Abstract
Parachlamydia acanthamoebae is a Chlamydia-related organism whose pathogenic role in pneumonia is supported by serological and molecular clinical studies and an experimental mouse model of lung infection. Toll-like receptors (TLRs) play a seminal role in sensing microbial products and initiating innate immune responses. The aim of this study was to investigate the roles of MyD88, TLR2, and TLR4 in the interaction of Parachlamydia with macrophages. Here, we showed that Parachlamydia entered bone-marrow derived macrophages (BMDMs) in a TLR-independent manner but did not multiply intracellularly. Interestingly, compared to live bacteria, heat-inactivated Parachlamydia induced the production of substantial amounts of tumor necrosis factor alpha (TNF), interleukin-6 (IL-6), and IL-12p40 by BMDMs and of TNF and IL-6 by peritoneal macrophages as well as RAW 264.7 and J774 macrophage cell lines. Cytokine production by BMDMs, which was partially inhibited upon trypsin treatment of Parachlamydia, was dependent on MyD88, TLR4, and, to a lesser extent, TLR2. Finally, MyD88−/−, TLR4−/−, and TLR2−/− mice were as resistant as wild-type mice to lung infection following the intratracheal instillation of Parachlamydia. Thus, in contrast to Chlamydia pneumoniae, Parachlamydia acanthamoebae weakly stimulates macrophages, potentially compensating for its low replication capacity in macrophages by escaping the innate immune surveillance.
Parachlamydia acanthamoebae is a strict intracellular bacterium which naturally infects free-living amoebae. Like other members of the Chlamydiales order, it exhibits a two-stage developmental cycle with infectious elementary bodies and metabolically active replicating reticulate bodies (25). Several pieces of evidence support the role of P. acanthamoebae as a new agent of lower respiratory tract infection (reviewed in references 18 and 32). The first hint was provided by the recovery of P. acanthamoebae strain Hall's coccus from the water of a humidifier associated with an outbreak of fever and the presence of anti-Parachlamydia antibodies among exposed individuals (2). Additional serological studies demonstrated a higher seropositivity rate among patients with pneumonia than among controls (20, 33). Furthermore, parachlamydial DNA was detected by PCR in mononuclear cells of a patient with bronchitis and in sputa and bronchoalveolar lavage samples from patients with lower respiratory tract infections (11, 12, 19, 37). Moreover, Parachlamydia infects human pneumocytes and macrophages in vitro (7, 23, 24). Finally, we recently developed an experimental model in which mice injected with living Parachlamydia showed signs of severe pneumonia and bacterial localization in cells that were likely pneumocytes and macrophages (9, 10).
The sensing of invasive pathogens by innate immune cells relies on their capacity to sense microbial molecular motifs through pattern recognition receptors. Toll-like receptors (TLRs) expressed on the surface or in the endosomes of immune cells allow the detection of microbially derived molecular structures such as lipids, proteins, and nucleic acids. TLR4 is an obligate partner for the host response to bacterial lipopolysaccharide (LPS) (endotoxin) and most Gram-negative bacteria (1). TLR2, which generates heterodimers in combination with either TLR1 or TLR6, has been reported to recognize a broad range of microbial compounds, among which are lipopeptides, lipoproteins, peptidoglycan subcomponents, and β-glucans (29). The activation of the intracellular signaling pathways upon microbial recognition by TLRs engages several adaptor molecules. Myeloid differentiation primary response gene 88 (MyD88) specifies most TLRs and the TLR4 MyD88-dependent signaling pathway, which is involved in the activation of mitogen-activated protein kinases and nuclear factor κB and in the generation of proinflammatory cytokines and immune-related genes (35).
