Abstract
The enteric protozoan parasite Entamoeba histolytica causes amebic colitis through disruption of the mucus layer, followed by binding to and destruction of epithelial cells. However, it is not known whether ameba infections or ameba components can directly affect the enteric nervous system. Analysis of mucosal innervations in the mouse model of cecal amebiasis showed that axon density was diminished to less than 25% of control. To determine whether amebas directly contributed to axon loss, we tested the effect of either E. histolytica secreted products (Eh-SEC) or soluble components (Eh-SOL) to an established coculture model of myenteric neurons, glia, and smooth muscle cells. Neuronal survival and axonal degeneration were measured after 48 h of exposure to graded doses of Eh-SEC or Eh-SOL (10 to 80 μg/ml). The addition of 80 μg of either component/ml decreased the neuron number by 30%, whereas the axon number was decreased by 50%. Cytotoxicity was specific to the neuronal population, since the glial and smooth muscle cell number remained similar to that of the control, and was completely abrogated by prior heat denaturation. Neuronal damage was partially prevented by the cysteine protease inhibitor E-64, showing that a heat-labile protease was involved. E. histolytica lysates derived from amebas deficient in the major secreted protease EhCP5 caused a neurotoxicity similar to that of wild-type amebas. We conclude that E. histolytica infection and ameba protease activity can cause selective damage to enteric neurons.
Entamoeba histolytica is a protozoan enteric parasite of humans that colonizes the colon, where it typically causes asymptomatic luminal infections. However, in ca. 10% of individuals, the parasite invades the mucosa to cause amoebic colitis, characterized by ulcerative lesions, diarrhea, and fever and, in severe cases can disseminate to soft organs (39). Although normally seldom fatal, the consequences of ameba infection become significant in the immunosuppressed. The mechanism of infection in the intestine is complex and involves dissolution of the mucus layer by motile trophozoites, followed by adhesion and lysis of epithelial cells and invading leukocytes (8, 9).
Cysteine proteases are important in the differentiation and pathogenicity of E. histolytica, and its genome contains about 50 genes coding for cysteine peptidases (36). A number of in vivo and in vitro studies have implicated cysteine protease activity as a major mechanism of cell death of infected cells, as well as degradation of the extracellular matrix and activation of the complement system (31). Although cell-cell contact is thought to be required for intestinal invasion by E. histolytica trophozoites, amebic proteins have been shown to cause cellular responses in vitro. For example, E. histolytica trophozoite-secreted products caused mucin degradation by proteolytic degradation of cysteine domains (28). In addition, incubation of secreted products and soluble proteins with cultured intestinal epithelial cells resulted in the upregulation of interleukin-8 mRNA to a similar extent as live trophozoites (11, 41). This suggests that both secreted products and direct contact serve important roles in the course of infection.
The consequences to the enteric nervous system (ENS) of E. histolytica infection are unknown but may constitute an important part of its pathogenicity. The epithelial layer of the intestine is innervated by axons extending from the submucosal ganglia of the ENS. This innervation is structurally and functionally poised to respond to factors affecting the integrity of the epithelial barrier, such as amoebic invasion and the release of cysteine proteases. In addition, intestinal inflammation can lead to permanent damage to the enteric nervous system in human disease, as well as in animal models. Neuronal hypertrophy and myenteric and submucosal plexitis are among the featured characteristics observed in patients with Crohn's disease (12), and models of colitis in the rat and other rodents show neuronal death and axonal degeneration in both the myenteric and the submucosal plexuses (24, 34). Since inflammation of the colon due to amebic invasion can resemble that seen in inflammatory bowel disease (30), we hypothesized that the enteric nervous system will also be damaged during amebic colitis.
To study this, we analyzed the effect of E. histolytica infection on axon integrity in an established model of invasive murine cecal amebiasis (18). The infected intestine showed a substantial decrease in axon number compared to the control, which was inversely correlated with the extent of tissue damage. We also used an in vitro model of intestinal neurons, smooth muscle, and glia (23) and examined the effects of either amebic secreted products (Eh-SEC) or soluble components (Eh-SOL) on neuronal survival and axonal structure. We found that a population of enteric neurons was targeted by Entamoeba-derived cysteine proteases, leading to selective damage in vitro. This shows that the innervation of the mucosa by the ENS is a potential target of E. histolytica intestinal invasion.
MATERIALS AND METHODS
Infection with E. histolytica trophozoites.
Six- to ten-week-old male CBA/J mice were purchased from The Jackson Laboratory. Trophozoites for intracecal injections were mouse-passaged amebas cultivated in antibiotic-supplemented media as previously described (18). A total of 2 × 106 trophozoites in 150 μl of TYI-S-33 were injected intracecally into each mouse according to the protocol described previously (18). The cecum was removed 15 days postinfection, fixed in Bouin's solution (Sigma, St. Louis, MO), paraffin embedded, and stained with hematoxylin and eosin (H&E). Histopathology was scored in blinded fashion for inflammation score (0 to 5) and ameba score (0 to 5) as previously described (16). Briefly, the numbers of histologically visible amebas were scored (0, none; 1, present but difficult to locate; 2, occasional, up to 10% of the lumen occupied by ameba; 3, moderate, up to 25% of lumen occupied; 4, heavy, up to 50% of lumen occupied; and 5, virtually complete occupation of the lumen by ameba) and degree of inflammation was scored 0 to 5 (0, normal; 1, mucosal hyperplasia; 2, spotty infiltration of inflammatory cells not involving the entire thickness of the mucosa; 3, marked increase in inflammatory cells involving full thickness of mucosa; 4, marked increase in inflammatory cells of mucosa and submucosa, with tissue architecture intact; and 5, complete destruction of cecal architecture by inflammation).
ELISA protocol.
Upon sacrifice, ceca were bisected longitudinally, and half of the cecal contents were diluted in 500 μl of phosphate-buffered saline and tested for parasite antigen by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (E. histolytica II stool ELISA kit; Techlab, Blacksburg, VA).
Immunocytochemistry of tissue sections.
To identify axons in cecal tissue, tissue sections (4 μm) were labeled with antibodies to PGP 9.5 (UltraClone; 1:2,000) overnight at 4°C, followed by a 2-h incubation in goat anti-rabbit Alexa Fluor 555-conjugated secondary antibodies (Molecular Probes; 1:4,000). In three to six nonadjacent segments from each animal, the innervation of mucosal regions located above an overtly inflamed submucosal space was imaged by fluorescence microscopy (Olympus BX60). Images were captured by using digital image acquisition with a standard setting applied to each image (Image ProPlus 6.0; Media Cybernetics). The number of PGP 9.5-labeled axons was determined by using a standard threshold setting applied across all images. This included a red color integer range between 135 and 256 on a 24-bit image, and a minimum area of 2 pixels. The total area analyzed was then measured, and the innervation density was calculated from this. All measurements were performed by an investigator blinded to the tissue identity.
