Abstract
The polysaccharide capsule is a major virulence factor of Streptococcus pneumoniae; it affects complement resistance and shields the bacterium from phagocytes. Certain capsular serotypes appear to be better able to cause invasive disease than others. Serotypes 1 and 5 are common causes of invasive disease but are rarely isolated from healthy carriers, whereas serotypes 6B and 23F are more frequently isolated from carriage than invasive disease. We have recently shown that serotypes 6B and 19F differ in resistance to complement C3 deposition and opsonophagocytic killing. In this study we assessed the complement resistance and susceptibility to opsonophagocytosis of several other serotypes targeted by the pneumococcal conjugate vaccines. Clinical isolates of serotypes 1, 4, 5, 14, 18C, and 23F were tested along reference strains of corresponding capsular types. The concentration of anticapsular antibodies required for opsonophagocytic killing correlated inversely with C3 deposition on the serotype. Serotype 1 was the most resistant of the clinical isolates to C3 deposition and, along with serotypes 5 and 19F, required the highest concentration of capsule antibodies for opsonophagocytic killing, whereas serotype 23F was the most sensitive to opsonophagocytosis. Sensitivity to C3 deposition and opsonophagocytosis was associated with serotype-specific mortality of invasive pneumococcal disease, suggesting that the primary pathogens, such as serotypes 1 and 5, are more resistant to complement and require a higher concentration of capsule antibodies for opsonophagocytic killing than the opportunistic serotypes such as 6B and 23F, which are associated with a more severe disease outcome.
Streptococcus pneumoniae colonizes the nasopharynx (NP) of healthy individuals but occasionally breaks from its carriage habitat and causes diseases ranging from acute otitis media to more severe diseases such as pneumonia, sepsis, and meningitis. Isolates that lack the capsule rarely cause invasive disease in humans, and loss of the capsule greatly attenuates virulence in animal models of disease (31, 54). Each of the more than 90 known pneumococcal serotypes produces a biochemically distinct polysaccharide structure, which is a major determinant of pneumococcal virulence since only a small number of serotypes account for the majority of infections (18). The polysaccharide capsule shields the bacterium from phagocytosis by inhibiting recognition of opsonins adhered to the bacterial cell wall.
Certain serotypes are relatively common causes of invasive pneumococcal disease (IPD) with respect to their prevalence in carriage, whereas others are common colonizers of the NP but rarely cause disease (6, 16). The disease potential, or relative invasiveness, is a measure of the ability of pneumococci to progress from nasopharyngeal carriage to invasive disease in humans, similar to the attack rate, which is the risk of disease as a result of pathogen acquisition (6, 50). Most of the invasive property of pneumococci seems to be determined by their capsular serotype rather than genetic background. However, different clones of the same serotype can vary in an ability to cause IPD (6, 16, 45).
Variation in disease potential among serotypes has been reported in a number of epidemiological studies. In young children in England, the relative invasiveness of more prevalent carriage serotypes—6B, 19F, and 23F—was low compared to the high disease potential of serotypes 1, 4, 14, 18C, and 7F (6). In another United Kingdom study in infants <2 years old, serotypes 23F, 6A, 19F, 16F, 6B, and 15B/C were associated with low attack rates; serotypes 4, 14, 7F, 9V, and 18C were associated with relatively high attack rates; and serotypes 1, 5, and 9A were only isolated from IPD (50). In a study of Finnish children <2 years of age serotypes 19F and 23F showed a tendency to be more common in carriage and serotypes 14, 18C, 19A, and 6B were significantly more common in IPD (16). Even in populations with a high proportion of disease caused by serotypes 1 and 5, such as in the Gambia (2), serotypes 1 and 5 are rarely detected in nasopharyngeal carriage (19, 29, 47). In a meta-analysis of seven data sets, serogroups 1, 5, and 7 had the highest invasive disease potential (7).
The duration of carriage varies by capsular type and is inversely correlated with the attack rate (50). Capsular serotypes carried for a short duration and with a high attack rate, such as serotypes 1, 5, and 7F, behave like “primary pathogens,” which affect previously healthy individuals and are associated with lower mortality. Meanwhile, serotypes that are carried for a long duration, such as 6B, 19F, and 23F, behave like opportunistic pathogens causing disease in patients with an underlying disease and are associated with a more severe disease and higher mortality (49). This observation correlates with the outcome of analysis of the largest ever population-based study on the severity of IPD published recently by Harboe et al. Odds ratios (ORs) for the death as an outcome of IPD were higher for opportunistic serotypes compared to invasive capsular types (17).
Comparison of pneumococcal strains isolated from carriage and IPD suggests that carriage isolates are more heterogeneous and invasiveness is associated with clonality (6, 42). Isolates of serogroups 7 and 14 are clonal and rare among carriers, whereas isolates of serogroups 6, 19, and 23 are heterogeneous, suggesting that the clonality may represent an advantage for invasiveness (51). Molecular epidemiology studies reveal that IPD caused by serotype 1 in the Gambia (3) and serotypes 1 and 5 in Israel (37) resulted from expansion of single, virulent clones, in contrast to serotypes 6B and 23F, which showed a large diversity in their genotypic characteristics (37). Studies of serotype 1 disease cases in different geographic regions did not identify clones with distinct virulence properties (8), which could reflect either a high virulence potential of the serotype 1 capsule or selection of genotypes advantageous for invasiveness.
Variations in susceptibility to host immune defense mechanisms can contribute to the differences between capsular serotypes in invasiveness. Immune response to pneumococcus strongly depends on opsonization of the bacteria with complement C3 molecules (C3b and iC3b), the deposition of which may be influenced by the capsular polysaccharides (20). We have previously shown that significantly more C3 is deposited on serotype 6B than 19F isolates and that a significantly higher concentration of polysaccharide-specific antibodies is required for opsonophagocytic killing of serotype 19F isolates (33). The fact that less C3 was deposited on serotype 19F pneumococci in the presence or absence of antibodies suggests innate differences between capsular serotypes in resistance to the complement. Comparison of C3 deposition on capsule-switched mutants of TIGR4 demonstrated large differences between serotypes (22), which indicates that the capsular serotype alone can have a significant impact on the complement resistance of strains.
Weinberger et al. described recently an association between serotype prevalence in carriage and the amount of capsular polysaccharide produced in vitro by isolates of a particular serotype (55). The association linked capsule chemistry to the success of the serotype as a colonizer. This observation suggested that a similar correlation can be present between capsule and the invasiveness of the serotype. In the present study we compared the resistance to complement and opsonophagocytosis of clinical isolates of several different pneumococcal serotypes. Each serotype was represented by multiple different clones. Isolates from blood or cerebrospinal fluid, from serotypes 1, 4, 5, 14, 18C, and 23F, as well as mucosal isolates from serotypes 1, 14, and 23F, were analyzed, along with reference strains used in the standard opsonophagocytic assay. We analyzed the results in a context of the serotype-specific severity of invasive disease and the chemical structure of the polysaccharides.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The pneumococcal isolates analyzed in the present study are listed in Table 1 . Clinical isolates of selected vaccine serotypes were analyzed to explore potential variance within the serotypes. Pediatric invasive isolates of serotypes 4, 14, 18C, and 23F collected in Finland by National Reference Laboratory for Pneumococcus (National Institute for Health and Welfare, Oulu, Finland) and mucosal isolates of serotypes 14 and 23F isolated from middle ear fluid samples in the Finnish Otitis Media cohort study (26) were selected based on previously published typing results to represent different multilocus sequence types (MLSTs) (16). Since serotypes 1 and 5 are uncommon in Finland isolates of these two capsular types representing different MLSTs were selected from pneumococci isolated from Gambian children during a vaccine trial and IPD projects in the Gambia (1, 11).