Although Parachlamydia may represent an emerging agent of pneumonia (18, 26), very little is known about its recognition by innate immune cells. Unfortunately, the mechanisms involved in the sensing of Chlamydia are most likely irrelevant for Parachlamydia given the specificities of this bacterium, such as its ability to replicate within amoebae (22) and its genome size, which is more than twice that of Chlamydia (21). In addition, the bioinformatics-based annotation of the genome of the Parachlamydia-related symbiont UWE25 indicates that it likely possesses a truncated LPS and lacks most immunogenic outer membrane proteins present in the Chlamydiaceae (27). Considering the central role played by TLRs in microbial sensing, we studied the contributions of MyD88, TLR2, and TLR4 in the recognition of P. acanthamoebae by macrophages in vitro and in the outcome of parachlamydial infection in an experimental model of pneumonia in mice. Our results show that living P. acanthamoebae enters but does not multiply in macrophages and that heat-inactivated P. acanthamoebae stimulates cytokine production by macrophages in a MyD88/TLR4-dependent manner and to a lesser extend through TLR2. Furthermore, MyD88−/−, TLR4−/−, and TLR2−/− mice are resistant to P. acanthamoebae infection. Taken together, these data indicate that P. acanthamoebae weakly stimulates the innate immune system, which may allow the bacterium to survive in infected cells despite its low replication capacity.
MATERIALS AND METHODS
Mice.
Eight- to 12-week-old female BALB/c and C57BL/6 mice were obtained from Charles River Laboratories (L'arbesle, France) and acclimatized for at least 1 week before experimentation. MyD88−/−, TLR2−/−, and TLR4−/− mice have been described previously (28, 30, 45). All animal procedures were approved by the Office Vétérinaire du Canton de Vaud, Lausanne, Switzerland (authorization no. 876.5, 877.5, and 1860.1), and performed according to our institutional guidelines for animal experiments.
Cells and reagents.
P. acanthamoebae strain Hall's coccus was propagated in Acanthamoeba castellanii (strain ATCC 30010; American Type Culture Collection, Manassas, VA) in peptone-yeast extract-glucose broth medium (PYG) at 32°C. After 6 days of incubation, cultures were harvested and the bacteria were purified using a sucrose barrier, as previously described (7). Bacteria were inactivated by heating for 1 h at 95°C. In selected experiments, the supernatant and the insoluble material from heat-inactivated P. acanthamoebae were obtained by centrifugation (10 min at 20,800 × g). The insoluble pellet was treated at 37°C for 1 h with trypsin (50 μg/ml) or for 8 h with DNase I (100 μg/ml) (Sigma-Aldrich, St. Louis, MO). Salmonella enterica serovar Minnesota ultrapure LPS was from List Biologicals Laboratories (Campbell, CA) and palmitoyl-cys((RS)-2,3-di(palmitoyloxy)-propyl)-Ser-Lys-Lys-Lys-Lys-OH trifluoroacetate salt (Pam3CSK4) from EMC Microcollections (Tuebingen, Germany).
Bone marrow-derived macrophages (BMDMs) and thioglycolate-elicited peritoneal macrophages were obtained as previously described (15, 41). BMDMs were cultured in Iscove's modified Dulbecco's medium (IMDM) (Invitrogen, Basel, Switzerland) containing 10% fetal calf serum (FCS) (Seromed, Berlin, Germany) and 50 μM 2-mercaptoethanol. Thioglycolate-elicited peritoneal macrophages and RAW 264.7 and J774 macrophage cell lines (ATCC) were cultured in RPMI 1640 medium (Invitrogen) containing 2 mM glutamine and 10% FCS.
Phagocytosis.
