Abstract
Streptococcus suis is an important swine and human pathogen responsible for septicemia and meningitis. In vivo research in mice suggested that in the brain, microglia might be involved in activating the inflammatory response against S. suis. The aim of this study was to better understand the interactions between S. suis and microglia. Murine microglial cells were infected with a virulent wild-type strain of S. suis. Two isogenic mutants deficient at either capsular polysaccharide (CPS) or hemolysin production were also included. CPS contributed to S. suis resistance to phagocytosis and regulated the inflammatory response by hiding proinflammatory components from the bacterial cell wall, while the absence of hemolysin, a potential cytotoxic factor, did not have a major impact on S. suis interactions with microglia. Wild-type S. suis induced enhanced expression of Toll-like receptor 2 by microglial cells, as well as phophotyrosine, protein kinase C, and different mitogen-activated protein kinase signaling events. However, cells infected with the CPS-deficient mutant showed overall stronger and more sustained phosphorylation profiles. CPS also modulated inducible nitric oxide synthase expression and further nitric oxide production from S. suis-infected microglia. Finally, S. suis-induced NF-κB translocation was faster for cells stimulated with the CPS-deficient mutant, suggesting that bacterial cell wall components are potent inducers of NF-κB. These results contribute to increase the knowledge of mechanisms underlying S. suis inflammation in the brain and will be useful in designing more efficient anti-inflammatory strategies for meningitis.
Streptococcus suis is one of the most important swine pathogens worldwide, as well as an important agent of zoonosis. So far, 35 serotypes have been described, although serotype 2 is still the most frequently isolated from both swine and humans. In swine, meningitis is the most striking feature of the infection, although other pathologies, such as septicemia, endocarditis, pneumonia, and arthritis, have been described (35). Although the most common pathology associated with S. suis infection in humans is also meningitis, cases of septicemia with septic shock, endocarditis, and several other clinical manifestations have been reported (69, 71). As a zoonosis, S. suis infection has been traditionally considered an occupational hazard, since most cases described in Western countries have occurred in people working in close contact with pigs or raw pork products. The situation in Asian countries is completely different, as the common population is affected. In addition to an important human outbreak in China caused by S. suis in 2005 (71), the pathogen has recently been reported to be the most frequent cause of bacterial meningitis in adults in Vietnam (29) and the third most common culture-confirmed cause of community-acquired bacterial meningitis in Hong Kong. People who survive S. suis infection may be handicapped, as severe postinfection sequels, such as deafness, may develop (67, 68).
In recent years, a number of important studies describing the proposed virulence factors of S. suis serotype 2 have been published (4, 29). However, few candidates have been shown to be critical for virulence. Among them, the capsular polysaccharide (CPS) is considered an important antiphagocytic factor (13). Although not essential for virulence, a hemolysin (suilysin) produced by most virulent strains in Eurasia has also been shown to be toxic for cells of murine, human, and swine origin (12, 14, 55, 66).
The pathogenesis of S. suis infection has been partially elucidated. In swine, infection occurs through the respiratory route with subsequent colonization of the tonsils, while in humans, access is mainly through skin cuts and/or the oral route (3, 28, 29). Once S. suis reaches the bloodstream, it travels either free or associated with monocytes (27), with invasion of different tissues and organs. The high mortality observed at this stage of the disease may be associated with septic shock with an exacerbated release of proinflammatory cytokines (18, 19). However, if the host overcomes septicemia, S. suis may still invade the central nervous system (CNS) and cause meningitis and, in some cases, encephalitis. The mechanisms used by the pathogen to gain access to the brain and induce local inflammation are still under debate. It is likely that access is by transcytosis and/or toxicity to brain microvascular endothelial cells (66) and/or choroid plexus epithelial cells that are part of the blood brain barrier (BBB) (64). Increase of BBB permeability due to inflammation cannot be ruled out (27).
Recently, our laboratory developed an in vivo mouse model of meningitis/encephalitis after S. suis infection via the intraperitoneal route (18). Using this model, an important inflammatory response in the CNS with expression of different proinflammatory genes, including Toll-like receptor 2 (TLR2), CD14, IκBα (an index of NF-κB expression), interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), and monocyte chemoattractant protein 1 (MCP-1), was observed. Interestingly, the expression of these genes and bacterial antigens was found to probably be associated with microglia and, to a lesser extent, with astrocytes (18). Microglia, the macrophage-like population within the CNS, represent the first line of defense against invading pathogens and have proinflammatory effector functions (49). Although previous findings draw attention to the implication of these cells in the development of meningitis and encephalitis, it is critical to dissect how the cells initiate key proinflammatory mechanisms in order to respond to S. suis infection. Hence, the goal of this study was to explore the murine microglial response to a virulent strain of S. suis, as well as isogenic mutants defective in either CPS or suilysin production. The ability of microglia to internalize S. suis, to activate TLRs, and to secrete different proinflammatory mediators, as well as to activate important inflammatory intracellular signaling pathways, was evaluated.
MATERIALS AND METHODS
Cell culture.
The murine microglial cell line BV-2 was kindly provided by S. Rivest (Université Laval, Quebec, Canada). This cell line exhibits morphological and functional characteristics of microglia (7, 8). It has recently been shown that the cell line is a valid substitute for primary microglial cells (34). BV-2 microglia were maintained in vitro in high-glucose Dulbecco's modified Eagle's medium (DMEM) (Wisent Inc., St-Jean Baptiste, Quebec, Canada) containing 10% heat-inactivated fetal bovine serum and 2% penicillin-streptomycin (both from Invitrogen, Carlsbad, CA). The cells were kept in 75-cm2 Falcon flasks at 37°C in a humidified atmosphere containing 5% CO2. Experiments were performed in 24-well plates unless otherwise specified, with a concentration of 5 × 105 cells/well. Absence of cell toxicity with the different S. suis strains and concentrations tested was evaluated by measuring the release of lactate dehydrogenase enzyme with the Cyto Tox 96 Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI), as previously described (66).
Bacterial strains and growth conditions.
Three strains of S. suis capsular serotype 2 were used, the virulent wild-type (WT) suilysin-positive strain 31533, originally isolated from a case of porcine meningitis and widely used in previous studies (18, 19, 42, 58, 59), as well as two isogenic mutants obtained by allelic exchange, the suilysin-negative (SLY−) mutant SX911 (45) and the nonencapsulated (CPS−) mutant B218 (23). Bacteria were grown overnight on sheep blood agar plates at 37°C, and isolated colonies were inoculated into 5 ml of Todd-Hewitt broth (THB) (Difco Laboratories, Detroit, MI), which was incubated for 8 h at 37°C with agitation. Working cultures were prepared by transferring 10 μl of 1/1,000 dilutions of 8-h cultures into 30 ml of THB, which was incubated for 16 h at 37°C with agitation. Stationary-phase bacteria were washed twice in phosphate-buffered saline (PBS), pH 7.3. For experiments, the bacterial pellet was resuspended and diluted in cell culture medium to a final concentration of 1 × 106 CFU/ml, unless otherwise specified. When necessary, the WT and CPS− S. suis strains were heat killed, as previously described, at 60°C for 45 min (the minimal experimental conditions required for S. suis killing) (57).
Phagocytosis assays.
Phagocytosis assays were performed as previously described (60) with some modifications. Briefly, microglial cells were infected by removing the culture medium and adding different S. suis strains resuspended in cell culture media at a multiplicity of infection (MOI) of 2:1. In selected experiments, bacterial opsonization was performed by incubating bacteria in the presence of 20% (vol/vol) complete normal or heat-inactivated mouse serum in PBS for 30 min at 37°C with shaking prior to cell infection. Serum from C57BL/6 mice was inactivated by incubation for 30 min at 56°C. After 15, 30, and 60 min of infection, the cell monolayers were washed twice with warm culture medium and reincubated for 1 h with medium containing penicillin G (5 μg/ml) and gentamicin (100 μg/ml) to kill extracellular bacteria (both antibiotics were from Sigma-Aldrich, Oakville, Ontario, Canada). Previous studies with S. suis showed that this concentration of antibiotics is able to kill any remaining extracellular bacteria (56, 60). In addition, supernatant controls were used in every test to confirm antibiotic efficacy. After antibiotic treatment, the cell monolayers were washed three times, and the medium was replaced with 1 ml of sterile distilled water to lyse microglial cells. After vigorous pipetting to ensure complete cell lysis, viable intracellular streptococci were determined by quantitative plating of serial dilutions of the lysates on THB agar. All samples were plated using an Autoplate 400 Automated Spiral Plater (SpiralBiotech Inc., Norwood, MA). Each test was repeated four times in independent experiments, and the number of CFU recovered per well (mean number ± standard error of the mean [SEM]) was determined.
Phagocytosis was confirmed by confocal microscopy. Microglial cells were plated on coverslips and infected with WT and CPS− S. suis strains. After 2 h of bacterium-cell contact, the coverslips were washed with culture medium to remove nonassociated bacteria, and the cells were fixed with methanol/acetone (80:20) for 20 min at −20°C, washed, and blocked with PBS containing 2% fetal bovine serum for 10 min. The coverslips were incubated for 1 h with optimal dilutions of rabbit hyperimmune anti-S. suis serum, produced as described previously (36), and with rat anti-LAMP1 antibody (Developmental Studies Hybridoma Bank, IA). After being washed, the coverslips were incubated with the secondary antibodies Alexa Fluor 488-goat anti-rabbit IgG and Alexa Fluor 568-goat anti-rat IgG (Invitrogen) for 30 min, washed, and mounted on glass slides with Mowiol containing DABCO (1,4-diazabicyclo-[2,2,2]-octane) and DAPI (4′,6-diamidino-2-phenylindole) to stain the nuclei. Samples were observed with an IX-80 confocal microscope integrated into the FV-1000 imagery system and analyzed using Fluoview software (Olympus, Markham, Ontario, Canada).
