Abstract
Understanding the regulation of human immune responses is critical for vaccine development and treating infectious diseases. We have previously shown that simultaneous engagement of the T cell receptor (TCR) and complement regulator CD46 on human CD4+ T cells in the presence of interleukin-2 (IL-2) induces potent secretion of the immunomodulatory cytokine IL-10. These T cells mediate IL-10-dependent suppression of bystander CD4+ T cells activated in vitro with anti-CD3 and anti-CD28 costimulation, reflecting a T regulatory type 1 (Tr1)-like phenotype. However, CD46-mediated negative regulation of pathogen-specific T cells has not been described. Therefore, we studied the ability of CD46-activated human CD4+ T cells to suppress T cell responses to Mycobacterium bovis BCG, the live vaccine that provides infants protection against the major human pathogen Mycobacterium tuberculosis. Our results demonstrate that soluble factors secreted by CD46-activated human CD4+ T cells suppress mycobacterium-specific CD4+, CD8+, and γ9δ2 TCR+ T cells. Dendritic cell functions were not downregulated in our experiments, indicating that CD46-triggered factors directly suppress pathogen-specific T cells. Interestingly, IL-10 appeared to play a less pronounced role in our system, especially in the suppression of γ9δ2 TCR+ T cells, suggesting the presence of additional undiscovered soluble immunoregulatory factors. Blocking endogenous CD46 signaling 3 days after mycobacterial infection enhanced BCG-specific T cell responses in a subset of volunteers. Taken together, these results indicate that CD46-dependent negative regulatory mechanisms can impair T cell responses vital for immune defense against mycobacteria. Therefore, modulating CD46-induced immune regulation could be integral to the development of improved tuberculosis therapeutics or vaccines.
Regulation of the immune system is critical for the resolution of immune responses and for the prevention of autoimmunity. FoxP3+ regulatory T cells have taken center stage in recent years, but other pathways of immune regulation are also under intense investigation. That the adaptive immune system regulates the effector arms of innate immunity is a central paradigm of immunology. No less crucial is the fact that innate immune mechanisms, such as pattern recognition receptors and the complement system, also instruct and regulate adaptive immunity (18, 25).
CD46, also known as membrane cofactor protein (MCP), is primarily recognized for its role in complement regulation and as a receptor for multiple human pathogens (7, 29, 41). Almost all nucleated cells express this type I transmembrane glycoprotein. When the cell-attached opsonins C3b and C4b, the major complement effector molecules, bind to CD46, the plasma serine protease factor I inactivates these fragments by limited proteolysis, thereby preventing unwanted complement activation on host tissue. Pathogens known to utilize CD46 as a receptor—perhaps to usurp its immunoregulatory properties (7)—include measles virus, human herpesvirus 6, certain group A and D adenoviruses, Streptococcus pyogenes, and pathogenic Neisseria. CD46 may also help to orchestrate certain key biological processes (27, 39). For example, this molecule is intimately involved in limited and highly regulated complement activation on self at the inner acrosomal membrane of spermatozoa following acrosomal activation (40). Importantly, CD46 also has signaling capabilities and can transmit intracellular signals in multiple cell types when cross-linked at the cell surface (25). Either of its two possible cytoplasmic tails can mediate the activation of distinct signaling pathways upon engagement of the extracellular domain (27, 41; reviewed in reference 39). Monoclonal antibodies (MAbs), complement activation fragments (i.e., C3b and C4b dimers), or pathogen components can act as cross-linking ligands for CD46 to initiate signaling.
In CD4+ T cells specifically, CD46 cross-linking initiates phosphorylation of its cytoplasmic domain by Lck (51), the activation of Vav and Rac (55), and phosphorylation of the mitogen-activated protein kinase Erk and the adapter proteins p120CBL and LAT (2). In the presence of interleukin-2 (IL-2) and T cell receptor (TCR) stimulation, this CD46 costimulation induces strong proliferation as well as a characteristic profile of cytokine expression. The latter includes a high level of IL-10 production, by which these cells suppress the proliferation of purified bystander T cells (4, 26). However, these studies analyzed the T cell-immunomodulatory effects of CD46-activated T cells only on CD3/CD28-activated purified CD4+ T cells or in mixed lymphocyte reactions. The impact of CD46-induced regulatory mechanisms on antigen-specific T cells in an autologous system of pathogen infection—a more physiological setting—has not been analyzed.
To accomplish this, we infected peripheral blood mononuclear cells (PBMC) from persons vaccinated with live Mycobacterium bovis bacille Calmette-Guérin (BCG) or from healthy individuals with evidence of latent tuberculosis (TB) infection in the presence of supernatants derived from CD46-activated CD4+ T cells. This experimental system demonstrated that soluble factors produced by CD46-activated CD4+ T cells suppress both proliferation and cytokine production of mycobacterium-specific CD4+, CD8+, and γδ T cells, thus limiting three major subsets of T cells that protect against M. tuberculosis.
Our reasoning for selecting this specific experimental in vitro system to address CD46 biology is the following: first, detailed functional in vivo characterization of CD46-induced regulatory pathways is hampered by the lack of a suitable small animal model. Neither mice nor rats express CD46 on their somatic cells (39, 47), and an analogous molecule with such immunomodulatory function has not yet been described in rodents. In addition, CD4+ T cells from mice transgenic for human CD46 do not demonstrate increased IL-10 production upon concurrent TCR and human CD46 stimulation (31; C. Kemper, unpublished observations), which argues that downstream mediators of a corresponding pathway in the mouse are likely not engaged properly by human CD46. Second, transferring supernatants from CD46-activated CD4+ T cells allows us to observe regulation of pathogen-specific T cell responses without engaging CD46 on the responding antimycobacterial T cells themselves. And third, we have an established model of infection that has previously been used in a clinical trial to enumerate proliferating and cytokine-producing effector T cell subsets known to protect against the major human pathogen Mycobacterium tuberculosis (11, 20). Importantly, this system allows for the analysis of autologous, antigen-presenting cell (APC)-mediated activation of antigen/pathogen-specific T cells through major histocompatibility complex (MHC)-TCR interactions.
MATERIALS AND METHODS
Ethics statement.
Blood from healthy donors was collected and used according to the guidelines of the Washington University Medical Center Human Studies Committee. The protocol for leukapheresis was approved by the Saint Louis University Institutional Review Board. Written informed consent was obtained from all donors. PBMC were obtained by Ficoll-Paque (Amersham) centrifugation of leukapheresis samples from healthy volunteers who were positive for the purified protein derivative tuberculin skin test (PPD+; response of ≥10-mm induration 48 to 72 h after a 5-tubercullin units test). PBMC were aliquoted and stored frozen in liquid nitrogen.
Antibodies, media, and reagents.
CD4+ T cell cultures were maintained in RPMI 1640 medium (Gibco Invitrogen) with 10% fetal calf serum and 200 mM l-glutamine in the presence of 25 U/ml recombinant human IL-2 (BioSource International, Camarilla, CA). RPMI supplemented with normal 10% pooled human serum, l-glutamine, and 50 U penicillin-50 mg streptomycin per ml was used to culture PBMC from PPD+ volunteers.
The hybridoma line expressing the MAb reactive with human CD3 (clone OKT3) used for T cell activation was obtained from ATCC (Manassas, VA), and the MAb was purified by the Rheumatic Diseases Core Center, Washington University School of Medicine. The CD46-activating MAb utilized in this study, TRA-2-10, recognizes an epitope within the first complement control repeat (28). The CD28-activating MAb (CD28.2) and the MAbs used to neutralize human IL-10 (JES3-9D7) (4), granulocyte-macrophage colony-stimulating factor (GM-CSF; BVD2-21C11), tumor necrosis factor alpha (TNF-α; MAb1), and Fas ligand (FasL; Nok-2) were purchased from BD Biosciences (San Jose, CA). The human CD40L/CD154 (MAb 24-31) and transforming growth factor β (TGF-β; MAb 2463) neutralizing antibodies were obtained from eBioscience (San Diego, CA) and R&D Systems (Minneapolis, MN), respectively. Neutralizing polyclonal antibodies to the chemokines macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and RANTES were obtained from Abcam, Inc. (Cambridge, MA).
Fluorophore-labeled MAbs directed against human CD25 (M-A251), CD4 (SK3), CD8 (SK1), CD3 (SK7), γδ TCR (11F2), gamma interferon (IFN-γ; B27), and granzyme B (GB11) were obtained from BD Biosciences. The mouse nonspecific isotype-matched control MAb MOPC 31c (IgG1) was obtained from Sigma-Aldrich (St. Louis, MO). All other isotype controls were purchased from BD Biosciences. Carboxyfluorescein succinimidyl ester (CFSE; Vybrant CFDA SE cell tracer kit) was obtained from Invitrogen Molecular Probes (Eugene, OR). Phorbol myristate acetate (PMA; Sigma-Aldrich, St. Louis, MO), ionomycin (Sigma), and a Cytofix/Cytoperm kit (BD Biosciences) were used for restimulation and intracellular staining.
