Abstract
Francisella tularensis is a highly virulent Gram-negative bacterium and is the etiological agent of the disease tularemia. IclR, a presumed transcriptional regulator, is required for full virulence of the animal pathogen, F. tularensis subspecies novicida U112 (53). In this study, we investigated the contribution of IclR to the intracellular growth, virulence, and gene regulation of human pathogenic F. tularensis subspecies. Deletion of iclR from the live vaccine strain (LVS) and SchuS4 strain of F. tularensis subsp. holarctica and F. tularensis subsp. tularensis, respectively, did not affect their abilities to replicate within macrophages or epithelial cells. In contrast to F. tularensis subsp. novicida iclR mutants, LVS and SchuS4 ΔiclR strains were as virulent as their wild-type parental strains in intranasal inoculation mouse models of tularemia. Furthermore, wild-type LVS and LVSΔiclR were equally cytotoxic and induced equivalent levels of interleukin-1β expression by infected bone marrow-derived macrophages. Microarray analysis revealed that the relative expression of a limited number of genes differed significantly between LVS wild-type and ΔiclR strains. Interestingly, many of the identified genes were disrupted in LVS and SchuS4 but not in their corresponding F. tularensis subsp. novicida U112 homologs. Thus, despite the impact of iclR deletion on gene expression, and in contrast to the effects of iclR deletion on F. tularensis subsp. novicida virulence, IclR does not contribute significantly to the virulence or pathogenesis of F. tularensis LVS or SchuS4.
Francisella tularensis is a Gram-negative bacterium and the etiological agent of tularemia, or “rabbit fever.” While zoonotic hosts include small mammals, such as rabbits and voles, F. tularensis is also found in ticks, mosquitoes, and flies and can replicate within amoebae as well (29). Human infection with F. tularensis can occur by several routes, including bites by arthropod vectors (4, 5, 34), contact with contaminated tissues, ingestion of contaminated food or water (28, 43), or inhalation of aerosolized bacteria (18, 48). F. tularensis is considered a select agent by the Centers for Disease Control and Prevention (CDC) due to its low infectious dose (as few as 10 organisms) via the pulmonary route and its potential as a biological threat agent (15, 46).
There are two F. tularensis subspecies that are most commonly associated with disease in humans: F. tularensis subsp. tularensis (type A) and F. tularensis subsp. holarctica (type B). The live vaccine strain (LVS) of F. tularensis subsp. holarctica is a useful model for studying the virulent F. tularensis subspecies because it causes disease in mice, is attenuated in humans (19), and shares genomic and proteomic similarity with F. tularensis subsp. holarctica and F. tularensis subsp. tularensis (51). F. tularensis subsp. novicida, which does not cause disease in healthy humans, has significant similarity with F. tularensis subsp. holarctica and F. tularensis subsp. tularensis and is also used as model organism for studying F. tularensis pathogenesis. Although there are reports of F. tularensis subsp. novicida causing disease, these cases are commonly associated with immunocompromised individuals (2, 9, 24, 32). However, F. tularensis subsp. novicida does cause a severe disease in in vivo mouse models (40).
Francisella is known to predominately infect and replicate within macrophages but also infects and replicates within neutrophils (37), dendritic cells (3), and type II alveolar epithelial cells (23). After phagocytosis, F. tularensis escapes the phagosome and replicates within the cytoplasm of host cells (1, 10). Numerous in vitro and in vivo screens have identified virulence factors required for this intracellular life cycle (13, 14, 27, 30, 35, 41, 47, 49, 53); however, many of the identified virulence factors have little or no similarity to known proteins of other bacteria, and their functions remain, for the most part, unknown.
Weiss et al. recently identified a locus (FTN_0720) in F. tularensis subsp. novicida U112 that is important for virulence in mice as determined by an in vivo competition assay between a FTN_0720 deletion mutant and wild-type U112 (53). FTN_0720 encodes a protein with homology to the IclR family of transcriptional regulators. IclR family members activate and repress genes in a wide range of bacteria, including genes involved in sporulation, metabolism, drug efflux pumps and organic solvent tolerance, and phytopathogenicity (39). Given the close genetic relationship among the F. tularensis subspecies, the phenotype of the F. tularensis subsp. novicida iclR deletion strain suggests that IclR may be involved in the pathogenicity of the F. tularensis subsp. holarctica and F. tularensis subsp. tularensis subspecies. We investigated the contribution of IclR homologs in the pathogenicity of F. tularensis subsp. holarctica and F. tularensis subsp. tularensis by evaluating the role of IclR in gene expression, host cell interactions, and virulence of F. tularensis subsp. holarctica LVS (FTL_1364) and F. tularensis subsp. tularensis SchuS4 (FTT_0748) strains.
MATERIALS AND METHODS
Bacterial strains.
F. tularensis subsp. holarctica LVS was obtained from the CDC, Atlanta, GA. F. tularensis subsp. tularensis SchuS4 was obtained from BEI Resources. F. tularensis subsp. novicida U112 was obtained from the American Type Culture Collection (ATCC). An iclR transposon mutant was one of two mutants of the transposon mutant library (21) and was received as a gift from Colin Manoil. All strains were maintained on chocolate agar supplemented with 1% IsoVitaleX (Becton-Dickson), brain heart infusion (BHI) broth supplemented with 1% IsoVitaleX, or Chamberlain's defined medium (CDM) (6). Escherichia coli TOP10 cells (Invitrogen) were used for cloning purposes. E. coli was propagated in Luria broth supplemented with hygromycin at 200 μg/ml or kanamycin at 20 μg/ml as necessary for antibiotic selection. All cultures were grown at 37°C.
Cell culture.
J774A.1 (ATCC TIB-67) cells are a macrophage-like cell line derived from mouse sarcoma reticulum cells and were cultured in Dulbecco's minimal essential medium (DMEM) with 4.5 g/liter glucose, 10% fetal bovine serum, and 2 mM l-glutamine. TC-1 (ATCC CRL-2785) cells are a tumor cell line derived from mouse primary lung epithelial cells and were cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 1.5 g/liter sodium bicarbonate, 10 mM HEPES, and 0.1 mM nonessential amino acids. Bone marrow-derived macrophages were generated by flushing bone marrow cells from C57BL/6 mouse femurs, and recovered cells were incubated for 6 days on 15 cm2 non-tissue culture-treated dishes in L929 cell-conditioned DMEM. Nonadherent cells were removed by washing with phosphate-buffered saline (PBS), and bone marrow-derived macrophages were recovered from the dishes by using 1 mM EDTA in PBS.
Molecular techniques and allelic exchange.
For both LVS and SchuS4 the iclR deletion was generated by splice overlap extension (SOE) PCR using primers designed to amplify the 5′ and 3′ regions of the iclR locus, which were then annealed to their complementary, homologous genomic DNA tags (20, 25). The subsequent deletion left only the first 6 amino acids and the stop codon of iclR. Each construct was cloned into the pCR-Blunt II TOPO vector (Invitrogen), verified by DNA sequence analysis, and subsequently cloned into pMP590 (sacB Kanr) using BamHI and NotI restriction sites (20, 33). For allelic exchange, plasmids were electroporated into LVS or SchuS4 and integrants were selected on chocolate agar containing kanamycin (10 μg/ml). Kanr strains were grown overnight and plated on 10% sucrose for counterselection (loss of plasmid) (20, 33). Both the LVS and SchuS4 iclR deletion strains were confirmed for loss of iclR by PCR. For complementation of the iclR deletion in LVS, iclR and its predicted promoter were PCR amplified and subcloned into the pCR-Blunt II TOPO vector (Invitrogen). After MluI/EcoRV restriction digestion, the construct was ligated into the pMP633 low-copy-number Francisella shuttle vector (20) and electroporated into LVSΔiclR. Complementation was determined by detection of iclR in the complementation strain via PCR as well as demonstration of increased iclR transcript levels via microarray analysis (data not shown).