Cell cultures.
Intestinal tissue was isolated from neonatal rats according to previously described methods (23), with a few modifications. The muscularis externa was removed from animals between postnatal days 4 and 8 and incubated in 0.25% trypsin II (Sigma, St. Louis, MO) in HEPES-buffered Hanks' saline (pH 7.35) for 45 to 65 min. The tissue was resuspended in Dulbecco modified Eagle medium (DMEM) containing 5% fetal calf serum (FCS) and triturated to yield a cell suspension that was plated onto glass-bottom culture plates previously coated with rat tail collagen I (0.002%; Sigma). After 48 h, the medium was replaced with serum-free DMEM for 24 h before addition of test substances.
Secreted (Eh-SEC) or soluble (Eh-SOL) amebic proteins were prepared as described previously (41) and added to the cocultures at concentrations from 10 to 80 μg/ml. In some cases, the protease inhibitor E-64 [trans-epoxysuccinyl-l-leucylamido(4-guanidino)butane, l-trans-3-carboxyoxiran-2-carbonyl-l-leucylagmatine, N-(trans-epoxysuccinyl)-l-leucine 4-guanidino-butylamide] was added just prior to amebic protein addition. The cultures were fixed 48 h later and processed for immunocytochemistry as described below. In some experiments, cultures were fixed at earlier time points (1 to 24 h) to detect the presence of degenerating cells in coculture. For some experiments, an ameba genetically deficient in EhCP5−/− was used (5; a gift from David Mirelman). Secreted cysteine proteinase activities in EhCP5−/− amebas were reduced by 99% compared to wild-type vector controls by using a specific assay to detect cleavage of z-Arg-Arg-pNA substrate (27).
Immunocytochemistry of cocultures.
Immunocytochemistry was used to detect neurons and axons in enteric cocultures as described previously (23). Cultures were fixed for 10 min in 4% neutral buffered formalin and labeled with antibodies to the neuronal cell body protein HuD (1:500; Molecular Probes) or the pan-axonal marker SNAP-25 (1:2,000; Sigma). After incubation for 2 h with fluorescent secondary antibodies (1:2,000 Alexa Fluor 555-conjugated goat anti-rabbit IgG or Alexa Fluor 488-conjugated goat anti-mouse IgG; Molecular Probes), staining was visualized for imaging and quantification by fluorescence microscopy. Some control cocultures were treated with antibodies to the axonal structural marker βIII-tubulin (1:1,000; Chemicon) or the neuronal secretory vesicle component synaptophysin (1:8,000; Sigma) to confirm that changes in axonal number were not due to altered expression of SNAP-25. In some experiments, cultures were colabeled with antibodies to HuD and nNOS (1:500; Eurodiagnostica), followed by incubation with secondary antibodies as described above.
To identify glial cells, cohort control and treated wells were labeled with antibodies to glial fibrillary acidic protein (GFAP; 1:500; Sigma), followed by the appropriate secondary antibodies, and GFAP-positive cells were counted as described below. To detect degenerating cells, propidium iodide (PI; 10−7 M) was added to the cocultures at the time of addition of Eh-SEC or Eh-SOL, fixed at later times (1 to 48 h), and labeled with anti-HuD antibodies. Alternatively, wells were processed for immunocytochemistry as described above, followed by nuclear staining with Hoechst 33352 (10−7 M). Some cohort wells were treated with trypan blue (0.4%; Sigma) to confirm the selectivity of the amebic protein damage.
Neuron and axon counts.
After labeling with HuD or HuD/nNOS, the neuron number was determined by counting all of the neurons in every third field of view from edge to edge of the midline of each culture (×60, NA1.20), which was then repeated in the perpendicular axis. The number of GFAP-labeled glia was determined by using a similar method. The axon number was quantified by counting the number of axon intersections with an arbitrary midline intersector in the same regions as described above and then multiplied to yield a value proportional to the number of axon intersections per area. These were averaged to give a representative axon number per condition.
Proliferation assay.
[3H]thymidine incorporation assays were used to evaluate the potential growth effect of amebic proteins on smooth muscle cells in coculture, as described previously (3). Cultures were maintained in serum-free medium for 72 h before addition of culture medium alone (control) or medium containing 30 μg of Eh-SEC or Eh-SOL/ml. The addition of 5% fetal calf serum was used as a positive control. [3H]thymidine was added 17 h later for 5 h before processing for determination of uptake by scintillation counting. Outcomes were normalized to cohort negative controls and expressed as the fold increase over control.
Statistics.
Values are expressed as the average ± the standard error of the mean of n animals. The statistical significance was assumed for P ≤ 0.05 using one-way analysis of variance.
RESULTS
Axonal damage in the cecal model of amebiasis.
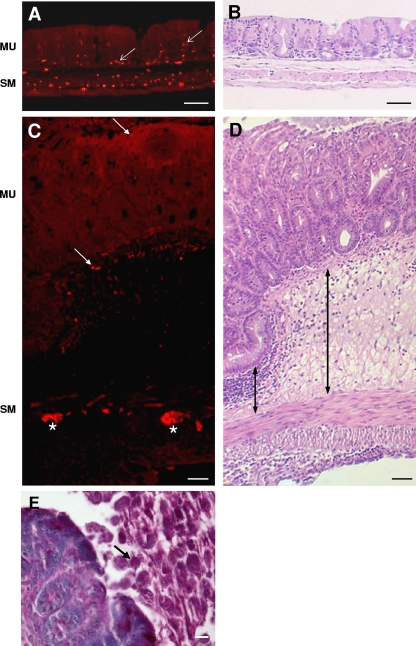
To determine whether the progressive development of E. histolytica lesions in the cecum caused axonal damage, we studied the mucosal innervation of tissue sections labeled with the pan-neuronal marker PGP9.5. In control cecal tissue, PGP9.5-positive axons were evident throughout both the smooth muscle layers and the mucosa (Fig. 1A, arrows). The average axonal area of the cecal mucosa was 0.84% ± 0.10% (n = 4) of the total area analyzed (Table 1). Similar sections incubated with secondary antibodies only or with anti-PGP 9.5 antibodies, followed by mismatched secondary antibodies (goat anti-mouse Alexa-555) showed no labeling (not shown).
FIG. 1.
Immunocytochemistry for PGP9.5 showing that mucosal innervation is reduced in the cecum of mice infected with E. histolytica trophozoites. (A) Image of section of control cecum showing the presence of axons throughout the mucosa (red; arrows). (B) Similar section stained with H&E. (C) Image of infected cecum showing reduced axon number in the mucosa (arrows), while axons and neurons in the smooth muscle/myenteric plexus region directly below this region retained prominent expression of PGP9.5 (asterisks). (D) Adjacent section stained with H&E showing extensive submucosal infiltrate (e.g., arrows). MU, mucosa; SM, smooth muscle layers. (E) Section of inflamed cecum stained with periodic-acid Schiff showing the presence of E. histolytica in the intestinal lumen (arrow). Scale bars: A to D, 50 μm; E, 20 μm.
