Abstract
Type III secretion systems (T3SSs) are key determinants of virulence in many Gram-negative bacterial pathogens. Upon cell contact, they inject effector proteins directly into eukaryotic cells through a needle protruding from the bacterial surface. Host cell sensing occurs through a distal needle “tip complex,” but how this occurs is not understood. The tip complex of quiescent needles is composed of IpaD, which is topped by IpaB. Physical contact with host cells initiates secretion and leads to assembly of a pore, formed by IpaB and IpaC, in the host cell membrane, through which other virulence effector proteins may be translocated. IpaB is required for regulation of secretion and may be the host cell sensor. It binds needles via its extreme C-terminal coiled coil, thereby likely positioning a large domain containing its hydrophobic regions at the distal tips of needles. In this study, we used short deletion mutants within this domain to search for regions of IpaB involved in secretion regulation. This identified two regions, amino acids 227 to 236 and 297 to 306, the presence of which are required for maintenance of IpaB at the needle tip, secretion regulation, and normal pore formation but not invasion. We therefore propose that removal of either of these regions leads to an inability to block secretion prior to reception of the activation signal and/or a defect in host cell sensing.
Shigella flexneri is the causative agent of shigellosis. There is still no vaccine available for this disease, which is responsible for 1.1 million deaths each year, especially among children under 5 years in developing countries (19). Shigellosis is characterized by the destruction of the colonic epithelium provoked by this Gram-negative bacterium's invasion of the mucosa and the inflammatory response that is induced to control the infection (36).
A type III secretion system (T3SS), encoded by a 31-kb fragment of the S. flexneri large virulence plasmid, is necessary and sufficient to trigger entry into epithelial cells and apoptosis of macrophages (9, 36). T3SSs are protein transport devices used by many Gram-negative bacteria to inject effector proteins, involved in modulating eukaryotic cell function to aid infection, directly into host cells upon contact (10, 14). Recent studies suggest that Shigella delivers the following two different sets of effectors: (i) early effectors, such as IpaA and IpgD, which are involved in entry into polarized epithelial cells in the early stage of infection, and (ii) late effectors, such as IpaH family proteins and VirA, which enable the bacteria to survive intracellularly, promote intra- and intercellular movement, and modulate the host inflammatory response (27, 32). Structurally, the S. flexneri T3S machinery is composed of a basal body anchored in the bacterial membranes and an external needle through which effector proteins are secreted (6).
After needle completion, three proteins known as the “translocators,” IpaD, IpaB and IpaC, are themselves exported by the T3S machinery and used for the delivery of effectors from the bacterium to the host cell cytoplasm by forming the translocon, a pore in the host cell membrane (5). In addition to their roles as translocators, membrane-inserted IpaC and IpaB play effector roles in mediating the actin rearrangements required for bacterial uptake and controlling host cell replication and survival, respectively (36). IpaD and IpaB preassociate with mature needle tips to form the “tip complex” (12, 13, 18, 26, 28, 37, 38). While others detected IpaB associated with the needle tip only after growth of bacteria in the presence of bile salts (28, 37), we found that IpaB is associated with the needle tip even upon growth in their absence (34, 38). We and others have also found that IpaC binds to needle tips upon activation of secretion (12, 38). Though pore formation requires the hydrophilic IpaD protein, only the hydrophobic IpaB and IpaC proteins are inserted in the host cell membrane to form a 25-Å pore, and IpaB is probably inserted first (5, 38). The formation of a pore in the host cell membrane is also the cause of contact-dependent hemolysis induced when Shigella is centrifuged onto sheep red blood cells (5).
The mechanism by which host cell sensing, occurring at the tip of the needle, is transmitted to the base of the secretion apparatus is not well understood. However, the interaction between IpaB, IpaD, and the needle tip seems key to this event (11, 18, 38). Structural and biochemical evidence suggests that the S. flexneri tip complex consists of four IpaD and one IpaB molecules (8, 18, 38). Furthermore, it has been proposed that the quiescent needle tip has an “off” conformation and turns “on” upon host cell sensing, which leads to a conformational change in the pentamer to allow export and assembly of a pore, formed by IpaB and IpaC, in the host cell membrane, through which other virulence effector proteins may be translocated (18, 34, 38).
IpaB's hydrophobic properties and predicted large-scale conformational changes as it moves from the bacterial cytoplasm into the host membrane mean that the atomic structure for any conformation of full-length IpaB will be hard to obtain (20). However, the protein is predicted to contain several conserved secondary structures (Fig. 1), as follows: a chaperone binding domain (amino acids [aa] 51 to 72) (20), two long coiled-coil regions (aa 110 to 170 and aa 514 to 580) (17), a putative amphipathic α-helical domain (aa 240 to 280) that is important for proper folding and stability of intrabacterial IpaB (15), two hydrophobic transmembrane domains (aa 313 to 333 and aa 399 to 419), which are supposed to penetrate the host membrane bilayer, allowing the intervening hydrophilic region to cross the membrane (17), and a IpaC binding domain (aa 367 to 458) (29). As we have shown that the extreme C terminus of IpaB is involved in binding it to needles, this model positions the predicted large C-terminal globular domain of IpaB, which lies between the two coiled-coil-forming regions, at the most distal point from the needle. This is the likely point of contact with the host cell and may also be the site of action of the small amphipathic dye molecule Congo red (CR), an artificial inducer of the Shigella T3SS (3, 38).
FIG. 1.
(A) Schematic representation of full-length Shigella flexneri IpaB. Predicted secondary structures are shown, as follows: the IpaC (chaperone) binding domain (gridded region, aa 51 to 72), coiled-coil domains (hatched regions, aa 110 to 170 and aa 514 to 580), amphipathic α-helix domain (dotted region, aa 240 to 280), transmembrane domains (filled regions, aa 313 to 333 and 399 to 419), and IpaC binding domain (dash-outlined region, aa 367 to 458). (B) Schematic representation of mutations generated in IpaB. Seven internal deletions of either 10 or 8 residues were generated, and the starting and ending amino acids deleted in each truncate are shown in the boxes.
Guichon and colleagues previously investigated the regions of IpaB that are necessary for invasion, phagosome escape, caspase-1 binding, and cytotoxicity (15). In this study, we regenerated some of same ipaB mutants they used using a similar strategy, repeated a few experiments, including protein expression and invasion, and performed mostly other, totally different functional assays to delineate the regions of IpaB involved in regulating secretion and sensing host cells. We found that small deletions within ipaB affect the needle tip composition, secretion regulation, contact-dependent hemolysis, translocon insertion in red blood cell (RBC) membranes, and consequently, Shigella's ability to adhere to and invade HeLa cells. This analysis not only is complementary to the previous work in generating a better understanding of the phenotype of these ipaB mutants but also has allowed us to identify two regions within the large globular C-terminal domain of IpaB that are directly or indirectly involved in regulating secretion.
MATERIALS AND METHODS
Bacterial strains and cell culture.
All bacterial strains used in this study are listed in Table 1. Shigella flexneri strains were maintained and selected on CR agar plates (23) and grown at 37°C in Trypticase soy broth (Becton Dickinson) supplemented with antibiotics when necessary (100 μg of ampicillin ml−1, 50 μg of kanamycin ml−1, 20 μg of chloramphenicol ml−1). There are 3 × 108 bacteria in 1 ml of broth at an optical density at 600 nm (OD600) of 1. HeLa cells were cultured at 37°C with 5% CO2 in Dulbecco modified Eagle medium (DMEM; Sigma) supplemented with 10% decomplemented fetal calf serum (Gibco) and 50 μg/ml each of penicillin and streptomycin.
TABLE 1.