Gentamicin protection assays.
Gentamicin protection assays were performed as described previously (20, 23). Briefly, J774A.1 murine macrophages, TC-1 murine lung epithelial cells, or bone marrow-derived macrophages were infected with LVS or SchuS4 at a multiplicity of infection (MOI) of 100. Cells were incubated with the bacterial inoculum for 2 h (J774A.1 and bone marrow-derived macrophages) or 4 h (TC-1) and then incubated with medium containing 25 μg/ml gentamicin for an additional 2 h to kill extracellular bacteria. At time points of 4 h (or 6 h for TC-1) and 24 h, medium was removed and cells were washed with PBS and then scraped from the dish, and the bacteria were serially diluted and plated to determine the number of viable bacteria.
Mouse infections.
Six- to 8-week-old C57BL/6 mice were anesthetized intraperitoneally (i.p.) with avertin and then inoculated intranasally (i.n.) with bacteria suspended in 50 μl PBS by application to the nares of each mouse or inoculated intradermally (i.d.) by injection into the tail using the same volume. Concentrations of LVS and U112 were determined based on Klett units and concentrations of SchuS4 were determined by using a spectrophotometer (based on the optical density at 600 nm [OD600]), and inocula were serially diluted and plated on chocolate agar to confirm the CFU administered. At the designated time points, mice were euthanized and the lungs, liver, and spleen of each mouse were removed and homogenized. Serial dilutions of the homogenates were plated on chocolate agar to enumerate the bacterial organ burdens. Statistical significance between strains in each organ and at each time point was determined by the Mann-Whitney nonparametric test using GraphPad Prism v.5 software. All animal experiments were performed according to the animal care and use guidelines as established by IACUC-approved protocols.
IL-1β ELISAs and cytotoxicity assays.
Bone marrow-derived macrophages were seeded in 12-well dishes at 1 × 106 cells per well, infected with bacteria at an MOI of 500 in a final volume of 1 ml of medium per well, and incubated at 37°C. After 24 h, the supernatants from each well were collected, centrifuged to pellet cellular debris, and stored at −20°C. The interleukin-1β (IL-1β) enzyme-linked immunosorbent assay (ELISA) was performed using the BD OptEIA mouse IL-1β ELISA kit (BD Biosciences) according to the manufacturer's protocol. The OD450 was read using a TECAN Infinite M200 and analyzed using Magellan version 6 software. Cytotoxicity assays were performed using the ToxiLight BioAssay kit (Lonza) following the manufacturer's protocol for cytokine detection from supernatants, and the luminescence was read using a TECAN Infinite M200 instrument and analyzed using Magellan version 6 software. Statistical significance between each strain was determined with Student's t test using the GraphPad Prism version 5 software.
Microarrays.
RNA was obtained using the RiboPure bacteria kit (Ambion) according to the manufacturer's protocol. Briefly, bacteria were grown to early mid-log phase in CDM and pelleted. Cells were disrupted by suspension in TRIzol and vortexing with 0.1-mm glass beads. Purified RNA was recovered by chloroform extraction followed by treatment with DNase I to remove DNA. Microarray analysis was performed following the guidelines provided by the Venter Institute for Genomic Research (SOPM007; M008). Briefly, aminoallyl (aa)-labeled cDNA was generated from 2 μg total RNA using SuperScript III reverse transcriptase (Invitrogen), random hexamers, and deoxynucleoside triphosphates containing aa-UTP. After removal of unincorporated aa-dUTP and free amines, labeled cDNA was coupled to Cy3 or Cy5 monoreactive dye (GE Healthcare). The Francisella microarray slides (Pathogen Functional Genomics Resource Center [PFGRC]) contained 2,331 70-mer oligonucleotides in quadruplicates of the F. tularensis SchuS4 genome and several LVS genes as well as quadruplicates of 70-mer oligonucleotides for 500 Arabidopsis thaliana oligonucleotides as controls. Slides were prehybridized in 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 10% SDS, and 1% bovine serum albumin, washed, and then hybridized with cDNA probes at 42°C. After posthybridization washes, the slides were scanned using the GenePix 4000B scanner and GenePix Pro version 6.0 software. The microarray data were normalized using the TIGR program MIDAS version 2.22 and analyzed using the TIGR Multiexperiment viewer version 4.2.1 (MeV) as part of the TM4 suite software (45). Using MeV, pooled, normalized Cy5/Cy3 intensities from wild-type LVS control arrays were compared to pooled, normalized Cy5/Cy3 intensities from LVS ΔiclR arrays. This list was filtered by statistical significance using the significance analysis for microarrays (SAM) provided in MeV after an 80% cutoff filter and using a false-discovery rate of 5%.
Quantitative RT-PCR.
Quantitative reverse transcriptase PCR (RT-PCR) was performed in a 96-well format using the SensiMix SYBR & Fluorescein one-step kit (Bioline) following the manufacturer's protocol. Briefly, 50 ng of RNA isolated from wild-type or iclR mutant strains was mixed with SensiMix SYBR & Fluorescein, RNase inhibitors, and designated primers in a 20-μl volume. A genomic DNA ladder and a no-RT control were analyzed using the SensiMix SYBR & Fluorescein kit following the manufacturer's protocol with primers to gyrA. Thermocycling and detection were performed using the iCycler thermal cycler (Bio-Rad). All starting quantity (SQ) values were normalized to the mean SQ value for gyrA.
Antibiotic sensitivity assays.
F. tularensis LVS was grown to mid-log phase in BHI broth supplemented with 1% IsoVitaleX, the bacterial suspension was spread onto chocolate agar plates, and antibiotic-containing filter paper discs were placed in the center of each plate. Rifampin (5 μg), tetracycline (30 μg), and colistin (10 μg) discs were purchased preloaded from Becton Dickinson. The ampicillin and polymyxin B discs were self-prepared by adding a 10-μl or 20-μl volume of antibiotic per disc with 10 μg ampicillin or 20 μg polymixin B. Bacteria were grown for 36 h, and the diameter of the zone of inhibition was measured.
Microarray data accession numbers.
The raw and normalized microarray data are available on the GEO database under the following accession numbers: GSM574374, GSM574375, GSM574376, GSM574377, GSM574379, GSM574380, and GSE23454.
RESULTS
Comparison of iclR alleles among F. tularensis subspecies and construction of iclR deletion mutants.
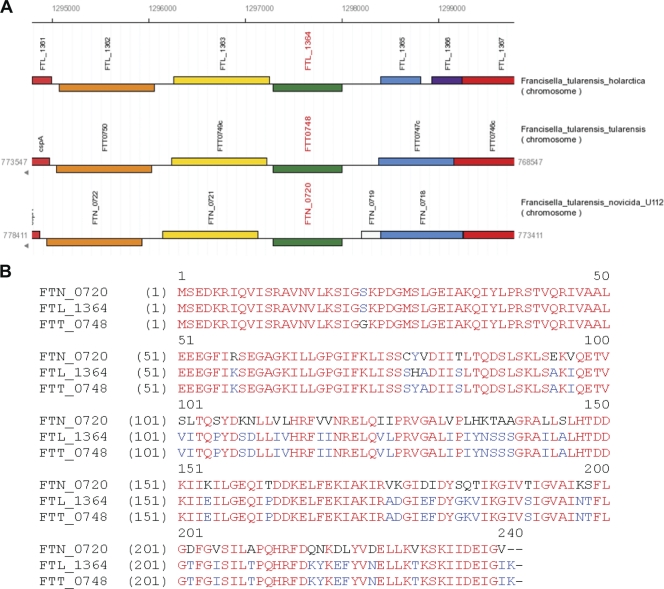
The locus FTL_1364 is annotated as a hypothetical protein in NCBI; however, some of its homologs in other Francisella species are annotated as proteins belonging to the IclR family of transcriptional regulators. A search for conserved domains found within FTL_1364 resulted in several related hits, including a helix-turn-helix (HTH) domain conserved among IclR family members. Additionally, Francisella IclR has a C-terminal domain with high similarity to the IclR family profile Pfam01614. A recent publication described a highly specific IclR family member profile that lies outside the HTH domain and covers less than 100 amino acids in the central region toward the C-terminal end (31). Those authors classified current Pfam01614 members as belonging to the IclR family, based on the new profile. Furthermore, BLAST analysis of F. tularensis LVS or SchuS4 IclR revealed high similarity to IclR family proteins found across many bacterial species. F. tularensis IclR proteins share considerable amino acid identity (30 to 40%) and amino acid similarity (60%) with non-Francisella IclR family proteins. Overall, the bioinformatic analysis strongly suggests that Francisella FTL_1364 and its homologous loci in other Francisella species encode a protein belonging to the IclR family of transcriptional regulators.
