condition was normalized to the maximum CTLL-2 assay response, and normalized values from three independent experiments were assessed for statistical significance by an unpaired Student's t test. Differences were statistically significant for LPS microvesicles without peptide versus with peptide (as in A; P < 0.001), LPS exosomes without peptide versus with peptide (as in B; P < 0.005), M. tuberculosis microvesicles without peptide versus with peptide (as in C; P < 0.005), M. tuberculosis exosomes without peptide versus with peptide (as in D; P < 0.001), and fixed macrophages with or without M. tuberculosis exosomes (as in E; P < 0.001).
We next considered the hypothesis that these exosomes might stimulate BB7 T hybridoma T cells if their Ag presentation functions were enhanced by an association with the surface of APCs (so-called “indirect presentation,” although it is the peptide-MHC-II complexes on the exosomes that are recognized by the T cells). It was demonstrated previously that the association of exosomes with the surface of cells (48) or larger particles (52) provides enhanced T-cell recognition of exosomal peptide-MHC-II complexes. Exosomes from M. tuberculosis-infected C57BL/6 macrophages were incubated with live or fixed macrophages from CBA/J mice, which express I-Ak molecules that cannot present the M. tuberculosis Ag 85B(241-256) epitope. When C57BL/6 exosomes were associated with fixed CBA/J macrophages, they were able to weakly but consistently present endogenously processed M. tuberculosis Ag 85B(241-256)-I-Ab complexes to T hybridoma cells (Fig. 4E). Live CBA/J macrophages were unable to enhance presentation by exosomes, possibly due to the internalization and degradation of the exosomes (data not shown).
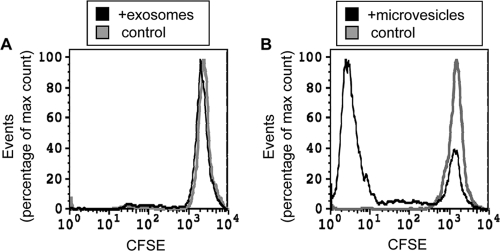
The capacity of M. tuberculosis-induced exosomes and microvesicles to activate P25 TCR-transgenic T cells that recognize M. tuberculosis Ag 85B(240-254) bound to I-Ab was additionally investigated (Fig. 5). Naïve CFSE-labeled P25 T cells were activated with M. tuberculosis-induced exosomes (20.8 μg or 10.4 μg per well) or microvesicles (2.7 μg, 5.4 μg, or 10.8 μg per well). After 6 days of incubation, the percentage of T cells undergoing proliferation was assessed by CFSE dilution as determined by flow cytometry. The results demonstrated that naïve T cells were activated by microvesicles (maximum response observed with 5.4 μg), whereas exosomes did not activate naïve T cells (Fig. 5).
FIG. 5.
Naïve T cells are stimulated by endogenously processed M. tuberculosis Ag 85B presented by microvesicles but not exosomes from M. tuberculosis-infected macrophages. Naïve CFSE-labeled P25 CD4+ T cells were activated with M. tuberculosis-induced microvesicles (10.8 μg, 5.4 μg, or 2.7 μg per well) or exosomes (20.8 μg or 10.4 μg per well) for 6 days, and the percentage of cells undergoing CFSE dilution was assessed by flow cytometry. (A) Proliferation of naïve CFSE-labeled P25 T cells incubated with or without 2.7 μg microvesicles per well. Limiting amounts of material (and decreased primary T-cell survival in the absence of a stimulus) limited the number of events that could be analyzed to 5,116 for T cells stimulated with exosomes and 1,877 for control T cells (CFSE labeled, no stimulus). To compare the extents of proliferation of the two T-cell populations, event counts are normalized to the maximum event count under the condition. Since the experiment was designed to allow ample time for T cells to divide to enhance sensitivity, peaks for early stages of CFSE dilution are not present, and proliferating T cells have divided six or more times to dilute the CFSE label to the background fluorescence level. (B) Proliferation of naïve CFSE-labeled P25 T cells incubated with or without 20.8 μg exosomes per well. Results were analyzed as described above for A, with 6,654 events for the microvesicle conditions and 1,454 events for the control conditions (CFSE labeled, no stimulus). Results of both panels represent a single flow cytometry sample from one experiment that is representative of data from three independent experiments.