Shigella flexneri strains used in this study
| Strain | Genotype (strain; plasmid[s]) | Reference |
|---|---|---|
| WT | Wild-type M90T, serotype 5a | 35 |
| ΔipaD | SF622 | 24 |
| ΔmxiD | SF401 | 2 |
| ΔmxiH | SH116 | 6 |
| ΔipaB | SF620 | 24 |
| ΔipaB complemented | SF620; pDR1 | 34 |
| ipaBΔ167-176 | SF620; pUC19B167 | This study |
| ipaBΔ227-236 | SF620; pUC19B227 | This study |
| ipaBΔ257-266 | SF620; pUC19B257 | This study |
| ipaBΔ297-306 | SF620; pUC19B297 | This study |
| ipaBΔ307-316 | SF620; pUC19B307 | This study |
| ipaBΔ325-334 | SF620; pUC19B325 | This study |
| ipaBΔ410-417 | SF620; pUC19B410 | This study |
| WT mxiH | Wild-type M90T; pACT3mxiH | This study |
| ΔipaD mxiH | SF622; pACT3mxiH | This study |
| ΔipaB mxiH | SF620; pACT3mxiH | This study |
| ΔipaB-complemented mxiH | SF620; pDR1, pACT3mxiH | This study |
| ipaBΔ167-176 mxiH | SF620; pUC19B167, pACT3mxiH | This study |
| ipaBΔ227-236 mxiH | SF620; pUC19B227, pACT3mxiH | This study |
| ipaBΔ257-266 mxiH | SF620; pUC19B257, pACT3mxiH | This study |
| ipaBΔ297-306 mxiH | SF620; pUC19B297, pACT3mxiH | This study |
| ipaBΔ307-316 mxiH | SF620; pUC19B307, pACT3mxiH | This study |
| ipaBΔ325-334 mxiH | SF620; pUC19B325, pACT3mxiH | This study |
| ipaBΔ410-417 mxiH | SF620; pUC19B410, pACT3mxiH | This study |
Cloning and mutagenesis of ipaB.
We initiated this study upon receiving a kind gift of all the IpaB deletion mutants generated by Guichon et al. (15). However, further work in our laboratory showed that every one of these strains, including the ΔipaB-complemented strain, contained a plasmid encoding IpaB with three nonconservative substitutions (F224S, T255S, and V416A) in addition to the desired deletions, where applicable. As this ΔipaB-complemented strain did not fully complement the IpaB mutant in terms of restoring wild-type (WT) secretion patterns, we eventually regenerated a set of identical IpaB deletion mutants without the additional substitutions. The selection of mutants was based on our preliminary data and made to cover all observed phenotypes. We used a two-step PCR strategy to regenerate the selected internal deletions in ipaB as previously described (15). In the first step, 5′ and 3′ fragments of ipaB were amplified from pDR1 (34) by using the primers listed in Table 2. In the second step, using the primer pair ipaB-F and ipaB-R, the mixture of 5′ and 3′ fragments was used as the template to amplify each ipaB gene with an internal deletion, which was then cloned into pUC19 by ligation to the HindIII and PstI sites of the polylinker, and the resultant plasmid was transformed into the ΔipaB strain (24) to obtain ipaB internal deletion mutants (Table 1). All deletions were confirmed by DNA sequencing of the entire ipaB gene (Eurofins MWG Operon). However, as we reconstructed the ipaB mutants in the manner previously described (15), we did not realize initially that 8 amino acids (MTMITPSL, confirmed by N-terminal sequencing) are added at the N terminus of IpaB because it has been cloned in frame with lacZ in pUC19. As such an ipaB gene totally restored a wild-type phenotype in the ΔipaB-complemented strain, we consider that the 8 additional amino acids at the N terminus of ipaB do not detectably alter its function. These 8 additional amino acids are also present in the ipaB constructs made by Roehrich et al. (34).
TABLE 2.
Primer sequences used in this study
| Primer | Sequencea |
|---|---|
| ipaB-F | 5′-GGGGAAGCTTGATGCATAATGTAAGCACCACAAC-3′ |
| ipaB-R | 5′-GGGGCTGCAGTCCTTATTTGTATCAAGCAGTAGT-3′ |
| d167-176b | 5′-CCAAATTCAAACAAGATTATCCAAATTAAGCCGGGAAGAAATAC-3′ |
| d227-236b | 5′-GAAATAGACTCTTTTTCTGCATCAACCCAGCAGAAATCATTAAC-3′ |
| d257-266b | 5′-CTCAATTGATGGCAACCTTTTCTTTAAAAAATGATCTGGC-3′ |
| d297-306b | 5′-CTGATGAGTATGCTGCTGAAATGGGTTGTGTTGGGAAAATAC-3′ |
| d307-316b | 5′-GCAGAAGAACTCAACAGAGTACTTTTAACTATCGTTAGT-3′ |
| d325-334b | 5′-CTATCGTTAGTGTTGTTGCACTGGCAGCTGTTGGTTTAGC-3′ |
| d410-417b | 5′-GGGGCAATCGCAGGCGCTCTCGTAGCCACTGTTGG-3′ |
Underlined letters represent non-ipaB sequences and restriction endonuclease sites generated to facilitate cloning mutants.
These primers were paired with ipaB-R to amplify the 3′ fragment of ipaB, and their reverse complementary sequences, which are not listed, were paired with ipaB-F to amplify the 5′ fragment of ipaB.
Protein analysis.
Overnight leakage of the Ipa proteins and CR-induced protein secretion were performed as described (3, 31). Antibodies used in this study include the mouse monoclonal antibodies against IpaC K24 (33), IpaB H16 (4), and MxiG (39) and the rabbit polyclonal sera against IpaB (4), IpaD (24), IpaH (22), and MxiH (21). Since IpaB H16 could hardly detect IpaB with a deletion of aa 167 to 177, where its epitope is therefore likely to be localized (4), a polyclonal antiserum against IpaB (4) was used, except in immunofluorescence microscopy. Horseradish peroxidase-labeled goat anti-rabbit or goat anti-mouse antibodies were used as secondary antibodies and visualized by enhanced chemiluminescence (Amersham).
Adhesion assay.
The evaluation of S. flexneri adhesion to HeLa cells was performed as described by Roehrich et al. (34). Briefly, HeLa cells seeded 1 day previously in a 24-well culture plate containing coverslips were infected with 4.5 × 107 exponentially grown bacteria at a multiplicity of infection (MOI) of 100, centrifuged, and incubated for 30 min at 25°C. The coverslips were then washed, fixed, and stained with Giemsa (Sigma). For each duplicated sample, 6 randomly chosen images were taken using a Leica DM IRB inverted microscope at 1,000× magnification. Both the numbers of HeLa cells and cell-associated bacteria in each image were counted, and the average number of bacteria per 100 HeLa cells was determined from 3 independent assays.
Invasion assay.
HeLa cell invasion was assessed with a gentamicin protection assay as previously described (1). Briefly, HeLa cells were infected with 4.5 × 107 exponentially grown bacteria at an MOI of 100 and incubated for 30 min at 37°C. After being washed, cells were further incubated in DMEM supplemented with 100 μg ml−1 gentamicin (Gibco). After 1.75 h at 37°C, the gentamicin solution was aspirated, and 0.1% (vol/vol) Triton X-100 (Sigma) was added to detach and lyse HeLa cells. Serial dilutions of the cell lysate were plated on Luria-Bertani agar plates, and the number of colonies was counted the next day. Invasion percentages are relative to that of the wild type (set to 100%), and normalization relative to adhesion was obtained by dividing by corresponding adhesion level. All samples were tested in duplicate, and data presented are the average results from three independent experiments.
Contact hemolysis and osmoprotection.
These assays were performed as previously described (5). Briefly, 100 μl (5 × 108 ml−1) of freshly prepared RBCs (in triplicate) was mixed at an MOI of 20 with 100 μl of exponentially grown bacteria in the presence or absence of 30 mM osmoprotectant, polyethylene glycol 400 (PEG 400) through PEG 3000 (Fluka), in a round-bottom 96-well plate, which was then centrifuged and incubated at 37°C for 1 h. The cells were resuspended and centrifuged, and 100 μl of supernatants was transferred to a flat-bottom 96-well plate, where the optical density at 595 nm was measured. The percentage of hemolysis and the pore size were then calculated. All samples were tested in triplicate, and data presented were the average results from three independent experiments.
RBC membrane isolation.