Cytokine and chemokine detection by ELISA.
Microglial cells were infected with the S. suis WT and mutant strains included in the study at an MOI of 2:1. Purified Escherichia coli lipopolysaccharide (LPS) at 1 μg/ml (Sigma-Aldrich) was used as a positive control. After 12 h of incubation (the optimal incubation time as observed in kinetic studies [data not shown]), the supernatant was recovered to measure levels of IL-1β, IL-6, TNF-α, MCP-1, and the chemokine CXC chemokine ligand 10/interferon-inducible protein of 10 kDa (CXCL10/IP-10) by a sandwich enzyme-linked immunosorbent assay (ELISA), using commercially available antibody pairs (R&D Systems, Minneapolis, MN), as previously described (58). Standard curves were included in each ELISA plate (Nunc, Ville Mont Royal, Quebec, Canada) as 2-fold dilutions of recombinant mouse IL-1β (1,000 to 39 pg/ml), IL-6 (1,250 to 39 pg/ml), TNF-α (1,000 to 8 pg/ml), MCP-1 (500 to 8 pg/ml), or IP-10 (4,000 to 31 pg/ml) (R&D Systems). Supernatant dilutions giving optical density readings in the linear portion of the appropriate standard curve were used to determine the level of each cytokine in the samples. Standard and sample dilutions were added in duplicate wells to each ELISA plate.
Analysis of TLR gene expression by real-time reverse transcriptase-quantitative PCR.
Microglial cells were infected with WT and CPS− S. suis strains at an MOI of 2:1 for 0, 1, 2, 4, and 8 h. Following infection, the medium was removed and the cells were washed with cell culture medium. Total cellular RNA was prepared from the cells using Trizol (Invitrogen) following standard procedures. Next, 1 μg of total RNA was reverse transcribed with the QuantiTect reverse transcription kit (Qiagen, Mississauga, Ontario, Canada), and the resulting cDNA was amplified using the SsoFast EvaGreen Supermix kit (Bio-Rad, Hercules, CA). The PCR amplification program for all cDNA samples consisted of an enzyme activation step of 3 min at 98°C, followed by 40 cycles of a denaturing step for 3 s at 98°C and an annealing/extension step for 5 s at 57°C. Each 10-μl reaction mixture contained 250 nM (each) forward and reverse primers. The primers used for amplification of the different target cDNAs are listed in Table 1 and were all tested to achieve an amplification efficiency between 90% and 110%. The primer sequences were all designed from NCBI GenBank mRNA sequences using the Web-based software Primerquest from Integrated DNA Technologies. The Bio-Rad CFX-96 sequence detector was used for amplification of target cDNAs of various TLRs, and quantitation of differences between the different groups was calculated using the 2−ΔΔCt method. β-Actin and β2 microglobulin (β2m) genes were used as normalizing genes to compensate for potential differences in cDNA amounts between the various samples. These two genes were selected from candidate normalizing genes using the Normfinder V19 algorithm (2), as their expression was found to be the most stable under the experimental conditions. The noninfected BV-2 microglial group was used as the calibrator reference in the analysis.
TABLE 1.
Primers used in real-time quantitative PCR for TLR amplification and detection
| Gene | Primer |
Amplicon size (bp) | |
|---|---|---|---|
| Forward | Reverse | ||
| TLR1 | CACAGCTCCTTGGTTTTAATG | TGGGTATAGGACGTTTCTGTAG | 102 |
| TLR2 | TGGAGCATCCGAATTGCATCACCG | GAGCGGCCATCACACACCCC | 193 |
| TLR3 | CGGGGGTCCAACTGGAGAACCT | GGGCGTTGTTCAAGAGGAGGGC | 198 |
| TLR4 | GCCTCCCTGGCTCCTGGCTA | AGGGACTTTGCTGAGTTTCTGATCCA | 139 |
| TLR6 | CCGTCAGTGCTGGAAATAG | CGATGGGTTTTCTGTCTTGG | 108 |
| TLR9 | CAGTTTGTCAGAGGGAGC | ACTTCAGGAACAGCCAATTG | 198 |
| β-Actin | CCAACCGTGAAAAGATGACC | AGCATAGCCCTCGTAGATG | 170 |
| β2 m | ATGGCTCGCTCGGTGACCCT | TTCTCCGGTGGGTGGCGTGA | 110 |
Measurement of nitric oxide production.
Microglial cells were seeded and stimulated with killed bacteria as previously described (56), with some modifications. Briefly, heat-killed S. suis WT and CPS− strains (a concentration equivalent to 1 × 109 CFU/ml) were incubated with cells (5 × 105 cells/well) for 6, 12, 24, and 48 h. LPS (1 μg/ml) was used as a positive control. Nitric oxide (NO) production was determined by measuring nitrite, a stable end product of NO. The microglial culture supernatant was assayed by mixing it with Griess reagent (Promega, Madison, WI). Sulfonamide (1%) and N-1 [(1-naphthyl)-ethylenediamine dihydrochloride] (0.1%) were added to the supernatant. After 30 min of incubation at room temperature, the absorbance was read at 540 nm. The nitrite concentration (expressed as μM/ml) was calculated using standard solutions of sodium nitrite prepared in culture medium.
Signaling pathway analysis by immunoblotting.
Western blotting was performed to analyze the phosphorylation states of the protein kinase C (PKC) pathway and several mitogen-activated protein kinases (MAPKs), including stress-activated protein kinase/c-Jun NH2-terminal kinase (SAPK/JNK), extracellular signal-regulated kinase (ERK), and p38, as well as total tyrosine phosphorylation and inducible nitric oxide synthase (iNOS) expression, as previously described (38, 50). Briefly, cells were plated in a 6-well plate and stimulated with S. suis WT and CPS− strains at an MOI of 2:1. LPS (1 μg/ml) was used as a positive control. After different incubation times (see Results), the cells were lysed in cold buffer containing 0.5 M Tris (pH 6.8), 0.5 M EDTA, 1% (vol/vol) β-mercaptoethanol (Bio-Rad, Mississauga, Ontario, Canada), 10 mM EGTA, 10% (vol/vol) IGEPAL [(octylphenoxy)polyethoxyethanol], 1 mM sodium orthovanadate (Na3VO4) (all from Sigma-Aldrich), and the protease inhibitors aprotinin (10 μg/ml) (Sigma-Aldrich) and leupeptin (5 μg/ml) (Roche, Mississauga, Ontario, Canada). The lysates (60 μg/lane) were separated by SDS-PAGE, and the proteins were transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). The membranes were blocked in Tris-buffered saline-0.1% Tween containing 1% bovine serum albumin (or 5% skim milk for anti-α-actin) for 1 h at room temperature. The membranes were then washed and incubated overnight at 4°C with one of the following antibodies: anti-phospho-ERK (p-ERK) 1/2 (Thr202/Tyr204), anti-phospho-p38 (Thr180/Tyr182), anti-phospho-(Ser) PKC substrate antibody (all from Cell Signaling, Danvers, MA), anti-phospho SAPK/JNK 1/2 (Thr183/Tyr185) (Invitrogen), anti-phospho-Tyr (clone 4G10; Upstate, Lake Placid, NY), or anti-iNOS (BD Systems, San Jose, CA). After being washed, the membranes were incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse antibody (Sigma-Aldrich) for 1 h, and the proteins were visualized with ECL Plus Western blotting reagent (Amersham, Arlington Heights, IL). The membranes were then stripped using the Restore Western Blot Stripping Buffer (Pierce, Rockford, IL), and blotting was repeated using anti-protein antibodies (according to the instructions of the manufacturer of the anti-phospho-protein). Blotting with anti-α-actin antibody (Sigma-Aldrich) was used as a loading contol for p-Tyr, p-PKC, and iNOS blots.
MAPK inhibition assays.
In selected experiments, microglial cells were pretreated with the ERK inhibitor apigenin (50 μM), the SAPK-JNK inhibitor SP600125 (50 μM), or the p38 inhibitor SB203580 (75 μM) (all from Biomol Research Laboratories, Plymouth Meeting, PA) 1 h prior to infection. Western blot analysis of the corresponding phosphorylated proteins was done as described above after 2 h or 4 h of incubation in the presence of bacteria. Cytokine measurement was performed as described above after 12 h of incubation. The inhibitors were used at maximal subcytotoxic concentrations as determined by the Cyto Tox 96 Non-Radioactive Cytotoxicity Assay. An inhibitor was considered cytotoxic if viability was <90% of that of the untreated control after 12 h.
NF-κB binding.