In conclusion, ATP-induced exosomes and microvesicles from M. tuberculosis-infected macrophages can present peptide-MHC-II complexes to T cells. Their ability to present endogenously processed MHC-II complexes with M. tuberculosis-derived peptides may be enhanced by an association with APCs, and they may be more efficient at stimulating memory or activated T cells than naïve T cells. These mechanisms may allow vesicles derived from M. tuberculosis-infected cells to traffic to sites distant from the infected cell and stimulate T cells in association with APCs at those sites (e.g., lymph nodes).
DISCUSSION
In contrast to the slow constitutive release of exosomes containing MHC-II by APCs, ATP induces a rapid shedding of MHC-II in both exosomes and microvesicles. Our prior study demonstrated that 15% of total cellular MHC-II is shed from macrophages within 90 min of stimulation with ATP (38). LPS-induced TLR4 signaling enhances ATP-stimulated shedding of MHC-II (38) (Fig. 1). Infection of macrophages with mycobacteria (M. tuberculosis H37Ra, M. tuberculosis H37Rv, or M. bovis BCG) also enhanced a rapid ATP-induced release of MHC-II (Fig. 1). Interestingly, M. tuberculosis also induced the release of MHC-II in the absence of exogenous ATP, although the maximum release was achieved with the combination of M. tuberculosis and ATP. The mechanism underlying the M. tuberculosis-induced release of MHC-II in the absence of an exogenously added ATP stimulus could involve either autocrine activation of P2X7 receptors by the M. tuberculosis-stimulated release of endogenous ATP or P2X7-independent signals entrained by M. tuberculosis infection. The latter possibility is supported by data reported previously by Kurenuma et al. (20), who observed that M. tuberculosis infection induced equivalent NLRP3 inflammasome activities in control and P2X7 knockout macrophages. Our previous study linked ATP-induced MHC-II release to the stimulation of the NLRP3 inflammasome. Thus, it is possible that M. tuberculosis infection is sufficient for MHC-II release via a similar inflammasome-related mechanism in the absence of ATP, and the activation of P2X7 signaling may further stimulate this response.
The recognition of M. tuberculosis by the innate immune system appears to be mediated by pathogen recognition receptors that include TLRs and nucleotide-binding oligomerization domain 2 (NOD2). M. tuberculosis-mediated MHC-II release was not dependent on TLR2, since no decrease in MHC-II release was observed for macrophages lacking TLR2 (data not shown). The involvement of other molecules such as NOD2 in this process needs further investigation.
Our observations demonstrate for the first time the antigen-presenting capabilities of microvesicles and exosomes released by ATP stimulation in combination with mycobacterial infection or LPS. Following stimulation with LPS and ATP, exosomes and microvesicles were both able to present exogenous peptide to T hybridoma cells. Infection of cells with M. tuberculosis allowed us to determine whether endogenous M. tuberculosis Ag 85B(241-256)-I-Ab complexes (generated by macrophage processing of M. tuberculosis) were presented by exosomes and microvesicles. Using a direct antigen presentation assay with organelles derived from M. tuberculosis-infected cells, BB7 T hybridoma cells detected endogenous M. tuberculosis Ag 85B(241-256)-I-Ab complexes in M. tuberculosis microvesicles but not in M. tuberculosis exosomes. M. tuberculosis exosomes were, however, able to directly present exogenous M. tuberculosis peptide Ag 85B(241-256), demonstrating that these exosomes were capable of antigen presentation but not with an adequate efficiency to directly present endogenous M. tuberculosis Ag 85B(241-256)-I-Ab complexes. With the “indirect” antigen presentation assay, the association of M. tuberculosis exosomes with fixed, MHC-II-mismatched macrophages allowed some T-cell detection of M. tuberculosis Ag 85B(241-256)-I-Ab complexes in M. tuberculosis exosomes, suggesting that M. tuberculosis exosomes do acquire low levels of these complexes. As an alternative to the BB7 T hybridoma, experiments were performed with naïve P25 TCR-transgenic T cells that also recognize M. tuberculosis Ag 85B(240-254)-I-Ab complexes. As assessed by proliferation, naïve P25 T cells were activated by M. tuberculosis-induced microvesicles but not exosomes (Fig. 5). In conclusion, both microvesicles and exosomes from M. tuberculosis-infected macrophages can activate T-cell responses, although indirect presentation may be important for boosting responses to exosomes.