This assay was performed as previously described (5). Briefly, after incubation with bacteria, RBCs were fully lysed using distilled water. After centrifugation, supernatants were collected and deposited on sucrose density gradients, which were centrifuged at 10,000 rpm for 16 h at 4°C. The material at the 44 to 25% sucrose interface was collected, diluted in phosphate-buffered saline (PBS), and centrifuged again at 100,000 rpm for 20 min at 4°C. The pellets were resuspended in about 50 μl of PBS. To assess the strength of association of Ipa proteins with RBC membranes, the above-mentioned membranes were divided into two aliquots and finally incubated at 4°C for 1 h in 200 μl PBS or PBS containing 5 M NaCl. A total of 600 μl of 63% sucrose was then mixed with the membranes that were deposited at the bottom of 1.5-ml tubes (catalogue number 357448; Beckman) and overlaid with 300 and 200 μl of 44 and 25% sucrose. The gradients were spun overnight at 4°C at 15,000 × g. The top 300 μl was collected from the gradients, diluted 1:10 in PBS, and concentrated by pelleting it in a TLA-55 rotor for 60 min at 135,000 × g at 4°C. The pellets were resuspended in 40 μl PBS. The protein concentrations of the samples were estimated using Bradford's assay (Bio-Rad), and an equivalent protein amount of each was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting.
Needle purification.
Needles were purified as previously described (38), using 0.2 mM IPTG (isopropyl-β-d-thiogalactopyranoside) to induce mxiH expression from pACT3mxiH (I. Martinez-Argudo, unpublished data), which overexpresses the needle protein MxiH and has a replication origin that is fully compatible with pDR1. Briefly, 400 ml of culture was grown, and 100 microliters of isolated needles were passed over a Superdex S200 HR 16/30 column equilibrated in 20 mM Tris-HCl, pH 7.4, and 150 mM NaCl. Fractions (1 ml) were collected, and 1.5 to 4 μl of the mixture of fractions 8 and 9 was finally analyzed by SDS-PAGE and visualized by silver staining (15% gels) to check for MixH or by Western blotting (10% gels) to check for IpaD, IpaB, and IpaC.
Immunofluorescence microscopy.
A total of 1 ml exponentially growing bacteria (OD600 = 1) was collected by centrifugation, washed in PBS, fixed in 1 ml 4% paraformaldehyde for 15 min at room temperature (RT), quenched in 1 ml 50 mM NH4Cl for 10 min, and blocked in 1 ml PBS containing 1.5% bovine serum albumin (BSA) at 4°C overnight. Bacteria were incubated with 200 μl anti-IpaB H16 mouse monoclonal antibodies (0.4 μg/ml) in PBS-BSA containing 0.1% Tween 20 for 45 min at RT, followed by incubation with biotin-conjugated goat anti-mouse IgG (0.7 μg/ml; Stratech) and DyLight 594-conjugated mouse anti-biotin IgG (0.7 μg/ml; Stratech). After being washed, bacteria were left to adhere to poly-l-lysine (Sigma)-coated glass-bottom dishes (MatTek) for 20 min, washed thrice in PBS-Tween 20 (PBST) and then in PBS, and kept in PBS. To be sure the signal was not due to nonspecifically adhered IpaB, some bacteria were further incubated for an hour in culture broth or sterile filtered supernatants of the WT or ΔipaD at 25°C before being fixed. Micrographs were captured using a Leica DMI6000 B inverted microscope with a Hamamatsu electron-multiplying charge-coupled-device (EM-CCD) camera at 1,000× magnification, using LAS AF software. Differential interference contrast and fluorescent images were merged in LAS AF. Several micrographs of each strain, containing at least 100 bacteria in total, were examined, and the numbers of the total bacteria, total signals, bacteria marked by a signal(s), and a signal(s) associated with bacteria were counted. The percentages of bacteria marked by a signal(s) and signals associated with bacteria were then calculated. The data presented are the average results from two independent experiments.
RESULTS
We constructed 7 mutants with internal deletions of regions encoding 10 or 8 aa between aa 167 and 417 (Fig. 1), as described in the Materials and Methods. Among these 7 mutants, ipaBΔ257-266 (ipaB with a deletion of the region encoding aa 257 to 266) was previously shown to be a largely nonfunctional deletion mutant (15) and, hence, was chosen as an additional control along with ΔipaB. In contrast, ipaBΔ167-176 showed nearly wild-type behavior (15) and was chosen as a positive control along with the ΔipaB-complemented strain.
ipaBΔ227-236, ipaBΔ257-266, ipaBΔ297-306, and ipaBΔ410- 417 result in high expression of late effector IpaH.
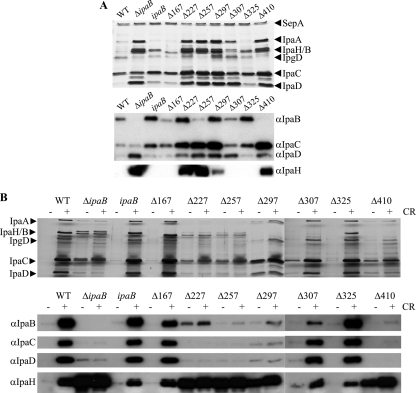
To make sure that the phenotypes we observed were not due to reduced expression levels or stability of IpaB truncates, we analyzed their levels in total culture by immunoblotting. In both overnight and exponential cultures, the expression levels of all these IpaB truncates, to a lesser extent for ipaBΔ167-176 and ipaBΔ257-266, are similar to that of the wild type (Fig. 2 and data not shown). We also examined the effect of the IpaB internal deletion mutants on the expression/stability of other translocators and effectors. All strains expressed similar levels of IpaC and IpaD. However, compared to the wild type, ΔipaB, ipaBΔ227-236, ipaBΔ257-266, ipaBΔ297-306, and ipaBΔ410-417 displayed higher to much higher levels of IpaH (Fig. 2 and data not shown), a late effector involved in the intracellular stage of the bacterium's infectious cycle (31). We conclude that all IpaB mutants have normal levels of IpaB, IpaC, and IpaD relative to the wild type but that ΔipaB, ipaBΔ227-236, ipaBΔ257-266, ipaBΔ297-306, and ipaBΔ410-417 have dramatically higher levels of expression of the late effector IpaH.
FIG. 2.
Expression of ipaB internal deletion mutants. Exponentially grown total cultures of the wild-type strain M90T, ΔipaB, ΔipaB expressing IpaB, or the indicated truncates were analyzed by immunoblotting with polyclonal rabbit antisera against IpaB and other antibodies as indicated. The data shown here are representative of results from at least 3 independent experiments. WT, wild type; ipaB, ΔipaB-complemented strain; Δ167, ipaBΔ167-176; Δ227, ipaBΔ227-236; Δ257, ipaBΔ257-266; Δ297, ipaBΔ297-306; Δ307, ipaBΔ307-316; Δ325, ipaBΔ325-334; Δ410, ipaBΔ410-417.
ipaBΔ227-236, ipaBΔ257-266, ipaBΔ297-306, and ipaBΔ410- 417 are constitutive secretors and insensitive to CR.
The Shigella T3SS displays three functional states. “Leakage” is a slow, low-level Ipa protein secretion prior to host cell contact whereby around 5% of Ipa proteins are secreted (21). “Induction” describes the burst of Ipa protein secretion upon host cell sensing (25) or CR addition (3). “Constitutive secretion” of Ipa proteins, which occurs in some mutants, represents an unregulated and higher-level secretion than leakage and involves not only the Ipa proteins but also the “late effectors” (7, 31). We previously reported that ΔipaB was a constitutive secretor and insensitive to CR. We therefore investigated the leakage profiles of ipaB truncates and their responsiveness to CR. Silver staining shows that ipaBΔ227-236, ipaBΔ257-266, ipaBΔ297-306, and ipaBΔ410-417 display a similar overnight leakage to ΔipaB, and in contrast, ipaBΔ167-176 and ipaBΔ325-334, together with the ΔipaB-complemented strain, are more or less similar to the wild type (Fig. 3A, top). ipaBΔ307-316 seems to display an intermediate phenotype. These observations are extended by Western blotting, which shows that ipaBΔ227-236, ipaBΔ257-266, ipaBΔ297-306, ipaBΔ307-316, and ipaBΔ410-417 secrete more translocators, such as IpaC and IpaD, and late effector IpaH (Fig. 3A, bottom). Although it seems that ipaBΔ227-236, ipaBΔ257-266, ipaBΔ297-306, and ipaBΔ410-417 secrete considerably more IpaC than ΔipaB, this is probably not the case. Indeed, our previous work showed that secreted IpaC aggregates in the absence of IpaB and then pellets with the bacteria during centrifugation (H. Nishioka and A. J. Blocker, unpublished data).
FIG. 3.