TABLE 1.
Bacterial strains used in this study
| Strain name in this study | Serotype | MLST | Sample type | Strain sourcea |
|---|---|---|---|---|
| MOPA1 | 1 | ND | Invasive | SPEC-1 (L82006), MOPA reference strain |
| INV1-1 | 1 | 618 | Invasive | PVT0002 |
| INV1-2 | 1 | 1331 | Invasive | PNI 0062 |
| INV1-3 | 1 | 3579 | Invasive | PNI 0213 |
| INV1-4 | 1 | 3574 | Invasive | PNI 0150 |
| CAR1-1 | 1 | 910 | Mucosal | CH0089 |
| CAR1-2 | 1 | 2084 | Mucosal | CH0053 |
| CAR1-3 | 1 | 3570 | Mucosal | PVT 13506 |
| MOPA4 | 4 | ND | Invasive | OREP-4 (DS2382), MOPA reference strain |
| INV4-1 | 4 | 176 | Invasive | IO11147 |
| INV4-2 | 4 | 205 | Invasive | IO11512 |
| INV4-3 | 4 | 205 | Invasive | IO12680 |
| MOPA5 | 5 | ND | Invasive | STREP-5 (DBL5) |
| INV5-1 | 5 | 289 | Invasive | PVT0020 |
| INV5-2 | 5 | 3313 | Invasive | PVT0027 |
| INV5-3 | 5 | 3338 | Invasive | PVT0018 |
| INV5-4 | 5 | 3339 | Invasive | PVT0026 |
| MOPA6B | 6B | ND | Invasive | SPEC-6B (BG25-9), MOPA reference strain |
| MOPA14 | 14 | ND | Invasive | STREP-14 (DS2214-94), MOPA reference strain |
| INV14-1 | 14 | 124 | Invasive | IO10163 |
| INV14-2 | 14 | 700 | Invasive | IO626 |
| INV14-3 | 14 | 9 | Invasive | IO12202 |
| MEF14-1 | 14 | 134 | Mucosal | IOKOR1832-5 |
| MEF14-2 | 14 | 156 | Mucosal | IOKOR2104-3 |
| MEF14-3 | 14 | 307 | Mucosal | IOKOR392-1 |
| MOPA18C | 18C | ND | Invasive | OREP-18C (GP116), MOPA reference strain |
| INV18C-1 | 18C | 496 | Invasive | IO10148 |
| INV18C-2 | 18C | 1016 | Invasive | IO10162 |
| INV18C-3 | 18C | 1073 | Invasive | IO632 |
| MOPA19F | 19F | ND | Invasive | SPEC-19F (DS2217-94), MOPA reference strain |
| MOPA23F | 23F | ND | Mucosal | EMC-23F (1212458), MOPA reference strain |
| INV23F-1 | 23F | 37 | Invasive | IO11697 |
| INV23F-2 | 23F | 36 | Invasive | IO10783 |
| INV23F-3 | 23F | 440 | Invasive | IO10961 |
| MEF23F-1 | 23F | 37 | Mucosal | IOKOR46-1 |
| MEF23F-2 | 23F | 515 | Mucosal | IOKOR1101-8 |
| MEF23F-3 | 23F | 535 | Mucosal | IOKOR1617-3 |
The MOPA reference strains were prepared by introducing drug resistance markers to the parent strains. The names of the parent strains are given in parentheses.
The reference strains of the multiplex opsonophagocytic killing assay (MOPA), which were compared in the present study, were all invasive isolates selected to carry a particular resistance marker (5, 9). Originally, the serotype 4, 14, 18C, and 19F reference strains were invasive blood isolates from the Centers for Disease Control and Prevention, Atlanta, GA (43). The serotype 1 and 6B strains came originally from D. Briles at the University of Alabama at Birmingham (9). The serotype 23F reference strain, a nasopharyngeal isolate, was naturally resistant to an antibiotic, whereas the drug resistances of the other MOPA reference strains were obtained by selective screening (5, 9).
Fresh pneumococcal cultures were used to measure C3 deposition on the pneumococcal surface in a flow cytometric assay. Bacteria were cultured to the early logarithmic growth phase in the Todd-Hewitt broth with 0.5% yeast extract (THYE) supplemented with 5% fetal bovine serum as described previously (33). Bacterial cells used in the opsonophagocytic assay were prepared exactly the same way except that, after reaching early logarithmic phase, the cultures were frozen slowly in 15% glycerol at −70°C and used immediately after thawing.
Serum samples used in the complement assays.
Sera from eight healthy adults who had not been immunized with any pneumococcal vaccine (normal human sera [NHS]) were used as a source of complement in the C3 deposition assay. Each serum was analyzed individually. Sera were (i) screened for antibodies to the corresponding capsular polysaccharides and protein antigens PspA (families 1 and 2), PspC (CbpA), and PhtD (Table 2) as previously described (48), (ii) aliquoted into small volumes, and (iii) stored at −70°C to preserve intact complement activity. Once thawed, the serum was immediately used in the assay.
TABLE 2.
Antibody concentrations to pneumococcal antigens in NHS
| Antigen | Geometric mean serum IgG concn in μg/ml (95% CI)a |
|---|---|
| Capsular polysaccharide | |
| 1 | 0.5 (0.2-1.5) |
| 4 | 0.2 (0.7-3.0) |
| 5 | 0.7 (0.2-2.9) |
| 6B | 0.2 (0.1-0.4) |
| 14 | 1.4 (0.4-4.7) |
| 18C | 0.3 (0.1-0.9) |
| 19F | 0.6 (0.1-2.5) |
| 23F | 2.8 (1.0-8.2) |
| Surface protein | |
| PspC | 13 (5.0-33) |
| PhtD | 24 (11-49) |
| PspA-1 | 1.8 (0.8-4.2) |
| PspA-2 | 2.0 (1.1-3.6) |
That is, the geometric mean antibody concentrations of the antigen-specific antibody concentrations of eight different normal human sera. The 95% confidence intervals (95% CI) are given in parentheses.
Serum samples used in the opsonophagocytic assay.
The functional activity of anticapsular antibodies on opsonophagocytosis was assessed by analyzing the pneumococcal isolates with pooled sera obtained from infants immunized with an 11-valent pneumococcal conjugate vaccine (59). Each serum pool was collected from post-booster sera of 5 to 11 different children. The capsule type-specific antibody concentrations of the serum pools (Table 3) were calculated based on concentrations of the individual sera previously measured with enzyme immunoassay (58). The ethics committee of the National Public Health Institute, Helsinki, Finland, reviewed the protocol and approved the use of the sera in the present study.
TABLE 3.
Serum antibody concentrations to capsular polysaccharides in pooled sera used in the opsonophagocytic assay
| Serotype | Geometric mean IgG concn in μg/ml in pooled sera (95% CI)a |
||
|---|---|---|---|
| Low | Medium | High | |
| 1 | 5.9 (3.2-11) | 6.6 (5.3-8.1) | 14 (11-20) |
| 4 | 1.4 (0.8-2.5) | 6.2 (3.4-11) | 15 (11-20) |
| 5 | 1.7 (1.0-2.8) | 6.4 (4.6-9.0) | 13 (8.5-20) |
| 6B | 1.2 (0.9-1.5) | 5.8 (4.9-7.0) | 15 (11-20) |
| 14 | 1.3 (0.7-2.4) | 5.8 (3.4-9.8) | 10 (5.2-19) |
| 18C | 1.2 (0.8-1.8) | 4.3 (2.5-7.5) | 16 (10-26) |
| 19F | 5.2 (5.0-5.4) | 7.2 (6.6-7.9) | 16 (14-17) |
| 23F | 1.3 (0.7-2.4) | 5.8 (3.4-9.8) | 10 (5.2-19) |
That is, the geometric mean IgG concentrations in pooled sera from infants immunized with an 11-valent pneumococcal conjugate vaccine. The 95% confidence intervals (95% CI) are given in parentheses. Each pool was collected from 5 to 11 different sera.
