Abstract
The human airway epithelium is constantly exposed to microbial products from colonizing organisms. Regulation of Toll-like receptor (TLR) expression and specific interactions with bacterial ligands is thought to mitigate exacerbation of inflammatory processes induced by the commensal flora in these cells. The genus Neisseria comprises pathogenic and commensal organisms that colonize the human nasopharynx. Neisseria lactamica is not associated with disease, but N. meningitidis occasionally invades the host, causing meningococcal disease and septicemia. Upon colonization of the airway epithelium, specific host cell receptors interact with numerous Neisseria components, including the PorB porin, at the immediate bacterial-host cell interface. This major outer membrane protein is expressed by all Neisseria strains, regardless of pathogenicity, but its amino acid sequence varies among strains, particularly in the surface-exposed regions. The interaction of Neisseria PorB with TLR2 is essential for driving TLR2/TLR1-dependent cellular responses and is thought to occur via the porin's surface-exposed loop regions. Our studies show that N. lactamica PorB is a TLR2 ligand but its binding specificity for TLR2 is different from that of meningococcal PorB. Furthermore, N. lactamica PorB is a poor inducer of proinflammatory mediators and of TLR2 expression in human airway epithelial cells. These effects are reproduced by whole N. lactamica organisms. Since the responsiveness of human airway epithelial cells to colonizing bacteria is in part regulated via TLR2 expression and signaling, commensal organisms such as N. lactamica would benefit from expressing a product that induces low TLR2-dependent local inflammation, likely delaying or avoiding clearance by the host.
The human respiratory epithelium is the first interface with airborne pathogens. Airway epithelial cells are protected by mucin that shields them from direct contact with microorganisms but are susceptible to activation by bacterial components from colonizing organisms. Upon activation, they produce antimicrobial molecules, proinflammatory cytokines, and chemokines for recruitment of immune cells to the airway epithelium via pattern recognition receptors (PRRs). These PRRs recognize conserved structural motifs expressed by microbial pathogens or PAMPs (pathogen-associated molecular patterns) (9, 26, 30). Among the PRRs, Toll-like receptors (TLRs) induce host innate immune responses and enhance adaptive immune responses to a variety of organisms (27). Although human airway epithelial cells express TLRs 1 to 10 (13, 36, 38), under physiological conditions they express lower levels of TLR2 than immune cells, express intracellular TLR4, and often lack necessary coreceptors for TLR signaling, such as MD-2 or CD36 (3, 5, 14, 16, 26, 33, 49). It has been proposed that this is a strategy to control local defense mechanisms and mitigate exacerbation of tissue inflammation induced by commensal organisms. Low TLR2 expression coincides with reduced responsiveness toward some TLR2 ligands (26, 28), and a correlation between the amount of TLR2 produced and of inflammatory mediators secreted, as well as phagocytosis of pathogens, has been established (1, 9, 36). TLR2 expression is upregulated under inflammatory conditions and by pathogen infection (35, 38).
Neisseria spp., a group of closely related Gram-negative bacteria, comprises pathogenic and nonpathogenic species commonly carried in the human upper respiratory tract (approximately 15% in the adult population) (7, 50). While reports of systemic infections due to N. lactamica are very rare (6, 12, 33), meningococcal strains can invade the host and cause meningitis and septicemia (41, 50), via the concerted action of a number of bacterial factors, i.e., type 4 pili (Tfp), Opa and Opc proteins, lipooligosaccharide (LOS), capsule, NMB1966, and porins (4, 10, 18, 19, 21, 32, 40, 46, 47, 48). All Neisseria organisms express the PorB porin, a trimeric protein with a β-barrel conformation and eight surface-exposed loops (8). PorB is a TLR2 agonist that activates cells via a TLR2/TLR1-dependent signaling pathway (22-24). Recently, it has been hypothesized that the interaction between PorB and TLR2 occurs through a ring of positively charged residues on the porin's surface-exposed regions and opposing negatively charged residues on the TLR2 ectodomain (43). However, PorB proteins from different Neisseria strains possess regions of high amino acid sequence variability, concentrated in the surface-exposed loops (8). Depending on the nature of the amino acid sequence diversity, this might affect the TLR2-specific interaction of different PorB molecules and their subsequent ability to induce TLR2-dependent cell activation.
The purpose of this study is to examine the effect of purified N. lactamica PorB and whole N. lactamica on induction of proinflammatory responses in human airway epithelial cells via TLR2-dependent interaction and signaling.
MATERIALS AND METHODS
Cell lines and culture conditions.
Adenovirus-12 simian virus 40 (SV40) hybrid virus-transformed, nontumorigenic human bronchial epithelial cells (BEAS-2B) (ATCC CRL-9609) were grown at 37°C/5% CO2 in Dulbecco modified Eagle medium (DMEM) F-12 supplemented with 5% fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin in flasks coated with 0.01 mg/ml bovine serum albumin (BSA), 0.03 mg/ml bovine collagen type I, and 0.01 mg/ml fibronectin. Prior to stimulation, BEAS-2B cells were serum starved overnight, followed by stimulation in FBS-free medium, unless otherwise specified. Cells of nasopharyngeal epithelial cell line Detroit 562 (ATCC CCL-138) were grown in MEM medium containing 5% FBS. Experiments were restricted to cells passaged 30 times from frozen stocks. Transfected HEK cells overexpressing TLR2 (24) were grown in DMEM with 5% FBS, 2 mM l-glutamine, and 10 μg/ml ciprofloxacin.
Bacterial strains and growth conditions.
N. lactamica strain Y92-1009 (ND:P1.ND,ND:F-ND:ST-3493, ST-613) (34) and strain 1341 (29) and N. meningitidis parent strain H44/76 (B:15;P1.7,16; L3,7,9, ST-32) (15), the PorA− and Rmp− variant (H44/76 Δ1Δ4) (45), and strain MC58 (B: P1.7,16-2: F1-5: ST-74 (cc32) (44) were subcultured from frozen stock on chocolate agar plates at 37°C for 16 to 18 h in 5% CO2. Single colonies were resuspended in GC medium containing Isovitalex and grown to exponential growth phase. Liquid cultures were adjusted to known concentrations (optical density at 660 nm [OD660] of 0.1 = 108 CFU per milliliter) prior to use. For some experiments, organisms were heat killed at 65°C for 1 h.
Porins and reagents.
Porins were purified from liquid cultures of N. lactamica Y92-1002 and N. meningitidis H44/76 Δ1Δ4 as previously described (25). For some experiments, N. lactamica PorB was labeled with the fluorescent dye Alexa Fluor-594 (Molecular Probes) or with biotin (24). Pam3CSK4 was purchased from EMC Microcollections (Tubingen, Germany); Escherichia coli lipopolysaccharide (LPS) (Sigma) was subjected to phenol extraction for removal of potentially contaminating lipopeptide. Anti-human-TLR2 blocking antibody clone TL2.1 was a kind gift from Egil Lien, University of Massachusetts (20). Soluble TLR2:Fc and TLR4:Fc were obtained as previously described (49).
Cytokine ELISA.
Production of interleukin 8 (IL-8), tumor necrosis factor α (TNF-α), and IL-6 was examined in supernatants of BEAS-2B cells (105/ml) incubated with live or heat-killed organisms (at a multiplicity of infection [MOI] of 10 bacteria/cell), N. lactamica PorB (0.1 to 10 μg/ml), N. meningitidis PorB (10 μg/ml), Pam3CSK4, and LPS (100 ng/ml) using an OptEIA enzyme-linked immunosorbent assay (ELISA) kit (BD Biosciences). LPS incubation was performed with or without FBS as specified in the text. For TLR2 blocking experiments, cells were pretreated with 10 μg/ml of human anti-TLR2 antibody (20) for 30 min at room temperature prior to stimulation.
Assessment of TLR surface expression.