Secretion profiles of ipaB internal deletion mutants. (A) Overnight leakage of the Ipa proteins analyzed by silver staining (top) and immunoblotting (bottom) using antisera against IpaB, IpaC, IpaD, and IpaH. (B) Induced secretion of Ipa proteins after the addition (+) or absence (−) of Congo red (CR) was analyzed by silver staining (top) and immunoblotting (bottom). The wild type (WT) and ΔipaB were used as controls, and bacterial numbers were normalized by optical density. The positions of the major Ipa proteins detected by silver staining and the antibodies used for the blots are indicated on the side. The data shown here are representative of 2 independent experiments giving similar results.
With respect to CR induction, ipaBΔ167-176, ipaBΔ307-316, ipaBΔ325-334, and the ΔipaB-complemented strain behave like the wild type, while the others are unresponsive (Fig. 3B, top and bottom). In the absence of CR induction, large amounts of IpaH and VirA were detected in ΔipaB, ipaBΔ227-236, ipaBΔ257-266, ipaBΔ297-306, and ipaBΔ410-417 (Fig. 3B, bottom, and data not shown). On the contrary, IpaH and VirA (not shown) were detected only in the presence of CR induction from the wild type, the ΔipaB-complemented strain, ipaBΔ167-176, ipaBΔ307-316, and ipaBΔ325-334. Taken together, these data indicate that ipaBΔ227-236, ipaBΔ257-266, ipaBΔ297-306, and ipaBΔ410-417 are constitutive secretors and insensitive to CR, while ipaBΔ167-176 and ipaBΔ325-334 are similar to the wild type and ipaBΔ307-316 shows an intermediate phenotype in terms of secretion regulation.
ipaBΔ227-236, ipaBΔ257-266, ipaBΔ297-306, and ipaBΔ410- 417 are hyperadhesive.
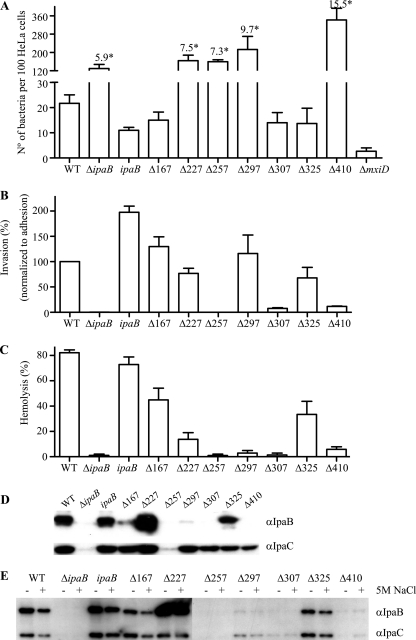
The ΔipaB mutant was previously reported to display increased adhesion to eukaryotic cells via an unknown mechanism (16). Therefore, HeLa cell adhesion was examined by incubating the bacteria with HeLa cells at 25°C, a temperature in which the T3SS is inactive (5). While ΔipaB is approximately 6-fold more adhesive than the wild type, we found that ipaBΔ227-236, ipaBΔ257-266, ipaBΔ297-306, and ipaBΔ410-417 were 7.5-, 7.3-, 9.7-, and 15.5-fold more adhesive than the wild type, respectively, and that ΔmxiD, a mutant with a defective T3SS (2), is much less adhesive than the wild type (Fig. 4A).
FIG. 4.
Adherence, invasiveness, hemolysis, and membrane insertion of ipaB mutants. (A to C) Adherence (A) and invasiveness (B) to HeLa cells and the hemolysis percentage of sheep RBC (C) were obtained, as described in Materials and Methods. The values are averages of results from 3 independent experiments performed in duplicate (or triplicate), and the errors given are standard deviations. “*” indicates the fold increase of mutants’ adhesion to HeLa cells compared with that of the wild type (WT). (D, E) Insertion of IpaB and IpaC into sheep RBC membranes after contact hemolysis (D) and further stripping (E) with PBS (−) or 5 M NaCl (+). The antibodies used for the Western blots are indicated on the right. The blots shown are representative of 2 independent assays giving similar results.
ipaBΔ227-236 and ipaBΔ297-306 remain invasive.
To evaluate the ability of the mutants to invade epithelial cells, the number of intracellular bacteria in infected HeLa cells was determined using a gentamicin protection assay. The invasion capacities of the mutants were expressed relative to that of the wild type, which was set to 100%. While ipaBΔ257-266 and ipaBΔ307-316 did not complement ΔipaB for HeLa cell invasion, ipaBΔ325-334 partially restored invasion (≈43%). ipaBΔ167-176 complemented ΔipaB as efficiently as ipaB, while ipaBΔ227-236, ipaBΔ297-306, ipaBΔ410-417 were 5.8-, 11.4-, and 1.8-fold more invasive than the wild type, respectively. Since the latter three mutants were also hyperadhesive, and we have shown that this can seemingly cause “hyperinvasiveness” (34), we normalized the invasiveness of all mutants to their adhesiveness (see Materials and Methods). In this mode of analysis, the ΔipaB-complemented strain and ipaBΔ167-176 are slightly more invasive than the wild type, while ipaBΔ227-236, ipaBΔ297-306, and ipaBΔ410-417 show 76.9%, 116.0%, and 11.6% of the invasiveness of the wild type, respectively (Fig. 4B). Taken together, the adhesion and invasion data indicate that ipaBΔ227-236 and ipaBΔ297-306 retain considerable functionality despite their deregulated secretion phenotypes.
ipaBΔ297-306, ipaBΔ307-316, and ipaBΔ410-417 are unable to perform contact hemolysis due to poor insertion of IpaB and IpaC into membranes.
Contact hemolysis is the ability of Shigella to lyse the RBCs with which they have been brought into contact by centrifugation. Hemolysis is due to the formation of a 25-Å pore by IpaB and IpaC within the RBC membrane (5). To study the effect of internal deletions of ipaB on pore formation, we investigated their contact hemolytic activity. As previously reported, ΔipaB was nonhemolytic (5), whereas complementation of ΔipaB with wild-type ipaB restored hemolysis to levels comparable to that of the wild type (Fig. 4C) (5). Similar to ΔipaB, ipaBΔ257-266, ipaBΔ297-306, ipaBΔ307-316, and ipaBΔ410-417 were nonhemolytic, while ipaBΔ167-176, ipaBΔ227-236, and ipaBΔ325-334 caused about 45%, 14%, and 33% hemolysis, respectively. To determine whether the loss of contact hemolytic activity observed in some ipaB truncates is due to their inability to insert translocon proteins in the RBC membrane, we examined the composition of the lysed RBC membrane isolated by floatation in a sucrose density gradient. As previously reported, IpaB and IpaC are present in these membranes when RBCs have been lysed by contact with the wild type (Fig. 4D) (5). No IpaB and little IpaC were detected from RBCs infected with ΔipaB or ipaBΔ257-266, a nonfunctional deletion mutant reported previously (15), supporting the notion that the insertion of IpaC into the RBC membrane is usually IpaB dependent (Fig. 4D) (5). In the membrane fractions of RBCs incubated with ipaBΔ167-176, ipaBΔ227-236, and ipaBΔ325-334, both IpaB and IpaC were found, though the amount of IpaB was not as same as that of the wild type (Fig. 4D). Specifically, ipaBΔ167-176 displayed considerably less IpaB in the lysed RBC membrane fraction, and ipaBΔ227-236 displayed considerably more IpaB. In the membrane fractions of RBCs incubated with ipaBΔ297-306, ipaBΔ307-316, and ipaBΔ410-417, the level of IpaC is comparable to that of the wild type, though the level of IpaC is relatively low in ipaBΔ410-417. However, in these samples, very little IpaB was detected, although we did observe some IpaB after longer exposure (Fig. 4D and data not shown).
To verify that IpaB and/or IpaC are indeed inserted into and not simply peripherally associated with RBC membranes, purified Shigella-lysed RBC membranes were further incubated with PBS or PBS containing 5 M NaCl to detach any proteins peripherally associated with the membranes through charge interactions (5). After “stripping” using 5 M NaCl, the wild type, the ΔipaB-complemented strain, ipaBΔ167-176, ipaBΔ227-236, and ipaBΔ325-334 retained the majority of IpaB and IpaC associated with RBC membrane; however, ipaBΔ297-306, ipaBΔ307-316, and ipaBΔ410-417 lost most of their IpaC after a second floatation, even when incubated only with PBS (Fig. 4E). Overall, these data suggest the following: (i) IpaB and IpaC are indeed inserted into RBC membranes by ipaBΔ167-176, ipaBΔ227-236, and ipaBΔ325-334; (ii) IpaBΔ227-236 inserts unusually efficiently into membranes, but this does not lead to a concomitant increase in IpaC insertion; and (iii) mutants ipaBΔ297-306, ipaBΔ307-316, and ipaBΔ410-417 do not insert IpaC into RBC membranes; instead, some of the IpaC protein which they secrete becomes only weakly peripherally associated with RBC membranes.