TABLE 1.
Correlation of ameba presence, inflammation, and innervation density in the cecal mucosa of mice infected with E. histolytica
| Ameba score | Inflammation score | ELISA ODa | Axon area/mucosa area (magnification, ×100) |
|
|---|---|---|---|---|
| Inflamed region | Adjacent region | |||
| 2 | 2 | 2.655 | 0.08 | 0.45 |
| 3 | 3 | 2.281 | 0.24 | 0.46 |
| 3 | 3 | 1.203 | 0.13 | 0.28 |
| 4 | 4 | 2.537 | 0.11 | 0.35 |
| 5 | 4 | 2.209 | 0.13 | NAb |
OD, optical density.
NA, not applicable.
Analysis of inflamed regions of the inoculated cecum showed a striking decrease in axon area compared to control (Fig. 1C). These mucosal regions were chosen based on their location above a submucosal infiltrate depth that was greater than 100 μm (arrow, Fig. 1D). This depth was arbitrarily chosen to reflect regions of most severe damage (e.g., (18). The axon area was 0.19% ± 0.05% of the total mucosal area analyzed, a decrease of >75% of the control values (P ≤ 0.05 relative to control; n = 8). As shown in Table 1, axon area was highest in tissue that showed minimal damage (0.29% and 0.50% of mucosal area) and was substantially reduced in tissue with higher inflammation scores (e.g., 0.11 and 0.13% of the mucosal area). Mucosal innervation was also determined in regions adjacent to overtly inflamed regions, defined by a submucosal infiltrate depth of <100 μm. Analysis showed a lessened axon loss compared to the inflamed regions, but this was still significantly reduced compared to control by almost 50% (0.42% ± 0.06%; n = 7). We conclude that E. histolytica lesions in the cecum led to a dramatic reduction in axon structure in both overtly inflamed and adjacent mucosa. Ameba were not present in the tissues but rather were localized in the cecal lumen and/or close to the mucosal lesions (Fig. 1E).
Effect of Entamoeba-secreted proteins (Eh-SEC) on enteric neurons in coculture.
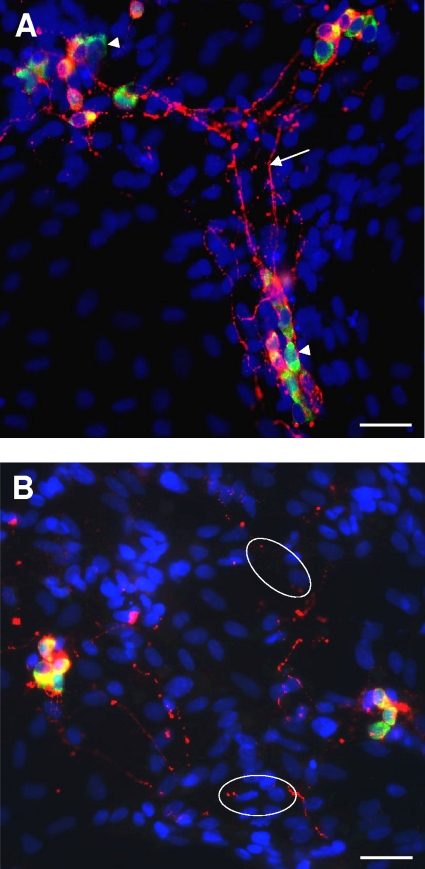
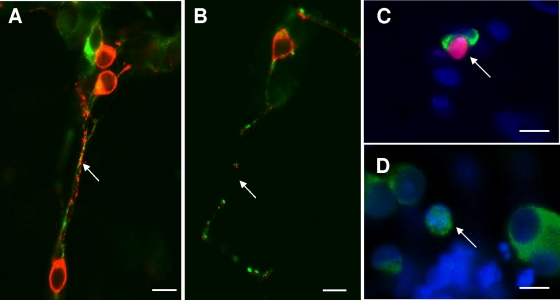
To assess whether axon damage resulted from amebic factors or from host-derived inflammatory mediators, we tested the effects of E. histolytica products on enteric neuron structure in vitro. Immunocytochemistry of control cocultures with antibodies to HuD and SNAP-25 showed that neurons were distributed throughout the dish, typically in clusters of 1 to 10 neurons, with SNAP-25-labeled axons extending widely to contact both neuronal and non-neuronal cells (Fig. 2A). However, the addition of secreted ameba proteins (30 μg/ml) caused evidence of neurodegeneration as early as 4 h after coincubation. Some HuD-labeled neuronal cell bodies were positive for nuclear PI labeling, a fluorescent vital stain that is normally excluded from healthy cells (Fig. 3C). This was indicative of early loss of plasma membrane integrity, while other neurons showed a markedly condensed morphology, an appearance previously correlated with cytotoxicity by neurotoxins (Fig. 3D) (23). Examination of axonal structure by labeling with anti-SNAP-25 antibodies showed fragmented and discontinuous axons (e.g., Fig. 2B and 3B).
FIG. 2.
Incubation of enteric cocultures with secreted components from E. histolytica (Eh-SEC) caused damage to axonal structure and loss of neurons. (A) Representative image of fluorescence immunocytochemistry of a control coculture labeled with antibodies to HuD to identity neuronal cell bodies (green; e.g., arrowheads) and SNAP-25 to label axon structure (red; arrow). Hoechst 33342 was added to revel all nuclei (blue). Scale bar, 50 μm. (B) Image of coculture after treatment with 30 μg of Eh-SEC/ml for 2 days, with immunocytochemistry as in panel A. Note the disruption in neurite structure (circles). Scale bar, 50 μm.
FIG. 3.
Onset of neurodegeneration in enteric neuronal cocultures exposed to secreted components from E. histolytica (Eh-SEC) for 4 h. (A and B) Higher-magnification view of control (A) and Eh-SEC-treated (B) cocultures showing coexpression of SNAP-25 (red) and the axon-specific intermediate filament βIII-tubulin (green) in axons. Both antibodies reveal prominent breaks in axon structure after treatment with Eh-SEC. Scale bar, 20 μm. (C) Example of uptake of the vital stain propidium iodide (red) at 4 h of treatment with Eh-SEC (30 μg/ml), indicating loss of viability. Cultures were subsequently labeled with anti-HuD antibodies (green) to identify neurons and the pan-nuclear fluorochrome Hoechst 333242 (blue) to identify all nuclei in the field. Scale bar, 20 μm. (D) Degenerating neuron identified with anti-HuD immunocytochemistry (green; arrow) and nuclear staining with Hoechst 33342 (blue), showing a condensed neuron with nuclear HuD labeling. Scale bar, 20 μm.