BEAS-2B cells (105/ml) were incubated with live or heat-killed organisms (MOI of 10 bacteria/cell), N. lactamica PorB, N. meningitidis PorB, Pam3CSK4, or E. coli LPS as described above. TLR2, TLR1, and TLR4 expression was determined with fluorescein-5-isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated anti-human TLR2 (clone TL2.1), TLR1 (clone GD2.F4) and TLR4 (clone HTA125) monoclonal antibodies (eBiosciences) by flow cytometry with a FACScan flow cytometer with gating to exclude cell debris associated with necrosis. PE- and FITC-labeled isotype antibodies were used as controls.
RT-PCR.
BEAS-2B cells (5 × 105/ml) were incubated as described above, and total mRNA was extracted using the Rneasy MiniKit and reverse transcriptase (RT) kit (Qiagen). The cDNA was amplified by PCR with specific primers for TLR1 (5′-ACCAAGTTGTCAGCGATGTGTT-3′, 3′-GATTGTCCCCTGCTTTTATTGA5′), TLR2 (5′-GAGTGAGTGGTGCAAGTATTGAAC-3′, 3′-GGGCCACTCCAGGTAGGTCT-5′), IL-8 (5′-CATGACTTCCAAGCTGGCCGTG-3′, 3′-GAGACACCATAGCTTCTTAGTCACT-5′), and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (5′-TCCATGACAACTTTGGTATCGTG-3′, 3′-AGGAGACTGAAGTTGTGCTGT-5′). The PCRs were carried out as follows: TLR1 and TLR2, 50°C for 30 min, 94°C for 1 min, 54°C for 1 min, and 72°C for 1 min for 30 cycles; IL-8 and GAPDH, 50°C for 30 min, 94°C for 1 min, 57°C for 1 min, and 72°C for 1 min for 30 cycles. The PCR products were analyzed on 1.2% agarose gels.
N. lactamica PorB binding assays.
A flow cytometry-based cell binding assay was used to determine binding of fluorescent N. lactamica PorB to the surface of BEAS-2B cells (106/ml, 100 μl/well plated in U-bottom 96-well plates) as previously described (24). Binding inhibition studies were performed using either a fixed excess amount (100 μg/ml) or increasing concentrations (0.2 to 50 μg/ml) of unlabeled competitors. The cells were analyzed with a FACScan flow cytometer with gating to exclude cell debris associated with necrosis. Cell-associated fluorescence is expressed as the mean fluorescence intensity (MFI) of triplicate wells from 10 experiments. For in vitro binding studies, a soluble TLR2:Fc chimera was used as previously described (24). The A450 of duplicate wells from triplicate experiments was measured as average readings ± standard deviation (SD). The binding affinity constant, Kd, was calculated empirically according to the law of mass action, which predicts the fractional receptor occupancy at equilibrium as a function of the ligand concentration in moles/liter.
Statistical analysis.
Statistical analysis and ligand-receptor binding parameters were calculated using GraphPad PRISM software.
RESULTS
IL-8 induction by purified N. lactamica PorB in human airway epithelial cells.
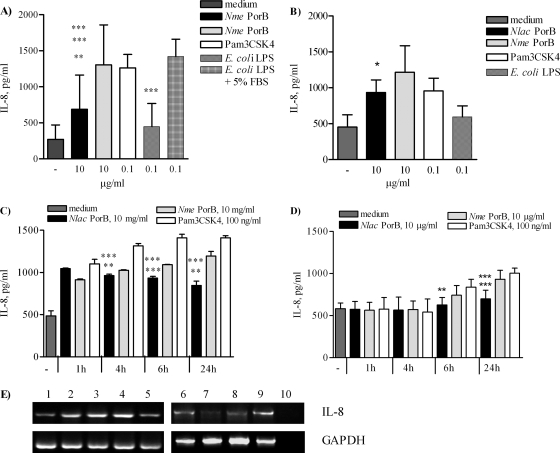
Previous work has characterized meningococcal PorB as a TLR2 agonist (22-24). The effect of PorB purified from the commensal Neisseria lactamica strain Y92-1009 (22, 34) (0.1, 1, and 10 μg/ml) was examined using BEAS-2B human airway epithelial cells and Detroit 562 cells (105/ml). IL-8 induction after 24 h of incubation in FBS-free culture medium was measured by ELISA of cell supernatants and was found to be dose dependent (not shown). The effect of N. lactamica PorB (10 μg/ml) incubation for 24 h was compared to those of other TLR2 and TLR4 ligands, such as N. meningitidis PorB (10 μg/ml), Pam3CSK4 (100 ng/ml), and LPS (100 ng/ml). N. lactamica PorB induced significantly lower IL-8 secretion than N. meningitidis PorB in BEAS-2B cells (Fig. 1A, black bar and gray bars, respectively; **, P = 0.008 by unpaired t test) and in Detroit 562 cells (Fig. 1B, black and gray bars, respectively; *, P = 0.026). In BEAS-2B cells, N. lactamica PorB induced lower IL-8 production than Pam3CSK4 (Fig. 1A, white bar; ***, P = 0.0008) while it was comparable in Detroit 562 cells (Fig. 1B, white bar). BEAS-2B cells lack expression of MD-2 and are hyporesponsive to LPS in FBS-free culture conditions (14, 15, 25, 33, 37, 49); under these conditions, minimal IL-8 production was induced by phenol-extracted E. coli LPS (100 ng/ml) (Fig. 1A, striped bar). However, in the presence of 5% FBS (a source of soluble MD-2), IL-8 induced by LPS (Fig. 1A, dashed bar) was significantly higher than that induced by N. lactamica PorB and by LPS in the absence of FBS (***, P = 0.0005 and ***, P < 0.0001, respectively). LPS induced low IL-8 secretion in Detroit 562 cells (Fig. 1B, dashed bar) despite the presence of FBS in the culture medium throughout the duration of the stimulation.
FIG. 1.
Induction of IL-8 by N. lactamica PorB. BEAS-2B cells (A) and Detroit 562 cells (B) incubated for 24 h with 10 μg/ml of N. lactamica PorB, 10 μg/ml of N. meningitidis PorB, 100 ng/ml of Pam3CSK4, and 100 ng/ml of phenol-extracted E. coli LPS in the absence or in the presence of FBS. For BEAS-2B cells, **, P = 0.008, ***, P = 0.0008, ***, P = 0.0005, and ***, P < 0.0001 by unpaired t test. For Detroit 562 cells, *, P = 0.026. (C) BEAS-2B cells incubated for 1 h, 4 h, 6 h, and 24 h with N. lactamica PorB, N. meningitidis PorB, and Pam3CSK4 as described above. **, P = 0.0044, ***, P = 0.0002, **, P = 0.0013 and ***, P < 0.0001 by unpaired t test. (D) Detroit 562 cells incubated as described above. ***, P = 0.0002, **, P = 0.0025, and ***, P = 0.0004. The results represent the average results of triplicate wells from a minimum of three independent experiments ± SD. (E) IL-8 mRNA expression in response to N. meningitidis PorB for 6 h and 24 h (lanes 2 and 3, respectively), N. lactamica PorB for 6 h and 24 h (lanes 4 and 5, respectively), Pam3CSK4 for 6 h and 24 h (lanes 6 and 7, respectively) in the absence of FBS and LPS plus FBS for 6 h and 24 h (lanes 8 and 9, respectively). Lane 1, medium control, 24 h; lane 10, no RNA. GAPDH, loading control.