By using the NCBI and the Francisella genome browser (www.francisella.org) for annotations and synteny analysis, we found that the iclR locus has shared characteristics among F. tularensis subsp. novicida U112, F. tularensis subsp. holarctica LVS, and F. tularensis subsp. tularensis SchuS4 strains (FTN_0720, FTL_1364, and FTT_0748, respectively) (Fig. 1A). On one side of iclR in each strain is a gene encoding a predicted protein with similarity to an esterase lipase (FTL_1363, FTN_0721, and FTT_0749). On the other side of iclR is a gene encoding a predicted protein with similarity to the multidrug efflux protein EmrA (FTL_1365-66, FTN_0718, and FTT_0747). One difference is that EmrA is divided into two ORFs in LVS. There are other differences in the length and coding sequences of this genetic region, including an additional open reading frame in U112 that encodes a predicted protein of unknown function, FTN_0719. Nevertheless, in each strain, iclR is located in a similar region of the genome.
FIG. 1.
Comparison of iclR in three Francisella strains. (A) Synteny diagram of the genomic organization of the iclR locus in F. tularensis subsp. novicida U112 (FTN_0720), F. tularensis subsp. holarctica LVS (FTL_1364), and F. tularensis subsp. tularensis SchuS4 (FTT_0748). (B) Amino acid sequence alignment of F. tularensis subsp. novicida U112, F. tularensis subsp. holarctica LVS, and F. tularensis subsp. tularensis SchuS4 IclR. Alignment was created using VectorNTI software and iclR sequences uploaded from NCBI annotated genomes of each strain and translated using VectorNTI. Red letters highlight residues conserved between all three strains. Blue letters highlight the residues conserved between two strains.
Additionally, iclR itself is highly conserved among the three F. tularensis strains U112, LVS, and SchuS4. SchuS4 iclR has three nucleotide differences compared to iclR from LVS that translate into two amino acid differences, S22G and H78Y, between LVS and SchuS4 IclR. U112 iclR has 95 nucleotide differences compared to LVS iclR and 94 nucleotide changes compared to SchuS4 iclR. Although this results in a 3-nucleotide truncation of U112 iclR, there is 80% amino acid identity between the U112 IclR and the SchuS4 and LVS IclR proteins (Fig. 1B). While these similarities suggest that IclR is conserved among the U112, LVS, and SchuS4 strains, there are a sufficient number of differences to account for possible functional deviations between these strains as well. Due to genetic similarity and the contribution of IclR to the virulence for F. tularensis subsp. novicida, we investigated the potential contribution of IclR to the virulence of F. tularensis subsp. holarctica and F. tularensis subsp. tularensis. To do this, we made a clean deletion of the iclR gene in the F. tularensis subsp. holarctica LVS (LVSΔiclR) and SchuS4 (SchuS4ΔiclR) by using SOE PCR and allelic exchange in LVS (FTL_1364). We also generated an iclR complementation strain by expression of iclR on a low-copy-number shuttle vector.
LVS and SchuS4 iclR deletion mutants are competent for intracellular replication.
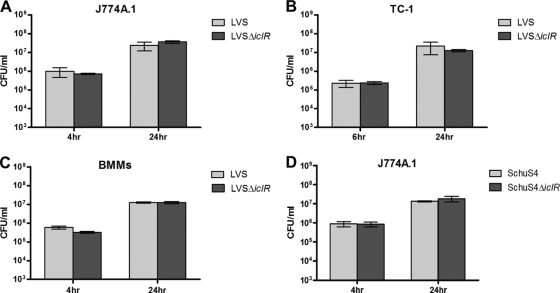
One method to assess the contribution of IclR to F. tularensis virulence is to determine what role IclR plays in intracellular replication. We used gentamicin protection assays in the J774A.1 murine macrophage-like cell line and the TC-1 murine lung epithelial cell-like cell line to assess intracellular replication by iclR deletion mutant strains. Both LVS ΔiclR and wild-type LVS replicated approximately 2 logs by 24 h in both J774A.1 and TC-1 cells (Fig. 2A and B). We also performed these assays in bone marrow-derived macrophages, and both wild-type LVS and LVS ΔiclR replicated intracellularly in these cells (Fig. 2C). Similarly, the intracellular replication of SchuS4ΔiclR was similar to wild-type SchuS4 in J774A.1 cells (Fig. 2D). These results demonstrate that IclR is not required for intracellular replication of LVS or SchuS4 in these cell types.
FIG. 2.
Intracellular replication of LVS ΔiclR and SchuS4 ΔiclR in murine macrophages or lung epithelial cells. Gentamicin protection assays were performed by infecting J774A.1 murine macrophages (A), TC-1 murine lung epithelial cells (B), and bone marrow-derived macrophages (BMMs) (C) with wild-type LVS or LVS ΔiclR at an MOI of 100. (D) A gentamicin protection assay was performed using J774A.1 cells infected with wild-type SchuS4 or SchuS4 ΔiclR. Bars represent the standard deviations of three replicate wells, and each graph is representative of two separate experiments.
LVS ΔiclR is not attenuated following intranasal or intradermal inoculation of mice.
Properties other than intracellular replication contribute to F. tularensis pathogenesis. We therefore determined whether IclR was required for LVS virulence in vivo. To test this, we used a mouse model of pulmonary tularemia in which we inoculated C57BL/6 mice i.n. with a lethal dose (1 × 105 CFU) of LVS or LVS ΔiclR. At 1, 3, 7, and 8 days postinoculation, the lungs, liver, and spleen were harvested to enumerate the bacterial organ burdens (Fig. 3A). These initial experiments revealed that there were no differences in the organ burdens at 1 or 3 days postinoculation. At day 7, there appeared to be slight differences in the organ burdens in the liver and spleen, and by day 8 the organ burdens in the liver and spleen had not increased. These initial experiments suggested that LVS ΔiclR may demonstrate enhanced clearance in the mouse. This would correlate with previously published data demonstrating a decrease in the competitive index in the spleen at 48 h for the F. tularensis subsp. novicida U112 iclR deletion mutant compared to wild-type F. tularensis subsp. novicida U112 (53).
FIG. 3.
Recovery of LVS ΔiclR mutant in mice following i.n. or i.d. inoculation. (A and B) C57BL/6 mice were inoculated with either wild-type LVS (circles) or LVS ΔiclR (triangles) i.n. at a lethal dose of ∼1 × 105 CFU (A) or a low dose of ∼1 × 103 CFU (B). (C) C57BL/6 mice were inoculated with either wild-type LVS (circles) or LVS ΔiclR (triangles) i.d. at a dose of ∼3 × 105 CFU. Each symbol represents data from a single mouse. There were no significant differences in recovery of mutant versus wild-type organisms from any organ at any time point as determined by the Mann-Whitney nonparametric test in the low-dose (B) and i.d. (C) experiments.