Soluble CD46 (sCD46) was prepared in the Rheumatic Diseases Core Center, Washington University School of Medicine, by cloning an Escherichia coli codon usage-optimized cDNA coding for short consensus repeat domains 1 to 4 of human CD46 into the pET15-b vector. BL21(DE3) bacteria were transfected with the construct. Recombinant sCD46 was then purified from inclusion bodies and refolded according to a method described by White et al. (52).
BCG strain and source.
The Danish strain of Mycobacterium bovis bacille Calmette-Guérin was obtained from the Statens Serum Institut (Copenhagen, Denmark).
Purification and stimulation of CD4+ lymphocytes.
CD4+ T lymphocytes were purified from whole blood by magnetic bead separation using CD4 microbeads (Miltenyi Biotec, Auburn, CA). In vitro stimulation of isolated CD4+ T cells was performed in flat-bottom 96-well plates coated with 2 μg/ml anti-CD3, anti-CD28, anti-CD46, or a matched IgG1 isotype control MAb. The wells were washed to remove unbound MAbs, and purified CD4+ T lymphocytes (1.5 × 105 to 2.0 × 105 cells/well) were added in 100 μl of culture medium supplemented with 25 U/ml recombinant human IL-2. The plates were centrifuged at 250 × g for 2 min and incubated at 37°C in 5% CO2. Supernatants were harvested on day 3. CD3/CD46 activation consistently resulted in approximately 50% IL-10/granzyme B/CD25 positive cells by flow cytometry as previously described (4, 26). In one set of experiments, soluble CD25, the high-affinity IL-2 receptor α chain (IL-2Rα), was removed from both control and regulatory supernatants by immunodepletion using anti-CD25 MAb from BD Biosciences coupled to Sepharose 4B beads (Pharmacia Biotech).
Cytokine and chemokine analyses.
IL-10, GM-CSF, soluble CD25 (IL-2Rα), and/or soluble CD40L present in the supernatants of CD3/CD28− or CD3/CD46-activated CD4+ T cells were measured using the appropriate enzyme-linked immunosorbent assay (ELISA) kits from BioSource International (Camarillo, CA) or BD Biosciences. Cytokine and chemokine levels produced over the course of 7-day culturing of PBMC with BCG and supernatant derived from CD3/CD46-activated human CD4+ T cells (3/46 SN) or supernatant derived from CD3/CD28-activated human CD4+ T cells (2/38 SN) were measured using cytometric bead array kits from BD Biosciences. Data collection was performed on an LSRII flow cytometer at the Saint Louis University Flow Cytometry Core Facility, and data analysis was done using FCAP Array software provided by BD Biosciences.
CFSE-based assay to measure BCG-specific T cell responses in vitro.
CFSE-labeled PBMC (1 × 106/ml) resuspended in 1 ml complete RPMI medium, control CD3/CD28 supernatant, or CD3/CD46 supernatant were stimulated with live BCG (multiplicity of infection [MOI], 1) for 7 days in a 5% CO2, 37°C humidified incubator. Before staining, the cells were restimulated with PMA (50 ng/ml) and ionomycin (750 ng/ml) in the presence of GolgiStop (0.7 μl/ml; Becton Dickinson) for 2 h. Cells were then washed, stained for T cell surface markers (CD3, CD4, CD8, and γδ TCR), fixed, permeabilized, and stained with MAb specific for IFN-γ. Flow cytometric acquisition was performed on the LSRII (BD Biosciences) of the Saint Louis University Flow Cytometry Core Facility, and analyses were carried out utilizing FlowJo software (TreeStar). A minimum of 10,000 events were acquired. Absolute numbers of CFSElo/IFN-γ+ T cells for each subset were obtained by multiplying viable cell counts per tube times the frequency of CFSElo/IFN-γ+ T cells for each gated T cell subset (CD4+, CD8+, or γδ TCR+) as determined by flow cytometry. For neutralization experiments, the appropriate function-neutralizing antibodies were added to parallel experimental and control supernatants 30 min prior to combining with infected PBMC cultures.
DC stimulation of mycobacterium-specific T cells.
Dendritic cells (DC) were generated from adherent monocytes by culture with human IL-4 and GM-CSF as described previously (46). DC were incubated overnight with BCG (5 CFU per DC) in the presence or absence of control or regulatory supernatants (0.2 ml). Infected DC were then irradiated and washed twice with fresh medium. CFSE-labeled nonadherent PBMC (1 × 106) and DC (2 × 104) were cultured for 7 days and analyzed as described above by flow cytometry to enumerate mycobacterium-specific T cell responses.
Statistical analysis.
Nonparametric statistics were applied to the results to determine statistical significance. The Wilcoxon matched pairs test was applied to matched pairs of data generated from cells derived from the same individual (e.g., treated with 3/28 SN or 3/46 SN). Medians are plotted on the bar graphs for the relevant figures, and single outliers were excluded using the extreme Studentized deviate test.
RESULTS
Experimental approach.
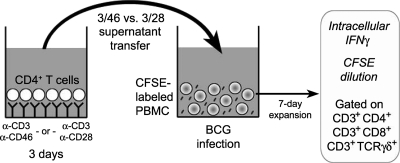
A schematic of our experimental approach is presented in Fig. 1. BCG stimulates CD4+, CD8+, and γδ T cell recall responses in human PBMC from BCG-vaccinated or PPD+ donors (20, 21, 53, 54). Previously, we quantified these pathogen-specific T cell responses by using multiparameter flow cytometry to detect proliferating T cells that secreted effector cytokines (20). Briefly, PBMC from immune donors were labeled with CFSE and infected with BCG. Proliferation (i.e., CFSE dilution), T cell surface markers, and effector cytokine secretion (i.e., intracellular cytokine staining for IFN-γ) were detected simultaneously for different T cell subsets. By measuring the frequencies of CFSElo/IFN-γ+ cells and counting total cells, we determined under various conditions absolute numbers of responding CD4+, CD8+, and TCR γδ+ T cells.
FIG. 1.
Generation and transfer of supernatants from CD3/CD46-activated CD4+ T cells. Suppression of mycobacterium-specific CD4+, CD8+, and γδ T cell responses was monitored following transfer of 3-day supernatants from CD46-activated CD4+ T cells (or from control CD28-activated CD4+ T cells) to BCG-infected CFSE-labeled PBMC from PPD+ donors. After 7 days, intracellular IFN-γ and dilution of CFSE were detected in the three T cell subsets by flow cytometry.
Concurrent engagement of CD3 and CD46 on human CD4+ T cells in the presence of IL-2 (>5 U/ml) results in the development of an IL-10-producing Tr1-like regulatory phenotype (4, 16, 17, 26, 38). However, these activation conditions also induce high granzyme B production as well as contact-dependent cytotoxic activity toward bystander T cells in CD4+ T cells (4, 16, 26). Thus, as a starting point in our study using the mycobacterial infection model, we first examined the cell contact-independent functions of CD46-activated CD4+ T cells (e.g., cytokines and chemokines) on BCG-specific T cell responses. T cell supernatants were generated as previously described (4, 16, 17, 26, 38) by stimulating purified human CD4+ T cells for 3 days with immobilized MAbs to CD3 and CD46 in the presence of recombinant IL-2. We confirmed the development of the regulatory phenotype by monitoring the expression of IL-10 and granzyme B, as well as suppressive activity. Supernatants were then transferred to PBMC cultures infected with BCG, and T cell responses were analyzed 7 days postinfection (Fig. 1). Supernatants derived from CD3/CD28-activated CD4+ T cells were used as a control in parallel cultures.
Supernatants of CD46-activated CD4+ T cells downregulate mycobacterium-specific T cell responses.