We noticed that ipaBΔ227-236 had only weak hemolytic activity, although more IpaB was found in the RBC membranes incubated with ipaBΔ227-236 than with the wild type (Fig. 4C to E). To investigate whether the low hemolytic activity of ipaBΔ227-236 is due to abnormal pore formation, we performed osmoprotection experiments. Previous studies showed that osmoprotectants, such as polyethylene glycol (PEG), induce a size-dependent increase of the half time of hemolysis that can be used to estimate pore size (5). The hemolysis caused by the wild type, the ΔipaB-complemented strain, ipaBΔ167-176, ipaBΔ227-236, and ipaBΔ325-334 gradually decreased with the size of the PEG (Fig. 5A), indicating that osmotic lysis, due to pore insertion rather than mere membrane disruption, was still occurring. A time course analysis of hemolysis caused by ipaBΔ227-236 and ipaBΔ325-334 in the presence of osmoprotectants allowed the estimation of the diameters of the pores formed by these mutants to be 22.0 ± 0.9 Å and 24.6 ± 0.6 Å, respectively (Fig. 5B). The difference in pore size between ipaBΔ227-236 and ipaBΔ325-334 is statistically significant (unpaired Student's t test; P = 0.0145), while that of ipaBΔ325-334 is not significantly different from that previously reported for the wild type (5).
FIG. 5.
Contact hemolysis of sheep RBC in the presence of osmoprotectants. (A) Hemolysis percentages in PBS containing 30 mM osmoprotectant were obtained, as described in Materials and Methods. The values are averages of results from 3 separate experiments performed in triplicate. Errors given are standard deviations. (B) The relative permeability of the molecule versus its size was determined as previously described (5). The best-fitting curve was obtained by using GraphPad Prism, and its intersection point with the x axis gave the estimated size of the pore. The curves shown are representative of results from 3 independent assays giving similar results. The diameters of the pores formed by ipaBΔ227-236 and ipaBΔ325-334 were 22.0 ± 0.9 Å and 24.6 ± 0.6 Å (n = 3), respectively, and the difference is statistically significant (unpaired t test; P = 0.0145).
ipaBΔ227-236, ipaBΔ257-266, ipaBΔ297-306, and ipaBΔ410- 417 are defective in their ability to interact with needles.
We previously reported that IpaB localizes to the tip of mature, quiescent needles. IpaC is also found in quiescent needles but substoichiometrically to both IpaB and IpaD and only to a significant degree in mutants mimicking an activated state. We showed that both IpaB and IpaC bind in an IpaD-dependent manner and independently of each other to quiescent needles (38). Recently we found that short C-terminal truncations of IpaB, which mimic an activated needle state, show decreased binding to needles but lead to an increased recruitment of IpaC to needles (34). We therefore wished to investigate the binding of our IpaB mutants to needles and their ability to recruit IpaC to this location. Since pRK2mxiH, the plasmid used to overexpress needles to examine their Ipa composition (38), is not fully compatible with the plasmid carrying our ipaB truncates, we used pACT3mxiH (I. Martinez-Argudo, unpublished data), which has an origin of replication that is compatible with those of the complementation plasmids, and transformed it into our strains to produce long needles. EM examination showed that more and longer needles were produced with the wild type containing pACT3mxiH (data not shown). In addition, all strains containing pACT3mxiH, except ipaBΔ167-176, showed similar expression levels of MxiG, an inner membrane secreton component, and MxiH (Fig. 6A), implying that they have similar numbers of secreton bases and needles of similar length. The higher expression of MxiH made it easier to detect IpaC in the wild type than that described in previous reports (Fig. 6B) (38). To investigate whether internal deletions in IpaB affect the binding of IpaBCD proteins to needles, purified long needles were prepared as previously described (38). We first checked the Ipa composition of needles isolated from ΔipaB, the ΔipaB-complemented strain, and ΔipaD. In needles derived from ΔipaD, little IpaB and IpaC were found compared to those found with the wild type. In needles derived from ΔipaB, IpaC was found at a level similar to that seen with the ΔipaB-complemented strain (Fig. 6B). These results corresponded to our previous findings (38), indicating that pACT3mxiH functions as well as pRK2mxiH for studying needle composition. The preparations were normalized according to the amount of MxiH and were then analyzed by Western blotting. In needles derived from ΔipaB, the ΔipaB-complemented strain, and all ipaB internal deletion mutants, IpaD was found at levels comparable to those found in wild-type needles (Fig. 6B), confirming that the binding of IpaD to needles is independent of IpaB (38). IpaC was detected at levels similar to or slightly lower than that detected for the ΔipaB-complemented strain in needles derived from all ipaB internal deletion mutants. In needles derived from ipaB truncates, little IpaB was detected with ipaBΔ227-236, ipaBΔ257-266, ipaBΔ297-306, and ipaBΔ410-417. Although IpaB was detected easily in needles from ipaBΔ167-176, ipaBΔ307-316, and ipaBΔ325-334, its amounts were clearly reduced compared to those detected with the ΔipaB-complemented strain and the wild type. Therefore, the decreased association of IpaC with needles in many of the mutants might be due to the decreased presence of IpaB, since we know that IpaB mutants with different levels of needle binding can lead to different levels of IpaC recruitment (34). Nevertheless, we were surprised that even internal IpaB truncates which retained substantial invasive and/or hemolytic properties, such as IpaBΔ227-236 and IpaBΔ297-306, demonstrated weak association with needles. We considered that this might reflect the high stringency of our biochemical assay.
FIG. 6.
Analysis of needle-associated proteins within ipaB internal deletion mutants. (A) Overnight total cultures from the wild type (WT), ΔipaB, ipaB internal deletion mutants, and ΔipaD-overexpressing MxiH were analyzed by immunoblotting with anti-MxiG and anti-MxiH antibodies. (B) Needles were purified and normalized for the amount of MxiH by silver-stained SDS-PAGE (bottom) and analyzed by Western blotting. The antibodies used for the blots are indicated on the right. The data shown are representative of 2 independent assays giving similar results.
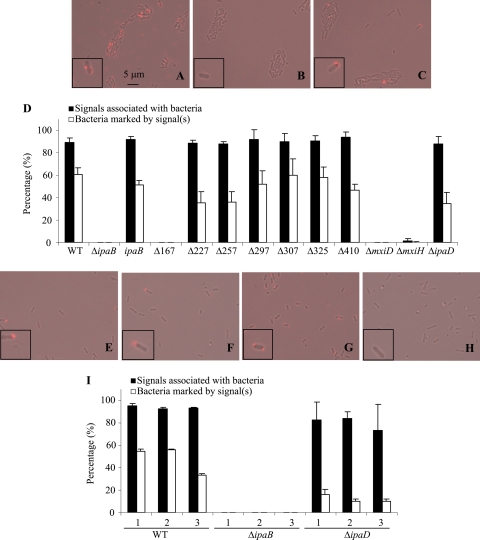
Immunofluorescence microscopy was previously used to show that both IpaD and IpaB localize on the surface of S. flexneri (13, 28). To determine whether the IpaB truncates were present on the S. flexneri surface, bacteria were incubated with anti-IpaB mouse monoclonal antibodies and then with fluorescent anti-mouse secondary antibodies. In these experiments, no signal was detected at the bacterial surface under normal culture conditions. This is consistent with previous reports (13, 28). However, we reasoned that this might be because only one molecule of IpaB is present at each needle tip, and with approximately 100 copies distributed over the entire cell surface (5), each secretion apparatus is sufficiently distant from its neighbors that no signal enhancement would occur through proximity. We therefore sought to enhance the signal using biotin-conjugated goat anti-mouse IgG and then fluorophore-conjugated mouse anti-biotin IgG (see Materials and Methods). Staining with the anti-IpaB monoclonal antibody H16 was not detectable on ΔmxiD, ΔipaB, ipaBΔ167-176, and ΔmxiH, as expected (Fig. 7A to D and data not shown). The nondetection of IpaB on ΔmxiD and ΔmxiH is due to the lack of functional secretion apparatuses, while the nonstaining of ipaBΔ167-176 is due to the removal of H16's epitope from this deletion mutant. In contrast, cell-associated anti-IpaB staining was seen in the wild type and all the other IpaB truncates, but approximately 50% fewer ipaBΔ227-236, ipaBΔ257-266, and ΔipaD cells were stained compared to that of wild-type cells (Fig. 7A to D and data not shown). Given that ipaBΔ227-236, ipaBΔ257-266, ipaBΔ297-306, ipaBΔ410-417, and ΔipaD are constitutive secretors, which heavily secrete Ipa proteins, including IpaB, the signals could be caused by secreted IpaB nonspecifically attaching to the cell surface. To ascertain whether or not this was the case, ΔipaB, ΔipaD, and WT bacteria were incubated for an hour at 25°C (where the secretion system is inactive) with the culture supernatants of ΔipaD before being fixed. Reassuringly, no signal was detected from ΔipaB treated in this way. In addition, less of a signal was detected from the WT incubated with the supernatant of ΔipaD, and even extra incubation of ΔipaD with Trypticase soy broth decreased the signal detected (Fig. 7D-I), suggesting that some loss of IpaB from needle tips occurred even during this short incubation time. Returning to analyze the mutants, we noted that those mutants that demonstrated reduced IpaB binding to needles were also those that demonstrated reduced surface association of IpaB. Therefore, we conclude that IpaB and probably all its internal truncates are present on the bacterial surface, possibly via association with the needle tip, with IpaBΔ227-236 and IpaBΔ257-266 (and to a lesser extent, IpaBΔ297-306 and IpaBΔ410-417) showing greater defects than others, in this respect.