An electrophoretic mobility shift assay (EMSA) was performed as previously described (50) with some modifications. Briefly, microglial cells were placed in a 25-cm2 flask and allowed to adhere overnight prior to infection with either an S. suis WT or CPS− strain at an MOI of 2:1. LPS (1 μg/ml) was used as a positive control. The cells were washed with ice-cold culture medium and scraped in 1 ml of culture medium. After centrifugation, the cells were resuspended in 400 μl of ice-cold buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol [DTT], and 1 mM phenylmethylsulfonyl fluoride [PMSF]) and incubated on ice for 15 min. Twenty-five microliters of 10% IGEPAL was then added. The tubes were vortexed for 10 s and centrifuged at maximum speed for 30 s. Nuclear fractions were resuspended in 50 μl of ice-cold buffer C (20 mM HEPES, pH 7.9, 400 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, and 1 mM PMSF) and incubated at 4°C on a shaking platform for 15 min. After centrifugation at 12,000 × g for 5 min at 4°C, the supernatants were stored at −70°C until further use. Then, 7 μg of these nuclear protein extracts was mixed with a γ-32P-labeled oligonucleotide containing a consensus binding sequence for NF-κB (Santa Cruz Biotechnology, Santa Cruz, CA). Complexes were resolved by electrophoresis on a 4% nondenaturing olyacrylamide gel. The gel was dried and visualized by autoradiography. The consensus sequence for NF-κB was 5′-AGT TGA GGG GAC TTT CCC AGG C-3′.
Statistical analysis.
Each test was done at least in triplicate biological repetitions. Differences were analyzed for significance by using the Student unpaired t test (two-tailed P value). A P value of <0.05 was considered significant. All data are presented as means ± standard errors of the mean (SEM). All statistical analyses were performed using Sigma Plot software (v.11; Systat Software, San Jose, CA).
RESULTS
The ability of murine microglia to phagocytose S. suis is modulated by CPS.
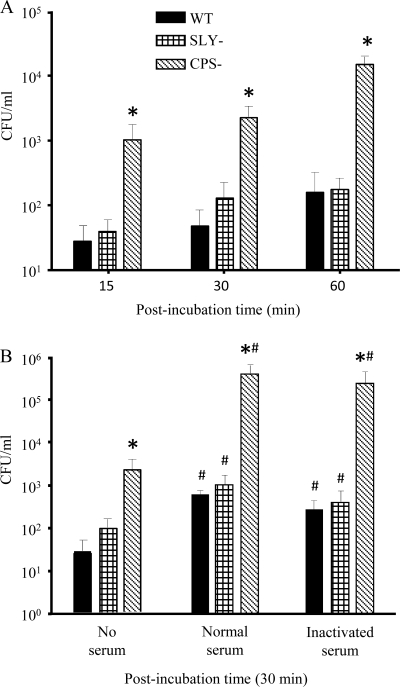
Figure 1A shows the kinetics of S. suis phagocytosis by murine microglia. The WT strain and its SLY− mutant were relatively poorly phagocytosed throughout the experiment, and no statistically significant differences were found between them (P > 0.05). This indicates that under nonopsonic conditions, the absence of suilysin, which is considered a putative S. suis virulence factor, does not have a major impact on phagocytosis by microglia. On the other hand, the CPS− mutant was quickly phagocytosed, and levels of ingested bacteria in comparison to the WT strain significantly increased with incubation time from 15 (P = 0.01) to 60 min (P = 0.002) (Fig. 1A). The possible effect of opsonization on S. suis phagocytosis by murine microglia was also assessed. All strains were preopsonized by incubation in the presence of 20% (vol/vol) complete normal or heat-inactivated serum in PBS for 30 min prior to microglial-cell infection for 30 min. As shown in Fig. 1B, S. suis opsonization with mouse serum increased the phagocytosis levels of all three strains in comparison to nonopsonizing conditions (P < 0.05). However, it is likely that factors present in the serum other than complement were responsible for this phenomenon, as the levels of phagocytosis for each strain opsonized with inactivated mouse serum were similar to those obtained with normal complete serum (P > 0.05).
FIG. 1.
Phagocytosis of S. suis by murine microglial cells. (A) Kinetics of phagocytosis of S. suis strains (1 × 106) by murine microglia after 15-, 30-, and 60-min infection times. *, P < 0.05 compared to phagocytosis levels obtained with the wild-type strain. (B) Effect of opsonization on phagocytosis at 30 min postinfection. Bacteria were nonopsonized (no serum) or preopsonized with 20% either normal or inactivated mouse serum. *, P < 0.05 compared to phagocytosis levels obtained with the wild-type strain; #, P < 0.05, indicating statistically significant differences between nonopsonized strains and their respective normal-serum- or inactivated-serum-opsonized counterparts. The numbers of internalized bacteria were determined by quantitative plating after 1 h of antibiotic treatment, and the results are expressed as CFU of recovered bacteria per ml (means plus SEM obtained from three independent experiments).
To confirm the intracellular location of bacteria, confocal microscopy was performed using hyperimmune serum against S. suis and an antibody specific for LAMP1, a protein enriched in phagolysosomes. Confocal analysis showed that only small numbers of WT S. suis bacteria were internalized by microglia (Fig. 2). However, the CPS− mutant was found in higher numbers, not only attached to the cell membrane, forming small chains, but also inside the cytoplasm and associated with numerous phagolysosomes of microglial cells (Fig. 2).
FIG. 2.
Interaction of murine microglial cells with S. suis. Microglia were infected with either the S. suis WT strain or the CPS− mutant for 2 h. The cells were then washed, and bacteria were visualized with rabbit anti-S. suis serum and Alexa Fluor 488-conjugated goat anti-rabbit IgG (green), while phagolysosomes from microglial cells were evidenced with rat anti-LAMP1 antibody and Alexa Fluor 568-conjugated goat anti-rat IgG (red). The cell nuclei were stained with DAPI (blue). The images were examined with a confocal laser scanning microscope.
S. suis induces production of proinflammatory cytokines and chemokines by murine microglia.
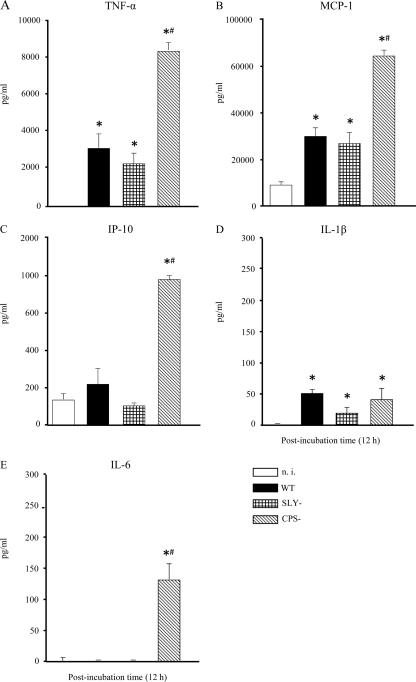
In S. suis meningitis, microglia have been suggested to be, at least in part, responsible for the high inflammatory reaction in the CNS (18). Therefore, microglial cells were stimulated with the S. suis WT strain and its isogenic mutants for 12 h, and levels of TNF-α, IL-1β, IL-6, MCP-1, and IP-10 production were analyzed by ELISA. Cell culture medium alone was used as a negative control, and purified E. coli LPS was used as a positive control. LPS induced high production levels of the aforementioned proinflammatory cytokines and chemokines (data not shown). S. suis induced different patterns of cytokine release by microglial cells (Fig. 3). The S. suis WT strain induced significantly higher levels of TNF-α and MCP-1 than in noninfected cells but failed to induce IP-10 production over basal levels (Fig. 3). The presence of suilysin was not involved in modulation of TNF-α, MCP-1, and IP-10 release, as the SLY− mutant induced levels of cytokines comparable to those observed for the WT strain (P > 0.05). On the other hand, the CPS− mutant induced significantly higher secretion of all three cytokines than the S. suis WT strain (P > 0.01).
FIG. 3.
Comparative study of cytokine production: TNF-α (A), MCP-1 (B), IP-10 (C), IL-1β (D), and IL-6 (E). Murine microglial cells were incubated with the different S. suis strains (1 × 106). The culture supernatants were harvested at 12 h poststimulation and analyzed for cytokine production by ELISA. The data are expressed as means plus SEM from at least three independent experiments. n.i., noninfected cells. *, P < 0.05, indicating significant differences from n.i. cells; #, P < 0.05, indicating significant differences from the WT S. suis strain.
Different results were obtained for IL-1β, as microglial cells produced low levels of the cytokine after infection with all S. suis strains tested (Fig. 3), although these levels were significantly higher than those observed with noninfected cells (P < 0.001). Interestingly, the production of IL-1β seemed not to be influenced by the absence of the capsule, as levels observed for the CPS− mutant were not significantly different from those found with the WT strain (P > 0.05). In the case of IL-6 (Fig. 3), microglial cells did not produce the cytokine after infection with the WT strain or the SLY− mutant. Only the CPS− mutant (and the positive-control LPS [data not shown]) was able to induce the release of IL-6 from these cells (P < 0.001).
As a whole, the results obtained with microglial cells for both phagocytosis and cytokine production were similar between the WT strain and the SLY− mutant. Important differences were observed only between the parental strain and its CPS− mutant. Therefore, we used only these two strains for further characterization of receptor activation and intracellular signaling pathways related to the S. suis-induced inflammatory response by murine microglia.