To further investigate the possibility of a more subtle phenotype of enhanced clearance, we used a low-dose (1 × 103 CFU) i.n. inoculation of groups of six wild-type C57BL/6 mice with LVS or LVS ΔiclR. At days 1, 3, 7, and 10 postinoculation, we again harvested the lungs, liver, and spleen to calculate the bacterial organ burdens. There were no significant differences between the bacterial organ burdens of LVS ΔiclR or wild-type LVS at any time point (Fig. 3B). This suggests that LVS ΔiclR is not attenuated in a mouse model of pulmonary tularemia.
Since the experiments with the F. tularensis subsp. novicida U112 iclR deletion mutant were performed using subcutaneous (s.c.) and i.p. inoculations, we investigated whether the role for iclR in pathogenesis may be route specific. Groups of six to seven wild-type C57BL/6 mice were infected i.d. with 3 × 105 CFU of LVS or LVS ΔiclR. The i.d. route has a comparable 50% lethal dose and is similar in nature to the s.c. route (17). At 1, 3, and 7 days postinoculation, we again harvested the lungs, liver, and spleen and determined bacterial organ burdens. At each time point and in each organ, there were no significant differences in the bacterial burdens between the LVS and LVS ΔiclR groups (Fig. 3C). These data indicate that for LVS, iclR is not required for pathogenesis in the mouse via the i.n. or i.d. route.
SchuS4 ΔiclR is not attenuated following intranasal inoculation of mice.
Although iclR does not appear to be required for LVS pathogenesis, it is possible that iclR plays a role in SchuS4 pathogenesis. We inoculated groups of four wild-type C57BL/6 mice i.n. with a lethal dose (100 CFU) of wild-type SchuS4 or SchuS4 ΔiclR. At 1 and 3 days postinoculation, the lungs, liver, and spleen were harvested to enumerate the bacterial organ burdens of infected mice (Fig. 4). At both time points and in each organ, there were no differences in bacterial burden between the wild-type SchuS4 and SchuS4 ΔiclR groups. These data suggest that IclR does not play a role in the in vivo virulence of SchuS4 when assessed in the mouse model of pulmonary tularemia.
FIG. 4.
Recovery of the SchuS4 ΔiclR mutant in mice following i.n. inoculation. C57BL/6 mice were inoculated with either wild-type SchuS4 (circles) or SchuS4 ΔiclR (triangles) i.n. at a dose of ∼100 CFU. No differences in recovery of mutant versus wild-type organisms from any organ at any time point were significant based on the Mann-Whitney nonparametric test.
An F. tularensis subsp. novicida U112 iclR transposon mutant is attenuated following intranasal inoculation of mice.
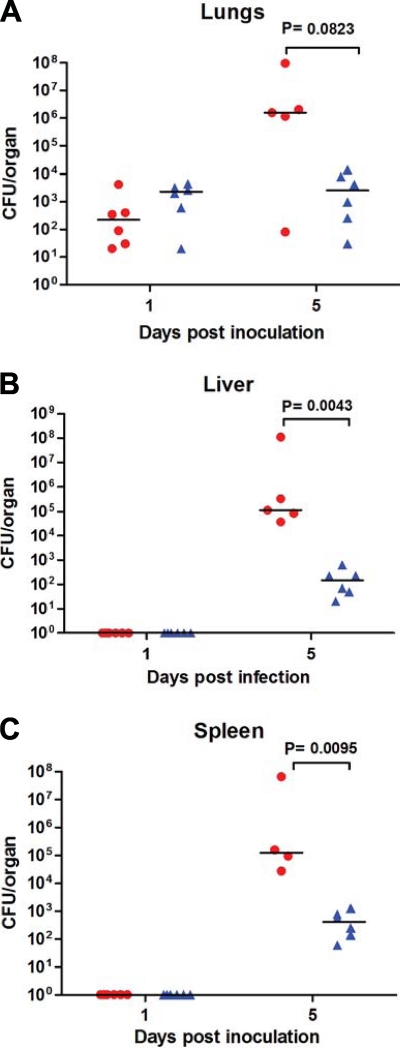
As noted above, an iclR deletion mutant in F. tularensis subsp. novicida U112 displays a decreased competitive index in the spleen following s.c. or i.p inoculation of mice (53). Therefore, we wanted to determine whether iclR is required for F. tularensis subsp. novicida U112 pathogenesis in a pulmonary mouse model. We inoculated groups of six wild-type C57BL/6 mice i.n. with a dose of approximately 10 CFU of wild-type U112 or a U112 iclR transposon mutant. At 1 and 5 days postinoculation, the lungs, liver, and spleen were harvested and the bacterial organ burdens were enumerated. Each organ had reduced burdens of the iclR transposon mutant compared to wild-type U112, and at day 5 these differences were statistically significant in the liver and spleen (Fig. 5). These data suggest that iclR is required for U112 pathogenesis via the i.n. route and correlate with the previously published data for the s.c. and i.p. routes.
FIG. 5.
Recovery of the U112 iclR transposon mutant in mice following i.n. inoculation. C57BL/6 mice were inoculated with either wild-type U112 (circles) or U112 iclR mutant (triangles) i.n. at a dose of ∼10 CFU. Differences in recovery of mutant versus wild-type organisms at day 5 for the liver and spleen were significant based on the Mann-Whitney nonparametric test.
Deletion of iclR does not affect IL-1β expression or cytotoxicity of infected cells.
To determine if there is an altered cellular response to LVS ΔiclR compared to wild-type LVS, we measured the production of proinflammatory cytokines by infected cells. Bone marrow-derived macrophages were infected at an MOI of 500 with LVS or LVS ΔiclR, and the supernatants were analyzed for IL-1β at 24 h postinfection (Fig. 6A). The levels of IL-1β measured in the supernatants of LVS ΔiclR-infected cells were similar to those in cells infected with wild-type LVS, and no differences between strains were statistically significant.
FIG. 6.
IL-1β release and cytotoxicity in murine bone marrow-derived macrophages infected with LVS ΔiclR. Infections were carried out at an MOI of 500 for wild-type LVS, LVS ΔiclR, and LVSΔiclR plus IclR (complementation). (A) IL-1β was quantified via ELISA, and (B) cytotoxicity was quantified via the ToxiLight bioassay (Lonza), both at 24 h postinfection. Graphs are representative of at least three separate experiments, with duplicate or triplicate wells for each strain per experiment. No differences were significant by any strain comparison based on Student's t test.
F. tularensis has also been reported to induce cytotoxicity of infected macrophages. To determine whether there was a change in cytotoxicity induced by LVS ΔiclR, we infected murine bone marrow-derived macrophages with LVS or LVS ΔiclR at an MOI of 500 and performed cytotoxicity assays on supernatants collected at 24 h postinfection. As shown in Fig. 6B, LVS ΔiclR induced cytotoxicity in infected cells to a level similar to that of wild-type LVS, and no differences between strains were statistically significant.
Effects of IclR on gene expression.
Due to its homology to transcriptional regulators, we used microarray analysis to determine what genes in LVS were affected by IclR by comparing gene expression between the LVS ΔiclR mutant and wild-type LVS. We grew LVS and LVS ΔiclR to mid-log phase to harvest RNA for reverse transcription and amino-allyl labeling of cDNA, and the labeled cDNA was hybridized to microarray slides. The slides are printed for every annotated ORF for SchuS4, plus LVS alleles that are either not present or are variant in SchuS4, but the slides are not tailored to F. tularensis subsp. novicida. Three separate microarrays from independent RNA samples were pooled, and statistically significant gene expression differences between LVS ΔiclR and wild-type LVS were determined by SAM (Table 1). Genes exhibiting significant changes in expression are listed by the provided locus annotations, LVS or SchuS4, as printed on the slides.
TABLE 1.