Complement C3 deposition assay.
Deposition of complement C3 on pneumococci was measured with a flow cytometric assay as described previously (33). In short, pneumococcal strains were incubated in 20% human serum with active complement components for 5 min and C3 molecules (C3b and iC3b) bound to the bacteria were detected with anti-C3c-fluorescein isothiocyanate-conjugated rabbit polyclonal anti-human complement C3c antibodies (Dako Immunoglobulins, Denmark). As a negative control in each analysis, bacteria were incubated with NHS in buffer containing 10 mM EDTA, which blocks both alternative and classical pathways of complement activation. The geometric mean fluorescence (GMF) intensity was analyzed for each sample by using a flow cytometer (FACSCalibur; Becton Dickinson).
Opsonophagocytic assay.
The functional activity of serum antibodies was measured by a standard opsonophagocytic killing assay (43) using differentiated HL-60 cells (promyelotic leukemia cells, CCL240; American Type Culture Collection, Rockville, MD), as described previously (33). Soon thereafter, polymorphonuclear cells were allowed to phagocytose bacteria in the presence of baby rabbit complement (Pell-Freez Biologicals/Dynal) and inactivated human serum or, as a control, complement only. The results were interpreted as the serum dilution that resulted in 50% of bacteria being killed compared to the bacteria present in the control well in which only complement, but no antibodies, was present. Each bacterial strain was analyzed with three different serum pools, which contained different concentrations of serotype-specific antibodies (Table 3).
Statistical methods.
The geometric mean serum antibody concentrations required for 50% opsonophagocytic killing and the GMF intensities of C3 deposition with 95% confidence intervals were analyzed. One-way analysis of variance (ANOVA) was applied in comparisons of serotypes, followed by Tukey's post-hoc test when appropriate. A Student t test was used in comparison of mucosal and invasive pneumococcal isolates within serotypes, as well as in comparison of reference strains with the clinical isolates. Pearson coefficient of correlation was calculated for C3 deposition and opsonophagocytic killing, as well as for C3 deposition and serum antibody concentration of the normal human sera used as the source of complement. The serotype-specific IPD mortality expressed as an adjusted OR estimates was compared to serotype-specific C3 deposition and the antibody concentration required for 50% opsonophagocytic killing by calculating Pearson correlation. The Pearson correlation was also calculated for serotype-specific C3 deposition and the number of hydroxyl groups per polysaccharide. Statistical analyses were performed on log-transformed data, except for the OR estimates and the number of hydroxyl groups, which were not transformed. In all analyses, P values of <0.05 were considered to indicate a statistically significant difference.
RESULTS
Capsular serotype affects resistance to deposition of C3 on the bacteria.
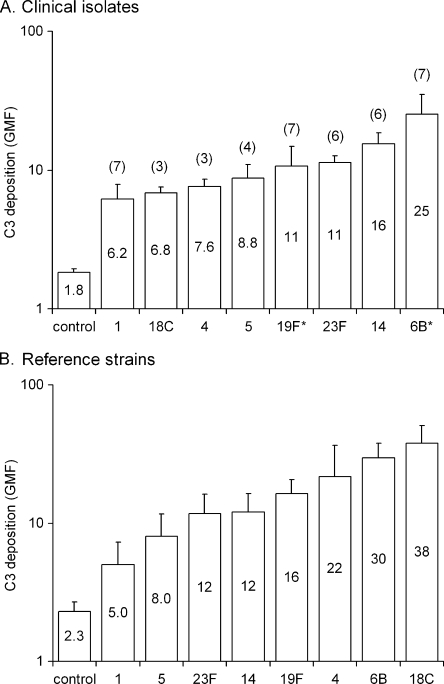
To investigate the role of the capsular serotype in sensitivity to complement, we measured C3 deposition on pneumococcal isolates using eight different NHS as the source of complement. Serotype 6B and 19F clinical isolates analyzed in our previous study (33) were also included in the comparison. Serotypes were ranked from most resistant serotype 1 to most susceptible 6B (clinical strains) or 18C (reference strains) according to C3 deposition, expressed as the GMF (Fig. 1). Among clinical isolates tested (Fig. 1A), the difference between two randomly selected serotypes was always significant unless they were next in the rank. The exceptions were a significant difference between close in rank serotypes 1 and 18C and nonsignificant between distant serotypes 5 and 18C, 5 and 23F, and 19F and 14 (one-way ANOVA, followed by Tukey's post-hoc test). Within a serotype, clinical isolates of capsular types 1, 14, 23F, and 18C varied significantly in sensitivity to complement. However, there was no difference in complement deposition between mucosal and invasive isolates of the same serotype when tested (Student t test). The differences between reference strains (Fig. 1B) were significant between serotype 1 and all other serotypes except type 5; between serotype 5 and serotypes 19F, 4, 6B, and 18C; between serotypes 23F and 14 and serotypes 6B and 18C; and between serotypes 19F and 18C (one-way ANOVA, followed by Tukey's post-hoc test). Compared to the clinical isolates, significantly more C3 was deposited on the reference strains of serotypes 4 and 18C (Student t test).
FIG. 1.
(A and B) Deposition of complement C3 on the surface clinical pneumococcal isolates (A) and pneumococcal reference strains used in the opsonophagocytic assay (B). C3 deposition is expressed as the geometric mean intensity of fluorescence (GMF) with 95% confidence intervals from analyses with eight different normal human sera (NHS). The number of clinical isolates analyzed per serotype is shown outside the bar, and the geometric mean of the GMF values is shown inside the bar. The serotype 6B and 19F clinical isolates, indicated with an asterisk, were analyzed in a previous study with four different normal human sera (33).
C3 deposition is associated with serum IgG to pneumococcal antigens.
We found an association between complement deposition and serum IgG concentration to PspC (r = 0.31, P < 0.01) and PspA family 2 (r = 0.27, P < 0.05, Pearson correlation), when the results for all isolates were combined. The associations depended on the isolate and not on the serotype. IgG concentration to PspC, PhtD, or PspA family 2 in the sera was significantly associated with increased C3 deposition on 15 of 39 isolates, and yet for most other isolates a trend toward association between resistance to complement and IgG concentration to one or two protein antigens was observed. A significant correlation between C3 deposition and antibody concentration to the corresponding capsular polysaccharide was seen only for a few isolates, of serotypes 1, 4, and 14.
Capsular serotype affects resistance to opsonophagocytic killing.