BMDMs (5 × 105) were grown overnight on 12-mm-diameter round coverslips in flat-bottom 24-well cell culture plates (Corning, Lowell, MA). After washing, BMDMs were incubated with living or heat-inactivated bacteria (bacterium-to-cell ratio, 100:1), centrifuged at 1,793 × g for 10 min at room temperature, and incubated for 15 min at 37°C. BMDMs were washed with RPMI medium and further incubated for different periods of time at 37°C in a 5% CO2 atmosphere. Coverslips were washed with phosphate-buffered saline (PBS). Cells were fixed with 3% paraformaldehyde, washed with PBS, and permeabilized in PBS containing 0.3% Tween (Merck-Schuchardt, Hohenbrunn, Germany). Coverslips were incubated with rabbit anti-Parachlamydia antibodies for 30 min at room temperature, washed, and incubated with Alexa 488-coupled anti-rabbit immunoglobulin antibodies (Bio-Rad, Rheinach, Switzerland) and 1% Evans blue (bioMérieux, Marcy-l'Etoile, France) for 30 min at room temperature. Primary and secondary antibodies were diluted in PBS containing 0.3% Tween and 10% FCS. The coverslips were washed successively with PBS-Tween and PBS and mounted onto glass slides. Cells were observed under an epifluorescence microscope (Axioplan 2; Zeiss, Feldbach, Switzerland) and a confocal microscope (Axioplan 2 LSM 510; Zeiss). The number of bacteria per macrophage was determined by counting three times the number of Parachlamydia organisms within about 50 macrophages.
Cytokine measurements.
Macrophages were plated at a density of 5 × 105 cells per well in 24-well culture plates (Costar). Cells were stimulated for 4 to 24 h with LPS (10 ng/ml), Pam3CSK4 (100 ng/ml), or P. acanthamoebae. The concentrations of tumor necrosis factor (TNF) and interleukin-6 (IL-6) in cell culture supernatants were measured using WEHI 164 clone 13 mouse fibrosarcoma cells (TNF) and 7TD-1 mouse hybridoma cells (IL-6) as described previously (40). IL-1β and IL-12p40 were quantified by enzyme-linked immunosorbent assay (ELISA) (RnD, Minneapolis, MN, and Becton Dickinson, San Diego, CA).
Mouse model of infection.
Mice were anesthetized by an intraperitoneal injection of ketamine (100 mg/kg) and midazolam (1 mg/kg). The trachea was uncovered, and 1.25 × 108 to 2.5 × 108 P. acanthamoebae bacteria were injected intratracheally using a sterile 26-gauge needle. Sham-operated mice were injected with PBS. The incision was closed using surgical staples. The body weight and the survival of mice were followed at least once daily. In selected experiments, blood was collected after 24 h to quantify cytokine levels. At selected time points, mice were euthanized by CO2 inhalation. Lungs were harvested and snap-frozen in liquid nitrogen. DNA was extracted and the copy number of the P. acanthamoebae genome determined by quantitative PCR as described previously (8). Parts of mouse lungs were minced in PBS for cytokine quantification or fixed with 4% paraformaldehyde for histology. Hematoxylin-eosin stained slides were read blindly by M.K.P. and G.G.
RESULTS
Parachlamydia acanthamoebae is internalized by macrophages but does not multiply inside macrophages.
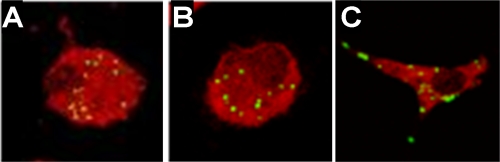
Entry of P. acanthamoebae into BMDMs was confirmed by confocal microscopy and found to be similar for wild-type, TLR2−/−, and TLR4−/− BMDMs (Fig. 1). No growth of Parachlamydia was observed in BMDMs. The mean numbers of bacteria per macrophage at 15 min after infection were 7.6, 11.0, and 7.9 and those 48 h later were 10, 8.6, and 13.3 in wild-type, TLR2−/−, and TLR4−/− BMDMs, respectively (P > 0.05 for all conditions).
FIG. 1.
Parachlamydia acanthamoebae is internalized by macrophages. Intracellular detection of P. acanthamoebae within wild-type (A), TLR2−/− (B), and TLR4−/− (C) BMDMs at 4 h postinfection is shown. Macrophages and P. acanthamoebae were stained with Evans blue and Alexa 488, respectively, and analyzed by confocal microscopy as described in Materials and Methods.