FIG. 7.
Localization of IpaB and its internal deletion variants on the Shigella surface. Bacteria grown into exponential phase were fixed and stained with anti-IpaB mouse monoclonal antibodies (H16), biotin-conjugated goat anti-mouse IgG, and DyLight 594-conjugated mouse anti-biotin IgG, as described in Materials and Methods. Merged images were obtained from contrasting one and IpaB labeling one. (A) WT; (B) ΔmxiD; (C) ΔipaD; (E) ΔipaD; (F) ΔipaD; (G) WT; (H) ΔipaB. Bacteria were incubated at 25°C with the Trypticase soy broth (E) or the supernatants of ΔipaD (F to H) for an hour before being fixed. (D and I) Percentages of signals associated with bacteria or bacteria labeled by signal(s) were calculated by counting bacteria in one or more fields containing at least 100 bacteria. The values are averages of results from 2 separate experiments, and errors given are standard deviations. Different strains of bacteria were incubated with the Trypticase soy broth (1), supernatants of WT (2), or supernatants of ΔipaD (3) before being fixed.
DISCUSSION
Summary.
We found that the deletion of small regions within ipaB affects the Ipa composition of needles, secretion regulation, contact-dependent hemolysis, translocon insertion in RBC membranes, and the ability of Shigella to adhere to and invade HeLa cells (Table 3). This now allows us to delineate two areas within the large globular C-terminal domain of IpaB that are involved in regulating type III secretion.
TABLE 3.
Summary of results obtained with this study
| Strain | Expressiona |
Constitutive secretiona | CR responsivenessa | % adhesion | % invasionc | % hemolysis | Membrane insertion | Tip composition | Mutant classb | |
|---|---|---|---|---|---|---|---|---|---|---|
| IpaB | IpaH | |||||||||
| WT | + | − | − | + | 22 ± 5.9 | 100 | 82.1 ± 4.0 | IpaBC | IpaDB(C) | NA |
| ΔipaB | − | +++ | + | − | 130 ± 31.1 | 0.3 ± 0.2 | 1.1 ± 1.7 | IpaD(C) | NA | |
| ΔipaB complemented | + | − | − | + | 11 ± 2.0 | 197.5 ± 21.2 | 72.9 ± 10.3 | IpaBC | IpaDB(C) | NA |
| ipaBΔ167-176 | ± | − | − | + | 15 ± 5.6 | 129.8 ± 33.5 | 45.0 ± 16.0 | IpaBC | IpaDB(C) | 1a |
| ipaBΔ227-236 | + | ++ | + | − | 164 ± 41.8 | 76.9 ± 17.5 | 13.7 ± 9.1 | IpaBC | IpaD(C) | 2a |
| ipaBΔ257-266 | ± | ++ | + | − | 160 ± 14.4 | 0.1 ± 0.1 | 0.9 ± 2.0 | IpaD(C) | 3 | |
| ipaBΔ297-306 | + | + | + | − | 213 ± 98.6 | 116.0 ± 63.4 | 2.9 ± 3.6 | IpaD(C) | 2a | |
| ipaBΔ307-316 | + | − | ± | + | 14 ± 6.9 | 7.7 ± 2.1 | 1.4 ± 2.6 | IpaDB(C) | 1b | |
| ipaBΔ325-334 | + | − | − | + | 14 ± 10.5 | 68.0 ± 35.9 | 33.4 ± 18.0 | IpaBC | IpaDB(C) | 1a |
| ipaBΔ410-417 | + | ++ | + | − | 342 ± 88.6 | 11.6 ± 1.2 | 5.8 ± 3.5 | IpaC | IpaD(C) | 2b |
Results are succinctly described as normal or positive (+), intermediate (±), and negative (−); refer to the text and corresponding figures for details.
NA, not applicable.
Normalized to adhesion.
Mutant classification.
In Table 3, we classified the mutants into two main phenotypic classes. Class 1 includes ipaBΔ167-176, ipaBΔ307-316, and ipaBΔ325-334, which regulate secretion (leakage and CR induction) in a quasi-wild-type fashion, while class 2 includes ipaBΔ227-236, ipaBΔ297-306, and ipaBΔ410-417, which do not. Both classes are subdivided into “a” and “b” subclasses, in which the “a” group (ipaBΔ167-176, ipaBΔ325-334, ipaBΔ227-236, and ipaBΔ297-306) retains substantial invasion and/or hemolytic capacities and the “b” group (ipaBΔ307-316 and ipaBΔ410-417) does not. ipaBΔ257-266, which phenotypically resembles ΔipaB by neither regulating secretion nor mediating any invasion/hemolysis, is in class 3. Therefore, our results agree with other findings that the putative amphipathic α-helix between aa 240 and 280, but none of the other IpaB areas here studied via the introduction of small deletions, is necessary to maintain IpaB in its native conformation(s) (15). Consequently, we assume that the changes we observe in secretion, adherence, invasion, hemolysis, membrane insertion, and needle tip composition are due to a more minor structural change(s), resulting from deletion of 8 to 10 aa.
Adhesion and invasion.
Hyperadhesion was previously demonstrated in Shigella ΔipaD and ΔipaB and some C-terminal ipaB deletion mutants (16, 24, 34). Shigella has no known adherence factors, and hence, its adhesion to host cells is not well understood (27, 36). All our hyperadhesive mutants are fast constitutive secretors, but our adhesion assay was carried out at 25°C, a temperature in which the T3SS is inactive (5). Therefore, it is unlikely that hyperadhesion is due to the constitutive secretion of Ipa proteins. Indeed, hyperadhesion is not correlated with slow constitutive secretion (34). Yet, the T3SS does seem to play a role in adhesion to HeLa cells (Fig. 4A) (24, 30). Perhaps modifications in the T3SS's activation state upregulate an adhesion mechanism that is compositionally distinct from the T3SS itself (34)? Although ipaBΔ227-236 and ipaBΔ297-306 are invasive and hyperadhesive, ΔipaB, ipaBΔ257-266, and ipaB Δ410-417, which are also hyperadhesive, are almost noninvasive. Therefore, there is no direct causal relationship between increased adhesion and invasion (34).
Pore insertion and invasion.
For the wild type, the ΔipaB-complemented strain, ipaBΔ167-176, ipaBΔ227-236, and ipaBΔ325-334, association of both IpaB and IpaC with RBC membranes accounts for the hemolysis and pore formation they cause (Fig. 4C to E). Although the association of IpaC with RBC membranes was initially observed in all strains except ΔipaB and ipaBΔ257-266, IpaC was not easily detected after stripping from strains devoid of contact hemolysis (Fig. 4D and E), confirming that insertion of both IpaB and IpaC is a prerequisite for pore formation (5).