Microarray gene expression in LVSΔiclR
| Gene regulation and locus | Fold change | Description or annotation (BLASTp)a | Result of gene comparison between strainsb |
||
|---|---|---|---|---|---|
| SchuS4 | LVS | U112 | |||
| Downregulated | |||||
| FTT0748 | 34.57 | iclR | Intact | Intact | Intact |
| FTT0980 | 5.02 | Hypothetical protein (aminotransferase class II) | Intact | Intact | Intact |
| FTT0987 | 2.84 | Hypothetical protein (membrane protein of unknown function) | Pseudogene | Pseudogene | Intact |
| FTL_1506 | 2.82 | Short-chain dehydrogenase (reductase family protein) | Intact | Pseudogene | Intact |
| FTT1082 | 2.64 | T1082 protein | Intact | Intact | Intact |
| FTL_0388/FTT0885 | 2.63/2.18 | Cation transporter (cobalt zinc cadmium cation transporter) | Pseudogene | Intact | Intact |
| FTL_1256 | 2.62 | Pseudogene (carbon-nitrogen hydrolase family protein) | Pseudogene | Pseudogene | Intact |
| FTL_1507 | 2.57 | 3-Oxoacyl-[acyl-carrier protein] reductase | Intact | Pseudogene | Intact |
| FTT1081 | 2.53 | Hypothetical protein (hemolysin-type binding protein) | Intact | Intact | Absent |
| FTT1507 | 2.37 | Hypothetical protein (thymosin beta-4 family protein) | Intact | Absent | Intact |
| FTL_1122 | 2.19 | Hypothetical membrane protein | Intact | Pseudogene | Absent |
| FTT0715 | 2.19 | Chitinase family 18 protein | Two large deletions | Two large deletions | Intact |
| FTT0389 | 2.16 | Acetyltransferase | Intactc | Intactc | Intactc |
| FTT0203 | 1.97 | Bifunctional purine biosynthesis protein (purH) | Intact | Intact | Intact |
| Upregulated | |||||
| FTL_1373/FTT0741 | 4.28/6.40 | Organic solvent tolerance protein | Pseudogene | Pseudogene | Intact |
| FTT1555 | 1.93 | RNase III (rnc) | Intact | Intact | Intact |
| FTT1554 | 1.88 | tRNA pseudouridine synthetase B (truB) | Intact | Intact | Intact |
| FTT0554 | 1.59 | Hypothetical protein | Intact | Intact | Intact |
Annotation based on the microarray; BLASTp results (shown in parentheses), when included, provided additional information.
Following alignments, genes were designated as intact, absent, or a pseudogene (introduced stop codon and two shorter predicted ORFs); genes those without such designations are discussed in the text.
Possible alternative start site (see text for details).
Using the above criteria, we identified 13 downregulated and 4 upregulated genes in LVS ΔiclR. The list of genes identified comprises diverse functional groups, suggesting that IclR does not impact expression of one specific functional group of proteins. There were several IclR-affected genes annotated as encoding hypothetical proteins. To get a better idea of what types of proteins these genes may be encoding and to possibly obtain insight on IclR function, we performed BLASTp analyses. Many of the proteins were only conserved in Francisella, with no similarity to proteins or conserved domains in other bacteria. However, there were several proteins with similarity to known proteins in other bacteria, and these are described in Table 1. Although most of the genes were represented exclusively by the SchuS4 allele, there were two cases where the SchuS4 and LVS homologs were both printed on the microarray slide and also appeared on the gene list as having significant expression changes in the absence of IclR. FTT_0741c and its FTL_1373 homolog were both upregulated in LVS ΔiclR, and both FTL_0388 and its homolog FTT_0885 were downregulated in LVS ΔiclR. Overall, although further studies need to be performed to demonstrate a function of IclR, both bioinformatic and microarray data suggest that the Francisella IclR may function as a transcriptional regulator.
Comparison of IclR-affected genes between LVS, SchuS4, and U112.
One explanation for the phenotypic differences observed for iclR mutants among the F. tularensis U112, LVS, and SchuS4 strains could be differences in the genes affected by IclR among the strains. To address this we performed a more detailed examination of the genes on our microarray list. First, we performed synteny analysis using the genome synteny tool at www.francisella.org to determine whether each gene was annotated in SchuS4, LVS, and U112. We observed that there were a few genes that were not annotated or not present in all three strains, as shown in Table 1. Second, we generated alignments and protein translations of the genes by using Vector NTI software based on the NCBI annotation or the putative loci of nonannotated genes from the synteny analysis, if they were found. For example, sequence alignments revealed that in LVS there is an unannotated ORF between FTL_1120 and FTL_1121 that bears homology to FTT_1082. Nearly half of the genes were similarly annotated and encoded one intact ORF in SchuS4, LVS, and U112. However, a significant percentage of genes displayed considerable sequence differences between strains, as shown in Table 1.
Of these genes, many were not intact in the virulent strains LVS and/or SchuS4, whereas the homologous genes in U112 were intact. For example, FTL_1506 and FTL_1507 are pseudogenes because they encode two ORFs, while their SchuS4 (FTT_0723c) and U112 (FTN_0634) genes encode only one ORF. One special case is FTT0715, which along with its LVS homolog FTL_1521, has two large deletions, 131 bp (119 bp in LVS) and 197 bp, compared to the U112 homolog FTN_0627. The significance of these deletions cannot be inferred, and although these genes are not pseudogenes, the fact that these large deletions are present only in SchuS4 and LVS is noteworthy. This also highlights that many of the intact genes on the microarray list have greater overall sequence differences between U112 and LVS or SchuS4 compared to the differences between LVS and SchuS4.
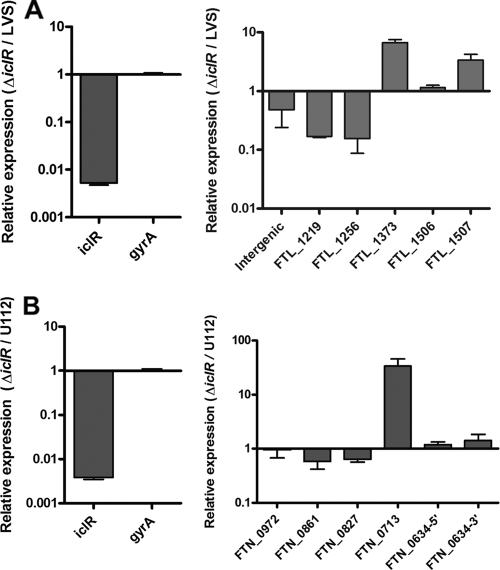
We next wanted to determine whether the set of genes that were changed in expression in LVS ΔiclR were also changed in the absence of IclR in U112. First, we performed quantitative RT-PCR on six genes that were differentially regulated in the microarray for LVS versus LVS ΔiclR (Fig. 7A), and we normalized the results to the housekeeping gene gyrA. We also included iclR. As expected, we detected a dramatic decrease in iclR transcript in LVS ΔiclR and a negligible change in gyrA. Of the six genes analyzed, four repeated the trend seen in the microarray analysis. For the two genes that did not, the primers appeared to amplify with similar efficiencies to other primers (data not shown). Overall, the qRT-PCR data support the fact that the genes identified in our microarray are changed in expression in the absence of IclR, based on a different method. We then tested the same set of gene homologs on RNA isolated from wild-type U112 and the U112 iclR transposon mutant (Fig. 7B). Quantitative RT-PCR first verified that iclR transcript levels were substantially lower in the transposon mutant. Overall, the six selected genes appeared to be changed to similar extents as their LVS homologs, suggesting a similar set of genes are affected by IclR in U112. These analyses did not account for any additional genes affected by the U112 IclR that were not affected by the LVS IclR as detected by microarray. Furthermore, these analyses alone are not sufficient to extrapolate any correlations in terms of IclR function or to determine which of the IclR-affected genes are likewise impacted at the protein or functional level.
FIG. 7.