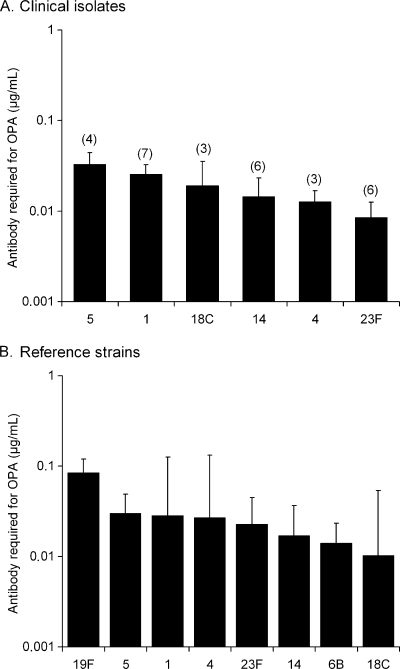
We measured the serotype specific antibody concentration required for 50% opsonophagocytic killing using pooled sera from immunized individuals. The clinical isolates of serotypes 1 and 5 required a significantly higher concentration of anticapsular antibodies than 23F for killing (P < 0.05, one-way ANOVA with Tukey's post-hoc test, Fig. 2A). Variation between clinical isolates of the same serotype in sensitivity to opsonophagocytic killing was statistically nonsignificant (one-way ANOVA). Unlike the complement deposition results, opsonophagocytosis of clinical serotype 6B and 19F isolates analyzed in our previous study (33) could not be compared to the serotypes analyzed in the present study because different sera were used and the opsonophagocytic titers of the two studies are not comparable. A significantly higher concentration of serotype-specific anticapsular antibodies was required for killing the serotype 19F than any other reference strain except those of serotype 1, 4, and 5 (Fig. 2B). Six times as much antibody was required for 50% opsonophagocytic killing of the serotype 19F as for the serotype 6B reference strain, a finding similar to our previous study (33). Serotype 1 and 5 reference strains required twice as much antibody as 6B, but the difference was not statistically significant. The serotype 23F reference strain was more resistant to opsonophagocytic killing than the serotype 23F clinical isolates (P < 0.05, Student t test).
FIG. 2.
(A and B) Opsonophagocytic killing of clinical pneumococcal isolates (A) and pneumococcal reference strains (B). Each strain was analyzed with three different serum pools from children immunized with a pneumococcal conjugate vaccine and the results are presented as the geometric mean anticapsular antibody concentration (GMC) required for 50% opsonophagocytic killing with 95% confidence intervals. The number of clinical isolates analyzed per serotype is shown in parentheses.
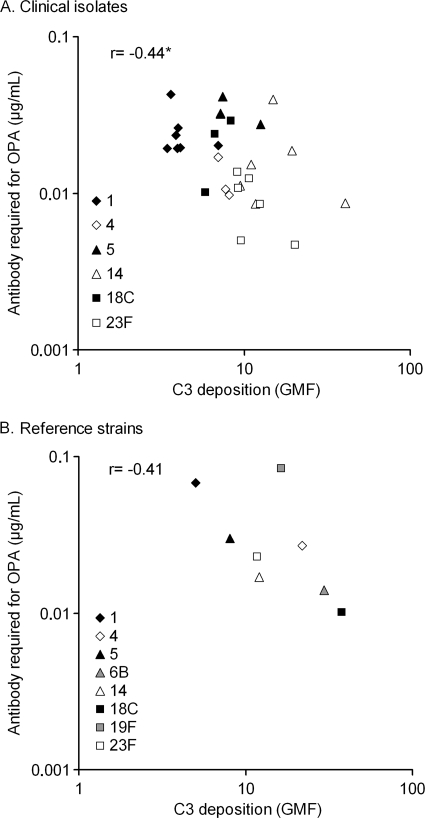
Resistance to complement deposition is associated with resistance to opsonophagocytic killing.
We found an association between complement deposition and opsonophagocytosis among the clinical isolates and the reference strains (Fig. 3). The clinical isolates most resistant to C3 deposition tended to require a higher concentration of anticapsular antibody for opsonophagocytic killing than the isolates least able to resist complement deposition. Reference strains of serotypes 1 and 5 were the two most resistant to complement, but they were less resistant to opsonophagocytosis than the serotype 19F reference strain. Serotype 19F reference strain deviates from the trend by showing a moderate resistance to complement and yet requiring the highest concentration of serotype-specific antibodies to be opsonophagocytosed.
FIG. 3.
Complement C3 deposition was associated with the anticapsular antibody concentration required for 50% opsonophagocytic killing of clinical isolates (A) and pneumococcal reference strains (B). Geometric mean intensities of fluorescence (GMF) and geometric mean anticapsular antibody concentrations are shown. *, P = 0.018 (Pearson correlation).
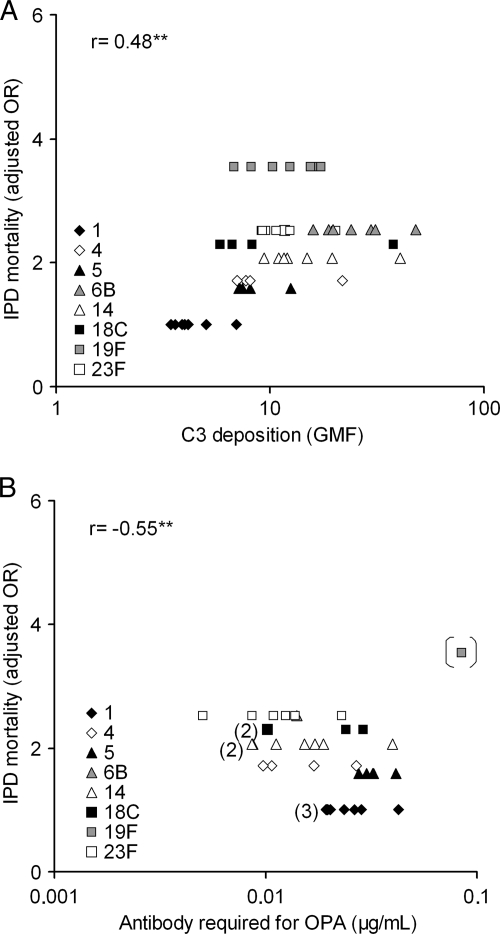
Resistance to complement deposition and opsonophagocytosis is related to a lower mortality of IPD.
Mortality following IPD caused by different serotypes has previously been assessed by Harboe et al. in a large population-based study by using a multivariate logistic regression analysis and OR estimates for the most frequent serotypes were calculated (17). We found for serotypes analyzed in the present study a significant association between serotype-specific IPD mortality (adjusted 30-day mortality OR estimates) and C3 deposition with the smallest amount of C3 deposited on the serotypes with the lowest mortality (Fig. 4A). There was also a trend between resistance to opsonophagocytic killing and IPD severity, which was statistically significant if serotype 19F was excluded (Fig. 4B). The serotypes with the lowest mortality required the highest concentration of antibodies to opsonophagocytic killing.
FIG. 4.
Complement C3 deposition (A) and concentration of anticapsular antibodies required for 50% opsonophagocytic killing (B) of pneumococcal clinical isolates and reference strains was associated with serotype-specific mortality of IPD in patients aged 5 years or older (17). The number of strains in the overlapping symbols is shown inside the brackets. Correlation of IPD severity with the antibody concentration required for opsonophagocytic killing was significant when serotype 19F reference strain was excluded. **, P < 0.01 (Pearson correlation).
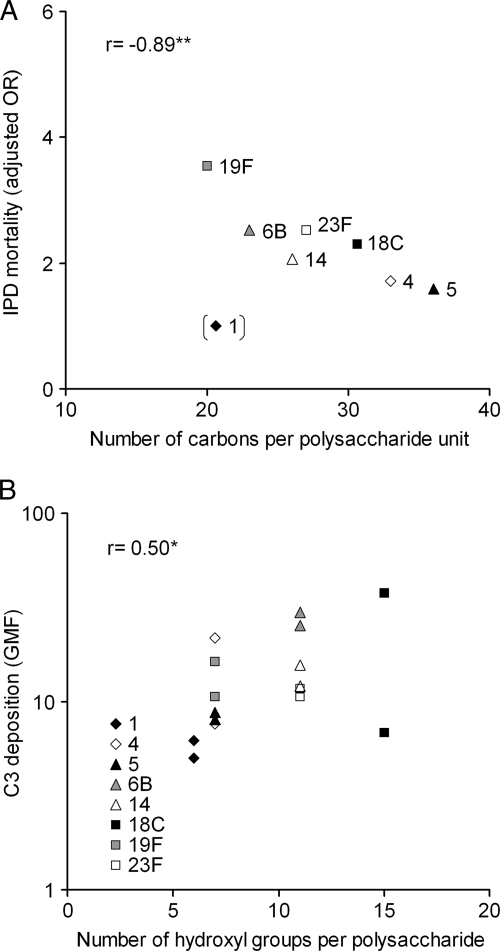
The polysaccharide structure is associated with serotype-specific IPD mortality and complement deposition.