S. suis infection induces TLR2 gene expression.
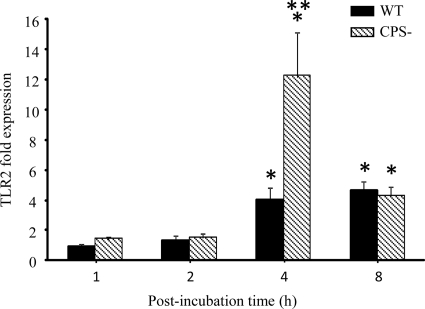
TLRs are receptors that play a key role in the innate immune response. Having observed differences in expression of cytokine levels following infection of microglia by the WT strain and the CPS− mutant, we were interested in examining whether gene expression of different TLRs was modified. To this end, microglial cells were infected with WT and CPS− strains of S. suis for different times, and TLR1, TLR2, TLR4, TLR6, and TLR9 gene expression was analyzed by real-time PCR. Microglial-cell infection by both S. suis strains failed to increase TLR1, TLR4, TLR6, and TLR9 gene expression over basal levels (data not shown). However, TLR2 gene expression was significantly increased in microglia between 4 and 8 h of incubation by either the WT strain or its CPS− mutant compared to noninfected cells (P < 0.05) (Fig. 4). Interestingly, the CPS− mutant was able to induce TLR2 gene expression in microglial cells at a higher level than the WT S. suis strain (P < 0.001). This finding is in agreement with the fact that the CPS− mutant was also associated with generally higher cytokine production by these cells.
FIG. 4.
Increase of TLR2 mRNA expression in murine microglial cells following S. suis infection. Microglia were stimulated for 1, 2, 4, and 8 h with 1 × 106 S. suis bacteria. Total RNA was isolated from the microglia at the indicated time points and analyzed for TLR2 mRNA expression by real-time quantitative PCR as described in Materials and Methods. The levels of TLR2 gene expression following S. suis infection were calculated after the cycle thresholds against the β-actin and β2 microglobulin housekeeping genes were normalized, using the 2−ΔΔCt method. The results are presented as fold induction relative to noninfected microglia. *, P < 0.05, indicating significant differences between infected and noninfected cells; **, P < 0.001, indicating significant differences between microglia stimulated with WT S. suis and cells infected with the S. suis CPS− mutant.The results are means plus SEM of three independent experiments.
S. suis induces iNOS expression and NO production in mouse microglia.
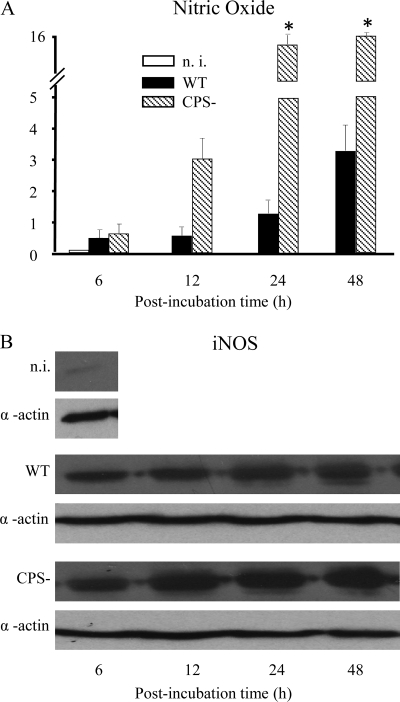
In the brain, following induction of iNOS, activated microglia release NO, which might lead to tissue destruction and degeneration (15, 46). Therefore, we sought to determine whether S. suis induces iNOS expression and NO release from murine microglia over time. Western blot analysis showed strong iNOS expression in microglial cells stimulated with both S. suis strains tested. iNOS expression was observed after as little as 6 h of bacterium-cell contact and reached a plateau at 24 h of incubation. The activation of iNOS was higher after infection of microglia with the CPS− mutant strain at all incubation times (Fig. 5). Accordingly, NO production increased over time and reached a plateau at 24 h of incubation. In agreement with iNOS results, NO production was significantly higher with the CPS− mutant strain (Fig. 5) (P < 0.05).
FIG. 5.
Time course of increase in nitric oxide production (A) and iNOS expression (B) by murine microglial cells treated with S. suis. The heat-killed S. suis WT or CPS− strain (1 × 109) was incubated with microglia for 6, 12, 24, and 48 h. (A) Microglial supernatants were collected to measure nitric oxide production by the Griess reaction method. The data are expressed as the means plus SEM (in μM/ml) of three independent experiments. *, P < 0.05, indicating significant differences versus WT S. suis. n. i., noninfected cells. (B) Representative Western blot analysis of murine microglial extracts using an iNOS-specific antibody. Blotting with anti-α-actin antibody was used as a loading control.
The microglial-cell profile of tyrosine phosphorylation is modulated during S. suis infection.
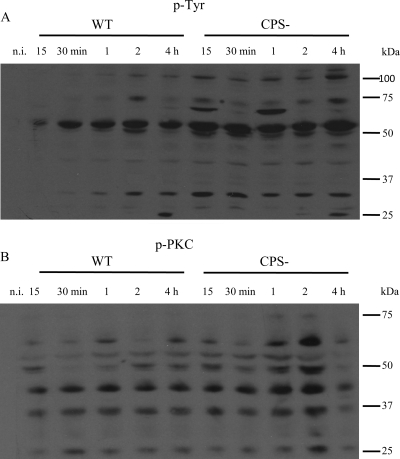
Studies with different pathogens have demonstrated that bacterial attachment can lead to activation of host signal transduction cascades, predominantly through Tyr phosphorylation of proteins that contribute to different signal transduction mechanisms, including internalization of pathogens in host cells (22). Thus, modulation of protein phosphotyrosine residues in response to S. suis infection and the effect of CPS on this process were assessed. As shown in Fig. 6A, the Tyr phosphorylation response to S. suis is considerably downmodulated by the presence of the capsule. Indeed, the WT strain induces a modest Tyr phosphorylation of some proteins, which is more apparent at 2 h postinfection, while the CPS− mutant leads to dramatic changes in the phosphorylation states of numerous proteins. Not only do these changes occur earlier than in the WT strain (15 min), but the level of phosphorylation is also stronger and generally increases over time. It is interesting that during the course of infection the phosphorylation levels of some proteins of ∼110, 70 to 75, and 25 to 30 kDa temporarily decreased, suggesting that either different bacterial components or different steps of the infection (adhesion versus internalization) may influence Tyr phosphorylation patterns (Fig. 6A).
FIG. 6.
S. suis-induced levels of tyrosine phosphorylation (p-Tyr) (A) and serine phosphorylation (p-PKC) (B). Murine microglial cells were infected for 15 or 30 min or 1, 2, or 4 h with either the WT strain or its CPS− mutant (1 × 106 bacteria). Cell lysates (total proteins) from noninfected cells and infected cells were subjected to Western blotting. p-Tyr and p-PKC protein levels were revealed by using anti-p-Tyr (clone 4G10) monoclonal antibody or anti-phospho-(Ser) PKC substrate antibody, respectively. The results are representative of three individual experiments.
Murine microglial cells display PKC activity after S. suis infection.
PKC represents a family of serine/threonine kinases that play central roles in multiple signaling events, such as regulation of the immune response by MAPKs and gene transcription activation (62). Using an antibody specific for PKC substrates containing phosphoserine, the phosphorylation levels of several proteins in microglial cells were shown to be different after infection with the WT strain than after infection with the CPS− mutant (Fig. 6B). In fact, the WT strain showed discrete phosphorylation of several proteins, ranging from ∼25 to 60 kDa, that occurred at different times postinfection depending on the protein. In contrast, the phosphorylation pattern of PKC substrates observed for the CPS− mutant was stronger and more stable, reaching maximal phosphorylation at 2 h postinfection for most of the proteins. Similarly to that observed for phosphotyrosine (Fig. 6A), PKC-dependent phosphorylation of some proteins showed a shift between the dephosphorylated and the nonphosphorylated states during the infection period.
S. suis infection activates MAPK phosphorylation in murine microglia.
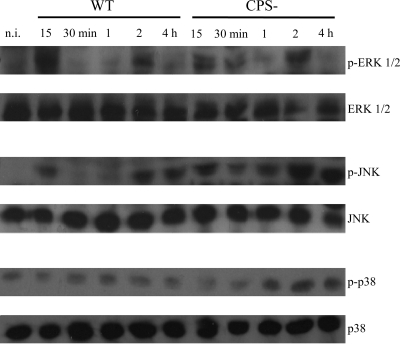
It is known that MAPK signal transduction pathways are involved in microglial activation, leading to production of different proinflammatory mediators that play essential roles in the host response to pathogens (6, 51). Therefore, the time course of phosphorylation of the three MAPK signaling pathways, ERK 1/2, SAPK/JNK, and p38, in microglial cells was investigated after stimulation with the S. suis WT strain and the CPS− mutant (Fig. 7). A specific phosphorylation pattern was observed for each MAPK evaluated, which seemed to also be influenced by the strain tested. ERK 1/2 activation in response to the WT strain was observed as early as 15 min postincubation; however, this phosphorylation was downregulated between 30 min and 1 h postincubation, though a second phase of phosphorylation was found at 2 h. No activation of this MAPK was found with longer incubation times (data not shown). A similar ERK 1/2 activation pattern was detected with the CPS− mutant. Similarly to the WT strain, there was dephosphorylation of ERK 1/2 at 1 h and a second and final phase of phosphorylation at 2 h. When cells were stimulated with WT S. suis, the phosphorylated form of JNK was also found at 15 min poststimulation, but similarly to ERK 1/2, phosphorylated JNK was downregulated from 30 min to 1 h. A second phase of activation was found at 2 h and extended up to 4 h of bacterium-cell contact. Interestingly, when the CPS− mutant was used, prompt and lasting JNK phosphorylation was detected from 15 min to 4 h of stimulation. This phosphorylation seemed to be slightly downregulated from 30 min to 1 h. In the case of p38, a very slight activation was found in WT strain-infected microglial cells between 30 min and 2 h postinfection. However, when microglial cells were stimulated with the CPS− mutant, p38 phosphorylation was more marked and extended from 1 h to 4 h postinfection. These results not only show that S. suis is capable of inducing activation of different MAPK signaling pathways, but also that cell wall components seem to be mainly implicated in this phenomenon.