The implication that exosomes from M. tuberculosis-infected cells may contain only low levels of M. tuberculosis peptide-MHC-II complexes fits with the observation that the late endosomal compartments that generate exosomes are also deficient in levels of these complexes. During the processing of M. tuberculosis by macrophages, M. tuberculosis Ag 85B(241-256)-I-Ab complexes were detected in phagosomes but not in late endosomal compartments (39). M. tuberculosis Ag 85B(241-256)-I-Ab complexes subsequently trafficked to the plasma membrane (39), from which microvesicles are derived, consistent with the greater T-cell responses to endogenous M. tuberculosis peptide-MHC-II complexes presented by microvesicles. Thus, peptide-MHC-II complexes generated by the phagosomal processing of M. tuberculosis may target more inefficiently for incorporation into exosomes than microvesicles. In addition, the higher levels of expression of costimulatory molecules (CD86 and ICAM-1) on microvesicles (Fig. 2C) may render them more efficient than exosomes at activating naïve T cells. Previous studies demonstrated that exosomes are better at activating primed T cells than naïve CD4+ T cells (27).
These studies have implications for potential physiological roles of exosomes during bacterial infection. While the role of the P2X7 receptor in tuberculosis is unclear (28), polymorphisms in P2X7 have been associated with an increased risk of mycobacterial disease (8, 13, 15, 21, 23, 29). The source of a physiological P2X7R ligand in the context of M. tuberculosis infection is unknown, but ATP released from infected injured or necrotic cells is one potential source. In addition, inflammatory mediators other than ATP may trigger or potentiate P2X7R activation; these include NAD (18), lysophosphatidylcholine (25), and antimicrobial cathelicidin peptides (11). Increased mycobacterial killing has been one hypothesis for the contribution of P2X7R to host resistance (7, 12, 14, 22, 41, 44, 45), but its involvement in the induction of MHC-II release via exosomes and microvesicles may also contribute. M. tuberculosis induced the release of exosomes even in the absence of ATP, but the ATP stimulation of M. tuberculosis-infected macrophages resulted in the highest level of MHC-II release in exosomes and microvesicles. These extracellular membranes may traffic to sites distant from the infected cell, e.g., lymph nodes, to generate CD4+ T-cell responses. Indirect presentation may be important, and the potential contributions of both microvesicles and exosomes should be considered and further investigated. It is interesting to speculate that microvesicles and exosomes may influence the differentiation or polarization of T-cell responses, due to their content of costimulatory molecules, potentially driving the differentiation of Th1 responses, a topic for future investigation (e.g., by studies of polarization of T-cell cytokine production resulting from the stimulation of naïve T cells with exosomes and microvesicles).
These studies also have implications for general therapeutic applications of exosomes. Exosomes are being investigated for potential use in cancer therapy, and the constitutively released exosomes used in these studies are generally collected from dendritic cells over a period of 48 to 72 h. Our studies suggest that ATP-induced exosomes could serve as an alternative to conventional exosomes. We have observed that ATP increases the release of exosomes from dendritic cells as well as macrophages (data not shown). ATP-induced exosomes can be generated very rapidly, and the yield is much higher than that obtained with the constitutive release of exosomes, potentially allowing significant time and cost advantages. Thus, ATP-induced exosomes may be an attractive alternative to conventional exosomes for therapeutic purposes.
Supplementary Material
Acknowledgments
We thank Hisashi Fujioka for electron microscopy sample preparation and image analysis, Alex Huang for use of facilities for confocal data analysis, and Daimon Simmons for assistance with flow cytometry analysis.