Analysis of the temporal induction of IL-8 by N. lactamica PorB (1 h, 4 h, 6 h, and 24 h) in BEAS-2B cells shows that this is an early-induced event that decreases over time (Fig. 1C, black bars). In contrast, N. meningitidis PorB and Pam3CSK4 induced a time-dependent increase of IL-8 secretion (Fig. 1C, gray bars and white bars, respectively). The level of N. lactamica PorB-induced IL-8 was significantly lower than that induced by N. meningitidis PorB at 4 h, 6 h and 24 h (**, P = 0.0044, ***, P = 0.0002, and **, P = 0.0013, respectively) and by Pam3CSK4 at the same time points (***, P < 0.0001 by unpaired t test). IL-8 induced by Pam3CSK4 peaked at 6 h and remained elevated at 24 h (Fig. 1C, white bars) similarly to E. coli LPS in the presence of FBS (not shown). In Detroit 562 cells, increase of basal IL-8 secretion was not detected before 6 h of incubation. N. lactamica PorB induced significantly lower levels of IL-8 than N. meningitidis PorB (Fig. 1D, black bar and gray bar; **, P = 0.0025) and Pam3CSK4 (Fig. 1D, white bar; **, P = 0.0025) at this time point as well as at 24 h (***, P = 0.0002; ***, P = 0.0004). These results suggest that N. lactamica PorB is a low inducer of IL-8 secretion in human airway epithelial cells.
To examine whether this is due to transcriptional regulation of the IL-8 gene, IL-8 mRNA was examined by RT-PCR in BEAS-2B cells. N. lactamica PorB incubation for 6 h increased IL-8 mRNA levels, but this was followed by a decrease after 24 h of incubation (Fig. 1E, lanes 4 and 5, respectively), consistent with IL-8 protein production. Although IL-8 protein secretion induced by Pam3CSK4 was sustained over time, a decrease of IL-8 mRNA at 24 h was also detected (Fig. 1E, lanes 6 and 7), as previously shown in other studies using these cells (2). N. meningitidis PorB (Fig. 1E, lanes 2 and 3) and LPS with FBS (Fig. 1E, lanes 8 and 9) induced increased IL-8 mRNA at 6 h and 24 h. Minimal IL-8 mRNA expression was induced by LPS without FBS (not shown). GAPDH was used as a loading control. These results correlate the kinetics of IL-8 protein secretion induced by N. lactamica PorB with decreased IL-8 mRNA production.
IL-8 induction by N. lactamica PorB is TLR2 dependent.
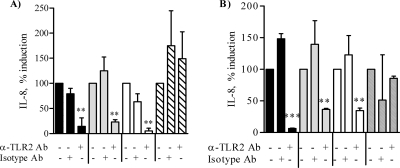
Proinflammatory cytokine secretion by airway epithelial cells is dependent on signaling via TLR2 (2, 27, 28, 36). The role of TLR2 in IL-8 production induced by N. lactamica PorB in BEAS-2B cells and Detroit 562 cells was examined by ELISA using a human anti-TLR2 blocking antibody (clone TL2.1) (20). A reduction of IL-8 of approximately 80% was observed by blocking TLR2 on the surface of BEAS-2B cells (Fig. 2A, black bars; **, P = 0.0047 by unpaired t test) and of Detroit 562 cells (Fig. 2 B, black bars; ***, P < 0.0001) compared to results with an isotype control antibody (Fig. 2A and B). Similar results were observed with N. meningitidis PorB (Fig. 2A and B, gray bars; **, P = 0.003 and **, P = 0.0088, respectively) and with Pam3CSK4 (Fig. 2A and B, white bars; **, P = 0.004 and **, P = 0.0078, respectively). Blocking of TLR2 had no effect on LPS stimulation in the presence of FBS (Fig. 2A and B, dashed bars). The results are expressed as percentages of IL-8 induction. Collectively, these results demonstrate that IL-8 secretion induced by N. lactamica PorB in human airway epithelial cells is TLR2 dependent.
FIG. 2.
TLR2-dependent IL-8 induction. BEAS-2B cells (A) and Detroit 562 cells (B) were incubated with human anti-TLR2 blocking antibody (10 μg/ml) or with isotype control antibody (10 μg/ml) for 30 min at room temperature, followed by addition of 10 μg/ml of N. lactamica PorB (black bars), 10 μg/ml of N. meningitidis PorB (gray bars), 100 ng/ml of Pam3CSK4 (white bars), or 100 ng/ml of LPS with FBS (dashed bars) for a further 24 h. IL-8 production was examined by ELISA of culture cell supernatants. The results are expressed as percent IL-8 induction and represent the average ± SD of results for triplicate wells from two independent experiments. For BEAS-2B cells, **, P < 0.0047, **, P = 0.003, and **, P = 0.004; for Detroit 562 cells, ***, P < 0.0001, **, P = 0.0088 and **, P = 0.0078 by unpaired t test.
TLR2 mRNA and protein expression by N. lactamica PorB.
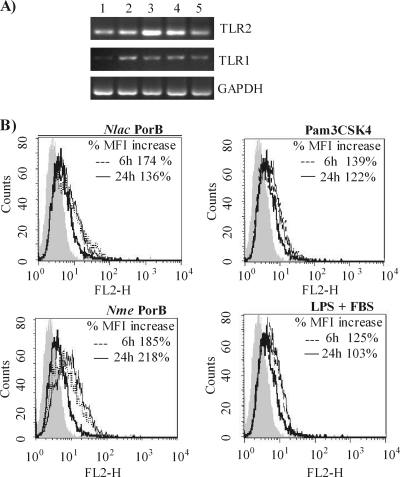
To examine whether the low IL-8 response induced by N. lactamica PorB in human airway epithelial cells results from regulation of TLR2 expression, both TLR2 mRNA and protein surface expression were measured in BEAS-2B cells. Cells were incubated with N. lactamica PorB or N. meningitidis PorB (10 μg/ml) for 6 h and 24 h, and TLR2 mRNA levels were examined by RT-PCR. N. lactamica PorB induced increased TLR2 mRNA at 6 h, which was followed by a decrease at 24 h (Fig. 3A, lanes 4 and 5). In contrast, N. meningitidis PorB induced increased TLR2 mRNA levels at both time points (Fig. 3A, lanes 2 and 3). GAPDH mRNA was used as a loading control. None of these stimuli had a significant effect on TLR1 mRNA production in BEAS-2B cells at the time points examined (Fig. 3A). Similar to N. meningitidis PorB, Pam3CSK4 and LPS (the latter in the presence of FBS) induced upregulation of TLR2 mRNA at both 6 h and 24 h (not shown).
FIG. 3.
TLR2 induction by N. lactamica (Nlac) PorB. (A) RT-PCR of TLR2 and TLR1 mRNA levels induced in BEAS-2B cells incubated with medium (lane 1), 10 μg/ml of N. meningitidis (Nme) PorB for 6 h and 24 h (lanes 2 and 3, respectively), and 10 μg/ml of N. lactamica PorB for 6 h and 24 h (lanes 4 and 5, respectively). GAPDH was used as a loading control. (B) Flow cytometry of TLR2 surface expression of BEAS-2B cells incubated as described above. The isotype control antibody is represented by the gray area; unstimulated cells are represented by the thin line, 6 h stimulation is represented by the dotted line; 24 h stimulation is represented by the thick line. The percent increase in MFI relative to that of cells incubated with medium alone is shown in the insets.
To determine whether regulation of TLR2 mRNA was accompanied by TLR2 surface protein expression, flow cytometry analysis was performed, and representative histograms are shown in Fig. 3B. Basal expression of TLR2 in BEAS-2B is indicated by the thick line in all the histograms, and the isotype control antibody is indicated by the gray area. Incubation with N. lactamica PorB for 6 h induced TLR2 surface expression (Fig. 3B, dotted line) which did not further increase at 24 h (Fig. 3B, thin line). The percent increase in MFI relative to that of cells incubated with medium alone is shown. Similar results were obtained with Pam3CSK4 for 6 h and 24 h (Fig. 3B, dotted line and thin line, respectively) and LPS with FBS for 6 h and 24 h (Fig. 3B, dotted line and thin line, respectively). No substantial TLR2 surface expression was induced by LPS without FBS (not shown). In contrast, N. meningitidis PorB induced higher levels of TLR2 surface protein expression at 6 h (Fig. 3B, dotted line) which was sustained at 24 h (Fig. 3B, thin line). None of the stimuli affected TLR1 surface expression (not shown), and a variable upregulation of TLR4 expression was detected (not shown). However, it is unlikely that TLR4 contributes to BEAS-2B cell activation by N. lactamica PorB, N. meningitidis PorB, or Pam3CSK4, since BEAS-2B cells lack expression of MD-2 (26), a necessary coreceptor for TLR4 signaling. In fact, our results show that FBS (as a soluble source of MD-2) is not required for the effect of N. lactamica PorB or N. meningitidis PorB in these cells. Furthermore, neither neisserial porins nor Pam3CSK4 is known to signal via TLR4. Collectively, these results show that N. lactamica PorB induces a low, transient upregulation of TLR2 expression in BEAS-2B cells, likely contributing to the observed low levels of cell activation.