FIG. 7.
Time course of phosphorylation of MAPKs in murine microglial cells. The cells were infected with either the S. suis WT strain or its CPS− mutant (1 × 106 bacteria). Cell extracts were recovered at 15 and 30 min and 1, 2, and 4 h postincubation and were subjected to Western blot analysis using antibodies specific for phospho-MAPKs (p-ERK, p-JNK, and p-p38). Following analysis, the blots were stripped and reprobed with an antibody specific for ERK, JNK, or p38 to verify uniformity in gel loading. The results are representative of three independent experiments. n. i., noninfected cells.
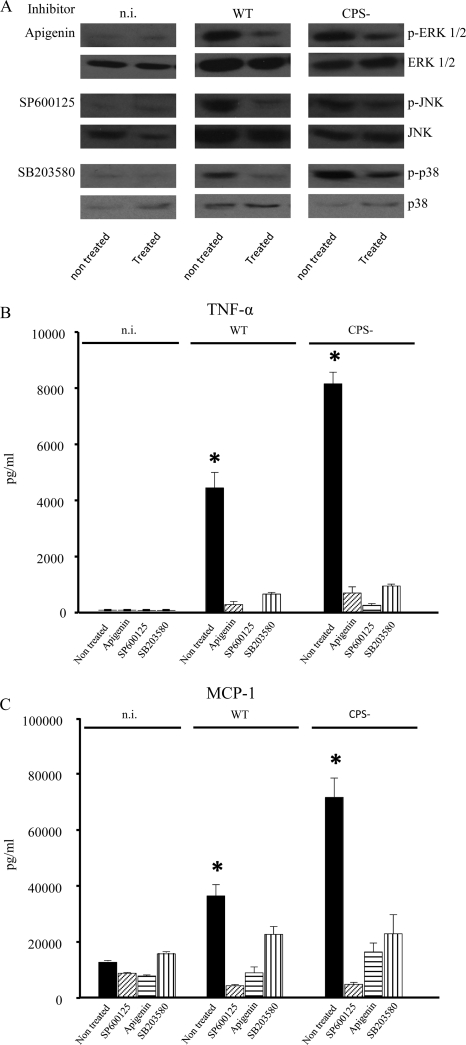
The involvement of ERK 1/2, JNK, and p38 in regulation of microglial production of proinflammatory cytokines and chemokines in response to S. suis infection was confirmed using specific inhibitors. For this purpose, microglial cells were treated with subcytotoxic doses of ERK 1/2 (Apigenin), JNK (SP600125), or p38 (SB203580) inhibitor and then infected with either the S. suis WT strain or its CPS− mutant. Figure 8A shows the results of Western blotting of this selective MAPK inhibition, with an evident abrogation in phosphorylation of all three MAPKs. In parallel, it was possible to confirm that MAPK activity was involved in cytokine and chemokine production, as microglia treated with MAPK inhibitors prior to S. suis infection showed a strong diminution of TNF-α and MCP-1 production (Fig. 8B and C) for either the WT strain or its CPS− mutant.
FIG. 8.
Pharmacologic inhibition of MAPKs. Murine microglial cells were treated with various inhibitors 1 h prior to infection with the S. suis WT strain or its CPS− mutant (1 × 106 bacteria). Apigenin (50 μM), SP600125 (50 μM), and SB203580 (75 μM) inhibit ERK 1/2, JNK, and p38, respectively. The inhibitors were all used at maximal subcytotoxic doses for a total of 13 h. (A) To confirm inhibition of MAPK phosphorylation, cell extracts were recovered after 2 h (p-ERK and p-JNK) or 4 h (p-p38) of bacterium-cell contact and then analyzed by Western blotting using specific antibodies for each of the proteins tested. The results are representative of three independent experiments. (B and C) To evidence inhibition in cytokine production, cells were infected for 12 h, and the supernatant was recovered for detection of TNF-α (B) and MCP-1 (C) production by ELISA. The data are expressed as means plus SEM from three independent experiments. *, P < 0.05, indicating significant differences from cells treated with MAPK inhibitors.
S. suis infection of murine microglia induces NF-κB activation.
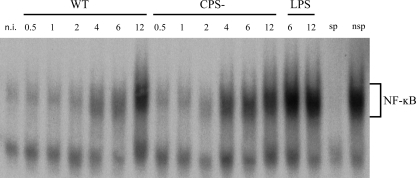
NF-κB is one of the most prominent transcription factors involved in the inflammatory response. As our previous in vivo research suggested NF-κB activation in the microglia of mice infected with S. suis (18), the ability of the pathogen to stimulate NF-κB in microglial cells was studied. Cells were incubated with either the WT or CPS− S. suis strain, and the time course of NF-κB translocation and its DNA binding activity were studied by EMSA. LPS, a potent inducer of NF-κB activity, served as a positive control. A basal DNA binding activity of NF-κB was observed in noninfected cells; however, in response to WT S. suis infection, an important induction of NF-κB binding activity was recorded at 4 h and increased over time, reaching its maximum at 12 h postinfection (Fig. 9). Although the S. suis CPS− mutant induced NF-κB translocation and DNA binding activity with a similar time course, this activity was overall stronger than the one recorded for the WT strain. The specificity of NF-κB DNA binding was confirmed by competition analysis with an excess of unlabeled specific or nonspecific oligonucleotides.
FIG. 9.
S. suis activates nuclear factor NF-κB in murine microglial cells. The cells were infected with either the S. suis WT strain or its CPS− mutant (1 × 106 cells) for 0.5 to 12 h. Noninfected cells were used as negative controls. LPS (1 μg/ml) served as a positive control. The cells were lysed, and nuclear extracts were subjected to EMSA. The presence of NF-κB-activated proteins in the cell nuclei was demonstrated by binding to oligonucleotide probes containing a single copy of the NF-κB motif 5′-AGT TGA GGG GAC TTT CCC AGG C-3′ end labeled with [γ-32P]ATP. The binding reaction mixtures were electrophoresed on native 4% polyacrylamide gels to separate bound and unbound DNA probe. sp, specific probe; nsp, nonspecific probe.
DISCUSSION
S. suis is an important agent of swine and human meningitis. Although research has significantly increased in recent years (29), knowledge of the pathogenesis of the infection is still scarce. Once S. suis arrives in the CNS, it encounters microglia (as well as meningeal and perivascular macrophages), major brain-resident innate immune effector cells. In fact, microglia play an ambiguous role, since they may protect neurons by preventing the entry of pathogens into the brain, but they can also be toxic to surrounding neurons by releasing NO, glutamate, and proinflammatory cytokines (10, 31, 72). In addition, activated microglia have been implicated in neurodegeneration resulting from bacterial meningitis (47). Using a well-standardized mouse model, it was shown that most S. suis-infected mice that survived septicemia later developed CNS clinical signs, such as locomotion problems, episthotonus, opisthotonus, lateral bending of the head, and walking in circles, which could be considered characteristic of brain inflammation. S. suis infection clearly induced inflammation and suppurative and necrotizing lesions in different anatomical sites of the brain parenchyma (18). Results from immunohistochemistry studies showed the presence of high bacterial antigen loads in association with cells that morphologically resembled microglia (18). It was hypothesized that these cells would be critically implicated in the CNS inflammatory response induced by S. suis (18).
Microglial cells have recently been shown to be able to phagocytose and kill Gram-positive bacteria, including well-encapsulated pathogenic Streptococcus pneumoniae (52). In the case of S. suis, previous studies carried out with murine, porcine, and human phagocytes indicated that the S. suis capsule is critical for bacterial resistance to phagocytosis (12, 56, 61). Similarly, phagocytosis and confocal microscopy results from the present study show that microglial cells hardly ingest well-encapsulated S. suis, whereas the CPS− mutant was significantly more often ingested than the WT strain. The absence of suilysin, a virulence factor present mainly in Eurasian strains (54), has been associated with partial susceptibility of encapsulated S. suis to killing by neutrophils and dendritic cells in the presence of complement (5, 12; M. P. Lecours, M. Gottschalk, M. Houde, P. Lemire, N. Fittipaldi, and M. Segura, submitted for publication). Since components of the complement cascade can be found in the brain (26), their possible effects on phagocytosis of S. suis by microglia were evaluated. Although the presence of serum significantly increased the phagocytosis rate of S. suis, complement components do not seem to be implicated in such a process. In addition, the encapsulated S. suis SLY− mutant behaved similarly to the WT strain, indicating a particular behavior of the microglial cells different from that of other phagocytes (5, 12). The observed increased rate of phagocytosis in the presence of serum might be due to other proteins, such as albumin and fibronectin (9).