To confirm that the decrease in SNAP-25-labeled profiles reflected axonal damage, some cocultures were colabeled with SNAP-25 and either βIII-tubulin (Fig. 3) or synaptophysin (not shown), markers of axonal and synaptic vesicle structure, respectively. In control cocultures, these were coexpressed with SNAP-25 in axonal processes, with prominent labeling throughout the axon length. Analysis of axon extensions using dual-label immunocytochemistry at 4 h after the addition of Eh-SEC showed evidence of degenerative changes with gaps along axonal extensions breaks, apparent with both antibodies (Fig. 3B). This confirmed the suitability of SNAP-25 detection as a marker of axon profiles, which was used to measure axonal number in subsequent experiments.
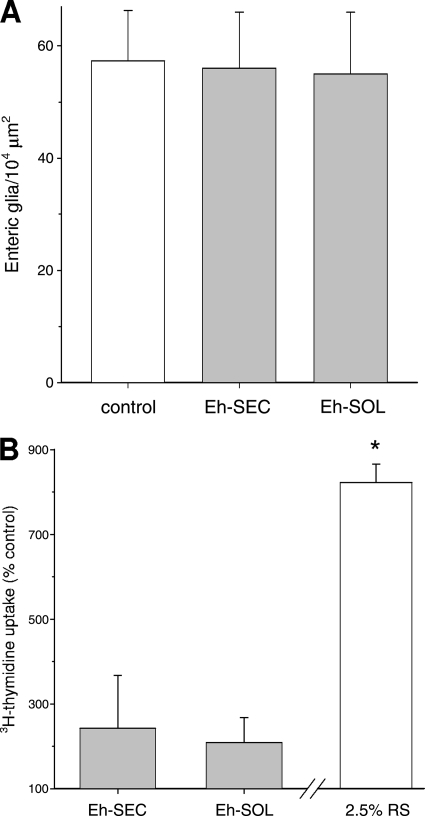
To investigate the possibility of general cytotoxicity in vitro, we next tested whether Eh-SEC and Eh-SOL products also caused damage to glia and smooth muscle cells in coculture. A trypan blue exclusion assay to measure cytotoxicity in cocultures treated with E. histolytica products for 4 or 48 h showed no difference from control vehicle-treated cocultures (not shown). To pursue this, immunocytochemistry was used to label glial cells with anti-GFAP antibodies in cocultures treated with 30 μg of Eh-SEC or Eh-SOL/ml 48 h earlier. Counts of GFAP-positive cells showed similar numbers in treated and control cocultures (Fig. 4A, P > 0.05, n = 4), suggesting that the amebic products did not target glial cells.
FIG. 4.
Characterization of the effect of E. histolytica products on non-neuronal cells in coculture. (A) Glial cell number was unchanged in cocultures treated for 48 h with 30 μg of Eh-SEC or Eh-SOL (n = 4), relative to cohort cocultures treated with control vehicle. Glia were identified by GFAP immunocytochemistry. (B) Eh-SEC or Eh-SOL (30 μg/ml) addition to enteric cocultures did not significantly increase [3H]thymidine incorporation in intestinal smooth muscle cells. Rat serum (2.5% RS) was as a positive control (*, P < 0.05 versus control; n = 3).
To further investigate whether E. histolytica can alter the integrity of smooth muscle cells, the effect on growth of intestinal smooth muscle cells in vitro was assessed by using a [3H]thymidine incorporation assay. [3H]thymidine uptake was slightly elevated but statistically similar between control cultures and cohort cultures exposed to 30 μg of Eh-SEC or Eh-SOL (Fig. 4B; P > 0.05, n = 3)/ml, indicating that these products, while not cytotoxic, do not promote the proliferation of smooth muscle cells.
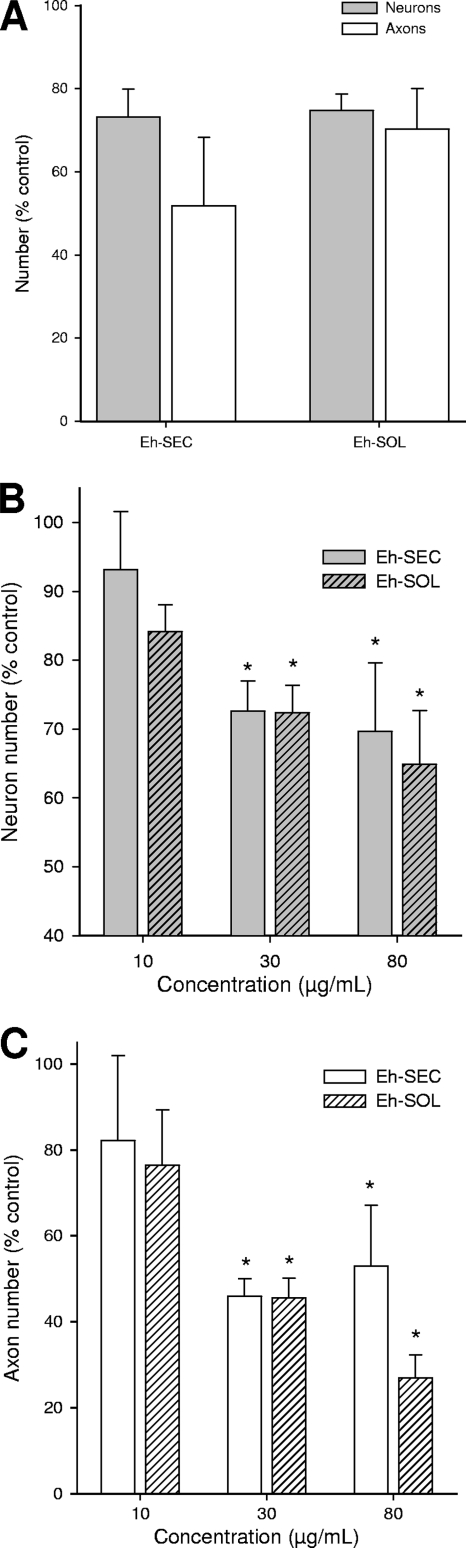
Overall, these experiments confirmed that the effects of E. histolytica components were limited to enteric neurons in these cocultures, and we then characterized the extent of damage caused by these agents. Direct counts of the number of neurons showed a significant decrease of >25% by 4 h after the addition of Eh-SEC (Fig. 5A, P < 0.05; n = 3 for each treatment), with a substantial decrease in axon number. To ensure that these secreted components reflected the full cytotoxic capability of E. histolytica, these experiments were repeated using solutions of the cytosolic proteins derived from cellular lysis (Eh-SOL). These soluble amebic proteins caused a similar damaging effect on the neurons in coculture. For example, neuron number was reduced to 74% ± 4% of that of the control by 4 h of coincubation with Eh-SEC, whereas the axon number was 70% ± 10% of that of the control at this time. This shows that E. histolytica protein products are able to cause selective early damage to enteric neurons in culture in the absence of live trophozoites.