MyD88, TLR4, and, to a lesser extent, TLR2 contribute to the sensing of heat-inactivated Parachlamydia acanthamoebae by macrophages.
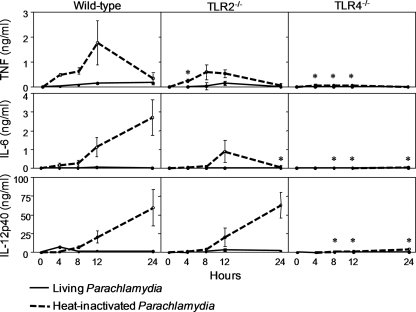
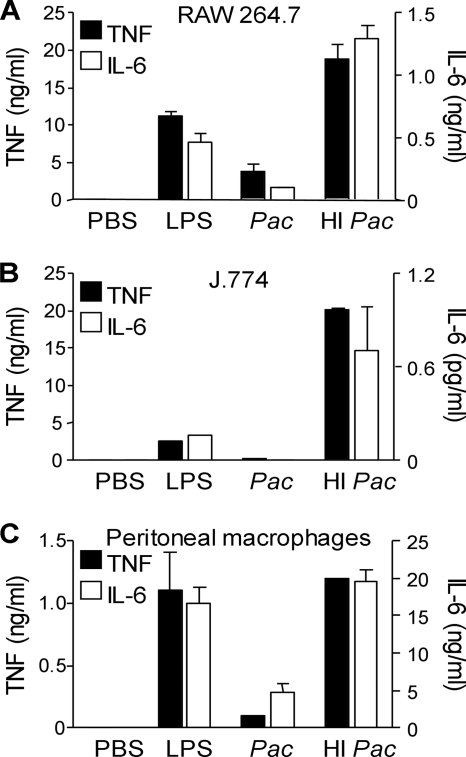
As a first step to investigate whether TLRs play a role in the sensing of P. acanthamoebae by innate immune cells, we compared the production of TNF, IL-6, and IL-12p40 by wild-type, TLR2−/−, and TLR4−/− BMDMs infected with P. acanthamoebae for 4, 8, 12, and 24 h. Experiments were performed using both living and heat-inactivated (1 h at 95°C) bacteria (Fig. 2). Overall, TLR2−/− BMDMs exposed to heat-inactivated P. acanthamoebae produced reduced levels of TNF and IL-6 compared to wild-type BMDMs, whereas TLR4−/− BMDMs produced almost no TNF, IL-6, or IL-12p40. Strikingly, living P. acanthamoebae slightly stimulated cytokine production by BMDMs. To substantiate this observation, we then tested the cytokine-inducing capacities of living and heat-inactivated P. acanthamoebae in peritoneal macrophages and in the RAW 264.7 and J774 macrophage cell lines. Recapitulating the results obtained with BMDMs, living bacteria induced much lower levels of TNF and IL-6 than heat-inactivated bacteria (P < 0.001) (Fig. 3).
FIG. 2.
Heat-inactivated Parachlamydia acanthamoebae stimulates the production of proinflammatory cytokines by macrophages through TLR4 and, to a lesser extent, TLR2. The kinetics of TNF, IL-6, and IL-12p40 production by wild-type, TLR2−/−, and TLR4−/− BMDMs infected with live and heat-inactivated P. acanthamoebae (multiplicity of infection [MOI], 100) are shown. Data are means ± standard deviations (SD) for triplicate samples from one experiment and are representative of three independent experiments. *, P < 0.05 versus result for wild-type BMDMs.
FIG. 3.