The results of cell activation by S. suis provide support for the relevance of microglia in the development of the inflammatory response against the pathogen, as shown by the production of proinflammatory cytokines and chemokines. This confirms previous in vivo findings in the brains of mice, where high mRNA expression levels of different proinflammatory mediators were observed in cells suspected to be microglia (18). Indeed, high levels of TNF-α and MCP-1, but relatively low levels of IL-1β, were observed after in vitro S. suis activation of microglial cells. Interestingly, the WT encapsulated strain did not induce IL-6 production. It has previously been shown that, although highly secreted in the bloodstream during the septicemic phase, IL-6 mRNA was not expressed in the brains of S. suis-infected mice (18). The results obtained in this study confirm this observation. For so far unknown reasons, the lack of IL-6 production by microglia differs from what was observed with other phagocytic cells and S. suis (30, 57, 58), as well as with other streptococci and microglial cells (44).
The pneumolysin produced by S. pneumoniae, which shows high homology with suilysin, was shown to play an active role in inflammation (37). This does not seem to be the case for the suilysin, since the S. suis SLY− mutant induced cytokine levels similar to those for the WT strain. On the other hand, the capsule seems to be critical for modulating production of proinflammatory mediators, as the CPS− mutant induced the release of significantly higher levels of all proinflammatory mediators. These findings support the assumption that several cell wall components, such as lipoteichoic acid (LTA), peptidoglycan (PG), and lipoproteins, partially masked by the capsule are potent proinflammatory inducers, as recently suggested (24, 25, 70). Finally, as low production of IP-10 for capsulated S. suis strains in comparison to the CPS− mutant was also noted, we hypothesize that the CPS might influence the onset of the adaptive inflammatory response, as recently shown for dendritic cells (Lecours et al., submitted). As a consequence, fewer T lymphocytes would be attracted to the site of infection.
It has recently been demonstrated that TLRs, which are crucial pattern recognition receptors in innate immunity, are expressed in microglia (49). TLR activation sets in motion a broad spectrum of intracellular events to initiate the inflammatory response, including MAPK signaling pathways, activation of NF-κB, and cytokine production (1). In the present work, it was observed that S. suis induces significant microglial TLR2 mRNA upregulation in a time-dependent fashion. As expected, and in agreement with the cytokine results, upregulation of TLR2 is influenced by direct exposure of cell wall components, as a significantly higher level of TLR2 expression was observed with the CPS− mutant. These findings confirm previous studies from our laboratory, where in vitro recognition of the pathogen by professional macrophages was shown to occur through TLR2 (30). In vivo, S. suis-infected mice showed clear upregulation of TLR2 in specific parts of the brain where microglial cells were present (18). Interestingly, our results differ slightly from those recently reported by Wichgers Schreur et al. (70), since those authors observed TLR2 upregulation after culturing transfected human epithelial cells with extracted lipoproteins but not with live or heat-killed S. suis. It should be noted, however, that interactions between S. suis and epithelial cells can differ greatly from those observed with phagocytic cells. The fact that no upregulation of TLR1, TLR4, TLR6, and TLR9 was observed should be regarded with caution, since relatively high constitutive expression levels of these mRNAs were observed, and no further upregulation could be observed using the respective positive controls (data not shown).
Sustained and therefore uncontrolled production of toxic products released from microglia may cause irreversible damage to neurons. NO plays a significant role in macrophage bactericidal functions; however, it is also involved in a variety of brain insults, including neurotoxicity, increase of intracranial pressure, and meningeal inflammation (41). It was observed that both S. suis WT and CPS− strains efficiently enhanced the expression of iNOS in a time-dependent manner, which was accompanied by the release of NO from microglia, although levels were again higher with the CPS− mutant. Cell wall components of other Gram-positive bacteria, such as LTA, have been reported to be involved in NO production by microglial cells (15).
In the present study, the modulation of classical PKC and Tyr phosphorylation events, which are involved in different processes of macrophage activation, such as phagocytosis, NO production, and cytokine production (11, 21, 32, 48) was examined. The results demonstrated a pattern of low and biphasic phosphorylation of PKC substrates and tyrosyl residues in microglial cells infected with the S. suis WT strain, while cells infected with the CPS− strain showed a stronger pattern of phosphorylation. This may indicate that once in contact with microglia, virulent encapsulated S. suis is able to modulate intracellular signaling events, most likely to avoid phagocytosis and delay the activation of the inflammatory response. These findings support previous research on S. suis modulation of murine macrophage functions, in which it was concluded that the capsule was responsible for weak activation of Akt and PKCα kinases, as well as activation of protein tyrosine phosphatases, which correlated with low levels of phagocytosis (56).
We also examined whether S. suis activates the three classical MAPK intracellular signaling pathways and if their phosphorylation was involved in the production of proinflammatory mediators. As expected, the CPS− mutant proved to be particularly potent in MAPK activation, as phosphorylation patterns were stronger and more sustained than those obtained with the WT strain. MAPK phosphorylation levels, in particular those of p-ERK 1/2 and p-JNK, followed a biphasic pattern. This noticeable dephosphorylation of MAPK proteins highlights the intimate cross talk between these signaling pathways and pathogen-derived components and likely has an impact on microglial proliferation and/or activation (20). Interestingly, the phosphorylation levels of p38, a MAPK that plays an important role in activation of inflammatory responses (53), were subtly increased by WT S. suis but more noticeably with the CPS− mutant, emphasizing the relevance of CPS in the regulation of proinflammatory events. It is likely that, again, hidden cell wall components are the principal mediators of MAPK pathway activation. In fact, recent studies revealed that a capsule-deficient mutant of S. suis was able to induce higher levels of transcriptional expression of different putative genes from the MAPK pathway than the parental strain (17). Furthermore, purified cell wall preparations from S. suis and other meningitis-causing bacteria were shown to trigger the phosphorylation of the MAPK signal transduction pathway (15, 63). The EstA cell surface protein is a recently described S. pneumoniae virulence factor that induces MAPK phosphorylation and NF-κB translocation (39). The estA gene is also found in S. suis (40), so we may hypothesize that activation of the MAPK pathway and other intracellular signaling pathways does not depend solely on a few S. suis constituents but that many of them participate in the activation of the proinflammatory machinery. The use of pharmacological MAPK inhibitors revealed almost complete abrogation of cytokine release from microglia infected with either the S. suis WT or CPS− strain, confirming the importance of ERK 1/2, JNK, and p38 in the inflammatory response against the pathogen. MAPK pathways are molecular targets for drug development, and their inhibitors will undoubtedly be one of the next groups of drugs developed for the treatment of human diseases (53), so these results may open the door to future studies using animal models of S. suis meningitis to evaluate the in vivo efficacy of such drugs.
NF-κB is a central mediator that is critical for driving the innate immune response against many pathogens that infect the brain (43). Both strains of S. suis tested were able to increase this DNA binding activity in a time-dependent fashion, yet the activity was faster and more apparent when cells were infected with the S. suis CPS− mutant. Similar to other reports, it is likely that cell wall components, in particular LTA, influence the activation of NF-κB (15). Moreover, these findings support previous research stating that the in vivo inflammatory response to S. suis in the brains of infected mice, as well as in vitro infection of porcine alveolar macrophages, involves NF-κB activation (17, 18). Activation of NF-κB in murine microglial cells infected with S. suis would lead to the production of different cytokines and chemokines, as well as production of neurotoxic products, such as NO, as previously demonstrated for other brain pathogens (39, 43).
Finally, it might also be argued that activation of microglial cells may be a direct consequence of phagocytosis. In fact, cytochalasin treatment significantly reduced cytokine release by S. suis-infected cells (data not shown). However, levels of cytokines produced by treated cells infected with the nonphagocytosed, well-encapsulated WT strain were also significantly reduced, suggesting that phagocytosis alone was not responsible for cell activation. Rearrangement of the actin cytoskeleton may be necessary for the formation of a fully active receptor (16) complex, which may indeed be affected by cytochalasin treatment, as reported in other systems (16, 33, 65). In fact, the regulation of inflammatory cytokine production is very complex and is controlled at transcriptional, posttranscriptional, and posttranslational levels. Alteration of cell receptors and/or actin networks could conceivably affect most of these levels of regulation by altering cell surface-mediated events. However, it is also possible that additional distinct mechanisms exist that are not related to alterations in receptor complexes and are probably mediated by phagolysosome “in-out” signals. Further studies are needed to address this issue.
In conclusion, the results obtained in the present study demonstrate that S. suis phagocytosis by microglia and consequent activation of these cells is highly influenced by the presence of the capsule and probably involves recognition of cell wall components that requires participation of a TLR2-dependent pathway, activation of different signaling pathways, translocation of NF-κB, and production of different proinflammatory mediators and neurotoxic metabolites. The results obtained may contribute to an understanding of the participation of microglia in the meningitis caused by S. suis and the genesis of brain injury associated with the pathogen.
Acknowledgments
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada to M.G. (no. 154280 and Discovery Accelerator Supplement 380299) and from the Canadian Institutes of Health Research to M.O. M.D.L.,C.D.P. and M.-P. L. are recipients of postgraduate scholarships from NSERC, and I.C. is the beneficiary of a postgraduate scholarship from CONACyT-Mexico.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 27 September 2010.