N. lactamica PorB binds to TLR2.
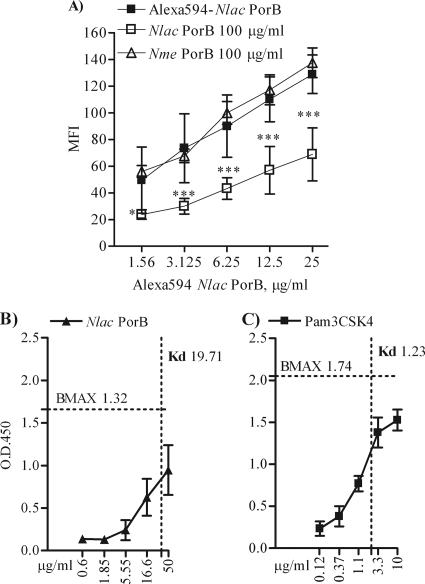
Our previous work has demonstrated the direct binding of N. meningitidis PorB TLR2 (24), but the interaction of N. lactamica PorB with TLR2 has not been characterized so far. To investigate whether N. lactamica PorB is also a TLR2 ligand, BEAS-2B cells (106/ml; 100 μl) were incubated for 1 h at 0°C with increasing concentrations of N. lactamica PorB labeled with the red fluorochrome Alexa Fluor-594, followed by quantification of cell-associated fluorescence by flow cytometry (24). N. lactamica PorB bound to the surface of BEAS-2B cells in a dose-dependent manner (Table 1). To determine whether N. lactamica PorB binding was dependent on TLR2, a fixed concentration of fluorescent N. lactamica PorB (12.5 μg/ml) was coincubated with increasing concentrations of anti-human TLR2 blocking antibody or with an isotype control antibody. As shown in Table 2, blocking TLR2 on the surface of BEAS-2B cells inhibited N. lactamica PorB cell association. The isotype control antibody failed to block N. lactamica PorB interaction with the cell surface (not shown). Fluorescent N. lactamica PorB binding was also subject to homologous competition by unlabeled N. lactamica PorB in a dose-dependent manner (Table 2). Dose-dependent homologous competition was also observed when increasing concentrations of fluorescent N. lactamica PorB (Fig. 4A, closed squares) were coincubated with an excess amount of unlabeled N. lactamica PorB (100 μg/ml) (Fig. 4A, open squares; ***, P = 0.0002 and *, P = 0.05 by unpaired t test). These results show that binding of N. lactamica PorB to BEAS-2B cells is specific and TLR2 dependent. In addition, N. lactamica PorB binding to TLR2 was also confirmed in a HEK cell TLR2-overexpression system (not shown), previously used to characterize TLR2-N. meningitidis PorB interactions (49). However, N. lactamica PorB binding was not inhibited by excess amount of unlabeled N. meningitidis PorB in either BEAS-2B cells (Fig. 4A, open triangles) or HEK cells overexpressing TLR2 (not shown).
TABLE 1.
N. lactamica PorB binding to BEAS-2B cells
| Nlaca PorB (μg/ml) | MFI ± SD |
|---|---|
| 200 | 165.2 ± 19 |
| 150 | 163.3 ± 16.7 |
| 100 | 164.5 ± 11.7 |
| 50 | 153.1 ± 12.9 |
| 25 | 128.9 ± 14.4 |
| 12.5 | 110.1 ± 16.8 |
| 6.25 | 90 ± 23.3 |
| 3.12 | 73.5 ± 25.8 |
| 1.6 | 49.5 ± 24.6 |
| 0 | 13.5 ± 3.9 |
Nlac, N. lactamica.
TABLE 2.
Alexa Fluor-594-N. lactamica PorB (12.5 μg/ml) binding competition
| Competitor (μg/ml) | MFI ± SDa |
|
|---|---|---|
| α-TLR2 | Nlac PorB | |
| 100 | 67.3 ± 11.6*** | |
| 50 | 79.5 ± 2.8*** | 78.4 ± 8.6*** |
| 25 | 89.8 ± 1.5*** | 95.1 ± 7.1*** |
| 12.5 | 97.3 ± 2.5** | 102 ± 3.44* |
| 6.25 | 103.1 ± 0.2* | 112 ± 8.1 |
| 3.12 | 112.8 ± 5.1 | 122 ± 6.2 |
| 0 | 110 ± 16.8 | 110 ± 16.8 |
***, P < 0.0001, **, P < 0.005 and *, P < 0.05 by unpaired t test with Welch correction.
FIG. 4.
N. lactamica PorB binding to TLR2. (A) Dose-dependent binding of fluorescent N. lactamica PorB to the surface of BEAS-2B cells (106/ml, 100 μl) measured by flow cytometry (closed squares) is inhibited by coincubation with an excess amount of unlabeled N. lactamica PorB (100 μg/ml, open squares) ***, P = 0.0002 and *, P = 0.05 by unpaired t test. Binding of N. lactamica PorB is not inhibited by excess amount of N. meningitidis PorB (open triangles). Binding affinities (Kd) of N. lactamica PorB (B) and PamCSK4 (C) for TLR2 in vitro measured by modified ELISA. Plated N. lactamica PorB (0.6 up to 50 μg/ml) and Pam3CSK4 (0.12 up to 10 μg/ml) were incubated with 2 μg/ml of soluble TLR2:Fc chimera. Specific binding is detected via the Fc tag using horseradish peroxidase (HRP)-conjugated anti-mouse IgG in duplicate wells from triplicate experiments and is expressed as average A450 ± SD. BMAX and Kd are calculated according to the law of mass action.
To determine the affinity of N. lactamica PorB for TLR2, a recombinant, soluble TLR2:Fc chimera (2 μg/ml) was used in an ELISA-like assay as previously described (49). A Kd of approximately 19 nM was calculated for N. lactamica PorB and approximately 1.2 nM for Pam3CSK4, according to the law of mass action (Fig. 4B and C). N. lactamica PorB binding affinity for TLR2 is approximately 2-fold lower than that previously shown for N. meningitidis PorB (24), suggesting a potentially different mechanism of interaction with TLR2. Soluble TLR4:Fc chimera was used as a negative control and did not show binding to PorB or Pam3SCK4 (not shown).
Induction of cytokines by N. lactamica organisms.
