Abstract
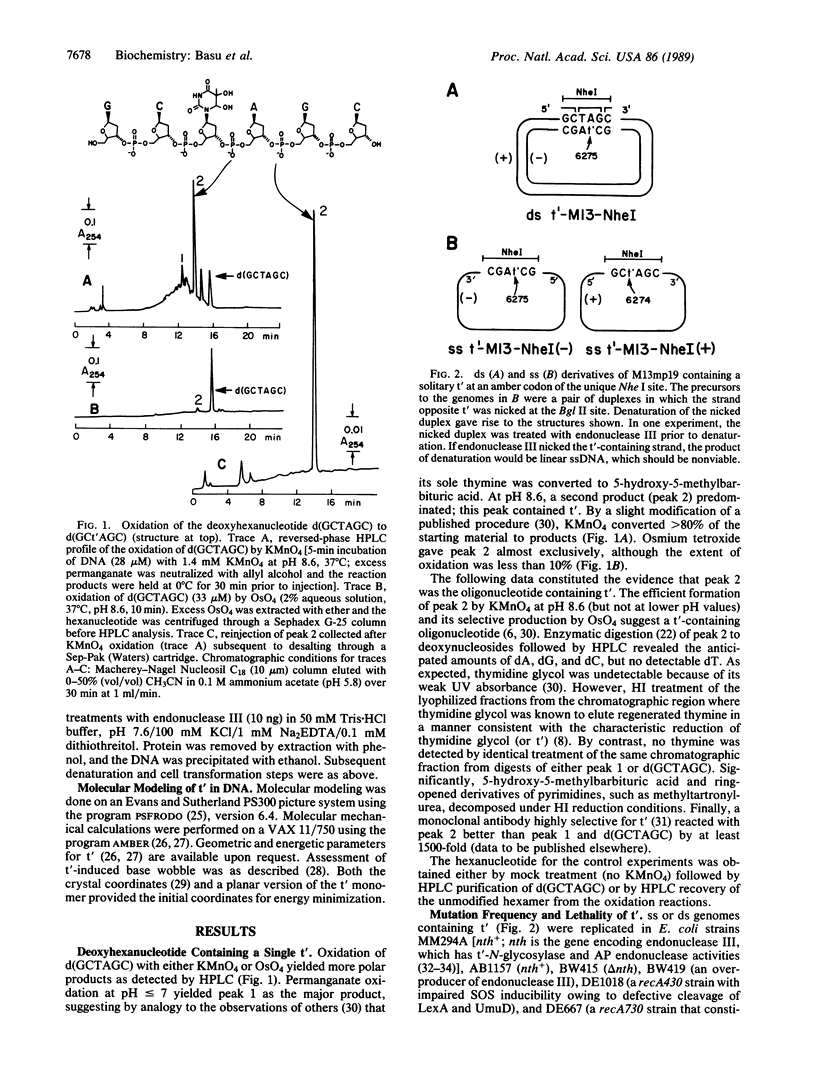
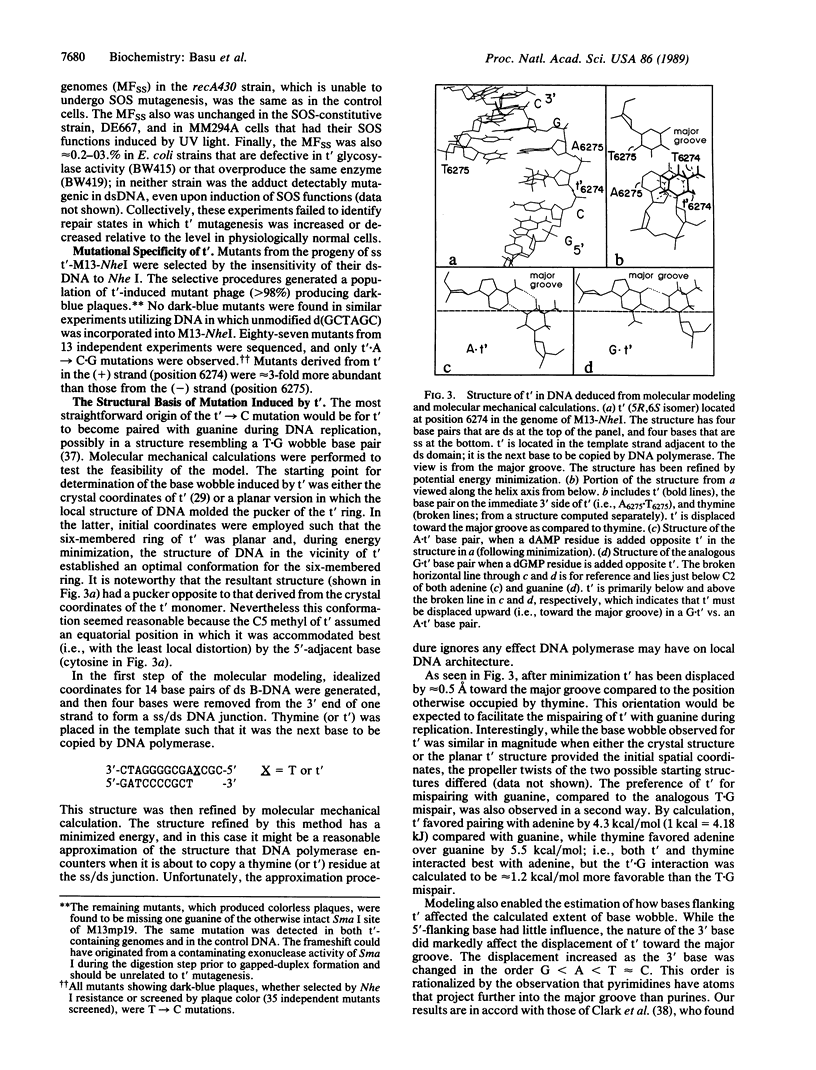
The mutational specificity and genetic requirements for mutagenesis by 5,6-dihydroxy-5,6-dihydrothymine (thymine glycol), one of the principal DNA lesions induced by oxidation and ionizing radiation, has been investigated in Escherichia coli. Thymine glycol was positioned at a unique site in the single-stranded genome of a bacteriophage M13mp19 derivative. Replication of the genome in E. coli yielded targeted mutations at a frequency of 0.3%; the mutations were exclusively T----C. Mutagenesis was independent of SOS and nth (nth encodes endonuclease III, a thymine glycol repair enzyme). The adduct was not detectably mutagenic in duplex DNA. A chemical rationalization for the mutation observed for thymine glycol was developed by applying molecular modeling and molecular mechanical calculations to the same DNA sequence studied in vivo. Modeling suggested that the 5R,6S isomer of cis-thymine glycol, when not base paired, was displaced laterally by approximately 0.5 A toward the major groove in comparison to the position that thymine would otherwise occupy. This perturbation of DNA structure should increase the likelihood of a guanine.thymine glycol wobble base pair during replication, which would explain the mutational specificity of the base observed in the genetic experiments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Ayaki H., Higo K., Yamamoto O. Specificity of ionizing radiation-induced mutagenesis in the lac region of single-stranded phage M13 mp10 DNA. Nucleic Acids Res. 1986 Jun 25;14(12):5013–5018. doi: 10.1093/nar/14.12.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A. K., Niedernhofer L. J., Essigmann J. M. Deoxyhexanucleotide containing a vinyl chloride induced DNA lesion, 1,N6-ethenoadenine: synthesis, physical characterization, and incorporation into a duplex bacteriophage M13 genome as part of an amber codon. Biochemistry. 1987 Sep 8;26(18):5626–5635. doi: 10.1021/bi00392a007. [DOI] [PubMed] [Google Scholar]
- Beer M., Stern S., Carmalt D., Mohlhenrich K. H. Determination of base sequence in nucleic acids with the electron microscope. V. The thymine-specific reactions of osmium tetroxide with deoxyribonucleic acid and its components. Biochemistry. 1966 Jul;5(7):2283–2288. doi: 10.1021/bi00871a017. [DOI] [PubMed] [Google Scholar]