REFERENCES
- 1.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, C. L., J. L. Jensen, and T. F. Ørntoft. 2004. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 64:5245-5250. [DOI] [PubMed] [Google Scholar]
- 3.Bahloul, H., A. Mofredj, A. Mrabet, G. Gineyt, and P. Rousselier. 2008. Streptococcus suis meningitis after oral contamination? Med. Mal. Infect. 38:281-282. [DOI] [PubMed] [Google Scholar]
- 4.Baums, C. G., and P. Valentin-Weigand. 2009. Surface-associated and secreted factors of Streptococcus suis in epidemiology, pathogenesis and vaccine development. Anim. Health Res. Rev. 10:65-83. [DOI] [PubMed] [Google Scholar]
- 5.Benga, L., M. Fulde, C. Neis, R. Goethe, and P. Valentin-Weigand. 2008. Polysaccharide capsule and suilysin contribute to extracellular survival of Streptococcus suis co-cultivated with primary porcine phagocytes. Vet. Microbiol. 132:211-219. [DOI] [PubMed] [Google Scholar]
- 6.Bhat, N. R., and F. Fan. 2002. Adenovirus infection induces microglial activation: involvement of mitogen-activated protein kinase pathways. Brain Res. 948:93-101. [DOI] [PubMed] [Google Scholar]
- 7.Blasi, E., R. Barluzzi, V. Bocchini, R. Mazzolla, and F. Bistoni. 1990. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J. Neuroimmunol. 27:229-237. [DOI] [PubMed] [Google Scholar]
- 8.Bocchini, V., R. Mazzolla, R. Barluzzi, E. Blasi, P. Sick, and H. Kettenmann. 1992. An immortalized cell line expresses properties of activated microglial cells. J. Neurosci. Res. 31:616-621. [DOI] [PubMed] [Google Scholar]
- 9.Brazeau, B., M. Gottschalk, S. Vincelette, and B. Martineau-Doizé. 1996. In vitro phagocytosis and survival of Streptococcus suis capsular type 2 inside murine macrophages. Microbiology 142:1231-1237. [DOI] [PubMed] [Google Scholar]
- 10.Brown, G. C. 2007. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem. Soc. Trans. 35:1119-1121. [DOI] [PubMed] [Google Scholar]
- 11.Castrillo, A., D. J. Pennington, F. Otto, P. J. Parker, M. J. Owen, and L. Boscá. 2001. Protein kinase C-epsilon is required for macrophage activation and defense against bacterial infection. J. Exp. Med. 194:1231-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chabot-Roy, G., P. J. Willson, M. Segura, S. Lacouture, and M. Gottschalk. 2006. Phagocytosis and killing of Streptococcus suis by porcine neutrophils. Microb. Pathog. 41:21-32. [DOI] [PubMed] [Google Scholar]
- 13.Charland, N., J. Harel, M. Kobisch, S. Lacasse, and M. Gottschalk. 1998. Streptococcus suis serotype 2 mutants deficient in capsular expression. Microbiology 144:325-332. [DOI] [PubMed] [Google Scholar]
- 14.Charland, N., V. Nizet, C. E. Rubens, K. S. Kim, S. Lacouture, and M. Gottschalk. 2000. Streptococcus suis serotype 2 interactions with human brain microvascular endothelial cells. Infect. Immun. 68:637-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chien, H., K. Y. Yeh, F. Jiang-Sjieh, I. W. Wei, C. Y. Chang, and C. H. Wu. 2005. Signal transduction pathways of nitric oxide release in primary microglial culture challenged with gram-positive bacterial constituent, lipoteichoic acid. Neuroscience 133:423-436. [DOI] [PubMed] [Google Scholar]
- 16.Dai, Q., and S. B. Pruett. 2006. Ethanol suppresses LPS-induced Toll-like receptor 4 clustering, reorganization of the actin cytoskeleton and associated TNF-alpha production. Alcohol Clin. Exp. Res. 30:1436-1444. [DOI] [PubMed] [Google Scholar]
- 17.de Greeff, A., L. Benga, P. J. Wichgers Schreur, P. Valentin-Weigand, J. M. J. Rebel, and H. E. Smith. 2010. Involvement of NF-κB and MAP-kinases in the transcriptional response of alveolar macrophages to Streptococcus suis. Vet. Microbiol. 141:59-67. [DOI] [PubMed] [Google Scholar]
- 18.Domínguez-Punaro, M. C., M. Segura, M. M. Plante, S. Lacouture, S. Rivest, and M. Gottschalk. 2007. Streptococcus suis serotype 2, an important swine and human pathogen, induces strong systemic and cerebral inflammatory responses in a mouse model of infection. J. Immunol. 179:1842-1854. [DOI] [PubMed] [Google Scholar]
- 19.Domínguez-Punaro, M. C., M. Segura, D. Radzioch, S. Rivest, and M. Gottschalk. 2008. Comparison of the susceptibilities of C57BL/6 and A/J. mouse strains to Streptococcus suis serotype 2 infection. Infect. Immun. 76:3901-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fettucciari, K., I. Fetriconi, A. Bartoli, R. Rossi, and P. Marconi. 2003. Involvement of mitogen-activated protein kinases in Group B Streptococcus-induced macrophage apoptosis. Pharmacol. Res. 47:355-362. [DOI] [PubMed] [Google Scholar]
- 21.Fiebich, B. L., R. D. Butcher, and P. J. Gebicke-Haerter. 1998. Protein kinase C-mediated inducible nitric oxide synthase expression in cultured microglial cells. J. Neuroimmunol. 92:170-178. [DOI] [PubMed] [Google Scholar]
- 22.Finlay, B. B., and P. Cossart. 1997. Exploitation of mammalian host cell functions by bacterial pathogens. Science 276:718-725. [DOI] [PubMed] [Google Scholar]
- 23.Fittipaldi, N., J. Harel, B. D'Amours, S. Lacouture, M. Kobisch, and M. Gottschalk. 2007. Potential use of an unencapsulated and aromatic amino acid-auxotrophic Streptococcus suis mutant as a live attenuated vaccine in swine. Vaccine 25:3524-3535. [DOI] [PubMed] [Google Scholar]
- 24.Fittipaldi, N., T. Sekizaki, D. Takamatsu, M. C. Domínguez-Punaro, J. Harel, N. K. Bui, W. Vollmer, and M. Gottschalk. 2008. Significant contribution of the pgdA gene to the virulence of Streptococcus suis. Mol. Microbiol. 70:1120-1135. [DOI] [PubMed] [Google Scholar]
- 25.Fittipaldi, N., T. Sekizaki, D. Takamatsu, J. Harel, M. C. Domínguez-Punaro, S. Von Aulock, C. Draing, C. Marois, M. Kobisch, and M. Gottschalk. 2008. d-Alanylation of lipoteichoic acid contributes to the virulence of Streptococcus suis. Infect. Immun. 76:3587-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francis, K., J. van Beek, C. Canova, J. W. Neal, and P. Gasque. 2003. Innate immunity and brain inflammation: the key role of complement. Expert Rev. Mol. Med. 23:1-19. [DOI] [PubMed] [Google Scholar]
- 27.Gottschalk, M., and M. Segura. 2000. The pathogenesis of the meningitis caused by Streptococcus suis: the unresolved questions. Vet. Microbiol. 76:259-272. [DOI] [PubMed] [Google Scholar]
- 28.Gottschalk, M., M. Segura, and J. Xu. 2007. Streptococcus suis infections in humans: the Chinese experience and the situation in North America. Anim. Health Res. Rev. 8:29-45. [DOI] [PubMed] [Google Scholar]
- 29.Gottschalk, M., J. Xu, C. Calzas, and M. Segura. 2010. Streptococcus suis: a new emerging or an old neglected zoonotic pathogen? Future Microbiol. 5:371-391. [DOI] [PubMed] [Google Scholar]
- 30.Graveline, R., M. Segura, D. Radzioch, and M. Gottschalk. 2007. TLR2-dependent recognition of Streptococcus suis is modulated by the presence of capsular polysaccharide which modifies macrophage responsiveness. Int. Immunol. 19:375-389. [DOI] [PubMed] [Google Scholar]
- 31.Hanisch, U. K. 2002. Microglia as a source and target of cytokines. Glia 40:140-155. [DOI] [PubMed] [Google Scholar]
- 32.Hanisch, U. K., M. Prinz, K. Angstwurm, K. G. Häusler, O. Kann, H. Kettenmann, and J. R. Weber. 2001. The protein tyrosine kinase inhibitor AG126 prevents the massive microglial cytokine induction by pneumococcal cell walls. Eur. J. Immunol. 31:2104-2115. [DOI] [PubMed] [Google Scholar]
- 33.Hawkes, D. J., and J. Mak. 2006. Lipid membrane: a novel target for viral and bacterial pathogens. Curr. Drug Targets 7:1615-1621. [DOI] [PubMed] [Google Scholar]
- 34.Henn, A., S. Lund, M. Hedtjärn, A. Schrattenholz, P. Pörzgen, and M. Leist. 2009. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX 26:83-94. [DOI] [PubMed] [Google Scholar]
- 35.Higgins, R., and M. Gottschalk. 2005. Streptococcal diseases, p. 769-783. In B. E. Straw, S. D'Allaire, W. L. Mengelin, and D. J. Taylor (ed.), Diseases of swine. Iowa State University Press, Ames, IA.