We examined the rank of serotypes according to the number of carbons required to generate a capsule polysaccharide unit published by Weinberger et al. (55) and found for serotypes analyzed in the present study an association between capsule synthesis metabolic cost and serotype dependent related severity of IPD published by Harboe et al. (17). The serotypes, which have the least carbons per polysaccharide repeat unit, tend to cause the most severe disease (Fig. 5A). The association was highly significant after serotype 1 exclusion. This particular capsule type is unique among pneumococci for its zwitterionic property of polysaccharide (10). We examined further published structures of capsular polysaccharides (27) in order to test whether there is a correlation between the number of hydroxyl groups in the polysaccharide repeat unit, known as potential targets for C3 deposition on the polysaccharide, and the susceptibility to complement among clinical strains of a particular serotype. We found a significant association between these two parameters. A high number of free hydroxyl groups was associated with a higher level of C3 deposition (Fig. 5B).
FIG. 5.
(A) Serotype-specific mortality to IPD was associated with the capsule structure (number of carbons per repeat unit of the polysaccharide) and the correlation was significant if serotype 1 was excluded. A high number of carbons tended to associate with a lower level of C3 deposition, but the correlation was not statistically significant even if serotype 1 was excluded (data not shown). (B) The degree of complement deposition on pneumococci was associated with the number of hydroxyl groups in the polysaccharide repeat unit. C3 deposition on reference strains and geometric mean of C3 deposition on clinical isolates of each serotype are shown in panel B. *, P < 0.05; **, P < 0.01 (Pearson correlation).
DISCUSSION
In our previous study we reported that serotypes 6B and 19F profoundly differ in susceptibility to complement deposition and to antibody-mediated opsonophagocytosis (33). In the present study we have broadened the comparison to include other clinically important capsular serotypes. We found significant differences among serotypes in their susceptibility to complement deposition and opsonophagocytic killing. Analysis of several pneumococcal strains suggests that isolates of primary pathogen serotypes 1, 4, 5, and 18C are better able to resist complement deposition, which could partially explain why these serotypes have a high invasive-disease potential (6, 16, 50). We also observed variation in C3 deposition within a serotype, including differences between clinical and reference strains, indicating the significance of serotype independent genetic background in resistance to complement. The lack of such differences between mucosal and invasive isolates suggests that there was no priming for higher resistance to complement in a serotype-independent manner.
The genetic background is likely to affect the relative importance of a surface protein to the virulence of the strain and how antibodies to the virulence protein induce complement deposition. Antibodies to protein antigens, which play a role in complement inhibition, may enhance C3 deposition on pneumococci even when the concentrations are relatively low. In a clinical study assessing PspA as a vaccine candidate the concentration of serum antibodies to PspA correlated with C3 deposition on pneumococci (35). We found in our previous study that the concentration of IgG to PspA present in normal human sera correlated significantly with C3 deposition on serotype 2, 3, and 19F isolates, but not with C3 deposition on a serotype 4 isolate (32). In the present study we found significant correlations between antibody concentration to PspA, PspC, and PhtD and capsular polysaccharides 1, 4, and 14 with complement deposition on individual pneumococcal strains. Of these, the PspC proteins have been shown to have complement inhibitory activity via binding of the alternative pathway inhibitor factor H (12, 23, 24), whereas the mechanism by which PspA inhibits C3 deposition (52) is suggested to be interference with the C1q initiation step of the classical pathway (30). The role of PhtD in the complement resistance of pneumococci is not clear, and it appears to depend on the genetic background of the strain (32, 36).
Similar polarization of serotypes as observed in the ability to resist complement deposition was seen with susceptibility to opsonophagocytosis. A relatively high concentration of antibodies was required for killing of serotype 1 and 5 isolates, whereas very low concentrations of polysaccharide-specific antibodies were sufficient to kill isolates of serotype 23F. There was a clear association between complement deposition and antibody concentration required for opsonophagocytosis, which suggests that the serotypes which are more resistant to complement require a higher concentration of capsule-specific antibodies for opsonophagocytic killing. Although there was significant variation within serotypes in complement deposition, the variation in sensitivity to opsonophagocytosis was nonsignificant, suggesting that the capsule type could be more important than the genetic background for determining resistance to opsonophagocytosis. Differences between serotypes in sensitivity to opsonophagocytosis could result from the complement deposited on the bacterial surface not being equally exposed to the phagocytes due to the different capsular structures and localization of the opsonins. Recent data suggest that the capsule inhibits phagocytosis of encapsulated bacteria by several mechanisms. It reduces the binding of IgG and CRP on the bacteria, thereby inhibiting activation of the classical pathway (21). In addition, the capsule inhibits phagocytosis mediated directly by IgG and nonopsonic phagocytic receptors (21) but also in an opsonin- and antibody-independent manner (55).
The difference in sensitivity to complement deposition is not likely to be the only factor to explain the different susceptibilities of the serotypes to opsonophagocytic killing. Despite moderate resistance to complement deposition, a very high concentration of serotype-specific antibodies was found here and previously (33) to be required for opsonophagocytosis of serotype 19F strains, suggesting inferior functional properties of antibodies. The data from clinical trials demonstrate a correlation of serotype-specific vaccine efficacy with opsonophagocytic activity of the antibodies (14, 46). The antibody concentration required for 50% opsonophagocytic killing depends on the capsular serotype and up to five times higher antibody concentrations have been reported to be required for killing serotype 19F compared to 6B (14, 33, 43, 46, 59). The 7-valent pneumococcal conjugate vaccine has been reported to efficiently protect against IPD caused by the serotypes included in the vaccine formula (4, 28, 34, 56). However, a case-control study in the United States suggests less efficient protection against serotype 19F (57). In the Gambia the efficacy of the 9-valent vaccine was significant against IPD caused by serotypes 5, 14, and 23F, whereas despite good immunogenicity the vaccine appeared to lack protection against serotype 1 (11, 44). The efficacy of conjugate vaccines against pneumococcal AOM was good against 6B and 23F but poor against 19F (15, 25), although the antibody concentrations after vaccination were the lowest for 6B and 23F and highest for 19F (13, 25). Lower efficacies against AOM caused by 19F have been reported in other studies as well (4, 38). Serotype 1 isolates analyzed in the present study and the serotype 19F isolates in our previous study (33) required high concentrations of capsule specific antibodies for opsonophagocytosis, which is in concordance with their less optimal vaccine efficacies. However, in the Gambia the conjugate vaccine offered a good protection against IPD caused serotype 5 (44), and yet in our study it required the highest concentration of capsule antibodies for killing in the opsonophagocytic assay.