Transcript levels of genes found significantly changed in microarray analysis comparing LVS and LVS ΔiclR. RNA was isolated from wild-type F. tularensis subsp. holarctica LVS and LVSΔiclR (A) or wild-type F. tularensis subsp. novicida U112 and a U112 iclR transposon mutant (B) and used in qRT-PCR analysis for several genes that were significantly changed in the microarray. Data are presented as the relative log change for the wild type versus the respective iclR mutant after normalization to gyrA. The graphs are representative of two or three experiments.
Effects of IclR on antibiotic resistance.
Other IclR family proteins are known to be involved in the regulation of multidrug efflux pumps (39). In all three F. tularensis subspecies, iclR is located near ORFs encoding hypothetical proteins that have homology to the EmrA multidrug efflux pump. In LVS, the two ORFs encoding proteins with EmrA homology that are found upstream of iclR were not changed in expression as determined by our microarray analysis. Nevertheless, the microarray data for LVS ΔiclR showed increased expression of a gene encoding a protein with homology to organic solvent tolerance proteins, suggesting that IclR may be involved in repression of some genes involved in drug efflux. Organic solvent tolerance is often associated with multidrug efflux pumps, most notably in Escherichia coli and Pseudomonas putida (42). Furthermore, our BLASTp analyses of hypothetical genes that appeared in the LVS ΔiclR microarray gene list also revealed proteins with homology to other transporter proteins. To determine whether iclR is involved in drug efflux, we performed disc diffusion assays using a panel of antibiotics. Antibiotics selected for analysis were chosen as representative of several classes of antibiotics targeting cell wall synthesis, protein synthesis, nucleic acid synthesis, and cell membrane integrity. There was no difference in antibiotic sensitivity between wild-type LVS and LVS ΔiclR using this method (Fig. 8).
FIG. 8.
Antibiotic sensitivity of LVS ΔiclR. Wild-type LVS and LVS ΔiclR were grown to mid-log phase, bacteria were spread on chocolate agar, and an antibiotic-containing paper disc was added to the center. Bacteria were grown for 36 h, and the diameter of the zone of inhibition was measured. The experiment was performed in triplicate, and the averages and standard deviations were calculated.
DISCUSSION
Herein we investigated the contribution of the putative transcriptional regulator IclR to F. tularensis pathogenicity. In this study, we found that the LVS ΔiclR was not attenuated for intracellular replication in J774A.1 macrophage-like cells, TC-1 epithelial cells, or bone marrow-derived macrophages. Similarly, SchuS4 ΔiclR was not attenuated for replication in J774A.1 cells. These data are consistent with published data by Weiss et al. for the F. tularensis subsp. novicida U112 iclR deletion mutant strain in bone marrow-derived macrophages (53).
Compared to wild-type LVS, LVS ΔiclR did not impact IL-1β induction or cytotoxicity of infected cells; these results are different from those of the published F. tularensis subsp. novicida studies (53). It is important to note that the methods used for these analyses were different between the two studies. The levels of IL-1β that we reported in this study are near but not below the limit of detection for the ELISA. The fact that the levels of IL-1β induced were low is consistent with other studies providing evidence that LVS suppresses the inflammatory response (26, 50). Furthermore, Weiss et al. used prestimulated bone marrow-derived macrophages, whereas we used naïve bone marrow-derived macrophages. Macrophages pretreated with LPS or heat-killed F. tularensis subp. novicida as well as thioglycolate-elicited macrophages produce higher levels of IL-1β in response to infection (11, 12, 36, 52). Another possibility is that there are strain-specific differences in the role of IclR, as evidenced by the results of the in vivo studies discussed below.
Unlike the F. tularensis subsp. novicida iclR deletion mutant, neither LVS ΔiclR nor SchuS4 ΔiclR was attenuated in mice following i.n. inoculation. There were differences in the experimental design between our studies and the F. tularensis subsp. novicida study. We initially inoculated mice i.n. and monitored the lungs, liver, and spleen over several days postinfection. Weiss et al. used s.c. and i.p. inoculations in competition assays and examined the spleen at 2 days postinfection. It is possible that the inoculation route may have an impact on the importance of IclR on establishing infection. To address the possibility that the phenotype is route specific, we performed a reciprocal analysis by evaluating the virulence of LVS and U112 iclR mutants in i.d. and i.n. infection models, respectively. The results confirmed that the Francisella virulence-specific properties of IclR are restricted to F. tularensis subsp. novicida.
It is not clear why IclR is required for virulence in U112 but not LVS and SchuS4. Based on our microarray analysis, the subspecies-specific sequence differences among IclR-affected genes could contribute to the functional differences we observed for IclR between subspecies. Many of the genes are intact in U112, but in LVS and/or SchuS4 the homologous gene(s) is a pseudogene or displayed significant sequence variation (e.g., two large deletions in FTT0715/FTL_1521). The virulent subspecies of F. tularensis are noted for their genome decay, as characterized by smaller genomes as well as increased numbers of pseudogenes, transposases, and gene rearrangements (51). Genome-wide analyses of Francisella strains support this idea, and many of the genes changed in LVS ΔiclR that we identified to be pseudogenes correlate with those found in other studies (7, 44). It is possible that IclR in F. tularensis subsp. novicida exerts its effects on genes that are intact, whereas in LVS and SchuS4, IclR affects genes that are similar to those in F. tularensis subsp. novicida but because of disruptions or changes to the ORFs, many of these genes are transcribed but do not encode functional proteins. We must also consider that there are two genes in the list that are absent in U112 that are present in LVS and SchuS4, and the absence of a gene affected by IclR in F. tularensis subsp. novicida could also contribute to the different phenotypes. Overall, analysis of the genes identified in our microarray suggests that the majority of genes affected by IclR have differences in sequence between the three subspecies and that this variation could contribute to the phenotypic disparities observed.
Taken together, our data suggest that IclR contributes to the virulence of U112 but not to that of LVS or SchuS4, highlighting the fact that there are significant differences among these strains. Another example of differences among strains is seen in the conserved acid phosphatases, AcpA, AcpB, and AcpC. These proteins have been shown to be required for the virulence of F. tularensis subsp. novicida, but not for the virulence of SchuS4 (8, 38). Even though IclR may not play a major role in SchuS4 or LVS virulence, there are other potential roles that IclR could have as a functional transcriptional regulator. Quite a few of the microarray-identified genes encode hypothetical proteins, but there are others that encode proteins with known functions or are homologous to proteins with known functions. Investigation into these proteins may provide additional understanding of the function of IclR in F. tularensis. For example, in Pseudomonas putida, the IclR family proteins TtgT and TtgV regulate operons encoding genes that form efflux pumps for organic solvent extrusion (16, 22). Although our antibiotic sensitivity assays showed no role for IclR in drug efflux by LVS, we cannot rule out the involvement of IclR in the regulation of a system specific for organic solvent efflux or the role of IclR in drug efflux in other F. tularensis subspecies. Finally, direct comparison of the complete transcriptional profiles of F. tularensis subsp. novicida, F. tularensis subsp. tularensis, and F. tularensis subsp. holarctica iclR deletion strains might reveal some clues to the properties that are responsible for the phenotypic differences. Unfortunately, the currently available microarrays do not contain targets for genes found exclusively in F. tularensis subsp. novicida.
Acknowledgments
This work was supported by NIH grants U54 AI057157 (T.K.) from the Southeastern Regional Center of Excellence for Emerging Infections and Biodefense and by R56 AI069339 (T.K.).
The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
The Francisella microarrays were obtained through NIAID's Pathogen Functional Genomics Resource Center, managed and funded by the Division of Microbiology and Infectious Diseases, NIAID, NIH, DHHS, and operated by the J. Craig Venter Institute. We give special thanks to Eric LoVullo, Jason Brunton, and Shaun Steele for their assistance performing the animal experiments and to John C. Braisted and Jianwei Li (J. Craig Venter Institute) for their assistance in using the TM4 Suite software.