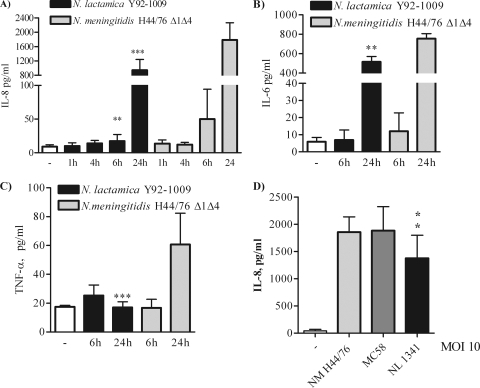
To examine whether the low TLR2-dependent IL-8 production induced by N. lactamica PorB may reflect a similar effect by N. lactamica, BEAS-2B cells were incubated with live organisms (MOI of 10 bacteria/cell) for up to 24 h. Since the meningococcal PorB and the N. lactamica PorB used in our study are purified from the N. meningitidis H44/76 Δ1Δ4 derivative strain (lacking PorA and Rmp [45]) and from the N. lactamica Y92-1009 strain (34), these strains were used in the following studies. Measurement of IL-8 in the coculture supernatants showed that live N. lactamica Y92-1009 induced low IL-8 secretion at 1 h, 4 h, and 6 h, followed by an increase at 24 h (Fig. 5A, black bars). However, compared to results with live N. meningitidis H44/76 Δ1Δ4, the levels of IL-8 induced by N. lactamica at 6 h and 24 h were significantly lower (Fig. 5A, gray bars; **, P = 0.0071 and ***, P < 0.0001 by unpaired t test). Similarly, live N. lactamica Y92-1009 induced lower levels of production of IL-6 (Fig. 5B, 24 h; **, P = 0.0053 by unpaired t test) and of TNF-α (Fig. 5C, 24 h; ***, P < 0.0001) compared to those of N. meningitidis H44/76 Δ1Δ4. These results show that N. lactamica is a poor inducer of proinflammatory mediators in human airway epithelial cells, similar to purified N. lactamica PorB. To expand the significance of these results to other Neisseria strains, the meningococcal strains H44/76 (parent) and MC58 (44) and the N. lactamica strain 1341 (29) were also tested. Although all Neisseria strains examined induce comparable levels of IL-8 production in a TLR2 and TLR4 overexpression HEK cell system (not shown), N. lactamica consistently induced lower IL-8 production in BEAS-2B cells than did meningococcal strains (Fig. 5D).
FIG. 5.
Induction of proinflammatory cytokines by N. lactamica. BEAS-2B cells were incubated with live N. lactamica Y92-1009 (black bars) and live N. meningitidis H44/76 Δ1Δ4 (gray bars) at an MOI of 10 bacteria/cell for 1 h, 4 h, 6 h, and 24 h, and cytokines were measured by ELISA of cell coculture supernatants. The results are expressed as pg/ml and are relative to results from triplicate wells from three independent experiments. For IL-8, ***, P = 0.0071 and P < 0.0001 (A), for IL-6, **, P = 0.0053 (B), and for TNF-α, ***, P < 0.0001 (C) by unpaired t test. (D) IL-8 induction by N. meningitidis strain H44/76 (parent) (gray bar), strain MC58 (dark gray bar) and N. lactamica strain 1341 (black bar). *, P = 0.02 and *, P = 0.04 by unpaired t test.
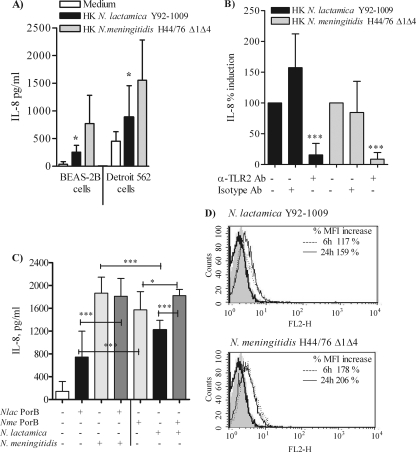
Analysis of bacterial growth curves showed comparable replication rates for all these organisms (not shown). However, variations of bacterial cell numbers due to different survival rates in airway epithelial cell cocultures might cause the observed reduced cytokine production by N. lactamica. Thus, heat-killed (HK) N. meningitidis H44/76 Δ1Δ4 and N. lactamica Y92-1009 strains (MOI of 10 bacteria/cell) were incubated with BEAS-2B cells and Detroit 562 cells for 24 h. HK N. lactamica consistently induced lower levels of IL-8 secretion than HK N. meningitidis in both cell types (Fig. 6A, black bars and gray bars, respectively; *, P = 0.024 and *, P = 0.026 by unpaired t test). Blockade of TLR2 in BEAS-2B cells decreased levels of IL-8 induced by HK N. lactamica as well as HK N. meningitidis (Fig. 6B, black bars and gray bars, respectively; ***, P < 0.0002 by Mann-Whitney test), in agreement with the previous results with purified N. lactamica PorB and N. meningitidis PorB.
FIG. 6.
Effect of heat-killed and live Neisseria organisms on IL-8 secretion and TLR2 expression. (A) BEAS-2B cells and Detroit 562 cells were incubated with heat-killed (HK) N. lactamica Y92-1009 and HK N. meningitidis H44/76 Δ1Δ4 at an MOI of 10 bacteria/cell for 24 h, and IL-8 was measured by ELISA of cell coculture supernatants. The results are expressed as pg/ml and are relative to results from triplicate wells from three independent experiments. For BEAS-2B cells, *, P = 0.024, and for Detroit 562 cells, *, P = 0.026 by unpaired t test. (B) BEAS-2B cells were incubated with HK neisserial organisms (MOI of 10) in the presence of human anti-TLR2 blocking antibody (10 μg/ml) or isotype control antibody (10 μg/ml) for 24 h. IL-8 secretion was measured in the coculture supernatants by ELISA. The results are expressed as percent IL-8 induction and represent the average ± SD of results from triplicate wells from two independent experiments. ***, P < 0.0002 by Mann-Whitney test. (C) BEAS-2B cells were incubated for 24 h as follows. (Left four bars) White bar, control; black bar, 10 μg/ml of N. lactamica PorB alone; light gray bar and dark gray bar, N. meningitidis H44/76 Δ1Δ4 (MOI of 10 bacteria/cell) alone or coincubated with N. lactamica PorB and N. meningitidis, respectively, for 24 h. ***, P < 0.0001 by unpaired t test. (Right three bars) Light gray bar, 10 μg/ml of N. meningitidis PorB alone; black bar and dark gray bar, N. lactamica Y92-1009 alone or coincubated with N. meningitidis PorB and N. lactamica. ***, P < 0.0001, and *, P = 0.04. For N. lactamica PorB (left, black bar) and N. meningitidis PorB (right, gray bar), ***, P = 0.0002; for N. lactamica (right, black bar) and N. meningitidis (left, gray bar), ***, P < 0.0001. IL-8 was measured in the coculture supernatants by ELISA of triplicate wells from three independent experiments. (D) Flow cytometry analysis of TLR2 surface expression induced by N. lactamica Y92-1009 (MOI of 10 bacteria/cell) and N. meningitidis H44-76 Δ1Δ4 (MOI of 10 bacteria/cell) for 6 h (dotted line) and 24 h (thin line) detected with a FITC-labeled anti-human TLR2 antibody. Basal expression of TLR2 is indicated by the thick line and isotype control antibody by the gray area. Gating was used to exclude dead cells and cell debris. The percent increase in MFI relative to that of cells incubated with medium alone is shown in the insets.
The effect of purified N. lactamica PorB on N. meningitidis-induced IL-8 was then examined. As shown in Fig. 6C, coincubation of BEAS-2B cells with N. lactamica PorB (10 μg/ml) and N. meningitidis H44/76 Δ1Δ4 (MOI of 10 bacteria/cell) (Fig. 6C, left, dark gray bar) induced IL-8 production comparable to that with N. meningitidis alone (Fig. 6C, left, light gray bar). This level was also significantly higher than that with N. lactamica PorB alone (Fig. 6C, left, black bar; ***, P < 0.0001 by unpaired t test), due to the effect of N. meningitidis organisms. In contrast, IL-8 production induced by coincubation with N. meningitidis PorB (10 μg/ml) and N. lactamica (MOI of 10 bacteria/cell) (Fig. 6C, right, dark gray bar) was significantly increased compared to that with N. lactamica alone (Fig. 6C, right, black bar; ***, P < 0.0001) as well as that with N. meningitidis PorB alone (Fig. 6C, right, light gray bar; *, P = 0.04 by unpaired t test), possibly due to an additive effect. As previously shown, the IL-8 level induced by N. lactamica PorB (Fig. 6C, left, black bar) was significantly lower than that induced by N. meningitidis PorB (Fig. 6C, right, gray bar) (***, P = 0.0002) and the IL-8 level induced by N. lactamica (Fig. 6C, right, black bar) was significantly lower than that induced by N. meningitidis (Fig. 6C, left, gray bar) (***, P < 0.0001).