We found an association between our results of serotype specific differences in susceptibility to C3 deposition and opsonophagocytic killing and the serotype-specific estimates for mortality due to IPD published by Harboe et al. (17), suggesting that the primary pathogens, which are associated with a lower mortality than the opportunistic serotypes, are more resistant to C3 deposition and require a higher concentration of capsule antibodies for opsonophagocytic killing. This could indicate that the primary pathogens, which are able to cause disease in healthy individuals and have a high potential to cause invasive disease (6, 7, 45, 49), are better able to resist host immunity than the opportunistic serotypes. The opportunistic serotypes commonly cause disease in immunocompromised patients, which could explain their association with more severe disease (17, 49). However, when the bias of underlying disease was eliminated, clones of primary pathogen serotypes were observed to cause a milder disease than clones belonging to the less invasive groups (49). Clones of the opportunistic serotypes also caused a more robust proinflammatory cytokine response in mice (49). Differences between serotypes in their ability to activate innate immune responses may contribute to disease severity, as well as bacterial clearance. The serotypes which are able to persist in the respiratory mucosa of healthy individuals are crucial for the survival and spread of the bacterium. The part of the capsular genome that regulates capsular expression has recently been found to fall into two highly divergent sequence clans, which interestingly seem to be associated with either serotypes common in carriage (such as serogroup 6 and serotype 23F) or invasive disease (1, 4, 5, 7F, and 14) (53). In an attempt to explain the dichotomy, it was speculated that the capsule expression of the more virulent clan could either be increased or more efficiently regulated through phase variation, which would provide an advantage for colonization (53).
The prevalence of a serotype in nasopharyngeal carriage seems to be determined by the metabolic cost the bacteria are investing in a capsule (55). The serotypes with a less complex and thus energy efficient structure (less carbons per polysaccharide repeat unit) were associated with a higher prevalence in carriage (55). We found that the number of carbons is also related to the serotype-specific mortality of IPD, which suggests that the serotypes which have a low number of carbons per repeat unit are associated with a high mortality. Serotypes 4 and 5, which have a high invasive disease potential but low mortality (49), had the highest number of carbons and the opposite was seen for serotypes 19F and 6B. Serotype 1, which was associated with the lowest mortality, deviated from the trend. The strong negative correlation between severity of IPD and metabolic cost of capsular polysaccharide unit synthesis could suggest that the metabolic cost of capsule production alone has a significant impact on the outcome of IPD, with serotypes of less metabolically costly capsule causing more severe disease. Interestingly, we also found that the ability of serotypes to restrict C3 deposition was associated with mortality of IPD, which suggests that the serotypes associated with a higher mortality (the opportunistic pathogens) are more sensitive to complement than the serotypes, which are associated with invasiveness but which cause a less severe disease.
Differences in the capsular polysaccharide structures (27) could partly explain the differences in complement deposition observed between serotypes. We found a significant association between C3 deposition and the number of free hydroxyl groups per polysaccharide repeat unit. Serotypes 1 and 5, which were the most resistant to C3 opsonization and opsonophagocytosis, have a smaller number of available targets (-OH or -NH2 groups) for C3b to make ester or amide linkages. The importance of acetyl groups to the antigenicity of pneumococcal polysaccharides was reported in a previous study in which the functional activity of antibodies was evaluated using an opsonophagocytic assay (41). It was shown that the target of vaccine induced antibodies was linked to an O-acetyl group present in the monosaccharide residues of serotype 15B and that these antibodies were not cross-reactive against serotype 15C, whose capsular polysaccharide is otherwise identical but not acetylated (41). In the repeating sugar units of serotype 1 there are 6 hydroxyls, three of which are in acidic carboxyls. There are two amino groups, one of which is acetylated. The sugar unit of serotype 5 has seven hydroxyl groups and three amino groups, all of which are acetylated. In contrast, serotypes 6B, 18C, and 23F have a high number of free hydroxyls (11 in serotype 6B, 15 in serotype 18C, and 11 in 23F). None of the hydroxyl groups of these three serotypes are in carboxylic acids and there is no acetylation. A low number of available targets for complement deposition and presence of acetyl groups in the amino and carboxyl groups of the sugars of the capsule polysaccharide seems to be advantageous to resisting recognition by innate immune mechanisms.
The results of the present study suggest that the serotype-dependent differences in resistance to complement deposition could explain why certain serotypes are able to efficiently resist the host innate immunity and act as primary pathogens. We also found that these serotypes require a high concentration of serotype-specific antibodies for opsonophagocytic killing. It is possible that the capsules of serotypes with a high invasive potential reduce the sensitivity of the serotype to opsonophagocytosis by efficiently restricting complement deposition on the pneumococcal surface. In contrast, low concentrations of polysaccharide-specific antibodies were sufficient for killing serotype of low invasive potential which also were less resistant to complement. We also identified exceptions from this general rule. Even though the complement resistance of serotype 19F was similar to serotypes of low invasive potential, isolates of this capsular type required concentration of anticapsular antibodies for killing higher than isolates of invasive serotypes 1 and 5, which suggest that the functionality of the antibodies to 19F may be inferior. It is clear from these data as well as from clinical studies that the threshold level for protection of vaccine induced anticapsular antibodies cannot be expected to be the same for all serotypes.
Acknowledgments
We thank Leena Tikkanen and Sanna Piipponen for skillful assistance in the laboratory analyses and statistician Mika Lahdenkari for advice in the statistical analysis of the data at The National Institute for Health and Welfare, Helsinki, Finland. We also thank William P. Hanage (Imperial College, London, United Kingdom) for sharing the MLST data of the Finnish clinical isolates.
Editor: A. Camilli
Footnotes
Published ahead of print on 20 September 2010.
REFERENCES
- 1.Adegbola, R. A., P. C. Hill, O. Secka, U. N. Ikumapayi, G. Lahai, B. M. Greenwood, and T. Corrah. 2006. Serotype and antimicrobial susceptibility patterns of isolates of Streptococcus pneumoniae causing invasive disease in The Gambia 1996-2003. Trop. Med. Int. Health 11:1128-1135. [DOI] [PubMed] [Google Scholar]