FIG. 5.
Treatment of cocultures with amebic proteins results in a concentration-dependent reduction in neuronal content. The majority of the losses in neuron and axon number was achieved by 4 h postaddition and was not significantly increased by 48 h. (A) Quantification of the number of neurons and axons present in cocultures treated with Eh-SEC or Eh-SOL for 4 h. In all cases, there was a significant decrease in neuron and axon number with either treatment relative to control (n = 4; P < 0.05). (B and C) Concentration-dependent decrease in the number of neurons (B) and axons (C) after 48 h of incubation with Eh-SEC or Eh-SOL (n = 3 to 13; *, P < 0.05).
Next, we studied the time course of the neurodegeneration caused by E. histolytica components to determine whether there was further damage or whether repair mechanisms would dominate. Cocultures were treated for 24 h (data not shown) or 48 h with Eh-SEC or Eh-SOL, and neuron and axon damage was characterized. Analysis of cocultures incubated with Eh-SEC or Eh-SOL (10 to 80 μg/ml) for 48 h showed a concentration-dependent decrease in the number of neurons and axons, with neuron number significantly reduced by treatment with 30 or 80 μg of secreted products or soluble components (Fig. 5B; n = 10 and 13, respectively; *, P < 0.05 relative to control)/ml. The axon number was reduced to an even larger extent, to 46% ± 4% and 46% ± 5% of control after treatment with 30 μg of Eh-SEC or Eh-SOL/ml, respectively (Fig. 5C).
To determine whether axons were selectively reduced after treatment (i.e., lost from surviving neurons), the ratio of number of axons per neuron was determined. This was decreased to 57% ± 7% and 63% ± 10% of control after treatment with Eh-SEC and Eh-SOL at 30 μg/ml, respectively, evidence of disproportionate damage to axons than to neuronal cell bodies. This value was decreased even further after addition of 80 μg of Eh-SEC or Eh-SOL/ml, with values of 27% ± 11% and 40% ± 8% of control, respectively. We conclude that E. histolytica products can cause axonal degeneration while cell body integrity remains intact, similar to that observed in other models of damage to enteric neurons in vitro (23) and during intestinal inflammation in vivo (24).
Damage to enteric neurons in intestinal inflammation induced by trinitrobenzene sulfonic acid (TNBS) has been shown to be nonspecific, with no preferential targeting of nitrergic (i.e., nNOS expressing) or cholinergic neuronal phenotypes (22). These are nonoverlapping markers of inhibitory and excitatory phenotypes, respectively, which together stain nearly all myenteric neurons (29, 35). To determine whether damage due to E. histolytica products resulted in loss of a specific phenotype, we selected nNOS as a principal subtype that represents almost 40% of all neurons in vivo and is similarly represented in vitro (23). The percentage of nNOS-positive neurons remaining after incubation with E. histolytica proteins for 48 h (37% ± 4%) was similar to control (34% ± 3%), suggesting that the damage caused by these components is not selective for a neuronal phenotype in vitro.
Physiochemical characterization of the E. histolytica cytotoxic factor.
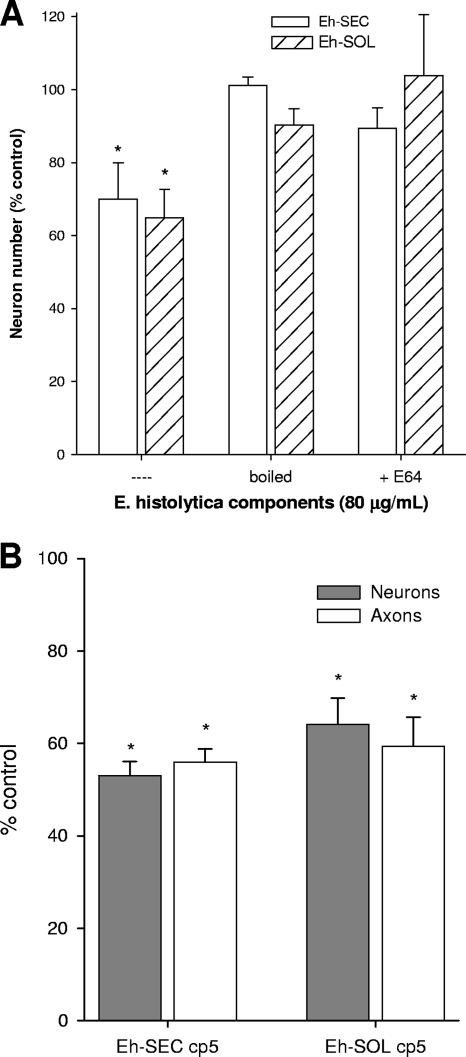
To characterize the nature of the cytotoxic factor in E. histolytica, Eh-SEC and Eh-SOL solutions were heat denatured by boiling before addition to enteric cocultures, and neuron and axon numbers determined after 48 h as before. Heat denaturation of Eh-SEC and Eh-SOL (30 μg/ml) removed all cytotoxic activity, with neuron and axon number similar to untreated controls (Fig. 6A). These data suggested that enzymes such as proteases might be responsible for the neuronal damage observed in enteric cocultures, particularly since studies using intestinal epithelial cells in vitro correlated cysteine proteinase activity of E. histolytica trophozoites with cell toxicity, which was sensitive to prior addition of the specific cysteine protease inhibitor E-64 (28). To determine whether neuron death in enteric cocultures was similarly due to protease activity in the amebic extracts, cohort cocultures were treated with E-64 just prior to addition of Eh-SEC or Eh-SOL, and neuron and axon numbers were determined 48 h later. Co-addition of 10 μM E-64 and 80 μg of Eh-SEC or Eh-SOL/ml prevented the damage seen in cohort cocultures receiving E. histolytica products alone (Fig. 6A). For example, neuronal cell body numbers were 89% ± 6% of control by 48 h after co-addition of Eh-SEC and E-64 (*, P < 0.05 relative to Eh-SEC-treated, n = 5). Addition of 10 μM E-64 alone had no effect on neuronal or axonal number. This shows that most of the neurotoxicity caused by E. histolytica components involves amoebic cysteine protease activity.
FIG. 6.
Secreted products and soluble components contain a heat-labile protease that is partly responsible for neuron damage in enteric cocultures. (A) The damaging effect of E. histolytica factors was abrogated by boiling (n = 4) or prior treatment with the protease inhibitor E64 (10 μM; n = 5) prior to addition to the cocultures. *, P < 0.05 versus untreated control. (B) Secreted or soluble factors from a CP5−/− E. histolytica (CP5) caused similar neuronal toxicity as wild-type vector products in enteric cocultures by 48 h posttreatment with Eh-SEC cp5 or Eh-SOL cp5 (30 μg/ml; n = 5). *, P < 0.05 relative to control.