In addition, the relatively low hemolysis caused by ipaBΔ167-176 and ipaBΔ325-334, which showed less IpaB insertion than the wild type, and ipaBΔ227-236, which had much more IpaB insertion than the wild type (Fig. 4C to E), implies that the quantity and structural intactness of IpaB also play important roles in pore formation. The smaller pore size induced by ipaBΔ227-236 may indicate an altered IpaB fold within the membrane and hence an altered pore structure. Of note, an amount of IpaB similar to that of the wild type was detected using ipaBΔ325-334, which lacks the region encoding 10 aa within the presumed first transmembrane domain (aa 313 to 333) (17). On the contrary, little IpaB was detected from the membranes of RBCs infected with ipaBΔ297-306, ipaBΔ307-316, and ipaBΔ410-417. This suggests that the second transmembrane domain (aa 399 to 419) and the region just upstream of the first transmembrane domain are more important for membrane insertion of IpaB than the first transmembrane domain. ipaBΔ325-334 is invasive, but so are ipaBΔ227-236 and ipaBΔ297-306. Short C-terminal deletion mutants of ipaB are also unable to perform contact hemolysis or insertion of IpaB and IpaC into RBC membranes, although they remain invasive and associate the translocators with HeLa cell membranes (34). Therefore, we presume that ipaBΔ227-236 and ipaBΔ297-306 also efficiently insert IpaB and IpaC into HeLa membranes.
Needle tip composition and regulation of secretion.
Long before the needle and tip complex were identified, IpaB and IpaD were proposed to “plug” secretion in the absence of the inducing signal (25). Since the constitutive secretion observed for ipaBΔ227-236, ipaBΔ257-266, ipaBΔ297-306, and ipaBΔ410-417 correlates with the absence of these truncates from needle preparations, our results continue to support a model in which an intact tip complex is a prerequisite for blocking secretion prior to activation of signal reception.
Yet, a large amount of IpaB is seen in RBC membranes lysed by contact with ipaBΔ227-236 (Fig. 4D and E), and both it and ipaBΔ297-306 retain substantial invasion capacity. These data are hard to reconcile with the notion of T3SS activation and translocation insertion occurring from the needle tip (8). However, IpaBΔ227-236, IpaBΔ257-266, IpaBΔ297-306, and IpaBΔ410-417 are present at the bacterial surface, although to a lesser degree than the other mutants. Therefore, lack of detection of some IpaB truncates in needle preparations might be because these truncates, due to their altered structure, are more easily detached from needles during their purification. Hence, we suggest that lack of secretion regulation in these mutants is due to their inability not to form but to maintain a complete tip complex.
All mutants studied here are altered in the proposed large C-terminal globular domain of IpaB (aa 171 to 513). It is also within the last third of this region that an interaction site with IpaC has been mapped using two-hybrid analysis (aa 367 to 458) (29). In this respect, it is notable that mutants ipaBΔ307-316, ipaBΔ325-334, and ipaBΔ410-417 show increasing defects in associating IpaC with the RBC membrane (Fig. 4D). In addition, this association is unstable (Fig. 4E), again suggesting that IpaC cannot be inserted into the RBC membrane without the help of intact IpaB.
ipaBΔ227-236 and, to a lesser extent, ipaBΔ297-306 insert near-wild-type quantities of IpaC in RBC membranes. This supports the notion that their primary defect is in maintenance of a tip complex. There are two reasons why they may not be able to respond to CR induction: (i) they are too strongly defective in tip complex maintenance and/or (ii) whatever tip complex is formed is in a structural state where it cannot respond to CR. Either option would lead to the fast constitutive secretion observed. As both ipaBΔ227-236 and ipaBΔ297-306 are invasive but show increased and decreased levels of IpaB insertion into RBC membranes, respectively, they may not be in the exact same functional state.
Comparison with other IpaB mutants.
Very short amino acid deletions from IpaB's C terminus impair its association with needles but increase recruitment of IpaC to them (34). In our present study, the deletion of 10 or 8 aa occasionally prevents the efficient association of truncated IpaB proteins with the needle. Yet, no increased IpaC was observed in purified needles derived from any of these mutants, although all of these secreted more IpaC than the WT. In fact, the only phenotypic characteristics that differentiate ipaBΔ227-236 and ipaBΔ297-306 (Table 3) from our C-terminal IpaB deletion mutants (34) are that the latter partially retain their ability to copurify with needles and recruit more IpaC to this location. The palette of phenotypes displayed by ipaBΔ227-236, ipaBΔ297-306, and the C-terminal truncates is also reminiscent of mxiHP44A+Q51A (9a, 18a, 38), which is a constitutive secretor, uninducible by CR but poorly invasive. ipaBΔ227-236 and ipaBΔ297-306 are also reminiscent of mutant mxiHP44A (18a, 38), which does not localize IpaB to needles efficiently yet remains sensitive to CR, hemolytic, and invasive. Together, this mutant set may delineate a series of conformational states within a signal transduction cascade, leading from IpaB in the needle tip and down the needle.
Yet, we failed to identify ipaB mutants that form a stable tip complex and are therefore not fast constitutive secretors but do not respond to CR or invade host cells. Such mutants would support the notion that IpaB is the T3SS sensor and further delineate how it may exert such a function. We now suspect that such mutants will need to be point mutants and are in the process of searching for them.
Acknowledgments
We thank Isabel Martinez-Argudo (University of Bristol) for constructing the plasmid pACT3mxiH and her and Dorothea Roehrich (University of Bristol) for discussions and critical comments on the manuscript. We thank Mark Jepson and Alan Leard of the Bioimaging facility (University of Bristol) for their suggestions and help with immunofluorescence microscopy. We thank Will Mawby (University of Bristol) for carrying out protein sequencing.
D.-K.S. was funded by United Kingdom Medical Research Council grant G0701243 to A.J.B., S.S. and H.N. were supported by the Guy G. F. Newton Senior Research Fellowship and a Research Career Development Award from the NIAID at the NIH (award K22AI001847), respectively, both awarded to A.J.B.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 11 October 2010.