This work was supported by NIH grants AI076792 to L.R.; AI035726, AI034343, and AI069085 to C.V.H.; AI027243 and HL055967 to W.H.B.; and GM36387 to G.R.D. K.T. received support from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 13 September 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Baroni, M., C. Pizzirani, M. Pinotti, D. Ferrari, E. Adinolfi, S. Calzavarini, P. Caruso, F. Bernardi, and F. Di Virgilio. 2007. Stimulation of P2 (P2X7) receptors in human dendritic cells induces the release of tissue factor-bearing microparticles. FASEB J. 21:1926-1933. [DOI] [PubMed] [Google Scholar]
- 2.Beatty, W. L., E. R. Rhoades, H. J. Ullrich, D. Chatterjee, J. E. Heuser, and D. G. Russell. 2000. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic 1:235-247. [DOI] [PubMed] [Google Scholar]
- 3.Beatty, W. L., and D. G. Russell. 2000. Identification of mycobacterial surface proteins released into subcellular compartments of infected macrophages. Infect. Immun. 68:6997-7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatnagar, S., and J. S. Schorey. 2007. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J. Biol. Chem. 282:25779-25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatnagar, S., K. Shinagawa, F. J. Castellino, and J. S. Schorey. 2007. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 110:3234-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianco, F., E. Pravettoni, A. Colombo, U. Schenk, T. Moller, M. Matteoli, and C. Verderio. 2005. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J. Immunol. 174:7268-7277. [DOI] [PubMed] [Google Scholar]
- 7.Biswas, D., O. S. Qureshi, W. Y. Lee, J. E. Croudace, M. Mura, and D. A. Lammas. 2008. ATP-induced autophagy is associated with rapid killing of intracellular mycobacteria within human monocytes/macrophages. BMC Immunol. 9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Britton, W. J., S. L. Fernando, B. M. Saunders, R. Sluyter, and J. S. Wiley. 2007. The genetic control of susceptibility to Mycobacterium tuberculosis. Novartis Found. Symp. 281:79-89; discussion 89-92, 208-209. [DOI] [PubMed] [Google Scholar]
- 9.Carlsson, F., J. Kim, C. Dumitru, K. H. Barck, R. A. Carano, M. Sun, L. Diehl, and E. J. Brown. 2010. Host-detrimental role of Esx-1-mediated inflammasome activation in mycobacterial infection. PLoS Pathog. 6:e1000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Virgilio, F. 2007. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol. Sci. 28:465-472. [DOI] [PubMed] [Google Scholar]
- 11.Elssner, A., M. Duncan, M. Gavrilin, and M. D. Wewers. 2004. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J. Immunol. 172:4987-4994. [DOI] [PubMed] [Google Scholar]
- 12.Fairbairn, I. P., C. B. Stober, D. S. Kumararatne, and D. A. Lammas. 2001. ATP-mediated killing of intracellular mycobacteria by macrophages is a p2x(7)-dependent process inducing bacterial death by phagosome-lysosome fusion. J. Immunol. 167:3300-3307. [DOI] [PubMed] [Google Scholar]
- 13.Fernando, S. L., B. M. Saunders, R. Sluyter, K. K. Skarratt, H. Goldberg, G. B. Marks, J. S. Wiley, and W. J. Britton. 2007. A polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 175:360-366. [DOI] [PubMed] [Google Scholar]
- 14.Fernando, S. L., B. M. Saunders, R. Sluyter, K. K. Skarratt, J. S. Wiley, and W. J. Britton. 2005. Gene dosage determines the negative effects of polymorphic alleles of the P2X7 receptor on adenosine triphosphate-mediated killing of mycobacteria by human macrophages. J. Infect. Dis. 192:149-155. [DOI] [PubMed] [Google Scholar]
- 15.Franco-Martinez, S., P. Nino-Moreno, S. Bernal-Silva, L. Baranda, M. Rocha-Meza, L. Portales-Cervantes, E. Layseca-Espinosa, R. Gonzalez-Amaro, and D. Portales-Perez. 2006. Expression and function of the purinergic receptor P2X7 in patients with pulmonary tuberculosis. Clin. Exp. Immunol. 146:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harding, C., J. Heuser, and P. Stahl. 1984. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur. J. Cell Biol. 35:256-263. [PubMed] [Google Scholar]