The E. histolytica genome codes for a number of cysteine proteases, which are known to be important for amoebic pathogenicity both in vivo and in vitro. Only a few cysteine proteases are significantly expressed in vitro, where studies have shown their importance in disruption of cell monolayers, hemolysis and mucin degradation (7, 20, 36). Of these, EhCP5 is considered a prime candidate responsible for cellular toxicity as trophozoites with reduced EhCP5 activity showed a decreased ability to damage epithelial cells (27). Conversely, overexpression of EhCP5 by trophozoites leads to increased cell damage in vitro and in vivo (37). The involvement of EhCP5 in E. histolytica-mediated neuronal degeneration in vitro was studied using secreted and cellular proteins derived from CP5−/− trophozoites (5). Interestingly, incubation with mutant E. histolytica CP5−/− lysates caused damage to a similar extent as the wild-type vector lysates. For example, neuron and axon number were reduced to 53% ± 3% and 32% ± 3% of vehicle-treated control by 48 h after the addition of CP5−/− Eh-SEC and Eh-SOL, respectively (n = 5). Overall, our results show that E. histolytica causes enteric neurotoxicity in vivo and in vitro through the activity of an as-yet-unidentified cysteine protease.
DISCUSSION
Even though E. histolytica infection in the colon is characterized by an acute inflammatory response with substantial tissue damage, the cellular pathology is poorly understood. We hypothesized that damage to the ENS could be an important aspect of this pathology and used the mouse model of cecal amebiasis (16, 18) to show a reduction in mucosal innervation at the infected and inflamed sites. To separate the direct effects of E. histolytica components from the associated inflammation, we tested the effects of both secreted and soluble components of amebas in an established coculture model of rat myenteric neurons, smooth muscle and glial cells (3, 23). This showed the neurons to be a selective target of amebic proteins with both components, with similar degrees of concentration-dependent loss of neurons with a parallel time course, which suggested the presence of the equivalent cytolytic factor in both soluble and secreted lysates. Although there was no evidence of toxicity to smooth muscle or glial cells in our cultures, we cannot exclude the possibility that an effect would occur with longer incubation in vitro.
Studies of affected individuals as well as in cultured cell lines suggest a pathogenic role for cysteine proteases from E. histolytica. For example, secreted protease activity was detected in all individuals showing symptoms of amoebiasis, but was found only occasionally in cases of nonsymptomatic infection (33). To investigate the importance of this to our model, we used the specific inhibitor of cysteine proteases, E64, which has been shown to completely prevent cysteine protease activity in cultured trophozoites, as well as inhibit the Entamoeba-mediated destruction of cell monolayers (27). Since E64 significantly reduced neuronal cell death due to the presence of E. histolytica products, it appears that protease-induced damage is the main factor responsible for neuronal degeneration in our model. It is also possible that amebic proteins might act indirectly to cause upregulation of host-derived, potentially neurotoxic factors such as the proinflammatory cytokines. However, this is the subject of ongoing research showing no evidence for neurotoxicity in the presence of these cytokines (see, for example, reference 14), thus tending to support the hypothesis that amebic proteins are directly involved.
Factors derived from E. histolytica cause death by apoptosis as well as necrosis. For example, the addition of trophozoites to a murine myeloid cell line resulted in cell death by a Bcl-2-independent apoptotic mechanism (32), while elsewhere, human myeloid cells have been shown to die by a necrotic (2) as well as apoptotic mechanism (19). An early decrease in neuron number by 4 h after addition of E. histolytica products to enteric cocultures was accompanied by PI-labeling of some neuronal nuclei that was not observed in control cocultures. Careful analysis of HuD-positive neurons colabeled with Hoechst 33342 in several cocultures treated for 2 to 48 h did not show the typical presence of condensed chromatin that is a feature of apoptotic cell death (40). This suggests that the damaging factor present in Entamoeba lysates or secreted proteins primarily causes neuronal cell death through a necrotic mechanism.
Although myenteric neurons were damaged by exposure to Entamoeba proteins in vitro, neither enteric glia nor intestinal smooth muscle cells were sensitive to this, with glia cell numbers unaffected by 48 h of incubation with E. histolytica lysates, and growth assays of smooth muscle cells showing a mild mitogenic effect. Glial cell sparing in the presence of neuronal death has been observed in other coculture models—hydrogen peroxide or paraquat-induced oxidative damage of retinal neuroglial cocultures caused the selective death of neurons with glial cell proliferation (1). We have previously shown the selective neurotoxicity of acrylamide or hydrogen peroxide in our enteric cocultures, suggesting that neurons are especially vulnerable to oxidative conditions (23). In vivo, TNBS-induced intestinal inflammation leads to significant neuronal death (21, 22. 34) but is associated with glial cell proliferation (6).
Although the effect of proteases on smooth muscle proliferation is not well known in the intestine, the growth of these cells is a consequence of intestinal inflammation in humans as well as in animal models (4). Extensive literature in the vascular system shows that protease activation is a hallmark of vascular injury and inflammation. For example, thrombin is a serine protease generated at the site of vascular injury (25) and acts through its proteinase-activated receptors (PARs) to cause the smooth muscle cell proliferation that is associated with vascular pathology. Since the features of amoebic colitis resemble that observed in inflammatory bowel disease, we tested whether E. histolytica products could act on smooth muscle cells directly to cause growth. However, our results showed that addition of Eh-SEC or Eh-SOL caused only a small but nonsignificant stimulation. Although not major growth-promoting influences, these factors were also clearly not cytotoxic unlike their actions on the enteric neurons, suggesting that protease activation during intestinal amebiasis is not likely to affect the smooth muscle cell layers directly, although indirect effects resulting from infiltration of immune cells to the infected colon may occur.
This implies that neurons are particularly sensitive to the actions of Entamoeba-derived proteins, which may be due in part to the large membrane areas present in their lengthy axon extensions, which were seen to be damaged in the present study and in other models of inflammatory or neurotoxic damage (23, 24, 34). Further, the innervation density of the mucosa was greatly reduced in cecal tissue infected with E. histolytica trophozoites. This showed that neurons may be particularly susceptible to amebiasis, since axon loss was most evident in overtly inflamed segments of the intestine, as well as in regions that were less obviously damaged. These actions could involve cysteine proteinases, since studies in both the central nervous system and peripheral nervous system implicate endogenous cysteine proteinases in the neuron death that occurs during ischemia and stroke through apoptotic or necrotic mechanisms (38). Also, upregulation of the cysteine protease calpain is correlated with optic neuritis (15) and degeneration of spinal cord neurons in models of multiple sclerosis (reviewed in reference 10). To pursue this, we used Eh-SEC and Eh-SOL from amebas deficient in the production and secretion of the cysteine protease CP5, which show a reduced ability to degrade colonic mucin and disrupt LS 174T epithelial cells (27). In the noninvasive strain E. dispar, CP5 is inactive (31), and this secreted and membrane-bound proteinase could be a key molecule in the invasive capacity of E. histolytica (13). However, the loss of neurons was similar upon incubation with wild-type versus CP5-deficient lysates, and so CP5 is clearly not the cysteine protease responsible for neuronal damage in our enteric cocultures. Other secreted proteases may also cause cell destruction, such as CP1, a protease that is also lost in E. dispar (7). Mice with human colon xenografts that were infected with virulent E. histolytica trophozoites developed intestinal amebiasis and the appearance of mucosal ulcers containing trophozoites, which could be prevented by preincubation of the trophozoites with the CP1 inhibitors K11777 or WRR483 (26). Further research into the outcomes of selective blockade of protease action is required to elucidate this.