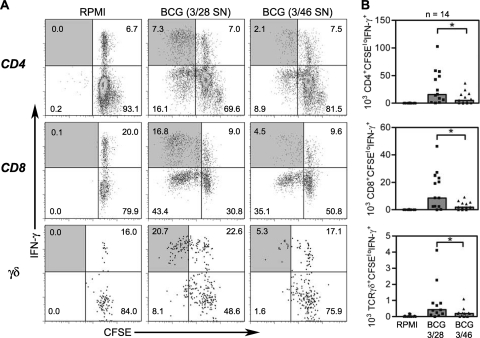
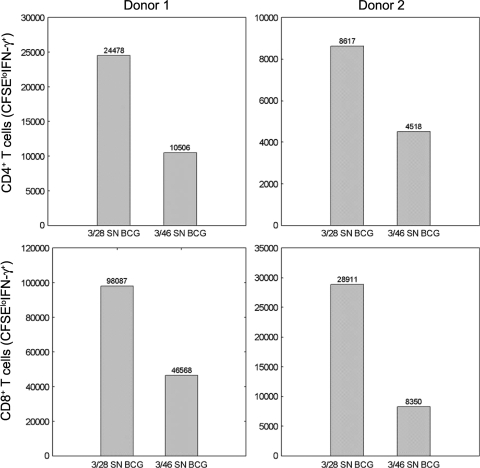
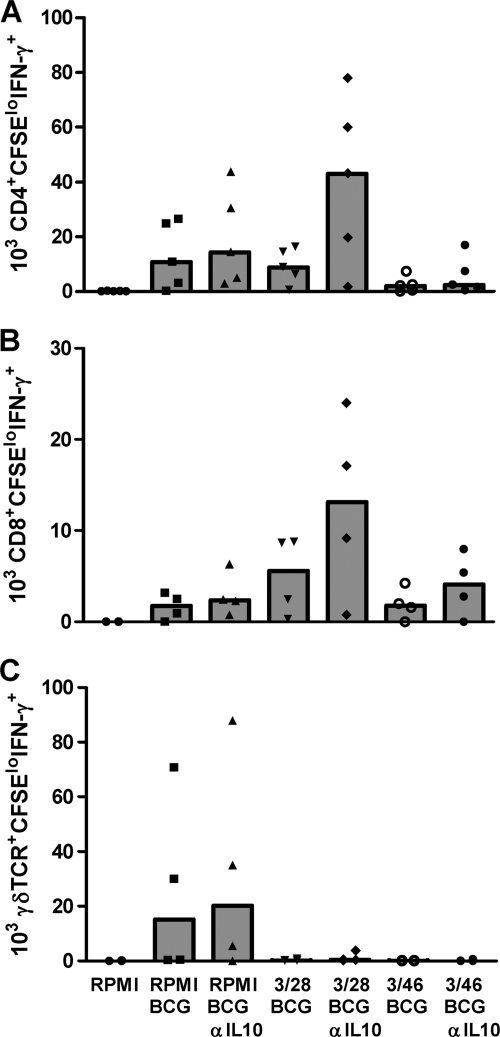
When 3/46 supernatants were transferred to cultures of PBMC infected with BCG for 7 days, we detected significantly lower numbers of responding BCG-specific T cells relative to parallel cultures in which control 3/28 supernatants were added. Representative dot plots and summary graphs for multiple PPD+ donors are shown in Fig. 2. On average, responding CD4+ T cells (CD3+/CD4+/CFSElo/IFN-γ+) were reduced 3-fold below control levels, while CD8+ (CD3+/CD8+/CFSElo/IFN-γ+) and γδ TCR+ responders (CD3+/TCR γδ+/CFSElo/IFN-γ+) were reduced at least 4-fold below the responses detected in control supernatants (P < 0.002 for all subsets), indicating that soluble factors from the CD46-induced T cells suppressed BCG-specific CD4+, CD8+, and γδ T cell responses.
FIG. 2.
Flow cytometry to detect antimycobacterial CD4+, CD8+, and γδ TCR+ T cells and suppression by 3/46 supernatants. (A) CFSE-labeled PBMC were cultured in RPMI complete medium (left), in control 3/28 SN cells infected with BCG (middle), or in cells from 3/46 SN and infected with BCG (right). (B) Responding CD3+ CD4+, CD3+ CD8+, or CD3+ γδ+ T cells were enumerated by determining absolute numbers of CFSElo IFN-γ+ T cells after 7 days in culture. Bar graphs indicate the medians of 14 experiments. Asterisks indicate significant differences from T cell responses in 3/28 SN (P < 0.002 for all T cell subsets by Wilcoxon matched pairs test).
CD3/CD46-activated CD4+ T cells exhibit a higher proliferation rate than CD3/CD28-activated CD4+ T cells (2, 26). To exclude the possibility that the different effects of 3/46 SN and 3/28 SN were due to unequal depletion of nutrients during the 3-day initial culture (Fig. 1, left), we concentrated these supernatants by using a 3-kDa cutoff cellulose membrane and reconstituted both with fresh medium. We then repeated the 7-day BCG infection of PBMC in the presence of these reconstituted supernatants and obtained similar results as before (Fig. 3). Thus, CD46-mediated suppression of pathogen-specific T cell responses was mediated by the generation of discrete soluble factors rather than increased depletion of nutrients in regulatory 3/46 SN.
FIG. 3.
3/46 supernatant reconstituted with fresh medium retains immunosuppressive activity. Purified CD4+ T cells were activated with plate-bound MAbs to CD3/CD28 or to CD3/CD46, and supernatants were harvested after 3 days. The supernatants were concentrated 10-fold using Amicon centrifugal filters with a 3-kDa molecular mass cutoff membrane and then reconstituted to the original volume using fresh serum-free medium. The reconstituted supernatants were then transferred to CFSE-labeled, BCG-infected PBMC from two donors. Absolute numbers of CD4 and CD8 responders (CFSElo IFN-γ+) were enumerated by flow cytometry after 7 days.
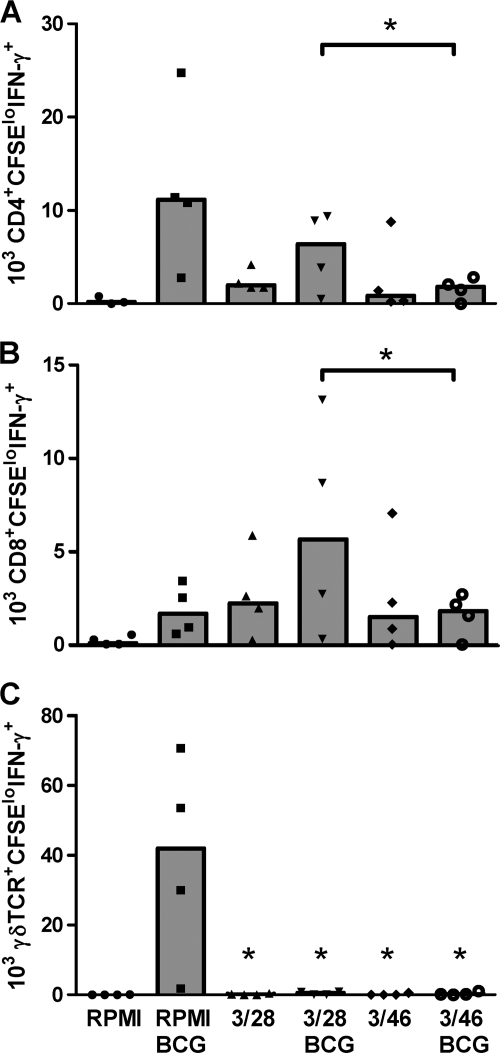
To further confirm that the apparent suppressive effects of 3/46 SN represented dominant inhibitory effects rather than the absence of growth-enhancing effects, we conducted experiments under additional control activation conditions, including PBMC cultures incubated with 3/46 SN or 3/28 control SN without BCG infection (Fig. 4). Analysis of these cultures after 7 days revealed that both supernatants provided some growth/stimulatory factors independent of BCG infection which acted on CD4+ and especially CD8+ T cells (compare RPMI findings with 3/28 and 3/46 findings in all panels), similar to our previous findings (26). However, the addition of BCG to 3/28 SN resulted in the expected antigen-specific increases in CD4+ and CD8+ T cell numbers (compare 3/28 and 3/28 BCG), while this antigen-specific induction of T cell responses was blocked when BCG and 3/46 SN were added to parallel cultures of the same PBMC (compare 3/46 to 3/46 BCG). These results confirmed that supernatants from CD46-activated CD4+ T cells have suppressive effects on BCG-specific T cell responses, compared with effector T cell supernatants (3/28 SN).
FIG. 4.
T cell responses to BCG infection exceeded background levels detected upon supernatant transfer alone and were suppressed by 3/46 SN. CD3+ CD4+ CFSElo IFN-γ+ T cells (A) and CD3+ CD8+ CFSElo IFN-γ+ T cells (B) were enumerated 7 days after CFSE-labeled PBMC from five PPD+ donors were added to medium (RPMI complete), 3/28 SN, or 3/46 SN with or without BCG infection. Both supernatants promoted some background stimulation, yet BCG enhanced that growth, and this enhancement was specifically suppressed by 3/46 SN. (C) Both 3/28 SN and 3/46 SN suppressed mycobacterium-specific γδ T cells relative to the control condition of PBMC infected with BCG in RPMI complete medium. In each graph (A to C), the data are plotted with medians indicated by bars. Asterisks indicate statistically significant suppression compared to the 3/28 BCG condition (A and B) or compared to RPMI with BCG infection (C) (n = 5; P < 0.05 by Wilcoxon matched pairs test).
Interestingly, we observed that BCG-specific γδ T cell responses were consistently suppressed by both 3/28 SN and 3/46 SN compared with BCG-infected cultures incubated in medium alone (Fig. 4C). Still, the suppressive effect of 3/46 SN on γδ T cells was stronger, compared with the control 3/28 SN condition (Fig. 2B), demonstrating that 3/46 SN provided maximal suppression of all three T cell subsets. In addition, supernatants from CD46-activated CD4+ T cells also suppressed γδ T cell responses in separate cultures stimulated with purified phosphoantigen (isopentenyl pyrophosphate) and IL-2 (conditions that specifically activate γδ T cells without concurrent CD4+ and CD8+ T cell stimulation), indicating direct suppression of γδ T cells, independent of cross talk with activated antigen-specific CD4+ and CD8+ T cells (data not shown).