REFERENCES
- 1.Adam, T., M. Arpin, M. C. Prevost, P. Gounon, and P. J. Sansonetti. 1995. Cytoskeletal rearrangements and the functional role of T-plastin during entry of Shigella flexneri into HeLa cells. J. Cell Biol. 129:367-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allaoui, A., P. J. Sansonetti, and C. Parsot. 1993. MxiD, an outer membrane protein necessary for the secretion of the Shigella flexneri lpa invasins. Mol. Microbiol. 7:59-68. [DOI] [PubMed] [Google Scholar]
- 3.Bahrani, F. K., P. J. Sansonetti, and C. Parsot. 1997. Secretion of Ipa proteins by Shigella flexneri: inducer molecules and kinetics of activation. Infect. Immun. 65:4005-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barzu, S., F. Nato, S. Rouyre, J. C. Mazie, P. Sansonetti, and A. Phalipon. 1993. Characterization of B-cell epitopes on IpaB, an invasion-associated antigen of Shigella flexneri: identification of an immunodominant domain recognized during natural infection. Infect. Immun. 61:3825-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blocker, A., P. Gounon, E. Larquet, K. Niebuhr, V. Cabiaux, C. Parsot, and P. Sansonetti. 1999. The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J. Cell Biol. 147:683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blocker, A., N. Jouihri, E. Larquet, P. Gounon, F. Ebel, C. Parsot, P. Sansonetti, and A. Allaoui. 2001. Structure and composition of the Shigella flexneri “needle complex,” a part of its type III secreton. Mol. Microbiol. 39:652-663. [DOI] [PubMed] [Google Scholar]
- 7.Blocker, A., K. Komoriya, and S. Aizawa. 2003. Type III secretion systems and bacterial flagella: insights into their function from structural similarities. Proc. Natl. Acad. Sci. U. S. A. 100:3027-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blocker, A. J., J. E. Deane, A. K. Veenendaal, P. Roversi, J. L. Hodgkinson, S. Johnson, and S. M. Lea. 2008. What's the point of the type III secretion system needle? Proc. Natl. Acad. Sci. U. S. A. 105:6507-6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchrieser, C., P. Glaser, C. Rusniok, H. Nedjari, H. D'Hauteville, F. Kunst, P. Sansonetti, and C. Parsot. 2000. The virulence plasmid pWR100 and the repertoire of proteins secreted by the type III secretion apparatus of Shigella flexneri. Mol. Microbiol. 38:760-771. [DOI] [PubMed] [Google Scholar]
- 9a.Cordes, F. S., S. Daniell, R. Kenjale, S. Saurya, W. L. Picking, W. D. Picking, F. Booy, S. M. Lea, and A. Blocker. 2005. Helical packing of needles from functionally Altered Shigella type III secretion systems. J. Mol. Biol. 354:206-211. [DOI] [PubMed] [Google Scholar]
- 10.Cornelis, G. R. 2006. The type III secretion injectisome. Nat. Rev. Microbiol. 4:811-825. [DOI] [PubMed] [Google Scholar]
- 11.Deane, J. E., P. Roversi, F. S. Cordes, S. Johnson, R. Kenjale, S. Daniell, F. Booy, W. D. Picking, W. L. Picking, A. J. Blocker, and S. M. Lea. 2006. Molecular model of a type III secretion system needle: implications for host-cell sensing. Proc. Natl. Acad. Sci. U. S. A. 103:12529-12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epler, C. R., N. E. Dickenson, A. J. Olive, W. L. Picking, and W. D. Picking. 2009. Liposomes recruit IpaC to the Shigella flexneri type III secretion apparatus needle as a final step in secretion induction. Infect. Immun. 77:2754-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espina, M., A. J. Olive, R. Kenjale, D. S. Moore, S. F. Ausar, R. W. Kaminski, E. V. Oaks, C. R. Middaugh, W. D. Picking, and W. L. Picking. 2006. IpaD localizes to the tip of the type III secretion system needle of Shigella flexneri. Infect. Immun. 74:4391-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galan, J. E., and H. Wolf-Watz. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444:567-573. [DOI] [PubMed] [Google Scholar]
- 15.Guichon, A., D. Hersh, M. R. Smith, and A. Zychlinsky. 2001. Structure-function analysis of the Shigella virulence factor IpaB. J. Bacteriol. 183:1269-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.High, N., J. Mounier, M. C. Prevost, and P. J. Sansonetti. 1992. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 11:1991-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hume, P. J., E. J. McGhie, R. D. Hayward, and V. Koronakis. 2003. The purified Shigella IpaB and Salmonella SipB translocators share biochemical properties and membrane topology. Mol. Microbiol. 49:425-439. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, S., P. Roversi, M. Espina, A. Olive, J. E. Deane, S. Birket, T. Field, W. D. Picking, A. J. Blocker, E. E. Galyov, W. L. Picking, and S. M. Lea. 2007. Self-chaperoning of the type III secretion system needle tip proteins IpaD and BipD. J. Biol. Chem. 282:4035-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Kenjale, R., J. Wilson, S. F. Zenk, S. Saurya, W. L. Picking, W. D. Picking, and A. Blocker. 2005. The needle component of the type III secretion system of Shigella regulates the activity of the secretion apparatus. J. Biol. Chem. 280:42929-42937. [DOI] [PubMed] [Google Scholar]
- 19.Kotloff, K. L., J. P. Winickoff, B. Ivanoff, J. D. Clemens, D. L. Swerdlow, P. J. Sansonetti, G. K. Adak, and M. M. Levine. 1999. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull. World Health Organ. 77:651-666. [PMC free article] [PubMed] [Google Scholar]
- 20.Lunelli, M., R. K. Lokareddy, A. Zychlinsky, and M. Kolbe. 2009. IpaB-IpgC interaction defines binding motif for type III secretion translocator. Proc. Natl. Acad. Sci. U. S. A. 106:9661-9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magdalena, J., A. Hachani, M. Chamekh, N. Jouihri, P. Gounon, A. Blocker, and A. Allaoui. 2002. Spa32 regulates a switch in substrate specificity of the type III secreton of Shigella flexneri from needle components to Ipa proteins. J. Bacteriol. 184:3433-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mavris, M., A. L. Page, R. Tournebize, B. Demers, P. Sansonetti, and C. Parsot. 2002. Regulation of transcription by the activity of the Shigella flexneri type III secretion apparatus. Mol. Microbiol. 43:1543-1553. [DOI] [PubMed] [Google Scholar]
- 23.Meitert, T., E. Pencu, L. Ciudin, M. Tonciu, I. Mihai, and S. Nicolescu. 1991. Correlation between Congo red binding as virulence marker in Shigella species and Sereny test. Roum. Arch. Microbiol. Immunol. 50:45-52. [PubMed] [Google Scholar]
- 24.Menard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menard, R., P. Sansonetti, and C. Parsot. 1994. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 13:5293-5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mueller, C. A., P. Broz, and G. R. Cornelis. 2008. The type III secretion system tip complex and translocon. Mol. Microbiol. 68:1085-1095. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa, M., Y. Handa, H. Ashida, M. Suzuki, and C. Sasakawa. 2008. The versatility of Shigella effectors. Nat. Rev. Microbiol. 6:11-16. [DOI] [PubMed] [Google Scholar]
- 28.Olive, A. J., R. Kenjale, M. Espina, D. S. Moore, W. L. Picking, and W. D. Picking. 2007. Bile salts stimulate recruitment of IpaB to the Shigella flexneri surface, where it colocalizes with IpaD at the tip of the type III secretion needle. Infect. Immun. 75:2626-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Page, A. L., M. Fromont-Racine, P. Sansonetti, P. Legrain, and C. Parsot. 2001. Characterization of the interaction partners of secreted proteins and chaperones of Shigella flexneri. Mol. Microbiol. 42:1133-1145. [DOI] [PubMed] [Google Scholar]
- 30.Pal, T., and T. L. Hale. 1989. Plasmid-associated adherence of Shigella flexneri in a HeLa cell model. Infect. Immun. 57:2580-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parsot, C., R. Menard, P. Gounon, and P. J. Sansonetti. 1995. Enhanced secretion through the Shigella flexneri Mxi-Spa translocon leads to assembly of extracellular proteins into macromolecular structures. Mol. Microbiol. 16:291-300. [DOI] [PubMed] [Google Scholar]
- 32.Parsot, C. 2009. Shigella type III secretion effectors: how, where, when, for what purposes? Curr. Opin. Microbiol. 12:110-116. [DOI] [PubMed] [Google Scholar]
- 33.Phalipon, A., J. Arondel, F. Nato, S. Rouyre, J. C. Mazie, and P. J. Sansonetti. 1992. Identification and characterization of B-cell epitopes of IpaC, an invasion-associated protein of Shigella flexneri. Infect. Immun. 60:1919-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roehrich, A. D., I. Martinez-Argudo, S. Johnson, A. J. Blocker, and A. K. Veenendaal. 2010. The extreme C terminus of Shigella flexneri IpaB is required for regulation of type III secretion, needle tip composition, and binding. Infect. Immun. 78:1682-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sansonetti, P. J., D. J. Kopecko, and S. B. Formal. 1982. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect. Immun. 35:852-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schroeder, G. N., and H. Hilbi. 2008. Molecular pathogenesis of Shigella spp.: controlling host cell signaling, invasion, and death by type III secretion. Clin. Microbiol. Rev. 21:134-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stensrud, K. F., P. R. Adam, C. D. La Mar, A. J. Olive, G. H. Lushington, R. Sudharsan, N. L. Shelton, R. S. Givens, W. L. Picking, and W. D. Picking. 2008. Deoxycholate interacts with IpaD of Shigella flexneri in inducing the recruitment of IpaB to the type III secretion apparatus needle tip. J. Biol. Chem. 283:18646-18654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veenendaal, A. K., J. L. Hodgkinson, L. Schwarzer, D. Stabat, S. F. Zenk, and A. J. Blocker. 2007. The type III secretion system needle tip complex mediates host cell sensing and translocon insertion. Mol. Microbiol. 63:1719-1730. [DOI] [PubMed] [Google Scholar]
- 39.Zenk, S. F., D. Stabat, J. L. Hodgkinson, A. K. Veenendaal, S. Johnson, and A. J. Blocker. 2007. Identification of minor inner-membrane components of the Shigella type III secretion system ‘needle complex.’ Microbiology 153:2405-2415. [DOI] [PubMed] [Google Scholar]