- 2.Antonio, M., H. Dada-Adegbola, E. Biney, T. Awine, J. O'Callaghan, V. Pfluger, G. Enwere, B. Okoko, C. Oluwalana, A. Vaughan, S. M. Zaman, G. Pluschke, B. M. Greenwood, F. Cutts, and R. A. Adegbola. 2008. Molecular epidemiology of pneumococci obtained from Gambian children aged 2-29 months with invasive pneumococcal disease during a trial of a 9-valent pneumococcal conjugate vaccine. BMC Infect. Dis. 8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonio, M., I. Hakeem, T. Awine, O. Secka, K. Sankareh, D. Nsekpong, G. Lahai, A. Akisanya, U. Egere, G. Enwere, S. M. Zaman, P. C. Hill, T. Corrah, F. Cutts, B. M. Greenwood, and R. A. Adegbola. 2008. Seasonality and outbreak of a predominant Streptococcus pneumoniae serotype 1 clone from The Gambia: expansion of ST217 hypervirulent clonal complex in West Africa. BMC Microbiol. 8:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, et al. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 5.Bogaert, D., M. Sluijter, R. De Groot, and P. W. Hermans. 2004. Multiplex opsonophagocytosis assay (MOPA): a useful tool for the monitoring of the 7-valent pneumococcal conjugate vaccine. Vaccine 22:4014-4020. [DOI] [PubMed] [Google Scholar]
- 6.Brueggemann, A. B., D. T. Griffiths, E. Meats, T. Peto, D. W. Crook, and B. G. Spratt. 2003. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J. Infect. Dis. 187:1424-1432. [DOI] [PubMed] [Google Scholar]
- 7.Brueggemann, A. B., T. E. Peto, D. W. Crook, J. C. Butler, K. G. Kristinsson, and B. G. Spratt. 2004. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J. Infect. Dis. 190:1203-1211. [DOI] [PubMed] [Google Scholar]
- 8.Brueggemann, A. B., and B. G. Spratt. 2003. Geographic distribution and clonal diversity of Streptococcus pneumoniae serotype 1 isolates. J. Clin. Microbiol. 41:4966-4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burton, R. L., and M. H. Nahm. 2006. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin. Vaccine Immunol. 13:1004-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi, Y. H., M. H. Roehrl, D. L. Kasper, and J. Y. Wang. 2002. A unique structural pattern shared by T-cell-activating and abscess-regulating zwitterionic polysaccharides. Biochemistry 41:15144-15151. [DOI] [PubMed] [Google Scholar]
- 11.Cutts, F. T., S. M. Zaman, G. Enwere, S. Jaffar, O. S. Levine, J. B. Okoko, C. Oluwalana, A. Vaughan, S. K. Obaro, A. Leach, K. P. McAdam, E. Biney, M. Saaka, U. Onwuchekwa, F. Yallop, N. F. Pierce, B. M. Greenwood, and R. A. Adegbola. 2005. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 365:1139-1146. [DOI] [PubMed] [Google Scholar]
- 12.Dave, S., A. Brooks-Walter, M. K. Pangburn, and L. S. McDaniel. 2001. PspC, a pneumococcal surface protein, binds human factor H. Infect. Immun. 69:3435-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekstrom, N., H. Ahman, J. Verho, J. Jokinen, M. Vakevainen, T. Kilpi, and H. Kayhty. 2005. Kinetics and avidity of antibodies evoked by heptavalent pneumococcal conjugate vaccines PncCRM and PncOMPC in the Finnish Otitis Media Vaccine Trial. Infect. Immun. 73:369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekstrom, N., M. Vakevainen, J. Verho, T. Kilpi, and H. Kayhty. 2007. Functional antibodies elicited by two heptavalent pneumococcal conjugate vaccines in the Finnish Otitis Media Vaccine Trial. Infect. Immun. 75:1794-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen, J. Haapakoski, E. Herva, A. Takala, H. Kayhty, P. Karma, R. Kohberger, G. Siber, and P. H. Makela. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403-409. [DOI] [PubMed] [Google Scholar]
- 16.Hanage, W. P., T. H. Kaijalainen, R. K. Syrjanen, K. Auranen, M. Leinonen, P. H. Makela, and B. G. Spratt. 2005. Invasiveness of serotypes and clones of Streptococcus pneumoniae among children in Finland. Infect. Immun. 73:431-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harboe, Z. B., R. W. Thomsen, A. Riis, P. Valentiner-Branth, J. J. Christensen, L. Lambertsen, K. A. Krogfelt, H. B. Konradsen, and T. L. Benfield. 2009. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLoS Med. 6:e1000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hausdorff, W. P., J. Bryant, P. R. Paradiso, and G. R. Siber. 2000. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin. Infect. Dis. 30:100-121. [DOI] [PubMed] [Google Scholar]
- 19.Hill, P. C., Y. B. Cheung, A. Akisanya, K. Sankareh, G. Lahai, B. M. Greenwood, and R. A. Adegbola. 2008. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian infants: a longitudinal study. Clin. Infect. Dis. 46:807-814. [DOI] [PubMed] [Google Scholar]
- 20.Hostetter, M. K. 1986. Serotypic variations among virulent pneumococci in deposition and degradation of covalently bound C3b: implications for phagocytosis and antibody production. J. Infect. Dis. 153:682-693. [DOI] [PubMed] [Google Scholar]
- 21.Hyams, C., E. Camberlein, J. M. Cohen, K. Bax, and J. S. Brown. 2010. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect. Immun. 78:704-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyams, C., J. Yuste, K. Bax, E. Camberlein, J. N. Weiser, and J. S. Brown. 2010. Streptococcus pneumoniae resistance to complement-mediated immunity is dependent on the capsular serotype. Infect. Immun. 78:716-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janulczyk, R., F. Iannelli, A. G. Sjoholm, G. Pozzi, and L. Bjorck. 2000. Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J. Biol. Chem. 275:37257-37263. [DOI] [PubMed] [Google Scholar]
- 24.Jarva, H., R. Janulczyk, J. Hellwage, P. F. Zipfel, L. Bjorck, and S. Meri. 2002. Streptococcus pneumoniae evades complement attack and opsonophagocytosis by expressing the pspC locus-encoded Hic protein that binds to short consensus repeats 8-11 of factor H. J. Immunol. 168:1886-1894. [DOI] [PubMed] [Google Scholar]
- 25.Kilpi, T., H. Ahman, J. Jokinen, K. S. Lankinen, A. Palmu, H. Savolainen, M. Gronholm, M. Leinonen, T. Hovi, J. Eskola, H. Kayhty, N. Bohidar, J. C. Sadoff, and P. H. Makela. 2003. Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: randomized, controlled trial of a 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine in 1,666 children. Clin. Infect. Dis. 37:1155-1164. [DOI] [PubMed] [Google Scholar]
- 26.Kilpi, T., E. Herva, T. Kaijalainen, R. Syrjanen, and A. K. Takala. 2001. Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of life. Pediatr. Infect. Dis. J. 20:654-662. [DOI] [PubMed] [Google Scholar]
- 27.Kim, J. S., E. R. Laskowich, R. G. Arumugham, R. E. Kaiser, and G. J. MacMichael. 2005. Determination of saccharide content in pneumococcal polysaccharides and conjugate vaccines by GC-MSD. Anal. Biochem. 347:262-274. [DOI] [PubMed] [Google Scholar]
- 28.Klugman, K. P., S. A. Madhi, R. E. Huebner, R. Kohberger, N. Mbelle, and N. Pierce. 2003. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N. Engl. J. Med. 349:1341-1348. [DOI] [PubMed] [Google Scholar]
- 29.Leimkugel, J., A. Adams Forgor, S. Gagneux, V. Pfluger, C. Flierl, E. Awine, M. Naegeli, J. P. Dangy, T. Smith, A. Hodgson, and G. Pluschke. 2005. An outbreak of serotype 1 Streptococcus pneumoniae meningitis in northern Ghana with features that are characteristic of Neisseria meningitidis meningitis epidemics. J. Infect. Dis. 192:192-199. [DOI] [PubMed] [Google Scholar]
- 30.Li, J., D. T. Glover, A. J. Szalai, S. K. Hollingshead, and D. E. Briles. 2007. PspA and PspC minimize immune adherence and transfer of pneumococci from erythrocytes to macrophages through their effects on complement activation. Infect. Immun. 75:5877-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malley, R., M. Lipsitch, A. Stack, R. Saladino, G. Fleisher, S. Pelton, C. Thompson, D. Briles, and P. Anderson. 2001. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect. Immun. 69:4870-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melin, M., E. Di Paolo, L. Tikkanen, H. Jarva, C. Neyt, H. Kayhty, S. Meri, J. Poolman, and M. Vakevainen. 2010. Interaction of pneumococcal histidine triad proteins with human complement. Infect. Immun. 78:2089-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melin, M., H. Jarva, L. Siira, S. Meri, H. Kayhty, and M. Vakevainen. 2009. Streptococcus pneumoniae capsular serotype 19F is more resistant to C3 deposition and less sensitive to opsonophagocytosis than serotype 6B. Infect. Immun. 77:676-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Brien, K. L., L. H. Moulton, R. Reid, R. Weatherholtz, J. Oski, L. Brown, G. Kumar, A. Parkinson, D. Hu, J. Hackell, I. Chang, R. Kohberger, G. Siber, and M. Santosham. 2003. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet 362:355-361. [DOI] [PubMed] [Google Scholar]