Induction of TLR2 expression by N. lactamica organisms.
Having established that N. lactamica induces TLR2-dependent IL-8 production less efficiently than N. meningitidis in BEAS-2B cells, the organisms' effect on TLR2 protein expression was examined by flow cytometry. Representative histograms in Fig. 6E show basal expression of TLR2 in BEAS-2B as the thick line and the isotype control antibody as the gray area. Increased TLR2 expression was observed in response to live N. lactamica (MOI of 10 bacteria/cell) after 6 h (Fig. 6E, dotted line) and was slightly decreased at 24 h (Fig. 6E, thin line). Incubation with live N. meningitidis for the same lengths of time induced a visibly greater increase of TLR2 expression at 6 h (Fig. 6E, dotted line), which remained elevated at 24 h (Fig. 6E, thin line). The percent increase in MFI relative to that of cells incubated with medium alone is shown. Similar results were detected by RT-PCR (not shown). Neither organism induced surface upregulation of TLR1 (not shown) and only incubation with N. meningitidis for 24 h induced a minor downregulation of TLR4 surface expression (not shown).
DISCUSSION
Regardless of their commensal or pathogenic nature, bacteria possess motifs called pathogen-associated molecular patterns (PAMPs), such as methylated CpG DNA motifs, LPS, and lipopeptide. PAMP recognition by host-specific extracellular and intracellular pattern recognition receptors (PRRs), including Toll-like receptors (TLRs) (27), leads to cell activation and secretion of proinflammatory mediators. Obviously, epithelial surfaces that are often colonized by numerous commensal organisms (i.e., the airways or the gut) would be in a constant activated and inflamed state if they were to respond to stimulation by any colonizing bacteria. It has been proposed that cells from these epithelia have diverged from immune cells in terms of microbe recognition. Although they use the same PRRs, their expression and responses are differentially regulated (14, 26). For example, the gut epithelium lacks expression of TLR4 and is thus mostly unresponsive to LPS (5, 31). Airway epithelial cells express very low levels of TLR2 under physiological conditions (16), do not express CD36 (a TLR2 coreceptor for lipopeptide signaling [28]) and coreceptors necessary for TLR4 signaling (26, 33, 49), and are mostly hyporesponsive to Gram-positive bacteria and certain bacterial products. TLR2 expression is upregulated in these cells by pathogenic organisms and infection by-products, such as gamma interferon (IFN-γ) and TNF-α (16, 35, 38). As a consequence of signaling via TLRs, airway epithelial cells secrete a variety of inflammatory mediators (2), including the neutrophil chemoattractant chemokine IL-8, often used as a hallmark of cell activation. Recent reports have demonstrated that production of cytokines and chemokines is also differentially regulated in airway epithelial cells via TLR expression and signaling. For example, IL-8 mRNA expression induced by poly(I·C), a TLR3 ligand, and by Pam3CSK4, a TLR2 ligand, peaks early during stimulation and drops at later time points, while IL-8 protein secretion by these agonists increases in a time-dependent manner possibly due to secondary cytokine production (2, 28). Thus, low levels of TLR2 surface expression and a TLR2-mediated signaling in human airway epithelial cells provide an initial mechanistic explanation for their low inflammatory responses to commensal organism. Collectively, these observations stress the importance of TLR-mediated differential signaling in the human airway epithelium.
The human nasopharynx is the natural habitat for N. lactamica and N. meningitidis. While N. lactamica is not known to cause disease, meningococci can cause severe infections. Previously, we established that meningococcal PorB porin is a TLR2 ligand that induces cell activation via a TLR2- and MyD88-dependent mechanism (23, 24, 39). We have examined whether PorB purified from the commensal N. lactamica Y92-1009 strain (34) has similar properties and demonstrate that this protein induces lower levels of IL-8 secretion than N. meningitidis PorB in human airway epithelial cells. In addition, N. lactamica PorB-induced IL-8 peaks at 6 h and decreases at 24 h, contrary to the situation with N. meningitidis PorB and other TLR ligands (i.e., Pam3CSK4, LPS). Consistent with secreted IL-8, N. lactamica PorB induces increased IL-8 gene expression at 6 h followed by a decrease at 24 h. N. meningitidis PorB induces a sustained IL-8 mRNA production up to 24 h. Pam3CSK4 also induces a transient increase of IL-8 mRNA, which is consistent with previous studies of IL-8 posttranscriptional and/or posttranslational regulation in airway epithelial cells by this TLR2 agonist (26). Since IL-8 induction by N. lactamica PorB is greatly reduced by a blocking anti-TLR2 antibody TLR2, it is concluded that TLR2 is involved in N. lactamica PorB signaling in these cells. Based on the current literature on human airway epithelial cell activation and TLR2 (26, 28, 33), the effect of N. lactamica PorB on TLR2 expression was examined. As with IL-8 production, early upregulation of TLR2 surface expression and of TLR2 mRNA by N. lactamica PorB was observed, but this was not sustained at 24 h. Collectively, our results suggest that PorB purified from N. lactamica is a low inducer of human airway epithelial cell activation via TLR2.
It is known that N. meningitidis PorB directly interacts with TLR2 in vitro and on the cell surface (24). The interaction of N. lactamica PorB with TLR2 was then examined. Our results show that N. lactamica PorB binds to TLR2 on the surface of BEAS-2B cells in a dose-dependent manner. This is subject to homologous competition by unlabeled N. lactamica PorB and is also inhibited by a human anti-TLR2 blocking antibody, demonstrating that N. lactamica PorB is a TLR2 ligand. Surprisingly, we did not observe heterologous binding competition using unlabeled N. meningitidis PorB. This could indicate that N. lactamica PorB and N. meningitidis PorB differentially interact with TLR2. Our in vitro analysis of N. lactamica PorB-TLR2 binding kinetics utilizing a soluble chimeric TLR2 receptor (49) has revealed a Kd of approximately 20 nM for N. lactamica PorB. This is higher than what has been previously established for N. meningitidis PorB (∼10 nM) (24), suggesting a different affinity for TLR2. The mechanism(s) of PorB binding to TLR2 is not known. Recent work by Tanabe et al. (42, 43) has proposed a model of electrostatic interactions between negatively charged residues on TLR2 and positively charged residues on the surface-exposed loop regions of the porin. This modality of interaction differs from those of the TLR2-ligand interactions that have been characterized so far (i.e., Pam3CSK4 [17]), but it might explain the difference in N. lactamica PorB and N. meningitidis PorB binding specificities for TLR2. In fact, Neisseria porin amino acid sequence variations that convey differences in the charge of the regions that interact with TLR2 could influence the strength of TLR2 binding. This hypothesis is being examined by our group at a molecular level and is the focus of a separate study. The contribution and the importance of accessory coreceptors for N. lactamica PorB (and N. lactamica) signaling remain to be determined.
Downregulation of proinflammatory mediators in the airway tissue by commensal organisms is possibly a strategy to avoid inflammation and bacterial clearance (26). Since control of TLR2 expression is one of the mechanisms employed by epithelial cells in nonsterile compartments to mitigate exacerbated responses to the commensal flora, we examined the effect of whole N. lactamica or N. meningitidis organisms in human airway epithelial cells. Consistent with our observations with purified N. lactamica PorB, live N. lactamica is a low inducer of the proinflammatory cytokines IL-8, IL-6, and TNF-α compared to N. meningitidis strains such as the MC58 strain (44), the H44/76 parent strain (15), and the H44/76 Δ1Δ4 derivative strain lacking Rmp and PorA (45). Similar results in human meningeal cells have been previously reported (11). Our data also show that IL-8 production by N. lactamica alone is enhanced by coincubation with N. meningitidis PorB to levels comparable to or higher than that with N. meningitidis PorB alone. In contrary, N. lactamica PorB did not affect IL-8 secretion induced by N. meningitidis, which is efficiently induced by this pathogen. Like N. lactamica PorB, whole N. lactamica organisms also induce a low TLR2 cell surface expression, suggesting a potential negative feedback mechanism on TLR2-dependent human airway epithelial cell activation.