- 36.Higgins, R., and M. Gottschalk. 1990. An update on Streptococcus suis identification. J. Vet. Diagn. Invest. 2:249-252. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs, A. A., P. L. Loeffen, A. J. van den Berg, and P. K. Storm. 1994. Identification, purification, and characterization of a thiol-activated hemolysin (suilysin) of Streptococcus suis. Infect. Immun. 62:1742-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaramillo, M., D. Channe Gowda, D. Radzioch, and M. Olivier. 2003. Hemozoin increases IFN-γ-inducible macrophage nitric oxide generation through extracellular signal-regulated kinase-and NF-κB-dependent pathways. J. Immunol. 171:4243-4253. [DOI] [PubMed] [Google Scholar]
- 39.Kang, E. H., E. Gebru, M. H. Kim, H. Cheng, and S. C. Park. 2009. EstA protein, a novel virulence factor of Streptococcus pneumoniae, induces nitric oxide and pro-inflammatory cytokine production in RAW 264.7 macrophages through NF-κB/MAPK. Microb. Pathog. 47:196-201. [DOI] [PubMed] [Google Scholar]
- 40.Kim, M. H., B. S. Kang, S. Kim, K. J. Kim, C. H. Lee, B. C. Oh, S. C. Park, and T. K. Oh. 2008. The crystal structure of the estA protein, a virulence factor from Streptococcus pneumoniae. Proteins 70:578-583. [DOI] [PubMed] [Google Scholar]
- 41.Kim, Y. S., and M. G. Täuber. 1996. Neurotoxicity of glia activated by Gram-positive bacterial products depends on nitric oxide production. Infect. Immun. 64:3148-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobish, M., M. Gottschalk, P. Morvan, R. Cariolet, G. Bénévent, and J. P. Joloy. 1995. Experimental infection of SPF piglets with Streptococcus suis serotype 2. J. Rech. Porcine France 27:97-102. [Google Scholar]
- 43.Lee, J., S. Shin, C. H. Teng, S. J. Hong, and K. S. Kim. 2005. FimH adhesin of Escherichia coli K1 type 1 fimbriae activates BV-2 microglia. Biochem. Biophys. Res. Commun. 334:917-923. [DOI] [PubMed] [Google Scholar]
- 44.Liu, X., V. S. Chauhan, A. B. Young, and I. Marriott. 2010. NOD2 mediates inflammatory responses of primary murine glia to Streptococcus pneumoniae. Glia 58:839-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lun, S., J. Perez-Casal, W. Connor, and P. Willson. 2003. Role of the suilysin in pathogenesis of Streptococcus suis capsular type 2. Microb. Pathog. 34:27-37. [DOI] [PubMed] [Google Scholar]
- 46.Marques, C. P., M. C. Cheeran, J. M. Palmquist, S. Hu, and J. R. Lokensgard. 2008. Microglia are the major cellular source of inducible nitric oxide synthase during experimental herpes encephalitis. J. Neurovirol. 14:229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neher, J. J., and G. C. Brown. 2007. Neurodegeneration in models of Gram-positive bacterial infections of the central nervous system. Biochem. Soc. Trans. 35:1166-1167. [DOI] [PubMed] [Google Scholar]
- 48.Newton, A. C. 1995. Protein kinase C: structure, function, and regulation. J. Biol. Chem. 270:28495-28498. [DOI] [PubMed] [Google Scholar]
- 49.Olson, J. K., and S. D. Miller. 2004. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 173:3916-3924. [DOI] [PubMed] [Google Scholar]
- 50.Pouliot, P., I. Plante, M.-A. Raquil, P. A. Tessier, and M. Olivier. 2008. Myeloid-related proteins rapidly modulate macrophage nitric oxide production during innate immune response. J. Immunol. 181:3539-3601. [DOI] [PubMed] [Google Scholar]
- 51.Pyo, H., I. Jou, S. Jung, S. Hong, and E. H. Joe. 1988. Mitogen-activated protein kinases activated by lipopolysaccharide and beta-amyloid in cultured rat microglia. Neuroreport 30:871-874. [DOI] [PubMed] [Google Scholar]
- 52.Ribes, S., S. Ebert, T. Regen, A. Agarwal, S. C. Tauber, D. Czesnik, A. Spreer, S. Bunkowski, H. Eiffert, U. K. Hanisch, S. Hammerschmidt, and R. Nau. 2010. Toll-like receptor stimulation enhances phagocytosis intracellular killing of nonencapsulated and encapsulated Streptococcus pneumoniae by murine microglia. Infect. Immun. 78:865-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schindler, J. F., J. B. Monahan, and W. G. Smith. 2007. p38 pathway kinases as anti-inflammatory drug targets. J. Dent. Res. 86:800-811. [DOI] [PubMed] [Google Scholar]
- 54.Segers, R. P., T. Kenter, L. A. de Haan, and A. A. Jacobs. 1998. Characterisation of the gene encoding suilysin from Streptococcus suis and expression in field strains. FEMS Microbiol. Lett. 167:255-261. [DOI] [PubMed] [Google Scholar]
- 55.Segura, M., and M. Gottschalk. 2002. Streptococcus suis interactions with the murine macrophage cell line J774: adhesion and cytotoxicity. Infect. Immun. 70:4312-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Segura, M., M. Gottschalk, and M. Olivier. 2004. Encapsulated Streptococcus suis inhibits activation of signaling pathways involved in phagocytosis. Infect. Immun. 72:5322-5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Segura, M., J. Stankova, and M. Gottschalk. 1999. Heat-killed Streptococcus suis capsular type 2 strains stimulate tumor necrosis factor alpha and interleukin-6 production by murine macrophages. Infect. Immun. 67:4646-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Segura, M., N. Vadeboncoeur, and M. Gottschalk. 2002. CD14-dependent and -independent cytokine and chemokine production by human THP-1 monocytes stimulated by Streptococcus suis capsular type 2. Clin. Exp. Immunol. 127:243-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Segura, M., G. Vanier, D. Al-Numani, S. Lacouture, M. Olivier, and M. Gottschalk. 2006. Proinflammatory cytokine and chemokine modulation by Streptococcus suis in a whole-blood culture system. FEMS Immunol. Med. Microbiol. 47:92-106. [DOI] [PubMed] [Google Scholar]
- 60.Segura, M. A., P. Cléroux, and M. Gottschalk. 1998. Streptococcus suis and group B Streptococcus differ in their interactions with murine macrophages. FEMS Immunol. Med. Microbiol. 21:189-195. [DOI] [PubMed] [Google Scholar]
- 61.Smith, H. E., M. Damman, J. van der Velde, F. Wagenaar, H. J. Wisselink, N. Stockhofe-Zurwieden, and M. A. Smits. 1999. Identification and characterization of the cps locus of Streptococcus suis serotype 2: the capsule protects against phagocytosis and is an important virulence factor. Infect. Immun. 67:1750-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tan, S. L., and P. J. Parker. 2003. Emerging and diverse roles of protein kinase C in immune cell signalling. Biochem. J. 376:545-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanabe, S., M. Gottschalk, and D. Grenier. 2008. Hemoglobin and Streptococcus suis cell wall in synergy to potentiate the inflammatory response of monocyte-derived macrophages. Innate Immun. 14:357-363. [DOI] [PubMed] [Google Scholar]
- 64.Tenenbaum, T., R. Adam, I. Eggelnpohler, D. Malaton, A. Seibt, G. E. Novotny, H. J. Galla, and H. Schroten. 2005. Strain-dependent disruption of blood-cerebrospinal fluid barrier by Streptoccocus suis in vitro. FEMS Immunol. Med. Microbiol. 44:25-34. [DOI] [PubMed] [Google Scholar]
- 65.Triantafilou, M., K. Miyake, D. T. Golenbock, and K. Triantafilou. 2002. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J. Cell Sci. 115:2603-2611. [DOI] [PubMed] [Google Scholar]
- 66.Vanier, G., M. Segura, P. Friedl, S. Lacouture, and M. Gottschalk. 2004. Invasion of porcine brain microvascular endothelial cells by Streptococcus suis serotype 2. Infect. Immun. 72:1441-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wangkaew, S., R. Chaiwarith, P. Tharavichitkul, and K. Supparatpinyo. 2006. Streptococcus suis infection: a series of 41 cases from Chiang Mai University Hospital. J. Infect. 52:455-460. [DOI] [PubMed] [Google Scholar]
- 68.Wangsomboonsiri, W., T. Luksananun, S. Saksornchai, K. Ketwong, and S. Sungkanuparph. 2008. Streptococcus suis infection and risk factors for mortality. J. Infect. 57:392-396. [DOI] [PubMed] [Google Scholar]
- 69.Wertheim, H. F. L., H. D. Trung Nghia, W. Taylor, and C. Schultsz. 2009. Streptococcus suis: an emerging human pathogen. Clin. Infect. Dis. 48:617-625. [DOI] [PubMed] [Google Scholar]
- 70.Wichgers Schreur, P. J., J. M. Rebel, M. A. Smits, J. P. van Putten, and H. E. Smith. 2010. Differential activation of the Toll-like receptor 2/6 complex by lipoproteins of Streptococcus suis serotypes 2 and 9. Vet. Microbiol. 143:363-370. [DOI] [PubMed] [Google Scholar]
- 71.Yu, H., H. Jing, Z. Chen, H. Zheng, X. Zhu, H. Wang, S. Wang, L. Liu, R. Zu, L. Luo, N. Xiang, H. Liu, X. Liu, Y. Shu, S. S. Lee, S. K. Chuang, Y. Wang, J. Xu, and W. Yang. 2006. Human Streptococcus suis outbreak, Sichuan, China. Emerg. Infect. Dis. 12:914-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao, W., W. Xie, W. Le, D. R. Beers, Y. He, J. S. Henkel, E. P. Simpson, A. A. Yen, Q. Xiao, and S. H. Appel. 2004. Activated microglia initiate motor neuron injury by a nitric oxide and glutamate-mediated mechanism. J. Neuropathol. Exp. Neurol. 63:964-977. [DOI] [PubMed] [Google Scholar]