- 17.Harding, C., J. Heuser, and P. Stahl. 1983. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 97:329-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong, S., N. Schwarz, A. Brass, M. Seman, F. Haag, F. Koch-Nolte, W. P. Schilling, and G. R. Dubyak. 2009. Differential regulation of P2X7 receptor activation by extracellular NAD and ecto-ARTs in murine macrophages and T cells. J. Immunol. 183:578-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kincaid, E. Z., A. J. Wolf, L. Desvignes, S. Mahapatra, D. C. Crick, P. J. Brennan, M. S. Pavelka, Jr., and J. D. Ernst. 2007. Codominance of TLR2-dependent and TLR2-independent modulation of MHC class II in Mycobacterium tuberculosis infection in vivo. J. Immunol. 179:3187-3195. [DOI] [PubMed] [Google Scholar]
- 20.Kurenuma, T., I. Kawamura, H. Hara, R. Uchiyama, S. Daim, S. R. Dewamitta, S. Sakai, K. Tsuchiya, T. Nomura, and M. Mitsuyama. 2009. The RD1 locus in the Mycobacterium tuberculosis genome contributes to activation of caspase-1 via induction of potassium ion efflux in infected macrophages. Infect. Immun. 77:3992-4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kusner, D. J., and J. Adams. 2000. ATP-induced killing of virulent Mycobacterium tuberculosis within human macrophages requires phospholipase D. J. Immunol. 164:379-388. [DOI] [PubMed] [Google Scholar]
- 22.Lammas, D. A., C. Stober, C. J. Harvey, N. Kendrick, S. Panchalingam, and D. S. Kumararatne. 1997. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity 7:433-444. [DOI] [PubMed] [Google Scholar]
- 23.Li, C. M., S. J. Campbell, D. S. Kumararatne, R. Bellamy, C. Ruwende, K. P. McAdam, A. V. Hill, and D. A. Lammas. 2002. Association of a polymorphism in the P2X7 gene with tuberculosis in a Gambian population. J. Infect. Dis. 186:1458-1462. [DOI] [PubMed] [Google Scholar]
- 24.MacKenzie, A., H. L. Wilson, E. Kiss-Toth, S. K. Dower, R. A. North, and A. Surprenant. 2001. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity 15:825-835. [DOI] [PubMed] [Google Scholar]
- 25.Michel, A. D., and E. Fonfria. 2007. Agonist potency at P2X7 receptors is modulated by structurally diverse lipids. Br. J. Pharmacol. 152:523-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishra, B. B., P. Moura-Alves, A. Sonawane, N. Hacohen, G. Griffiths, L. F. Moita, and E. Anes. 2010. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell. Microbiol. 12:1046-1063. [DOI] [PubMed] [Google Scholar]
- 27.Muntasell, A., A. C. Berger, and P. A. Roche. 2007. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J. 26:4263-4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myers, A. J., B. Eilertson, S. A. Fulton, J. L. Flynn, and D. H. Canaday. 2005. The purinergic P2X7 receptor is not required for control of pulmonary Mycobacterium tuberculosis infection. Infect. Immun. 73:3192-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nino-Moreno, P., D. Portales-Perez, B. Hernandez-Castro, L. Portales-Cervantes, V. Flores-Meraz, L. Baranda, A. Gomez-Gomez, V. Acuna-Alonzo, J. Granados, and R. Gonzalez-Amaro. 2007. P2X7 and NRAMP1/SLC11 A1 gene polymorphisms in Mexican mestizo patients with pulmonary tuberculosis. Clin. Exp. Immunol. 148:469-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noss, E. H., R. K. Pai, T. J. Sellati, J. D. Radolf, J. Belisle, D. T. Golenbock, W. H. Boom, and C. V. Harding. 2001. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19 kD lipoprotein of Mycobacterium tuberculosis. J. Immunol. 167:910-918. [DOI] [PubMed] [Google Scholar]
- 31.Pai, R. K., M. Convery, T. A. Hamilton, W. H. Boom, and C. V. Harding. 2003. Inhibition of IFN-gamma-induced class II transactivator expression by a 19-kDa lipoprotein from Mycobacterium tuberculosis: a potential mechanism for immune evasion. J. Immunol. 171:175-184. [DOI] [PubMed] [Google Scholar]
- 32.Pai, R. K., M. E. Pennini, A. A. Tobian, D. H. Canaday, W. H. Boom, and C. V. Harding. 2004. Prolonged Toll-like receptor signaling by Mycobacterium tuberculosis and its 19-kilodalton lipoprotein inhibits gamma interferon-induced regulation of selected genes in macrophages. Infect. Immun. 72:6603-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan, B. T., and R. Johnstone. 1984. Selective externalization of the transferrin receptor by sheep reticulocytes in vitro. J. Biol. Chem. 259:9776-9782. [PubMed] [Google Scholar]
- 34.Pan, B. T., and R. M. Johnstone. 1983. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33:967-978. [DOI] [PubMed] [Google Scholar]