N. lactamica is not considered an invasive organism. It could be argued that lack of signaling via additional intracellular PRRs might contribute to the observed low induction of proinflammatory cytokines by N. lactamica. However, consistent with results with live organisms, heat-killed N. lactamica also induce a low IL-8 production. This is inhibited by blocking TLR2 on the cell surface, further stressing the relevance of TLR2 signaling in activation of human airway epithelial cells by these organisms. We speculate that the observed low airway epithelial cell responses induced by N. lactamica PorB might favor colonization by N. lactamica in the absence of a host inflammatory response.
Obviously, assuming that PorB is the only neisserial outer membrane component involved in human airway epithelial cell activation would be an oversimplification. Although PorB is the major neisserial outer membrane protein (OMP), other Neisseria TLR ligands such as lipooligosaccharide (LOS) and lipoprotein induce TLR-mediated cell activation. However, since BEAS-2B cells do not express CD36 and MD-2 (26, 49), the effects of Neisseria LOS and lipoprotein are minimized in our studies. In conclusion, our results suggest a major role for PorB in regulation of human airway epithelial cell activation via TLR2 and provide new insights on the potential mechanism(s) of cellular inflammatory responses to pathogenic and commensal Neisseria organisms.
Acknowledgments
This work was supported by NIH grant R01 AI40944. L.O.N. was supported by CNPq/CAPES and by Adalberto Pessoa Junior and Marco Antonio Stephano.
We also thank Caroline Genco for critical reading of the manuscript.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 11 October 2010.
REFERENCES
- 1.Abreu, M. T., E. T. Arnold, L. S. Thomas, R. Gonsky, Y. Zhou, B. Hu, and M. Arditi. 2002. TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J. Biol. Chem. 277:20431-20437. [DOI] [PubMed] [Google Scholar]
- 2.Berube, J., C. Bourdon, Y. Yao, and S. Rousseau. 2009. Distinct intracellular signaling pathways control the synthesis of IL-8 and RANTES in TLR1/TLR2, TLR3 or NOD1 activated human airway epithelial cells. Cell. Signal. 21:448-456. [DOI] [PubMed] [Google Scholar]
- 3.Bihl, F., L. Salez, M. Beaubier, D. Torres, L. Lariviere, L. Laroche, A. Benedetto, D. Martel, J. M. Lapointe, B. Ryffel, and D. Malo. 2003. Overexpression of Toll-like receptor 4 amplifies the host response to lipopolysaccharide and provides a survival advantage in transgenic mice. J. Immunol. 170:6141-6150. [DOI] [PubMed] [Google Scholar]
- 4.Capecchi, B., J. Adu-Bobie, F. Di Marcello, L. Ciucchi, V. Masignani, A. Taddei, R. Rappuoli, M. Pizza, and B. Arico. 2005. Neisseria meningitidis NadA is a new invasin which promotes bacterial adhesion to and penetration into human epithelial cells. Mol. Microbiol. 55:687-698. [DOI] [PubMed] [Google Scholar]
- 5.Cario, E., I. M. Rosenberg, S. L. Brandwein, P. L. Beck, H. C. Reinecker, and D. K. Podolsky. 2000. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 164:966-972. [DOI] [PubMed] [Google Scholar]
- 6.Cartwright, K. A., J. M. Stuart, D. M. Jones, and N. D. Noah. 1987. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol. Infect. 99:591-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denning, D. W., and S. S. Gill. 1991. Neisseria lactamica meningitis following skull trauma. Rev. Infect. Dis. 13:216-218. [DOI] [PubMed] [Google Scholar]
- 8.Derrick, J. P., R. Urwin, J. Suker, I. M. Feavers, and M. C. Maiden. 1999. Structural and evolutionary inference from molecular variation in Neisseria porins. Infect. Immun. 67:2406-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamond, G., D. Legarda, and L. K. Ryan. 2000. The innate immune response of the respiratory epithelium. Immunol. Rev. 173:27-38. [DOI] [PubMed] [Google Scholar]
- 10.Exley, R. M., R. Sim, L. Goodwin, M. Winterbotham, M. C. Schneider, R. C. Read, and C. M. Tang. 2009. Identification of meningococcal genes necessary for colonization of human upper airway tissue. Infect. Immun. 77:45-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler, M. I., K. Y. Yin, H. E. Humphries, J. E. Heckels, and M. Christodoulides. 2006. Comparison of the inflammatory responses of human meningeal cells following challenge with Neisseria lactamica and with Neisseria meningitidis. Infect. Immun. 74:6467-6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold, R., I. Goldschneider, M. L. Lepow, T. F. Draper, and M. Randolph. 1978. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J. Infect. Dis. 137(2):112-121. [DOI] [PubMed] [Google Scholar]
- 13.Greene, C. M., and N. G. McElvaney. 2005. Toll-like receptor expression and function in airway epithelial cells. Arch. Immunol. Ther. Exp. (Warsz.) 53:418-427. [PubMed] [Google Scholar]
- 14.Guillot, L., S. Medjane, K. Le Barillec, V. Balloy, C. Danel, M. Chignard, and M. Si-Tahar. 2004. Response of human pulmonary epithelial cells to lipopolysaccharide involves Toll-like receptor 4 (TLR4)-dependent signaling pathways: evidence for an intracellular compartmentalization of TLR4. J. Biol. Chem. 279:2712-2718. [DOI] [PubMed] [Google Scholar]
- 15.Holten, E. 1979. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J. Clin. Microbiol. 9:186-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homma, T., A. Kato, N. Hashimoto, J. Batchelor, M. Yoshikawa, S. Imai, H. Wakiguchi, H. Saito, and K. Matsumoto. 2004. Corticosteroid and cytokines synergistically enhance Toll-like receptor 2 expression in respiratory epithelial cells. Am. J. Respir. Cell Mol. Biol. 31:463-469. [DOI] [PubMed] [Google Scholar]
- 17.Jin, M. S., S. E. Kim, J. Y. Heo, M. E. Lee, H. M. Kim, S. G. Paik, H. Lee, and J. O. Lee. 2007. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130:1071-1082. [DOI] [PubMed] [Google Scholar]
- 18.Jones, D. M., R. Borrow, A. J. Fox, S. Gray, K. A. Cartwright, and J. T. Poolman. 1992. The lipooligosaccharide immunotype as a virulence determinant in Neisseria meningitidis. Microb. Pathog. 13:219-224. [DOI] [PubMed] [Google Scholar]
- 19.Li, M. S., N. Y. Chow, S. Sinha, D. Halliwell, M. Finney, A. R. Gorringe, M. W. Watson, J. S. Kroll, P. R. Langford, and S. A. Webb. 2009. A Neisseria meningitidis NMB1966 mutant is impaired for invasion of respiratory epithelial cells, survival in human blood and for virulence in vivo. Med. Microbiol. Immunol. 198:57-67. [DOI] [PubMed] [Google Scholar]
- 20.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 21.Litt, D. J., S. Savino, A. Beddek, M. Comanducci, C. Sandiford, J. Stevens, M. Levin, C. Ison, M. Pizza, R. Rappuoli, and J. S. Kroll. 2004. Putative vaccine antigens from Neisseria meningitidis recognized by serum antibodies of young children convalescing after meningococcal disease. J. Infect. Dis. 190:1488-1497. [DOI] [PubMed] [Google Scholar]