Editor: A. Camilli
Footnotes
Published ahead of print on 4 October 2010.
REFERENCES
- 1.Anthony, L. D., R. D. Burke, and F. E. Nano. 1991. Growth of Francisella spp. in rodent macrophages. Infect. Immun. 59:3291-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birdsell, D. N., T. Stewart, A. J. Vogler, E. Lawaczeck, A. Diggs, T. L. Sylvester, J. L. Buchhagen, R. K. Auerbach, P. Keim, and D. M. Wagner. 2009. Francisella tularensis subsp. novicida isolated from a human in Arizona. BMC Res. Notes 2:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosio, C. M., and S. W. Dow. 2005. Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J. Immunol. 175:6792-6801. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2005. Tularemia transmitted by insect bites—Wyoming, 2001-2003. MMWR Morb. Mortal. Wkly. Rep. 54:170-173. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2009. Tularemia—Missouri, 2000-2007. MMWR Morb. Mortal. Wkly. Rep. 58:744-748. [PubMed] [Google Scholar]
- 6.Chamberlain, R. E. 1965. Evaluation of live tularemia vaccine prepared in a chemically defined medium. Appl. Microbiol. 13:232-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Champion, M. D., Q. Zeng, E. B. Nix, F. E. Nano, P. Keim, C. D. Kodira, M. Borowsky, S. Young, M. Koehrsen, R. Engels, M. Pearson, C. Howarth, L. Larson, J. White, L. Alvarado, M. Forsman, S. W. Bearden, A. Sjostedt, R. Titball, S. L. Michell, B. Birren, and J. Galagan. 2009. Comparative genomic characterization of Francisella tularensis strains belonging to low and high virulence subspecies. PLoS Pathog. 5:e1000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Child, R., T. D. Wehrly, D. Rockx-Brouwer, D. W. Dorward, and J. Celli. 2010. Acid phosphatases do not contribute to the pathogenesis of type A Francisella tularensis. Infect. Immun. 78:59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarridge, J. E., III, T. J. Raich, A. Sjosted, G. Sandstrom, R. O. Darouiche, R. M. Shawar, P. R. Georghiou, C. Osting, and L. Vo. 1996. Characterization of two unusual clinically significant Francisella strains. J. Clin. Microbiol. 34:1995-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemens, D. L., B. Y. Lee, and M. A. Horwitz. 2004. Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect. Immun. 72:3204-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole, L. E., K. L. Elkins, S. M. Michalek, N. Qureshi, L. J. Eaton, P. Rallabhandi, N. Cuesta, and S. N. Vogel. 2006. Immunologic consequences of Francisella tularensis live vaccine strain infection: role of the innate immune response in infection and immunity. J. Immunol. 176:6888-6899. [DOI] [PubMed] [Google Scholar]
- 12.Cole, L. E., A. Santiago, E. Barry, T. J. Kang, K. A. Shirey, Z. J. Roberts, K. L. Elkins, A. S. Cross, and S. N. Vogel. 2008. Macrophage proinflammatory response to Francisella tularensis live vaccine strain requires coordination of multiple signaling pathways. J. Immunol. 180:6885-6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowley, S. C., C. J. Gray, and F. E. Nano. 2000. Isolation and characterization of Francisella novicida mutants defective in lipopolysaccharide biosynthesis. FEMS Microbiol. Lett. 182:63-67. [DOI] [PubMed] [Google Scholar]
- 14.Deng, K., R. J. Blick, W. Liu, and E. J. Hansen. 2006. Identification of Francisella tularensis genes affected by iron limitation. Infect. Immun. 74:4224-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dennis, D. T., T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, M. Layton, S. R. Lillibridge, J. E. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, K. Tonat, and the Working Group on Civilian Biodefense. 2001. Tularemia as a biological weapon: medical and public health management. JAMA 285:2763-2773. [DOI] [PubMed] [Google Scholar]
- 16.Duque, E., A. Segura, G. Mosqueda, and J. L. Ramos. 2001. Global and cognate regulators control the expression of the organic solvent efflux pumps TtgABC and TtgDEF of Pseudomonas putida. Mol. Microbiol. 39:1100-1106. [DOI] [PubMed] [Google Scholar]
- 17.Elkins, K. L., R. K. Winegar, C. A. Nacy, and A. H. Fortier. 1992. Introduction of Francisella tularensis at skin sites induces resistance to infection and generation of protective immunity. Microb. Pathog. 13:417-421. [DOI] [PubMed] [Google Scholar]
- 18.Feldman, K. A., R. E. Enscore, S. L. Lathrop, B. T. Matyas, M. McGuill, M. E. Schriefer, D. Stiles-Enos, D. T. Dennis, L. R. Petersen, and E. B. Hayes. 2001. An outbreak of primary pneumonic tularemia on Martha's Vineyard. N. Engl. J. Med. 345:1601-1606. [DOI] [PubMed] [Google Scholar]
- 19.Fortier, A. H., M. V. Slayter, R. Ziemba, M. S. Meltzer, and C. A. Nacy. 1991. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect. Immun. 59:2922-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuller, J. R., R. R. Craven, J. D. Hall, T. M. Kijek, S. Taft-Benz, and T. H. Kawula. 2008. RipA, a cytoplasmic membrane protein conserved among Francisella species, is required for intracellular survival. Infect. Immun. 76:4934-4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallagher, L. A., E. Ramage, M. A. Jacobs, R. Kaul, M. Brittnacher, and C. Manoil. 2007. A comprehensive transposon mutant library of Francisella novicida, a bioweapon surrogate. Proc. Natl. Acad. Sci. U. S. A. 104:1009-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guazzaroni, M. E., W. Teran, X. Zhang, M. T. Gallegos, and J. L. Ramos. 2004. TtgV bound to a complex operator site represses transcription of the promoter for the multidrug and solvent extrusion TtgGHI pump. J. Bacteriol. 186:2921-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall, J. D., R. R. Craven, J. R. Fuller, R. J. Pickles, and T. H. Kawula. 2007. Francisella tularensis replicates within alveolar type II epithelial cells in vitro and in vivo following inhalation. Infect. Immun. 75:1034-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollis, D. G., R. E. Weaver, A. G. Steigerwalt, J. D. Wenger, C. W. Moss, and D. J. Brenner. 1989. Francisella philomiragia comb. nov. (formerly Yersinia philomiragia) and Francisella tularensis biogroup novicida (formerly Francisella novicida) associated with human disease. J. Clin. Microbiol. 27:1601-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61-68. [DOI] [PubMed] [Google Scholar]
- 26.Huang, M. T., B. M. Mortensen, D. J. Taxman, R. R. Craven, S. Taft-Benz, T. M. Kijek, J. R. Fuller, B. K. Davis, I. C. Allen, W. Brickey, D. Gris, H. Wen, T. H. Kawula, and J. P. Ting. 4 October 2010. Deletion of ripA alleviates suppression of the inflammasome and MAP kinase by Francisella tularensis. J. Immunol. doi: 10.4049/jimmunol.1002154. [DOI] [PMC free article] [PubMed]
- 27.Kadzhaev, K., C. Zingmark, I. Golovliov, M. Bolanowski, H. Shen, W. Conlan, and A. Sjostedt. 2009. Identification of genes contributing to the virulence of Francisella tularensis SCHU S4 in a mouse intradermal infection model. PLoS One. 4:e5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kandemir, B., I. Erayman, M. Bitirgen, E. T. Aribas, and S. Guler. 2007. Tularemia presenting with tonsillopharyngitis and cervical lymphadenitis: report of two cases. Scand. J. Infect. Dis. 39:620-622. [DOI] [PubMed] [Google Scholar]