- Brandenburger A., Godson G. N., Radman M., Glickman B. W., van Sluis C. A., Doubleday O. P. Radiation-induced base substitution mutagenesis in single-stranded DNA phage M13. Nature. 1981 Nov 12;294(5837):180–182. doi: 10.1038/294180a0. [DOI] [PubMed] [Google Scholar]
- Breimer L. H., Lindahl T. DNA glycosylase activities for thymine residues damaged by ring saturation, fragmentation, or ring contraction are functions of endonuclease III in Escherichia coli. J Biol Chem. 1984 May 10;259(9):5543–5548. [PubMed] [Google Scholar]
- Breimer L. H., Lindahl T. Thymine lesions produced by ionizing radiation in double-stranded DNA. Biochemistry. 1985 Jul 16;24(15):4018–4022. doi: 10.1021/bi00336a032. [DOI] [PubMed] [Google Scholar]
- Brown T., Kennard O., Kneale G., Rabinovich D. High-resolution structure of a DNA helix containing mismatched base pairs. Nature. 1985 Jun 13;315(6020):604–606. doi: 10.1038/315604a0. [DOI] [PubMed] [Google Scholar]
- Cathcart R., Schwiers E., Saul R. L., Ames B. N. Thymine glycol and thymidine glycol in human and rat urine: a possible assay for oxidative DNA damage. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5633–5637. doi: 10.1073/pnas.81.18.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. M., Beardsley G. P. Thymine glycol lesions terminate chain elongation by DNA polymerase I in vitro. Nucleic Acids Res. 1986 Jan 24;14(2):737–749. doi: 10.1093/nar/14.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. M., Pattabiraman N., Jarvis W., Beardsley G. P. Modeling and molecular mechanical studies of the cis-thymine glycol radiation damage lesion in DNA. Biochemistry. 1987 Aug 25;26(17):5404–5409. doi: 10.1021/bi00391a028. [DOI] [PubMed] [Google Scholar]
- Demple B., Linn S. DNA N-glycosylases and UV repair. Nature. 1980 Sep 18;287(5779):203–208. doi: 10.1038/287203a0. [DOI] [PubMed] [Google Scholar]
- Ennis D. G., Fisher B., Edmiston S., Mount D. W. Dual role for Escherichia coli RecA protein in SOS mutagenesis. Proc Natl Acad Sci U S A. 1985 May;82(10):3325–3329. doi: 10.1073/pnas.82.10.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel K., Chrzan K., Troll W., Teebor G. W., Steinberg J. J. Radiation-like modification of bases in DNA exposed to tumor promoter-activated polymorphonuclear leukocytes. Cancer Res. 1986 Nov;46(11):5533–5540. [PubMed] [Google Scholar]