A recent study suggested that E. histolytica CP4 might play a significant role in intestinal invasion, as a specific inhibitor of EhCP4 significantly reduced tissue damage (17). Interestingly, EhCP4 has a unique preference for valine and isoleucine at P2 unlike the other secreted CPs that have a preference for arginine at the P2 position, which could lead to under-reporting of apparent total protease activity in the CP5 mutant. Thus, the residual proteases in the mutant could play a role in the neuronal damage observed in our study. Further research into the outcomes of selective blockade of protease action is required to elucidate the full range of involvement of CP4 in this model.
In summary, our findings add a novel dimension to the pathogenesis of amebiasis, showing that a secreted cysteine protease can cause selective death to enteric neurons in vitro. Although further research is needed, neural involvement in the infected colon may contribute to the development of colitis in affected individuals. The macroscopic appearance of the colon during amebiasis can resemble that observed in inflammatory bowel disease (30), and it is therefore possible that many of the pathological effects, including neurodegeneration and axon loss, may be similar between these two diseases.
Acknowledgments
We thank David Mirelman (Weizmann Institute of Science, Rehovot, Israel) for E. histolytica cultures deficient in CP5.
This study was supported by grants from the Crohn's and Colitis Foundation of Canada (M.G.B.) and the Canadian Institutes of Health Research (K.C.).
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 20 September 2010.
REFERENCES
- 1.Abrahan, C. E., M. F. Insua, L. E. Politi, O. L. German, and N. P. Rotstein. 2009. Oxidative stress promotes proliferation and dedifferentiation of retina glial cells in vitro. J. Neurosci. Res. 87:964-977. [DOI] [PubMed] [Google Scholar]
- 2.Berninghausen, O., and M. Leippe. 1997. Necrosis versus apoptosis as the mechanism of target cell death induced by Entamoeba histolytica. Infect. Immun. 65:3615-3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blennerhassett, M. G., and S. Lourenssen. 2000. Neural regulation of intestinal smooth muscle growth in vitro. Am. J. Physiol. Gastrointest. Liver Physiol. 279:G511-G519. [DOI] [PubMed] [Google Scholar]
- 4.Blennerhassett, M. G., P. Vignjevic, D. L. Vermillion, and S. M. Collins. 1992. Inflammation causes hyperplasia and hypertrophy in smooth muscle of rat small intestine. Am. J. Physiol. 262:G1041-G1046. [DOI] [PubMed] [Google Scholar]
- 5.Bracha, R., Y. Nuchamowitz, M. Anbar, and D. Mirelman. 2006. Transcriptional silencing of multiple genes in trophozoites of Entamoeba histolytica. PLoS Pathog. 2:e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley, J. S., Jr., E. J. Parr, and K. A. Sharkey. 1997. Effects of inflammation on cell proliferation in the myenteric plexus of the guinea pig ileum. Cell Tissue Res. 289:455-461. [DOI] [PubMed] [Google Scholar]
- 7.Bruchhaus, I., T. Jacobs, M. Leippe, and E. Tannich. 1996. Entamoeba histolytica and Entamoeba dispar: differences in numbers and expression of cysteine proteinase genes. Mol. Microbiol. 22:255-263. [DOI] [PubMed] [Google Scholar]
- 8.Chadee, K., and E. Meerovitch. 1984. The Mongolian gerbil (Meriones unguiculatus) as an experimental host for Entamoeba histolytica. Am. J. Trop. Med. Hyg. 33:47-54. [DOI] [PubMed] [Google Scholar]
- 9.Chadee, K., and E. Meerovitch. 1984. The pathogenesis of experimentally induced amebic liver abscess in the gerbil (Meriones unguiculatus). Am. J. Pathol. 117:71-80. [PMC free article] [PubMed] [Google Scholar]
- 10.Das, A., M. K. Guyton, J. T. Butler, S. K. Ray, and N. L. Banik. 2008. Activation of calpain and caspase pathways in demyelination and neurodegeneration in animal model of multiple sclerosis. CNS Neurol. Disord. Drug Targets 7:313-320. [DOI] [PubMed] [Google Scholar]
- 11.Dey, I., and K. Chadee. 2008. Prostaglandin E2 produced by Entamoeba histolytica binds to EP4 receptors and stimulates interleukin-8 production in human colonic cells. Infect. Immun. 76:5158-5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrante, M., G. de Hertog, T. Hlavaty, G. D'Haens, F. Penninckx, A. D'Hoore, S. Vermeire, P. Rutgeerts, K. Geboes, and G. van Assche. 2006. The value of myenteric plexitis to predict early postoperative Crohn's disease recurrence. Gastroenterology 130:1595-1606. [DOI] [PubMed] [Google Scholar]
- 13.Freitas, M. A., H. C. Fernandes, V. C. Calixto, A. S. Martins, E. F. Silva, J. L. Pesquero, and M. A. Gomes. 2009. Entamoeba histolytica: cysteine proteinase activity and virulence: focus on cysteine proteinase 5 expression levels. Exp. Parasitol. 122:306-309. [DOI] [PubMed] [Google Scholar]
- 14.Gougeon, P. Y., S. Lourenssen, A. Li, and M. G. Blennerhassett. 2010. TNF and IL-1 potentiate axonal growth of myenteric neurons via indirect action on smooth muscle cells. Can. J. Gastroenterol. 24(Suppl. A):A137. [Google Scholar]
- 15.Guyton, M. K., E. A. Sribnick, S. K. Ray, and N. L. Banik. 2005. A role for calpain in optic neuritis. Ann. N. Y. Acad. Sci. 1053:48-54. [DOI] [PubMed] [Google Scholar]
- 16.Hamano, S., A. Asgharpour, S. E. Stroup, T. A. Wynn, E. H. Leiter, and E. Houpt. 2006. Resistance of C57BL/6 mice to amoebiasis is mediated by nonhemopoietic cells but requires hemopoietic IL-10 production. J. Immunol. 177:1208-1213. [DOI] [PubMed] [Google Scholar]