- 35.Ochs, M. M., W. Bartlett, D. E. Briles, B. Hicks, A. Jurkuvenas, P. Lau, B. Ren, and A. Millar. 2008. Vaccine-induced human antibodies to PspA augment complement C3 deposition on Streptococcus pneumoniae. Microb. Pathog. 44:204-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogunniyi, A. D., M. Grabowicz, L. K. Mahdi, J. Cook, D. L. Gordon, T. A. Sadlon, and J. C. Paton. 2009. Pneumococcal histidine triad proteins are regulated by the Zn2+-dependent repressor AdcR and inhibit complement deposition through the recruitment of complement factor H. FASEB J. 23:731-738. [DOI] [PubMed] [Google Scholar]
- 37.Porat, N., R. Trefler, and R. Dagan. 2001. Persistence of two invasive Streptococcus pneumoniae clones of serotypes 1 and 5 in comparison to that of multiple clones of serotypes 6B and 23F among children in southern Israel. J. Clin. Microbiol. 39:1827-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prymula, R., P. Peeters, V. Chrobok, P. Kriz, E. Novakova, E. Kaliskova, I. Kohl, P. Lommel, J. Poolman, J. P. Prieels, and L. Schuerman. 2006. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typeable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 367:740-748. [DOI] [PubMed] [Google Scholar]
- 39.Quataert, S. A., C. S. Kirch, L. J. Wiedl, D. C. Phipps, S. Strohmeyer, C. O. Cimino, J. Skuse, and D. V. Madore. 1995. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin. Diagn. Lab. Immunol. 2:590-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quataert, S. A., K. Rittenhouse-Olson, C. S. Kirch, B. Hu, S. Secor, N. Strong, and D. V. Madore. 2004. Assignment of weight-based antibody units for 13 serotypes to a human antipneumococcal standard reference serum, lot 89-S(f). Clin. Diagn. Lab. Immunol. 11:1064-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajam, G., G. M. Carlone, and S. Romero-Steiner. 2007. Functional antibodies to the O-acetylated pneumococcal serotype 15B capsular polysaccharide have low cross-reactivities with serotype 15C. Clin. Vaccine Immunol. 14:1223-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson, D. A., K. M. Edwards, K. B. Waites, D. E. Briles, M. J. Crain, and S. K. Hollingshead. 2001. Clones of Streptococcus pneumoniae isolated from nasopharyngeal carriage and invasive disease in young children in central Tennessee. J. Infect. Dis. 183:1501-1507. [DOI] [PubMed] [Google Scholar]
- 43.Romero-Steiner, S., D. Libutti, L. B. Pais, J. Dykes, P. Anderson, J. C. Whitin, H. L. Keyserling, and G. M. Carlone. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin. Diagn. Lab. Immunol. 4:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saaka, M., B. J. Okoko, R. C. Kohberger, S. Jaffar, G. Enwere, E. E. Biney, C. Oluwalana, A. Vaughan, S. M. Zaman, L. Asthon, D. Goldblatt, B. M. Greenwood, F. T. Cutts, and R. A. Adegbola. 2008. Immunogenicity and serotype-specific efficacy of a 9-valent pneumococcal conjugate vaccine (PCV-9) determined during an efficacy trial in The Gambia. Vaccine 26:3719-3726. [DOI] [PubMed] [Google Scholar]
- 45.Sandgren, A., K. Sjostrom, B. Olsson-Liljequist, B. Christensson, A. Samuelsson, G. Kronvall, and B. Henriques Normark. 2004. Effect of clonal and serotype-specific properties on the invasive capacity of Streptococcus pneumoniae. J. Infect. Dis. 189:785-796. [DOI] [PubMed] [Google Scholar]
- 46.Schuerman, L., R. Prymula, I. Henckaerts, and J. Poolman. 2007. ELISA IgG concentrations and opsonophagocytic activity following pneumococcal protein D conjugate vaccination and relationship to efficacy against acute otitis media. Vaccine 25:1962-1968. [DOI] [PubMed] [Google Scholar]
- 47.Scott, J. A., A. J. Hall, A. Hannington, R. Edwards, S. Mwarumba, B. Lowe, D. Griffiths, D. Crook, and K. Marsh. 1998. Serotype distribution and prevalence of resistance to benzylpenicillin in three representative populations of Streptococcus pneumoniae isolates from the coast of Kenya. Clin. Infect. Dis. 27:1442-1450. [DOI] [PubMed] [Google Scholar]
- 48.Simell, B., M. Lahdenkari, A. Reunanen, H. Kayhty, and M. Vakevainen. 2008. Effects of ageing and gender on naturally acquired antibodies to pneumococcal capsular polysaccharides and virulence-associated proteins. Clin. Vaccine Immunol. 15:1391-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sjostrom, K., C. Spindler, A. Ortqvist, M. Kalin, A. Sandgren, S. Kuhlmann-Berenzon, and B. Henriques-Normark. 2006. Clonal and capsular types decide whether pneumococci will act as a primary or opportunistic pathogen. Clin. Infect. Dis. 42:451-459. [DOI] [PubMed] [Google Scholar]
- 50.Sleeman, K. L., D. Griffiths, F. Shackley, L. Diggle, S. Gupta, M. C. Maiden, E. R. Moxon, D. W. Crook, and T. E. Peto. 2006. Capsular serotype-specific attack rates and duration of carriage of Streptococcus pneumoniae in a population of children. J. Infect. Dis. 194:682-688. [DOI] [PubMed] [Google Scholar]
- 51.Takala, A. K., J. Vuopio-Varkila, E. Tarkka, M. Leinonen, and J. M. Musser. 1996. Subtyping of common pediatric pneumococcal serotypes from invasive disease and pharyngeal carriage in Finland. J. Infect. Dis. 173:128-135. [DOI] [PubMed] [Google Scholar]
- 52.Tu, A. H., R. L. Fulgham, M. A. McCrory, D. E. Briles, and A. J. Szalai. 1999. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect. Immun. 67:4720-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varvio, S. L., K. Auranen, E. Arjas, and P. H. Makela. 2009. Evolution of the capsular regulatory genes in Streptococcus pneumoniae. J. Infect. Dis. 200:1144-1151. [DOI] [PubMed] [Google Scholar]
- 54.Watson, D. A., and D. M. Musher. 1990. Interruption of capsule production in Streptococcus pneumonia serotype 3 by insertion of transposon Tn916. Infect. Immun. 58:3135-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weinberger, D. M., K. Trzcinski, Y. J. Lu, D. Bogaert, A. Brandes, J. Galagan, P. W. Anderson, R. Malley, and M. Lipsitch. 2009. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathog. 5:e1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, N. M. Bennett, R. Lynfield, A. Reingold, P. R. Cieslak, T. Pilishvili, D. Jackson, R. R. Facklam, J. H. Jorgensen, and A. Schuchat. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348:1737-1746. [DOI] [PubMed] [Google Scholar]
- 57.Whitney, C. G., T. Pilishvili, M. M. Farley, W. Schaffner, A. S. Craig, R. Lynfield, A. C. Nyquist, K. A. Gershman, M. Vazquez, N. M. Bennett, A. Reingold, A. Thomas, M. P. Glode, E. R. Zell, J. H. Jorgensen, B. Beall, and A. Schuchat. 2006. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 368:1495-1502. [DOI] [PubMed] [Google Scholar]
- 58.Wuorimaa, T., R. Dagan, M. Vakevainen, F. Bailleux, R. Haikala, M. Yaich, J. Eskola, and H. Kayhty. 2001. Avidity and subclasses of IgG after immunization of infants with an 11-valent pneumococcal conjugate vaccine with or without aluminum adjuvant. J. Infect. Dis. 184:1211-1215. [DOI] [PubMed] [Google Scholar]
- 59.Wuorimaa, T. K., R. Dagan, F. Bailleux, R. Haikala, N. Ekstrom, J. Eskola, M. Yaich, and H. Kayhty. 2005. Functional activity of antibodies after immunization of Finnish and Israeli infants with an 11-valent pneumococcal conjugate vaccine. Vaccine 23:5328-5332. [DOI] [PubMed] [Google Scholar]