- 29.Keim, P., A. Johansson, and D. M. Wagner. 2007. Molecular epidemiology, evolution, and ecology of Francisella. Ann. N. Y. Acad. Sci. 1105:30-66. [DOI] [PubMed] [Google Scholar]
- 30.Kraemer, P. S., A. Mitchell, M. R. Pelletier, L. A. Gallagher, M. Wasnick, L. Rohmer, M. J. Brittnacher, C. Manoil, S. J. Skerett, and N. R. Salama. 2009. Genome-wide screen in Francisella novicida for genes required for pulmonary and systemic infection in mice. Infect. Immun. 77:232-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krell, T., A. J. Molina-Henares, and J. L. Ramos. 2006. The IclR family of transcriptional activators and repressors can be defined by a single profile. Protein Sci. 15:1207-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leelaporn, A., S. Yongyod, S. Limsrivanichakorn, T. Yungyuen, and P. Kiratisin. 2008. Francisella novicida bacteremia, Thailand. Emerg. Infect. Dis. 14:1935-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LoVullo, E. D., L. A. Sherrill, L. L. Perez, and M. S. Pavelka, Jr. 2006. Genetic tools for highly pathogenic Francisella tularensis subsp. tularensis. Microbiology 152:3425-3435. [DOI] [PubMed] [Google Scholar]
- 34.Lubbert, C., C. Taege, T. Seufferlein, and R. Grunow. 2009. Prolonged course of tick-borne ulceroglandular tularemia in a 20-year-old patient in Germany: case report and review of the literature. Dtsch. Med. Wochenschr. 134:1405-1410. [DOI] [PubMed] [Google Scholar]
- 35.Maier, T. M., M. S. Casey, R. H. Becker, C. W. Dorsey, E. M. Glass, N. Maltsev, T. C. Zahrt, and D. W. Frank. 2007. Identification of Francisella tularensis Himar1-based transposon mutants defective for replication in macrophages. Infect. Immun. 75:5376-5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mariathasan, S., D. S. Weiss, V. M. Dixit, and D. M. Monack. 2005. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 202:1043-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCaffrey, R. L., and L. A. Allen. 2006. Francisella tularensis LVS evades killing by human neutrophils via inhibition of the respiratory burst and phagosome escape. J. Leukoc. Biol. 80:1224-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohapatra, N. P., S. Soni, T. J. Reilly, J. Liu, K. E. Klose, and J. S. Gunn. 2008. Combined deletion of four Francisella novicida acid phosphatases attenuates virulence and macrophage vacuolar escape. Infect. Immun. 76:3690-3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molina-Henares, A. J., T. Krell, M. Eugenia Guazzaroni, A. Segura, and J. L. Ramos. 2006. Members of the IclR family of bacterial transcriptional regulators function as activators and/or repressors. FEMS Microbiol. Rev. 30:157-186. [DOI] [PubMed] [Google Scholar]
- 40.Ojeda, S. S., Z. J. Wang, C. A. Mares, T. A. Chang, Q. Li, E. G. Morris, P. A. Jerabek, and J. M. Teale. 2008. Rapid dissemination of Francisella tularensis and the effect of route of infection. BMC Microbiol. 8:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin, A., and B. J. Mann. 2006. Identification of transposon insertion mutants of Francisella tularensis tularensis strain Schu S4 deficient in intracellular replication in the hepatic cell line HepG2. BMC Microbiol. 6:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramos, J. L., E. Duque, M. T. Gallegos, P. Godoy, M. I. Ramos-Gonzalez, A. Rojas, W. Teran, and A. Segura. 2002. Mechanisms of solvent tolerance in gram-negative bacteria. Annu. Rev. Microbiol. 56:743-768. [DOI] [PubMed] [Google Scholar]
- 43.Reintjes, R., I. Dedushaj, A. Gjini, T. R. Jorgensen, B. Cotter, A. Lieftucht, F. D'Ancona, D. T. Dennis, M. A. Kosoy, G. Mulliqi-Osmani, R. Grunow, A. Kalaveshi, L. Gashi, and I. Humolli. 2002. Tularemia outbreak investigation in Kosovo: case control and environmental studies. Emerg. Infect. Dis. 8:69-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rohmer, L., C. Fong, S. Abmayr, M. Wasnick, T. J. Larson Freeman, M. Radey, T. Guina, K. Svensson, H. S. Hayden, M. Jacobs, L. A. Gallagher, C. Manoil, R. K. Ernst, B. Drees, D. Buckley, E. Haugen, D. Bovee, Y. Zhou, J. Chang, R. Levy, R. Lim, W. Gillett, D. Guenthener, A. Kang, S. A. Shaffer, G. Taylor, J. Chen, B. Gallis, D. A. D'Argenio, M. Forsman, M. V. Olson, D. R. Goodlett, R. Kaul, S. I. Miller, and M. J. Brittnacher. 2007. Comparison of Francisella tularensis genomes reveals evolutionary events associated with the emergence of human pathogenic strains. Genome Biol. 8:R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saeed, A. I., V. Sharov, J. White, J. Li, W. Liang, N. Bhagabati, J. Braisted, M. Klapa, T. Currier, M. Thiagarajan, A. Sturn, M. Snuffin, A. Rezantsev, D. Popov, A. Ryltsov, E. Kostukovich, I. Borisovsky, Z. Liu, A. Vinsavich, V. Trush, and J. Quackenbush. 2003. TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34:374-378. [DOI] [PubMed] [Google Scholar]
- 46.Saslaw, S., H. T. Eigelsbach, J. A. Prior, H. E. Wilson, and S. Carhart. 1961. Tularemia vaccine study. II. Respiratory challenge. Arch. Intern. Med. 107:702-714. [DOI] [PubMed] [Google Scholar]
- 47.Schulert, G. S., R. L. McCaffrey, B. W. Buchan, S. R. Lindemann, C. Hollenback, B. D. Jones, and L. A. Allen. 2009. Francisella tularensis genes required for inhibition of the neutrophil respiratory burst and intramacrophage growth identified by random transposon mutagenesis of strain LVS. Infect. Immun. 77:1324-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siret, V., D. Barataud, M. Prat, V. Vaillant, S. Ansart, A. Le Coustumier, J. Vaissaire, F. Raffi, M. Garre, and I. Capek. 2006. An outbreak of airborne tularaemia in France, August 2004. Euro Surveill. 11:58-60. [PubMed] [Google Scholar]
- 49.Su, J., J. Yang, D. Zhao, T. H. Kawula, J. A. Banas, and J. R. Zhang. 2007. Genome-wide identification of Francisella tularensis virulence determinants. Infect. Immun. 75:3089-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Telepnev, M., I. Golovliov, T. Grundstrom, A. Tarnvik, and A. Sjostedt. 2003. Francisella tularensis inhibits Toll-like receptor-mediated activation of intracellular signalling and secretion of TNF-alpha and IL-1 from murine macrophages. Cell. Microbiol. 5:41-51. [DOI] [PubMed] [Google Scholar]
- 51.Titball, R. W., and J. F. Petrosino. 2007. Francisella tularensis genomics and proteomics. Ann. N. Y. Acad. Sci. 1105:98-121. [DOI] [PubMed] [Google Scholar]
- 52.Ulland, T. K., B. W. Buchan, M. R. Ketterer, T. Fernandes-Alnemri, D. K. Meyerholz, M. A. Apicella, E. S. Alnemri, B. D. Jones, W. M. Nauseef, and F. S. Sutterwala. 2010. Cutting edge: mutation of Francisella tularensis mviN leads to increased macrophage absent in melanoma 2 inflammasome activation and a loss of virulence. J. Immunol. 185:2670-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weiss, D. S., A. Brotcke, T. Henry, J. J. Margolis, K. Chan, and D. M. Monack. 2007. In vivo negative selection screen identifies genes required for Francisella virulence. Proc. Natl. Acad. Sci. U. S. A. 104:6037-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]