- Glickman B. W., Rietveld K., Aaron C. S. gamma-Ray induced mutational spectrum in the lacI gene of Escherichia coli: comparison of induced and spontaneous spectra at the molecular level. Mutat Res. 1980 Jan;69(1):1–12. doi: 10.1016/0027-5107(80)90171-2. [DOI] [PubMed] [Google Scholar]
- Green C. L., Loechler E. L., Fowler K. W., Essigmann J. M. Construction and characterization of extrachromosomal probes for mutagenesis by carcinogens: site-specific incorporation of O6-methylguanine into viral and plasmid genomes. Proc Natl Acad Sci U S A. 1984 Jan;81(1):13–17. doi: 10.1073/pnas.81.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosovsky A. J., de Boer J. G., de Jong P. J., Drobetsky E. A., Glickman B. W. Base substitutions, frameshifts, and small deletions constitute ionizing radiation-induced point mutations in mammalian cells. Proc Natl Acad Sci U S A. 1988 Jan;85(1):185–188. doi: 10.1073/pnas.85.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan P. V., Achey P. M., Cerutti P. A. Biological effect of thymine ring saturation in coliphage phiX174-DNA. Radiat Res. 1977 Feb;69(2):375–378. [PubMed] [Google Scholar]
- Hayes R. C., LeClerc J. E. Sequence dependence for bypass of thymine glycols in DNA by DNA polymerase I. Nucleic Acids Res. 1986 Jan 24;14(2):1045–1061. doi: 10.1093/nar/14.2.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes R. C., Petrullo L. A., Huang H. M., Wallace S. S., LeClerc J. E. Oxidative damage in DNA. Lack of mutagenicity by thymine glycol lesions. J Mol Biol. 1988 May 20;201(2):239–246. doi: 10.1016/0022-2836(88)90135-0. [DOI] [PubMed] [Google Scholar]
- Hutchinson F. Chemical changes induced in DNA by ionizing radiation. Prog Nucleic Acid Res Mol Biol. 1985;32:115–154. doi: 10.1016/s0079-6603(08)60347-5. [DOI] [PubMed] [Google Scholar]
- Ide H., Kow Y. W., Wallace S. S. Thymine glycols and urea residues in M13 DNA constitute replicative blocks in vitro. Nucleic Acids Res. 1985 Nov 25;13(22):8035–8052. doi: 10.1093/nar/13.22.8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida S., Hayatsu H. The permanganate oxidation of thymine. Biochim Biophys Acta. 1970 Jul 16;213(1):1–13. doi: 10.1016/0005-2787(70)90002-x. [DOI] [PubMed] [Google Scholar]