Mechanisms of CD46-mediated suppression of BCG-stimulated PBMC include other soluble factors in addition to IL-10.
IL-10 secretion is one major pathway of immunosuppression employed by natural and adaptive regulatory T cells (5, 22, 35). CD46-activated CD4+ T cells also utilize this mechanism to suppress polyclonally stimulated, purified CD4+ T cells (4, 26). Therefore, we assessed whether IL-10 was responsible for the regulatory effects of 3/46 SN in our mycobacterium-specific system. A function-neutralizing anti-IL-10 MAb (4, 16, 26) was added to PBMC cultures infected with BCG, and T cell responses were measured at day 7 (Fig. 5). In BCG-infected cultures with control 3/28 SN, IL-10 neutralization moderately increased BCG-specific CD4+ and CD8+ T cell numbers compared to those in BCG-infected cultures with medium alone (although these differences did not reach statistical significance). This is likely due to the combinatorial effects of growth factors present in the 3/28 SN (Fig. 4) and neutralization of the low levels of IL-10 generally observed in 3/28 T cell culture SN (produced by memory CD4+ T cells restimulated with plate-bound anti-CD3 and anti-CD28). Surprisingly, however, neutralization of IL-10 in 3/46 SN consistently resulted at best in only partial reversal of the suppression of BCG-specific CD4+ and CD8+ T cells (Fig. 5). These results indicate that an additional (nonidentified) soluble factor(s) in the 3/46 SN mediates suppression of pathogen-specific T cell responses, or perhaps that CD46-induced cytokine/chemokines have an impact on the cross talk between immunocompetent cells over the course of the 7-day PBMC culture with BCG. Also, the suppression of γδ T cell responses in our system was again distinct from that of CD4+ and CD8+ T cells in that neutralizing anti-IL-10 appeared to have no effect on the suppression mediated by 3/28 SN or 3/46 SN.
FIG. 5.
CD46-induced suppression of pathogen-specific T cell responses is not reversed by IL-10 blockade alone. CFSE-labeled PBMC from six PPD+ donors were infected with BCG and cultured in control 3/28 SN or 3/46 SN in the presence or absence of a monoclonal antibody to block IL-10 activity. Responding antimycobacterial CD4+, CD8+, and γδ T cells (A to C, respectively) were enumerated by gating on the appropriate CD3+ CFSElo IFN-γ+ subpopulation. For each graph, the data for six experiments are plotted, with medians indicated by bars.
To identify the additional soluble factors that might be acting in concert with IL-10 to regulate T cell responses in our system, we analyzed the cytokine/chemokine profiles present in the PBMC cultures at various time points during the 7-day BCG culture, with and without 3/46 and 3/28 SN (Table 1). Throughout the course of the infection, IL-10 and GM-CSF (produced by CD46-stimulated CD4+ T cells [4, 26]) remained elevated in cultures incubated with 3/46 SN compared with cultures incubated with 3/28 SN. Additionally, we observed that TNF-α and the chemokines MIP-1α, MIP-1β, and RANTES (all of which bind CCR5) were maintained at higher levels in cultures treated with 3/46 SN, while no significant differences were detected in IL-1β, IL-6, IL-12p70, IP-10, monocyte chemoattractant protein 1, and vascular endothelial growth factor (VEGF) production (data not shown). Thus, the ability of 3/46 SN to regulate T cell responses to BCG appeared to correlate with a unique cytokine/chemokine expression pattern.
TABLE 1.
Cytokine levels in BCG-infected PBMC cultures in the presence of 3/28 or 3/46 supernatants from activated CD4+ T cells
| Cytokine | Supernatant | Initial concn (pg/ml) | Culture condition | PBMC donor no. | Level (pg/ml) after infection for: |
|||
|---|---|---|---|---|---|---|---|---|
| 1 day | 3 days | 5 days | 7 days | |||||
| GM-CSF | Medium | 0 | BCG | 1 | 90 | 155 | 182 | 184 |
| 2 | 83 | 94 | 79 | 109 | ||||
| 3/28 SN | 622 | BCG-3/28 | 1 | 536 | 503 | 542 | 554 | |
| 2 | 514 | 488 | 498 | 531 | ||||
| 3/46 SN | 2,454 | BCG-3/46 | 1 | 2,109 | 1,945 | 2,002 | 1,967 | |
| 2 | 1,990 | 1,879 | 1,967 | 1,945 | ||||
| IL-10 | Medium | 0 | BCG | 1 | 132 | 69 | 32 | 22 |
| 2 | 166 | 98 | 43 | 48 | ||||
| 3/28 SN | 3,597 | BCG-3/28 | 1 | 2,313 | 1,518 | 1,059 | 1,048 | |
| 2 | 2,390 | 1,534 | 1,099 | 1,195 | ||||
| 3/46 SN | 11,312 | BCG-3/46 | 1 | 8,398 | 5,712 | 4,352 | 4,208 | |
| 2 | 7,699 | 5,396 | 4,114 | 4,068 | ||||
| TNF-α | Medium | 6 | BCG | 1 | 294 | 225 | 151 | 53 |
| 2 | 1,569 | 354 | 135 | 68 | ||||
| 3/28 SN | 193 | BCG-3/28 | 1 | 357 | 350 | 147 | 61 | |
| 2 | 518 | 344 | 141 | 101 | ||||
| 3/46 SN | 1,491 | BCG-3/46 | 1 | 870 | 349 | 518 | 407 | |
| 2 | 888 | 797 | 452 | 403 | ||||
| MIP-1α | Medium | 0 | BCG | 1 | 10,129 | 19,480 | 16,969 | 14,123 |
| 2 | 13,669 | 22,453 | 15,086 | 9,248 | ||||
| 3/28 SN | 3,696 | BCG-3/28 | 1 | 5,174 | 8,093 | 7,107 | 8,334 | |
| 2 | 3,987 | 6,811 | 4,810 | 4,654 | ||||
| 3/46 SN | 18,330 | BCG-3/46 | 1 | 18,172 | 18,489 | 17,558 | 17,862 | |
| 2 | 14,595 | 13,669 | 11,466 | 13,894 | ||||
| MIP-1β | Medium | 0 | BCG | 1 | 26,561 | 33,707 | 30,118 | 26,818 |
| 2 | 37,772 | 46,676 | 42,389 | 50,143 | ||||
| 3/28 SN | 16,609 | BCG-3/28 | 1 | 26,818 | 31,624 | 28,142 | 28,971 | |
| 2 | 35,587 | 40,921 | 32,727 | 31,316 | ||||
| 3/46 SN | 80,633 | BCG-3/46 | 1 | 69,361 | 60,449 | 38,151 | 45,735 | |
| 2 | 75,569 | 70,105 | 56,184 | 56,773 | ||||
| RANTES | Medium | 279 | BCG | 1 | 564 | 735 | 1,024 | 1,881 |
| 2 | 326 | 384 | 419 | 665 | ||||
| 3/28 SN | 352 | BCG-3/28 | 1 | 485 | 721 | 1,301 | 1,948 | |
| 2 | 303 | 491 | 798 | 984 | ||||
| 3/46 SN | 1,447 | BCG-3/46 | 1 | 1,167 | 911 | 1,055 | 1,301 | |
| 2 | 735 | 564 | 654 | 956 | ||||
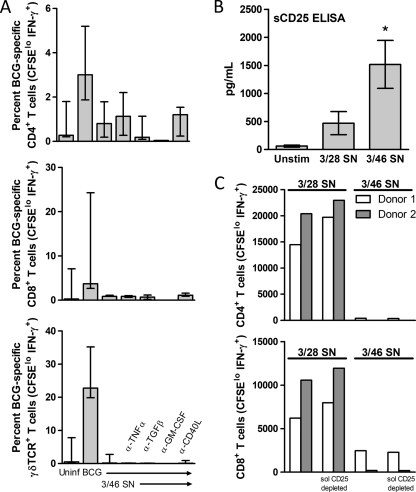
We next asked whether TNF-α or the CCR5 ligands might act synergistically with IL-10 to induce the observed potent downregulation of BCG-specific T cell responses. We blocked TNF-α activity in our system by using a neutralizing MAb and found no reversal of CD46-mediated suppression (Fig. 6A). However, the production of TNF-α may contribute to or be required for the initial induction of BCG-specific responses in vitro, a conclusion also suggested by TNF-α production during BCG infection of PBMC (Table 1, TNF-α data). We therefore could not definitively determine whether the higher level of TNF-α present in 3/46 SN is involved in the suppressive mechanism. Neutralizing antibodies to MIP-1α, MIP-1β, and RANTES did not affect the regulatory capacity of the 3/46 SN, either alone or in combination with neutralizing anti-IL-10 (data not shown). Similarly, neutralization of GM-CSF, TGF-β, CD40L (CD154), or FasL (CD178) in the 3/46 SN had no effect on the suppressive capacity of 3/46 SN (Fig. 6A and data not shown). An alternative mechanism of suppression by regulatory T cells is competition for prosurvival cytokines, most notably IL-2 (37). Interestingly, sCD25, the high-affinity IL-2 receptor, is present at high levels in the 3/46 SN (Fig. 6B). However, immunodepletion of sCD25 from 3/46 SN did not impact the ability to suppress BCG-specific responses (Fig. 6C).