- 35.Pan, B. T., K. Teng, C. Wu, M. Adam, and R. M. Johnstone. 1985. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 101:942-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pizzirani, C., D. Ferrari, P. Chiozzi, E. Adinolfi, D. Sandona, E. Savaglio, and F. Di Virgilio. 2007. Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood 109:3856-3864. [DOI] [PubMed] [Google Scholar]
- 37.Qu, Y., L. Franchi, G. Nunez, and G. R. Dubyak. 2007. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J. Immunol. 179:1913-1925. [DOI] [PubMed] [Google Scholar]
- 38.Qu, Y., L. Ramachandra, S. Mohr, L. Franchi, C. V. Harding, G. Nunez, and G. R. Dubyak. 2009. P2X7 receptor-stimulated secretion of MHC-II-containing exosomes requires the ASC/NLRP3 inflammasome but is independent of caspase-1. J. Immunol. 182:5052-5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramachandra, L., E. Noss, W. H. Boom, and C. V. Harding. 2001. Processing of Mycobacterium tuberculosis antigen 85B involves intraphagosomal formation of peptide-major histocompatibility complex II complexes and is inhibited by live bacilli that decrease phagosome maturation. J. Exp. Med. 194:1421-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raposo, G., H. W. Nijman, W. Stoorvogel, R. Liejendekker, C. V. Harding, C. J. Melief, and H. J. Geuze. 1996. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 183:1161-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saunders, B. M., S. L. Fernando, R. Sluyter, W. J. Britton, and J. S. Wiley. 2003. A loss-of-function polymorphism in the human P2X7 receptor abolishes ATP-mediated killing of mycobacteria. J. Immunol. 171:5442-5446. [DOI] [PubMed] [Google Scholar]
- 42.Schorey, J. S., and S. Bhatnagar. 2008. Exosome function: from tumor immunology to pathogen biology. Traffic 9:871-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segura, E., C. Nicco, B. Lombard, P. Veron, G. Raposo, F. Batteux, S. Amigorena, and C. Thery. 2005. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood 106:216-223. [DOI] [PubMed] [Google Scholar]
- 44.Shemon, A. N., R. Sluyter, S. L. Fernando, A. L. Clarke, L. P. Dao-Ung, K. K. Skarratt, B. M. Saunders, K. S. Tan, B. J. Gu, S. J. Fuller, W. J. Britton, S. Petrou, and J. S. Wiley. 2006. A Thr357 to Ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. J. Biol. Chem. 281:2079-2086. [DOI] [PubMed] [Google Scholar]
- 45.Sikora, A., J. Liu, C. Brosnan, G. Buell, I. Chessel, and B. R. Bloom. 1999. Purinergic signaling regulates radical-mediated bacterial killing mechanisms in macrophages through a P2X7-independent mechanism. J. Immunol. 163:558-561. [PubMed] [Google Scholar]
- 46.Sklar, M. D., A. Tereba, B. D. Chen, and W. S. Walker. 1985. Transformation of mouse bone marrow cells by transfection with a human oncogene related to c-myc is associated with the endogenous production of macrophage colony stimulating factor 1. J. Cell. Physiol. 125:403-412. [DOI] [PubMed] [Google Scholar]
- 47.Tamura, T., H. Ariga, T. Kinashi, S. Uehara, T. Kikuchi, M. Nakada, T. Tokunaga, W. Xu, A. Kariyone, T. Saito, T. Kitamura, G. Maxwell, S. Takaki, and K. Takatsu. 2004. The role of antigenic peptide in CD4+ T helper phenotype development in a T cell receptor transgenic model. Int. Immunol. 16:1691-1699. [DOI] [PubMed] [Google Scholar]
- 48.Thery, C., L. Duban, E. Segura, P. Veron, O. Lantz, and S. Amigorena. 2002. Indirect activation of naive CD4(+) T cells by dendritic cell-derived exosomes. Nat. Immunol. 3:1156-1162. [DOI] [PubMed] [Google Scholar]
- 49.Thery, C., L. Zitvogel, and S. Amigorena. 2002. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2:569-579. [DOI] [PubMed] [Google Scholar]
- 50.Trautmann, A. 2009. Extracellular ATP in the immune system: more than just a “danger signal.” Sci. Signal. 2:pe6. [DOI] [PubMed] [Google Scholar]
- 51.Verhoef, P. A., M. Estacion, W. Schilling, and G. R. Dubyak. 2003. P2X7 receptor-dependent blebbing and the activation of Rho-effector kinases, caspases, and IL-1 beta release. J. Immunol. 170:5728-5738. [DOI] [PubMed] [Google Scholar]
- 52.Vincent-Schneider, H., P. Stumptner-Cuvelette, D. Lankar, S. Pain, G. Raposo, P. Benaroch, and C. Bonnerot. 2002. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int. Immunol. 14:713-722. [DOI] [PubMed] [Google Scholar]
- 53.Zitvogel, L., A. Regnault, A. Lozier, J. Wolfers, C. Flament, D. Tenza, P. Ricciardi-Castagnoli, G. Raposo, and S. Amigorena. 1998. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat. Med. 4:594-600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.