- Kato T., Oda Y., Glickman B. W. Randomness of base substitution mutations induced in the lacI gene of Escherichia coli by ionizing radiation. Radiat Res. 1985 Feb;101(2):402–406. [PubMed] [Google Scholar]
- Koffel-Schwartz N., Maenhaut-Michel G., Fuchs R. P. Specific strand loss in N-2-acetylaminofluorene-modified DNA. J Mol Biol. 1987 Feb 20;193(4):651–659. doi: 10.1016/0022-2836(87)90348-2. [DOI] [PubMed] [Google Scholar]
- Kow Y. W., Wallace S. S. Mechanism of action of Escherichia coli endonuclease III. Biochemistry. 1987 Dec 15;26(25):8200–8206. doi: 10.1021/bi00399a027. [DOI] [PubMed] [Google Scholar]
- Leadon S. A., Hanawalt P. C. Monoclonal antibody to DNA containing thymine glycol. Mutat Res. 1983 Aug;112(4):191–200. doi: 10.1016/0167-8817(83)90006-8. [DOI] [PubMed] [Google Scholar]
- Loechler E. L. Adduct-induced base-shifts: a mechanism by which the adducts of bulky carcinogens might induce mutations. Biopolymers. 1989 May;28(5):909–927. doi: 10.1002/bip.360280502. [DOI] [PubMed] [Google Scholar]
- Loechler E. L., Green C. L., Essigmann J. M. In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6271–6275. doi: 10.1073/pnas.81.20.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran E., Wallace S. S. The role of specific DNA base damages in the X-ray-induced inactivation of bacteriophage PM2. Mutat Res. 1985 Nov;146(3):229–241. doi: 10.1016/0167-8817(85)90063-x. [DOI] [PubMed] [Google Scholar]
- Rouet P., Essigmann J. M. Possible role for thymine glycol in the selective inhibition of DNA synthesis on oxidized DNA templates. Cancer Res. 1985 Dec;45(12 Pt 1):6113–6118. [PubMed] [Google Scholar]
- Storz G., Christman M. F., Sies H., Ames B. N. Spontaneous mutagenesis and oxidative damage to DNA in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8917–8921. doi: 10.1073/pnas.84.24.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoule R., Bonicel A., Bert C., Cadet J., Polverelli M. Identification of radioproducts resulting from the breakage of thymine moiety by gamma irradiation of E. coli DNA in an aerated aqueous solution. Radiat Res. 1974 Jan;57(1):46–58. [PubMed] [Google Scholar]