FIG. 6.
Mechanistic investigations of the nature and identity of a 3/46-induced soluble factor(s) that inhibits BCG-specific T cell responses. (A) Neutralization of TNF-α, TGF-β, GM-CSF, or CD40L in 3/46 supernatants does not prevent suppression. Supernatant was generated by activation of purified CD4+ T cells with plate-bound MAbs to CD3 and CD46 for 3 days. After a 30-min preincubation with neutralizing MAbs to TNF-α, TGF-β, GM-CSF, or CD40L, the 3/46 SN was transferred to CFSE-labeled, BCG-infected PBMC from three donors. Controls included uninfected PBMC and infected PBMC without supernatant. Medians and ranges are plotted for percentages of CD4+, CD8+, and γδ TCR+ T cells (CFSElo IFN-γ+) detected by flow cytometry for each condition after 7 days. (B) Higher levels of soluble CD25 were produced in the presence of IL-2 by CD4+ T cells stimulated through CD3/CD46 than with CD3/CD28. Without MAbs (Unstim), minimal soluble CD25 was detected. Supernatants were collected after 3 days of stimulation, and soluble CD25 was assessed by ELISA. Means of eight experiments (±standard errors of the means) are shown. *, P < 0.05 compared to 3/28 SN-stimulated cells. (C) Depletion of soluble CD25 from 3/46 SN does not reverse suppression of BCG-specific T cell responses. Purified CD4+ T cells were activated with plate-bound MAbs to CD3/CD28 or to CD3/CD46, and supernatants were harvested after 3 days. Soluble CD25 was removed from both control and regulatory supernatants by immunodepletion using anti-CD25 MAb coupled to Sepharose beads. Supernatants were then transferred to CFSE-labeled, BCG-infected PBMC from two donors. Absolute numbers of responding CD4 and CD8 T cells (CFSElo IFN-γ+) were enumerated by flow cytometry after 7 days.
We conclude from our experiments that, although IL-10 is an expected contributing factor in the CD46-mediated downregulation of the pathogen-specific T cell responses in our in vitro model, additional required suppressive “agents” are yet to be identified.
Soluble mediators induced by CD46 signaling do not suppress DC maturation or antigen presentation.
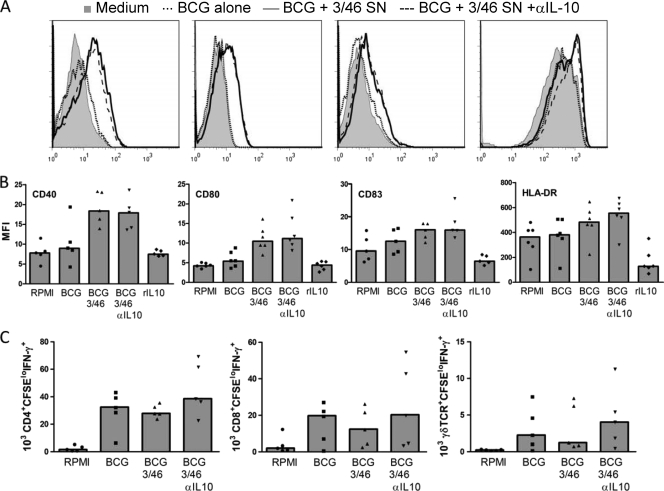
IL-10, depending on the biological context, has the capacity to suppress T cell activation and/or APC functions (34). Thus, we assessed whether 3/46 SN could negatively regulate APC maturation or presentation of BCG antigens. Immature DC were generated from peripheral blood monocytes using IL-4 and GM-CSF. DC were infected with BCG and incubated overnight in medium alone, in medium with recombinant IL-10, in 3/46 SN, or in 3/46 SN plus anti-IL-10 MAb and subsequently analyzed for the expression of DC maturation markers (Fig. 7 A and B). We found that culturing the DC in 3/46 SN did not suppress but rather enhanced maturation, as CD40, CD80, CD83, and HLA class II molecules were detected at higher levels than following stimulation with BCG alone. The addition of anti-IL-10 did not significantly alter marker upregulation. As expected, recombinant IL-10 (10 ng/ml) inhibited DC maturation. The amount of recombinant IL-10 in this control was comparable to that found in 3/46 SN (Table 1), indicating that the combination of soluble factors produced upon CD46 stimulation counteracts the usually inhibitory effects of IL-10 on DC maturation.
FIG. 7.
Dendritic cell maturation and antigen-presenting function are unaffected by 3/46 supernatants. DC were preincubated overnight in RPMI medium alone (no BCG), RPMI with BCG, 3/46 SN with BCG with or without neutralizing anti-IL-10, or RPMI with recombinant human IL-10 (10 ng/ml). DC were then analyzed by flow cytometry to measure cell surface expression of CD40, CD80, CD83, and HLA-DR as indicators of maturation. 3/46 SN did not prevent maturation of DC, as might be expected due to its high IL-10 content; in fact, the 3/46 SN increased expression of maturation markers. (A) Representative histograms indicate surface staining of DC incubated in medium alone (shaded histogram), with BCG (dotted line), BCG and 3/46 SN (solid line), or BCG plus 3/46 SN plus anti-IL-10 antibody (dashed line). (B) Mean fluorescence intensity (MFI) values for six individuals, with medians indicated by bars. (C) DC were infected with BCG overnight in the presence of RPMI or 3/46 SN with or without neutralizing anti-IL-10 antibody and then were washed to remove extracellular BCG. Autologous CFSE-labeled PBMC were then added to detect DC antigen presentation to mycobacterium-specific T cells. After 7 days, CD4+, CD8+, and γδ T cells responding to BCG (CFSElo IFN-γ+) were detected in similar absolute numbers whether or not DC were pretreated with 3/46 SN. Bars indicate medians for the data generated from six individuals.
Next, to determine whether 3/46 SN affects DC antigen presentation to autologous, pathogen-specific T cells, we incubated immature DC overnight with BCG in the presence of 3/46 SN, irradiated and washed the DC, and added autologous nonadherent PBMC from PPD+ donors. After 7 days, we enumerated proliferating, IFN-γ-producing CD4+, CD8+ and γδ T cells and found that DC presentation of BCG antigens was unaffected by preincubation with the 3/46 SN (Fig. 7C). Neutralization of IL-10 did not add significantly to the number of responders in any T cell subset analyzed. These data suggest that the regulation of T cell responses in our system occurs primarily at the level of the responding T cell and not at the level of APC function. This is in agreement with our previous findings demonstrating that DC that have matured in the presence of 3/46 SN retain the ability to induce potent allogeneic T cell responses in mixed lymphocyte reactions (4). In that previous study, soluble CD40L (CD154) and GM-CSF produced upon CD46 activation of CD4+ T cells abrogated the suppressive effects of IL-10 on DC, thus permitting APC function in the presence of CD46 signaling.
Endogenous CD46 ligands have differential effects on the activation and expansion phases of BCG-specific T cell responses.
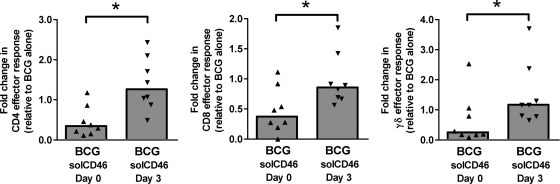
In preliminary experiments (results not shown), we found that BCG infection of PBMC in fresh medium, without CD46 SN addition, could induce a subset of CD4+ T cells phenotypically similar to CD46-activated T cells. Therefore, we wondered whether natural ligands for CD46 may be produced during PBMC stimulation with live BCG. The cognate interaction of human T cells and APCs has been reported to result in the secretion/generation of activated complement components, including the CD46 ligands C3b and C4b (45). Thus, it seemed feasible to expect that during the 7-day course of BCG infection of human PBMC, CD46 signaling on T cells may be induced through its locally produced ligands. To assess this possibility, we added soluble recombinant CD46 (which interferes with the cross-linking and activation of cell surface-expressed CD46) to PBMC cultures in fresh medium infected with mycobacteria (Fig. 8). Addition of the soluble CD46 reagent on day 0 of the culture had the unexpected effect of reducing the number of responding T cells detected on day 7. This observation implies that CD46 costimulation has an important positive impact on T cell responses if delivered at the initiation of T cell activation. In contrast, when soluble CD46 was added to the cultures 3 days postinfection with BCG, the numbers of responding T cells detectable at day 7 were increased in PBMC from at least a subset of volunteers, indicating a release from regulatory mechanisms induced by CD46 signaling. However, soluble CD46 added on day 3 of culture did not enhance T cell responses in PBMC obtained from some of the volunteers, which suggests that the kinetics of positive versus negative immunoregulatory effects mediated by CD46 signaling may vary between individuals. Although we have not yet been able to demonstrate functional consequences of CD46 cross-linking directly on APC or B cells (4, 14), we cannot exclude the possibility that the presence of soluble recombinant CD46 in these PBMC cultures affects other CD46-expressing cells in addition to the pathogen-specific T cells.