- 22.Liu, X., L. M. Wetzler, and P. Massari. 2008. The PorB porin from commensal Neisseria lactamica induces Th1 and Th2 immune responses to ovalbumin in mice and is a potential immune adjuvant. Vaccine 26:786-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massari, P., P. Henneke, Y. Ho, E. Latz, D. T. Golenbock, and L. M. Wetzler. 2002. Cutting edge: immune stimulation by neisserial porins is Toll-like receptor 2 and MyD88 dependent. J. Immunol. 168:1533-1537. [DOI] [PubMed] [Google Scholar]
- 24.Massari, P., A. Visintin, J. Gunawardana, K. A. Halmen, C. A. King, D. T. Golenbock, and L. M. Wetzler. 2006. Meningococcal porin PorB binds to TLR2 and requires TLR1 for signaling. J. Immunol. 176:2373-2380. [DOI] [PubMed] [Google Scholar]
- 25.Massari, P., C. A. King, H. Macleod, and L. M. Wetzler. 2005. Improved purification of native meningococcal porin PorB and studies on its structure/function. Protein Expr. Purif. 44:136-146. [DOI] [PubMed] [Google Scholar]
- 26.Mayer, A. K., and A. H. Dalpke. 2007. Regulation of local immunity by airway epithelial cells. Arch. Immunol. Ther. Exp. (Warsz.) 55:353-362. [DOI] [PubMed] [Google Scholar]
- 27.Medzhitov, R., and C. A. Janeway, Jr. 1999. Innate immune induction of the adaptive immune response. Cold Spring Harbor Symp. Quant. Biol. 64:429-435. [DOI] [PubMed] [Google Scholar]
- 28.Melkamu, T., D. Squillace, H. Kita, and S. M. O'Grady. 2009. Regulation of TLR2 expression and function in human airway epithelial cells. J. Membr. Biol. 229(2):101-113. [DOI] [PubMed] [Google Scholar]
- 29.Mietzner, T. A., R. C. Barnes, Y. A. JeanLouis, W. M. Shafer, and S. A. Morse 1986. Distribution of an antigenically related iron-regulated protein among the Neisseria spp. Infect. Immun. 51:60-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moranta, D., V. Regueiro, C. March, E. Llobet, J. Margareto, E. Larrate, J. Garmendia, and J. A. Bengoechea. 2010. Klebsiella pneumoniae capsule polysaccharide impedes the expression of β-defensins by airway epithelial cells. Infect. Immun. 78:1135-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naik, S., E. J. Kelly, L. Meijer, S. Pettersson, and I. R. Sanderson. 2001. Absence of Toll-like receptor 4 explains endotoxin hyporesponsiveness in human intestinal epithelium. J. Pediatr. Gastroenterol. Nutr. 32:449-453. [DOI] [PubMed] [Google Scholar]
- 32.Nassif, X., M. Marceau, C. Pujol, B. Pron, J. L. Beretti, and M. K. Taha. 1997. Type-4 pili and meningococcal adhesiveness. Gene 192:149-153. [DOI] [PubMed] [Google Scholar]
- 33.Ohnishi, T., M. Muroi, and K. Tanamoto. 2007. The lipopolysaccharide-recognition mechanism in cells expressing TLR4 and CD14 but lacking MD-2. FEMS Immunol. Med. Microbiol. 51:84-91. [DOI] [PubMed] [Google Scholar]
- 34.Oliver, K. J., K. M. Reddin, P. Bracegirdle, M. J. Hudson, R. Borrow, I. M. Feavers, A. Robinson, K. Cartwright, and A. R. Gorringe. 2002. Neisseria lactamica protects against experimental meningococcal infection. Infect. Immun. 70:3621-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Regueiro, V., D. Moranta, M. A. Campos, J. Margareto, J. Garmendia, and J. A. Bengoechea. 2009. Klebsiella pneumoniae increases the levels of Toll-like receptors 2 and 4 in human airway epithelial cells. Infect. Immun. 77:714-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmeck, B., S. Huber, K. Moog, J. Zahlten, A. C. Hocke, B. Opitz, S. Hammerschmidt, T. J. Mitchell, M. Kracht, S. Rosseau, N. Suttorp, and S. Hippenstiel. 2006. Pneumococci induced TLR- and Rac1-dependent NF-kappaB-recruitment to the IL-8 promoter in lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 290:L730-L737. [DOI] [PubMed] [Google Scholar]
- 37.Schulz, C., L. Farkas, K. Wolf, K. Kratzel, G. Eissner, and M. Pfeifer. 2002. Differences in LPS-induced activation of bronchial epithelial cells (BEAS-2B) and type II-like pneumocytes (A-549). Scand. J. Immunol. 56:294-302. [DOI] [PubMed] [Google Scholar]
- 38.Sha, Q., A. Q. Truong-Tran, J. R. Plitt, L. A. Beck, and R. P. Schleimer. 2004. Activation of airway epithelial cells by Toll-like receptor agonists. Am. J. Respir. Cell Mol. Biol. 31:358-364. [DOI] [PubMed] [Google Scholar]
- 39.Singleton, T. E., P. Massari, and L. M. Wetzler. 2005. Neisserial porin-induced dendritic cell activation is MyD88 and TLR2 dependent. J. Immunol. 174:3545-3550. [DOI] [PubMed] [Google Scholar]
- 40.Spinosa, M. R., C. Progida, A. Tala, L. Cogli, P. Alifano, and C. Bucci. 2007. The Neisseria meningitidis capsule is important for intracellular survival in human cells. Infect. Immun. 75:3594-3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stephens, D. S., L. H. Hoffman, and Z. A. McGee. 1983. Interaction of Neisseria meningitidis with human nasopharyngeal mucosa: attachment and entry into columnar epithelial cells. J. Infect. Dis. 148:369-376. [DOI] [PubMed] [Google Scholar]
- 42.Tanabe, M., and T. M. Iverson. 2009. Expression, purification and preliminary X-ray analysis of the Neisseria meningitidis outer membrane protein PorB. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 65(pt. 10):996-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanabe, M., C. M. Nimigean, and T. M. Iverson. 2010. Structural basis for solute transport, nucleotide regulation, and immunological recognition of Neisseria meningitidis PorB. Proc. Natl. Acad. Sci. U. S. A. 107:6811-6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 45.Tommassen, J., P. Vermeij, M. Struyve, R. Benz, and J. T. Poolman. 1990. Isolation of Neisseria meningitidis mutants deficient in class 1 (PorA) and class 3 (PorB) outer membrane proteins. Infect. Immun. 58:1355-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Townsend, R., L. Goodwin, T. M. Stevanin, P. B. Silcocks, A. Parker, M. C. Maiden, and R. C. Read. 2002. Invasion by Neisseria meningitidis varies widely between clones and among nasopharyngeal mucosae derived from adult human hosts. Microbiology 148(pt. 5):1467-1474. [DOI] [PubMed] [Google Scholar]
- 47.Vaughan, T. E., P. J. Skipp, C. D. O'Connor, M. J. Hudson, R. Vipond, M. J. Elmore, and A. R. Gorringe. 2006. Proteomic analysis of Neisseria lactamica and Neisseria meningitidis outer membrane vesicle vaccine antigens. Vaccine 24:5277-5293. [DOI] [PubMed] [Google Scholar]
- 48.Virji, M., K. Makepeace, D. J. Ferguson, M. Achtman, and E. R. Moxon. 1993. Meningococcal Opa and Opc proteins: their role in colonization and invasion of human epithelial and endothelial cells. Mol. Microbiol. 10:499-510. [DOI] [PubMed] [Google Scholar]
- 49.Visintin, A., K. A. Halmen, E. Latz, B. G. Monks, and D. T. Golenbock. 2005. Pharmacological inhibition of endotoxin responses is achieved by targeting the TLR4 coreceptor, MD-2. J. Immunol. 175:6465-6472. [DOI] [PubMed] [Google Scholar]
- 50.Yazdankhah, S. P., and D. A. Caugant. 2004. Neisseria meningitidis: an overview of the carriage state. J. Med. Microbiol. 53(pt. 9):821-832. [DOI] [PubMed] [Google Scholar]