- 17.He, C., G. P. Nora, E. L. Schneider, I. D. Kerr, E. Hansell, K. Hirata, D. Gonzalez, M. Sajid, S. E. Boyd, P. Hruz, E. R. Cobo, C. Le, W. T. Liu, L. Eckmann, P. C. Dorrestein, E. R. Houpt, L. S. Brinen, C. S. Craik, W. R. Roush, J. McKerrow, and S. L. Reed. 2010. A novel Entamoeba histolytica cysteine proteinase, EhCP4, is key for invasive amebiasis and a therapeutic target. J. Biol. Chem. 285:18516-18527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houpt, E. R., D. J. Glembocki, T. G. Obrig, C. A. Moskaluk, L. A. Lockhart, R. L. Wright, R. M. Seaner, T. R. Keepers, T. D. Wilkins, and W. A. Petri, Jr. 2002. The mouse model of amebic colitis reveals mouse strain susceptibility to infection and exacerbation of disease by CD4+ T cells. J. Immunol. 169:4496-4503. [DOI] [PubMed] [Google Scholar]
- 19.Huston, C. D., E. R. Houpt, B. J. Mann, C. S. Hahn, and W. A. Petri, Jr. 2000. Caspase 3-dependent killing of host cells by the parasite Entamoeba histolytica. Cell Microbiol. 2:617-625. [DOI] [PubMed] [Google Scholar]
- 20.Lejeune, M., J. M. Rybicka, and K. Chadee. 2009. Recent discoveries in the pathogenesis and immune response toward Entamoeba histolytica. Future Microbiol. 4:105-118. [DOI] [PubMed] [Google Scholar]
- 21.Lin, A., S. Lourenssen, R. D. Stanzel, and M. G. Blennerhassett. 2005. Selective loss of NGF-sensitive neurons following experimental colitis. Exp. Neurol. 191:337-343. [DOI] [PubMed] [Google Scholar]
- 22.Linden, D. R., J. M. Couvrette, A. Ciolino, C. McQuoid, H. Blaszyk, K. A. Sharkey, and G. M. Mawe. 2005. Indiscriminate loss of myenteric neurons in the TNBS-inflamed guinea-pig distal colon. Neurogastroenterol. Motil. 17:751-760. [DOI] [PubMed] [Google Scholar]
- 23.Lourenssen, S., K. G. Miller, and M. G. Blennerhassett. 2009. Discrete responses of myenteric neurons to structural and functional damage by neurotoxins in vitro. Am. J. Physiol. Gastrointest. Liver Physiol. 297:G228-G239. [DOI] [PubMed] [Google Scholar]
- 24.Lourenssen, S., R. W. Wells, and M. G. Blennerhassett. 2005. Differential responses of intrinsic and extrinsic innervation of smooth muscle cells in rat colitis. Exp. Neurol. 195:497-507. [DOI] [PubMed] [Google Scholar]
- 25.Martorell, L., J. Martinez-Gonzalez, C. Rodriguez, M. Gentile, O. Calvayrac, and L. Badimon. 2008. Thrombin and protease-activated receptors (PARs) in atherothrombosis. Thromb. Haemost. 99:305-315. [DOI] [PubMed] [Google Scholar]
- 26.Melendez-Lopez, S. G., S. Herdman, K. Hirata, M. H. Choi, Y. Choe, C. Craik, C. R. Caffrey, E. Hansell, B. Chavez-Munguia, Y. T. Chen, W. R. Roush, J. McKerrow, L. Eckmann, J. Guo, S. L. Stanley, Jr., and S. L. Reed. 2007. Use of recombinant Entamoeba histolytica cysteine proteinase 1 to identify a potent inhibitor of amebic invasion in a human colonic model. Eukaryot. Cell 6:1130-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moncada, D., K. Keller, S. Ankri, D. Mirelman, and K. Chadee. 2006. Antisense inhibition of Entamoeba histolytica cysteine proteases inhibits colonic mucus degradation. Gastroenterology 130:721-730. [DOI] [PubMed] [Google Scholar]
- 28.Moncada, D., K. Keller, and K. Chadee. 2003. Entamoeba histolytica cysteine proteinases disrupt the polymeric structure of colonic mucin and alter its protective function. Infect. Immun. 71:838-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neunlist, M., D. Reiche, K. Michel, H. Pfannkuche, S. Hoppe, and M. Schemann. 1999. The enteric nervous system: region and target specific projections and neurochemical codes. Eur. J. Morphol. 37:233-240. [DOI] [PubMed] [Google Scholar]
- 30.Pritt, B. S., and C. G. Clark. 2008. Amebiasis. Mayo Clin. Proc. 83:1154-1159. [DOI] [PubMed] [Google Scholar]
- 31.Que, X., and S. L. Reed. 2000. Cysteine proteinases and the pathogenesis of amebiasis. Clin. Microbiol. Rev. 13:196-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ragland, B. D., L. S. Ashley, D. L. Vaux, and W. A. Petri, Jr. 1994. Entamoeba histolytica: target cells killed by trophozoites undergo DNA fragmentation which is not blocked by Bcl-2. Exp. Parasitol. 79:460-467. [DOI] [PubMed] [Google Scholar]
- 33.Reed, S. L., W. E. Keene, and J. H. McKerrow. 1989. Thiol proteinase expression and pathogenicity of Entamoeba histolytica. J. Clin. Microbiol. 27:2772-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanovic, S., D. P. Lamb, and M. G. Blennerhassett. 1999. Damage to the enteric nervous system in experimental colitis. Am. J. Pathol. 155:1051-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schemann, M., and C. Schaaf. 1995. Differential projection of cholinergic and nitroxidergic neurons in the myenteric plexus of guinea pig stomach. Am. J. Physiol. 269:G186-G195. [DOI] [PubMed] [Google Scholar]
- 36.Tillack, M., L. Biller, H. Irmer, M. Freitas, M. A. Gomes, E. Tannich, and I. Bruchhaus. 2007. The Entamoeba histolytica genome: primary structure and expression of proteolytic enzymes. BMC Genomics 8:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tillack, M., N. Nowak, H. Lotter, R. Bracha, D. Mirelman, E. Tannich, and I. Bruchhaus. 2006. Increased expression of the major cysteine proteinases by stable episomal transfection underlines the important role of EhCP5 for the pathogenicity of Entamoeba histolytica. Mol. Biochem. Parasitol. 149:58-64. [DOI] [PubMed] [Google Scholar]
- 38.Vosler, P. S., C. S. Brennan, and J. Chen. 2008. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol. Neurobiol. 38:78-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walsh, J. A. 1986. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Rev. Infect. Dis. 8:228-238. [DOI] [PubMed] [Google Scholar]
- 40.Waters, E. M., and R. B. Simerly. 2009. Estrogen induces caspase-dependent cell death during hypothalamic development. J. Neurosci. 29:9714-9718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu, Y., and K. Chadee. 1997. Entamoeba histolytica stimulates interleukin 8 from human colonic epithelial cells without parasite-enterocyte contact. Gastroenterology 112:1536-1547. [DOI] [PubMed] [Google Scholar]