FIG. 8.
Endogenous CD46 ligands have differential effects on T cell activation and expansion phases of BCG-induced T cell responses. Soluble CD46 molecules were produced by refolding of bacterial inclusion body material and then purified to remove endotoxin. This recombinant protein was found to bind CD46 MAb. As described above, CFSE-labeled human PBMC were infected with BCG in fresh medium. Soluble CD46 (10 μg/ml) was added either at day 0 or day 3 of the 7-day culture period to saturate the culture with binding sites for potential ligands to CD46 (and thus block signaling). CD4+, CD8+, and γδ T cells responding to BCG (CFSElo IFN-γ+) were detected by flow cytometry. The data are normalized to the absolute number of responding T cells in each subset detected in parallel cultures infected with BCG. Asterisks indicate statistically significant differences between day 0 and day 3 groups (n = 8; P < 0.05 by Wilcoxon matched pairs test).
In conclusion, we show here for the first time in an APC-dependent, antigen-specific, and autologous human system that CD46-activated CD4+ T cells potently downregulate CD4+, CD8+, and TCR γδ+ T cell proliferation and IFN-γ production in response to infection with live mycobacteria. Soluble factors produced upon CD46 costimulation directly suppressed responding T cells but not APC function. Although IL-10 participated in suppression of conventional T cell responses (CD4+ and CD8+) to mycobacteria, key soluble mediators of CD46-induced regulation remain to be identified. Surprisingly, γδ T cell responses were negatively regulated by both CD46- and “classically” CD28-costimulated T cells, and this regulation was completely independent of IL-10. Further, we generated data suggesting that natural ligands (e.g., activated complement fragments) engage CD46 in our system and that CD46 engagement appears to promote the initial activation of antimycobacterial T cell responses but inhibit pathogen-specific T cell expansion at later time points.
DISCUSSION
TB, which is caused by the intracellular pathogen Mycobacterium tuberculosis, is a global public health concern (8, 43). Approximately one-third of the world's population is infected with M. tuberculosis, and significant challenges have hampered efforts to control its disease manifestations. Although standard treatment regimens based on five first-line drugs are available, increasing rates of drug resistance threaten to transform TB into a virtually untreatable disease (12, 49). The available BCG vaccines are effective in protecting against disseminated TB disease manifestations in children but provide only limited protection against pulmonary disease and the establishment of latent mycobacterial infection (10). Detailed examination of the interactions between this pathogen and relevant components of the immune system will facilitate the development of improved TB vaccines and/or treatments.
Immune protection against M. tuberculosis involves multiple immunocompetent cell types (19). CD4+ T cells recognize mycobacterial epitopes presented by MHC II molecules at the surface of infected macrophages, the preferred cell type for mycobacterial replication. IFN-γ produced by activated CD4+ T cells in turn stimulates infected macrophages, increasing their potential to kill intracellular mycobacteria, a critical effector mechanism for the control of mycobacterial infection (23, 24). CD8+ T cells also produce IFN-γ, thereby contributing to TB protection (13, 15). In addition, CD8+ T cells directly recognize and kill TB-infected macrophages (9, 53). In vivo, IFN-γ-producing CD8+ T cells have been identified within tissue granulomas from human lung biopsy specimens of patients with latent M. tuberculosis infection (48). TCR γ9δ2+ T cells also provide significant support for antimycobacterial immunity (19, 21, 44, 53). While γδ T cells normally comprise only 2 to 5% of total circulating lymphocytes, the number of γδ T cells with mycobacterium-specific cytolytic activity increases significantly following BCG vaccination (36). Furthermore, BCG-specific γδ T cells have the capacity to inhibit the growth of intracellular mycobacteria (44, 53). We report here that supernatants from CD4+ T cells activated through CD46 costimulation significantly dampen the proliferation and effector cytokine production of these three major protective T cell subsets: CD4+, CD8+, and γ9δ2 TCR+ T cells.
Despite the inhibitory effects of 3/46 supernatants on mycobacterium-specific CD4+, CD8+, and γ9δ2 TCR+ T cells, we found that the ability of DC to present mycobacterial antigens to these T cell subsets was not affected, indicating that 3/46 supernatants have direct inhibitory effects on target T cells. These results are similar to those we reported previously demonstrating that 3/46 supernatants did not prevent lipopolysaccharide-induced DC maturation or allogeneic stimulation (4). The 3/46 supernatants contain high levels of GM-CSF and sCD40L, which promote DC development and prevent the inhibitory effects of IL-10 on DC maturation, respectively (4). Our new data presented here are the first to show that 3/46 supernatants do not interfere with the ability of DC to present antigens expressed by an infectious pathogen. It is unlikely that the 7-day duration of the assays was an important mitigating factor preventing any possible inhibitory effect of the 3/46 supernatants on DC function. Previous reports have demonstrated that full activation of T cells requires only 4 to 6 h (50), indicating that the early period of our cocultures was probably the most critical for the initial DC effects on stimulated mycobacterium-specific T cells. Furthermore, the marked suppressive effects of IL-10 alone demonstrate that our assay system was able to detect inhibitory effects on DC if present. Therefore, despite the presence of IL-10 in 3/46 supernatants, high levels of GM-CSF and sCD40L (6) likely prevent the suppressive effects of IL-10 on the ability of DC to present mycobacterial antigens to pathogen-specific T cells.
Releasing negative regulation of the immune system may be a key approach to achieving more complete protection or even complete clearance of mycobacteria. Although the exact in vivo induction conditions and functional role(s) of CD46-induced suppressor T cells are currently not possible to create in a small animal model (see introduction), evidence for the physiological significance of the CD46 regulatory signaling pathway in humans is emerging. A recent report by Astier et al. established a connection between defects in the CD46-mediated induction of IL-10 in CD4+ T cells with multiple sclerosis (MS), which suggests that CD46 may indeed play a role in the prevention of autoimmunity in humans (3, 32). Similarly, dysfunctional CD46-mediated IL-10 production in T cells has now been connected with acute experimental autoimmune encephalomyelitis (EAE) in cynomolgus monkeys (which express CD46 naturally) (30).
CD46-activated CD4+ T cells may function in vivo preferentially at the host-environment interface. Mucosal homing surface markers and migration patterns have been detected upon CD46 costimulation (1). Furthermore, CD46 is a receptor for several human pathogens which may be encountered at mucosal surfaces (7, 29, 41). Utilization of CD46 as a receptor might allow pathogens to coopt its immunoregulatory properties to create a local anti-immunogenic milieu that would not only limit complement activation but also suppress T cell responses. For example, Bordetella pertussis was observed to induce clonal expansion of antigen-specific regulatory T cells (Treg) during infection of the respiratory tract (33). These Treg suppressed the protective local Th1 responses against the pathogenic bacteria in vivo and in vitro through production of IL-10. We have found that the binding of the streptococcal M protein to CD46 induces development of an adaptive Tr1-like phenotype (38), and our current results open up the possibility that CD46 signals might also be relevant during mycobacterial infection in the mucosa of the lung or upper respiratory tract.
In addition to the negative regulation of BCG-specific conventional and unconventional T cells through factors secreted by CD46-stimulated CD4+ T cells, we made another interesting observation: the blockage of “natural” CD46 engagement through C3b/C4b produced by activated immunocompetent cells in our system could have substantial effects on BCG-induced T cell responses. This effect was clearly dependent on the timing of the CD46 signaling blockade: inhibition of CD46 signals during the effector T cell induction phase blocked Th1 activation, while later blockade apparently reduced Treg/Tr1 induction and thereby supported Th1 responses in several donors. These findings are consistent with observations in the field that have been difficult to reconcile for a number of years. On one hand, CD3/CD46-activated T cells produce high levels of IL-10 and can potently suppress the proliferation of bystander T cells (4, 26). In contrast, CD46-activated CD4+ T cells also have many characteristics reminiscent of effector Th1 cells, including the following: (i) high proliferative capacity (26); (ii) high levels of TNF-α and IFN-γ production (4); (iii) a cytokine secretion profile (including secretion of GM-CSF and soluble CD40L) that supports the maturation of DC even in the presence of IL-10 (4); (iv) granzyme B and perforin synthesis with a concomitant capacity for contact-dependent cytotoxicity toward autologous activated T cells (17). In fact, Sanchez et al. (42) demonstrated that CD46 signaling induced strong Th1 induction in their system with only limited IL-10 secretion. In addition, we recently observed that the amount of recombinant IL-2 added to CD46-triggered cultures differentially regulates IFN-γ versus IL-10 production(6). It appears that the timing of CD46 blockade and the levels of IL-2 present in vitro can result in disparate and reciprocal effects on regulatory and effector T cell functions. Thus, CD46 signals in vivo might have divergent effects, depending on the timing of T cell activation and the biological context.
The suppression of γδ T cells by both 3/28 SN and 3/46 SN (Fig. 4 and 5) implies that a distinct mechanism is responsible for the suppression of these antimycobacterial γδ T cell responses. Understanding this mechanism may be crucial for designing vaccines that stimulate γδ T cell immunity. We have recently shown that γδ T cells provide helper functions for the induction of optimal adaptive αβ T cell responses (C. T. Spencer, G. Abate, and D. F. Hoft, submitted for publication). If the primary importance of mycobacterium-specific γ9δ2 T cells is to rapidly respond and inhibit early mycobacterial growth and then to facilitate definitive αβ T cell protective functions, it is feasible that αβ T cells may provide negative feedback to γδ T cells in order to maintain/reestablish immune homeostasis. This is one possible explanation for the suppression of γδ T cell responses by the supernatants from 3/28-activated CD4+ T cells. Further investigation is required to explore these relationships.
In summary, we have demonstrated that CD46-activated CD4+ T cells can suppress human mycobacterium-specific CD4+, CD8+, and γδ T cells through soluble mechanisms. The major suppressive effects were not reversed by neutralizing IL-10, indicating that additional regulatory mechanisms remain to be identified. We made rigorous attempts to elucidate the molecular mechanisms involved by adding neutralizing antibodies against IL-10, FasL, CD40L, and TGF-β, none of which reversed the suppression. Our data also argue against mechanisms of nutrient or growth factor depletion. We know the suppressive factor(s) is greater than 3 kDa, because the suppressor activities are maintained after concentration across a 3-kDa molecular mass cutoff membrane followed by reconstitution with fresh medium (Fig. 3). It also appears that endogenous ligands for CD46 are involved, based on our soluble CD46 blocking results. Furthermore, the factor(s) is relatively stable, retaining functional activity after a freeze-thaw cycle. On the other hand, in preliminary experiments, we found that heat inactivation of 3/46 supernatants reversed inhibitory effects, suggesting that proteins subject to heat-induced denaturation are involved. Future work is needed to further characterize the CD46-triggered inhibitory factors. Nevertheless, it is clear that CD46 can mediate potent suppression of antimycobacterial immunity in vitro. Now it will be key to determine whether regulatory signals triggered by CD46 are relevant in vivo during natural TB infection in humans. If so, it will be important to identify protocols for modulating this immune regulation. Specific blockade of immunoregulatory CD46 signals, as well as adaptive and natural regulatory T cell subsets, could lead to more effective vaccination or new immunotherapies for tuberculosis.
Acknowledgments
This work was supported by National Institutes of Health Vaccine Treatment and Evaluation Unit contract NO1-AI-25464 (D.F.H., coinvestigator); NIH grants R01 AI48391 (D.F.H.), U19 AI070489 (J.P.A. and C.K.), and R01 AI037618 (J.P.A.); grant P30 AR48335 Rheumatic Diseases Core Center (J.P.A.); and the Strategic Program for Asthma Research (http://www.spar-aaf.org; J.P.A.). The funders for this work had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
S.M.T., G.A., J.D.P., C.K., J.P.A., and D.F.H. conceived and designed the experiments. S.M.T., G.A., J.D.P., and C.K. performed the experiments. S.M.T., G.A., J.D.P., C.K., J.P.A., and D.F.H. analyzed the data. C.K., J.P.A., and D.F.H. contributed reagents, materials, and analysis tools. S.M.T., G.A., C.K., J.P.A., and D.F.H. wrote the manuscript.
We thank Anja Fuchs for contributions to experiments and edits to the manuscript.
We declare no competing interests.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 4 October 2010.
REFERENCES
- 1.Alford, S. K., G. D. Longmore, W. F. Stenson, and C. Kemper. 2008. CD46-induced immunomodulatory CD4+ T cells express the adhesion molecule and chemokine receptor pattern of intestinal T cells. J. Immunol. 181:2544-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astier, A., M. C. Trescol-Biemont, O. Azocar, B. Lamouille, and C. Rabourdin-Combe. 2000. Cutting edge: CD46, a new costimulatory molecule for T cells, that induces p120CBL and LAT phosphorylation. J. Immunol. 164:6091-6095. [DOI] [PubMed] [Google Scholar]
- 3.Astier, A. L., G. Meiffren, S. Freeman, and D. A. Hafler. 2006. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J. Clin. Invest. 116:3252-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barchet, W., J. D. Price, M. Cella, M. Colonna, S. K. MacMillan, J. P. Cobb, P. A. Thompson, K. M. Murphy, J. P. Atkinson, and C. Kemper. 2006. Complement-induced regulatory T cells suppress T-cell responses but allow for dendritic-cell maturation. Blood 107:1497-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bluestone, J. A., and A. K. Abbas. 2003. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 3:253-257. [DOI] [PubMed] [Google Scholar]
- 6.Cardone, J., F. G. Le, P. Vantourout, A. Roberts, A. Fuchs, I. Jackson, T. Suddason, G. Lord, J. P. Atkinson, A. Cope, A. Hayday, and C. Kemper. 2010. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat. Immunol. 11:862-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cattaneo, R. 2004. Four viruses, two bacteria, and one receptor: membrane cofactor protein (CD46) as a pathogens' magnet. J. Virol. 78:4385-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charles, M., and J. W. Pape. 2006. Tuberculosis and HIV: implications in the developing world. Curr. HIV AIDS Rep. 3:139-144. [DOI] [PubMed] [Google Scholar]
- 9.Cho, S., V. Mehra, S. Thoma-Uszynski, S. Stenger, N. Serbina, R. J. Mazzaccaro, J. L. Flynn, P. F. Barnes, S. Southwood, E. Celis, B. R. Bloom, R. L. Modlin, and A. Sette. 2000. Antimicrobial activity of MHC class I-restricted CD8+ T cells in human tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 97:12210-12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis: meta-analysis of the published literature. JAMA 271:698-702. [PubMed] [Google Scholar]
- 11.de Valliére, S., G. Abate, A. Blazevic, R. M. Heuertz, and D. F. Hoft. 2005. Enhancement of innate and cell-mediated immunity by antimycobacterial antibodies. Infect. Immun. 73:6711-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinal, M. A., S. J. Kim, P. G. Suarez, K. M. Kam, A. G. Khomenko, G. B. Migliori, J. Baez, A. Kochi, C. Dye, and M. C. Raviglione. 2000. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA 283:2537-2545. [DOI] [PubMed] [Google Scholar]
- 13.Flynn, J. L., M. M. Goldstein, K. J. Triebold, B. Koller, and B. R. Bloom. 1992. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. U. S. A. 89:12013-12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs, A., J. P. Atkinson, V. Fremeaux-Bacchi, and C. Kemper. 2009. CD46-induced human Treg enhance B-cell responses. Eur. J. Immunol. 39:3097-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Juarrero, M., O. C. Turner, J. Turner, P. Marietta, J. V. Brooks, and I. M. Orme. 2001. Temporal and spatial arrangement of lymphocytes within lung granulomas induced by aerosol infection with Mycobacterium tuberculosis. Infect. Immun. 69:1722-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grossman, W. J., J. W. Verbsky, W. Barchet, M. Colonna, J. P. Atkinson, and T. J. Ley. 2004. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity 21:589-601. [DOI] [PubMed] [Google Scholar]
- 17.Grossman, W. J., J. W. Verbsky, B. L. Tollefsen, C. Kemper, J. P. Atkinson, and T. J. Ley. 2004. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood 104:2840-2848. [DOI] [PubMed] [Google Scholar]
- 18.Hoebe, K., E. Janssen, and B. Beutler. 2004. The interface between innate and adaptive immunity. Nat. Immunol. 5:971-974. [DOI] [PubMed] [Google Scholar]
- 19.Hoft, D. F. 2008. Tuberculosis vaccine development: goals, immunological design, and evaluation. Lancet 372:164-175. [DOI] [PubMed] [Google Scholar]
- 20.Hoft, D. F., A. Blazevic, G. Abate, W. A. Hanekom, G. Kaplan, J. H. Soler, F. Weichold, L. Geiter, J. C. Sadoff, and M. A. Horwitz. 2008. A new recombinant bacille Calmette-Guérin vaccine safely induces significantly enhanced tuberculosis-specific immunity in human volunteers. J. Infect. Dis. 198:1491-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoft, D. F., R. Brown, and S. Roodman. 1998. Bacille Calmette-Guérin vaccination enhances human γδ T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J. Immunol. 161:1045-1054. [PubMed] [Google Scholar]
- 22.Ito, T., S. Hanabuchi, Y. H. Wang, W. R. Park, K. Arima, L. Bover, F. X. Qin, M. Gilliet, and Y. J. Liu. 2008. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity 28:870-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeevan, A., C. T. McFarland, T. Yoshimura, T. Skwor, H. Cho, T. Lasco, and D. N. McMurray. 2006. Production and characterization of guinea pig recombinant gamma interferon and its effect on macrophage activation. Infect. Immun. 74:213-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufmann, S. H. 2001. How can immunology contribute to the control of tuberculosis? Nat. Rev. Immunol. 1:20-30. [DOI] [PubMed] [Google Scholar]
- 25.Kemper, C., and J. P. Atkinson. 2007. T-cell regulation: with complements from innate immunity. Nat. Rev. Immunol. 7:9-18. [DOI] [PubMed] [Google Scholar]
- 26.Kemper, C., A. C. Chan, J. M. Green, K. A. Brett, K. M. Murphy, and J. P. Atkinson. 2003. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature 421:388-392. [DOI] [PubMed] [Google Scholar]
- 27.Liszewski, M. K., C. Kemper, J. D. Price, and J. P. Atkinson. 2005. Emerging roles and new functions of CD46. Springer Semin. Immunopathol. 27:345-358. [DOI] [PubMed] [Google Scholar]
- 28.Liszewski, M. K., M. Leung, W. Cui, V. B. Subramanian, J. Parkinson, P. N. Barlow, M. Manchester, and J. P. Atkinson. 2000. Dissecting sites important for complement regulatory activity in membrane cofactor protein (MCP; CD46). J. Biol. Chem. 275:37692-37701. [DOI] [PubMed] [Google Scholar]
- 29.Liszewski, M. K., T. W. Post, and J. P. Atkinson. 1991. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu. Rev. Immunol. 9:431-455. [DOI] [PubMed] [Google Scholar]
- 30.Ma, A., Z. Xiong, Y. Hu, S. Qi, L. Song, H. Dun, L. Zhang, D. Lou, P. Yang, Z. Zhao, X. Wang, D. Zhang, P. Daloze, and H. Chen. 2009. Dysfunction of IL-10-producing type 1 regulatory T cells and CD4(+)CD25(+) regulatory T cells in a mimic model of human multiple sclerosis in cynomolgus monkeys. Int. Immunopharmacol. 9:599-608. [DOI] [PubMed] [Google Scholar]
- 31.Marie, J. C., A. L. Astier, P. Rivailler, C. Rabourdin-Combe, T. F. Wild, and B. Horvat. 2002. Linking innate and acquired immunity: divergent role of CD46 cytoplasmic domains in T cell induced inflammation. Nat. Immunol. 3:659-666. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Forero, I., R. Garcia-Munoz, S. Martinez-Pasamar, S. Inoges, A. Lopez-Diaz de Cerio, R. Palacios, J. Sepulcre, B. Moreno, Z. Gonzalez, B. Fernandez-Diez, I. Melero, M. Bendandi, and P. Villoslada. 2008. IL-10 suppressor activity and ex vivo Tr1 cell function are impaired in multiple sclerosis. Eur. J. Immunol. 38:576-586. [DOI] [PubMed] [Google Scholar]
- 33.McGuirk, P., C. McCann, and K. H. Mills. 2002. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J. Exp. Med. 195:221-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore, K. W., M. R. de Waal, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 35.O'Garra, A., and P. Vieira. 2004. Regulatory T cells and mechanisms of immune system control. Nat. Med. 10:801-805. [DOI] [PubMed] [Google Scholar]
- 36.Olin, M. R., C. K. Hwa, J. Lee, and T. W. Molitor. 2005. γδ T lymphocyte cytotoxic activity against Mycobacterium bovis analyzed by flow cytometry. J. Immunol. Methods 297:1-11. [DOI] [PubMed] [Google Scholar]
- 37.Pandiyan, P., L. Zheng, S. Ishihara, J. Reed, and M. J. Lenardo. 2007. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat. Immunol. 8:1353-1362. [DOI] [PubMed] [Google Scholar]
- 38.Price, J. D., J. Schaumburg, C. Sandin, J. P. Atkinson, G. Lindahl, and C. Kemper. 2005. Induction of a regulatory phenotype in human CD4+ T cells by streptococcal M protein. J. Immunol. 175:677-684. [DOI] [PubMed] [Google Scholar]
- 39.Riley-Vargas, R. C., D. B. Gill, C. Kemper, M. K. Liszewski, and J. P. Atkinson. 2004. CD46: expanding beyond complement regulation. Trends Immunol. 25:496-503. [DOI] [PubMed] [Google Scholar]
- 40.Riley-Vargas, R. C., S. Lanzendorf, and J. P. Atkinson. 2005. Targeted and restricted complement activation on acrosome-reacted spermatozoa. J. Clin. Invest. 115:1241-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell, S. 2004. CD46: a complement regulator and pathogen receptor that mediates links between innate and acquired immune function. Tissue Antigens 64:111-118. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez, A., M. J. Feito, and J. M. Rojo. 2004. CD46-mediated costimulation induces a Th1-biased response and enhances early TCR/CD3 signaling in human CD4+ T lymphocytes. Eur. J. Immunol. 34:2439-2448. [DOI] [PubMed] [Google Scholar]
- 43.Sharma, S. K., and A. Mohan. 2006. Multidrug-resistant tuberculosis: a menace that threatens to destabilize tuberculosis control. Chest 130:261-272. [DOI] [PubMed] [Google Scholar]
- 44.Spencer, C. T., G. Abate, A. Blazevic, and D. F. Hoft. 2008. Only a subset of phosphoantigen-responsive γ9δ2 T cells mediate protective tuberculosis immunity. J. Immunol. 181:4471-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strainic, M. G., J. Liu, D. Huang, F. An, P. N. Lalli, N. Muqim, V. S. Shapiro, G. R. Dubyak, P. S. Heeger, and M. E. Medof. 2008. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity 28:425-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thurner, B., C. Roder, D. Dieckmann, M. Heuer, M. Kruse, A. Glaser, P. Keikavoussi, E. Kampgen, A. Bender, and G. Schuler. 1999. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J. Immunol. Methods 223:1-15. [DOI] [PubMed] [Google Scholar]
- 47.Tsujimura, A., K. Shida, M. Kitamura, M. Nomura, J. Takeda, H. Tanaka, M. Matsumoto, K. Matsumiya, A. Okuyama, Y. Nishimune, M. Okabe, and T. Seya. 1998. Molecular cloning of a murine homologue of membrane cofactor protein (CD46): preferential expression in testicular germ cells. Biochem. J. 330:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tully, G., C. Kortsik, H. Hohn, I. Zehbe, W. E. Hitzler, C. Neukirch, K. Freitag, K. Kayser, and M. J. Maeurer. 2005. Highly focused T cell responses in latent human pulmonary Mycobacterium tuberculosis infection. J. Immunol. 174:2174-2184. [DOI] [PubMed] [Google Scholar]
- 49.van Helden, P. D., P. R. Donald, T. C. Victor, H. S. Schaaf, E. G. Hoal, G. Walzl, and R. M. Warren. 2006. Antimicrobial resistance in tuberculosis: an international perspective. Expert Rev. Anti Infect. Ther. 4:759-766. [DOI] [PubMed] [Google Scholar]
- 50.van Stipdonk, M. J., E. E. Lemmens, and S. P. Schoenberger. 2001. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2:423-429. [DOI] [PubMed] [Google Scholar]
- 51.Wang, G., M. K. Liszewski, A. C. Chan, and J. P. Atkinson. 2000. Membrane cofactor protein (MCP; CD46): isoform-specific tyrosine phosphorylation. J. Immunol. 164:1839-1846. [DOI] [PubMed] [Google Scholar]
- 52.White, J., P. Lukacik, D. Esser, M. Steward, N. Giddings, J. R. Bright, S. J. Fritchley, B. P. Morgan, S. M. Lea, G. P. Smith, and R. A. Smith. 2004. Biological activity, membrane-targeting modification, and crystallization of soluble human decay accelerating factor expressed in E. coli. Protein Sci. 13:2406-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Worku, S., and D. F. Hoft. 2003. Differential effects of control and antigen-specific T cells on intracellular mycobacterial growth. Infect. Immun. 71:1763-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Worku, S., and D. F. Hoft. 2000. In vitro measurement of protective mycobacterial immunity: antigen-specific expansion of T cells capable of inhibiting intracellular growth of bacille Calmette-Guerin. Clin. Infect. Dis. 30(Suppl. 3):S257-S261. [DOI] [PubMed] [Google Scholar]
- 55.Zaffran, Y., O. Destaing, A. Roux, S. Ory, T. Nheu, P. Jurdic, C. Rabourdin-Combe, and A. L. Astier. 2001. CD46/CD3 costimulation induces morphological changes of human T cells and activation of Vav, Rac, and extracellular signal-regulated kinase mitogen-activated protein kinase. J. Immunol. 167:6780-6785. [DOI] [PubMed] [Google Scholar]