Heat-inactivated Parachlamydia acanthamoebae stimulates cytokine production by RAW 264.7, J774, and peritoneal macrophages. TNF and IL-6 production by RAW 264.7 (A), J774 (B), and thioglycolate-elicited peritoneal (C) macrophages cultured with PBS, LPS, and living (Pac) or heat-inactivated (HI Pac) P. acanthamoebae is shown. Data are means ± SD for triplicates samples from one experiment; P < 0.05 for living versus heat-inactivated P. acanthamoebae (A to C).
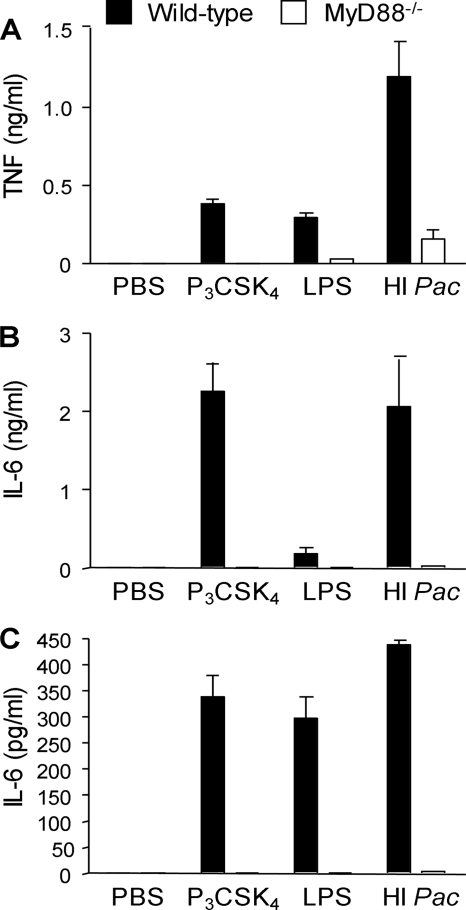
Considering that MyD88 is required for signaling through TLR2 and TLR4, we then compared the responses of wild-type and MyD88−/− peritoneal macrophages and BMDMs to infection with heat-inactivated P. acanthamoebae (Fig. 4). As expected, MyD88 deficiency strongly impaired TNF production and fully abrogated IL-6 production by macrophages, indicating that heat-inactivated P. acanthamoebae stimulated the release of cytokines in a MyD88-dependent manner.
FIG. 4.
MyD88 is required for the sensing of heat-inactivated Parachlamydia acanthamoebae by macrophages. TNF and IL-6 production by thioglycolate-elicited macrophages (A and B) and BMDMs (C) exposed to PBS, Pam3CSK4 (P3CSK4), LPS, and heat-inactivated P. acanthamoebae (HI Pac) is shown. Data are means ± SD for triplicate samples from one experiment and are representative of two independent experiments; P < 0.05 for wild-type versus MyD88−/− BMDMs for all conditions.
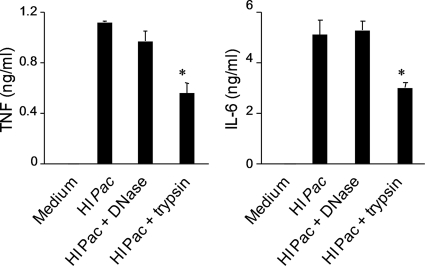
To gain insight into the nature of the molecular structures involved in the activation of macrophages by P. acanthamoebae, the supernatant and the insoluble material obtained after centrifugation of heat-inactivated P. acanthamoebae preparations were treated with DNase and trypsin and tested for their ability to stimulate TNF, IL-6, and IL-12p40 release by BMDMs (Fig. 5 and data not shown). Whereas the supernatant of heat-inactivated bacteria did not induce cytokine release by BMDMs, the bacterial pellet recovered in PBS stimulated cytokine production to the same extent as the original bacterial preparation. Trypsin treatment reduced TNF and IL-6 production induced by the bacterial pellet by 2-fold, whereas DNase treatment had no impact.
FIG. 5.
Heat-inactivated Parachlamydia acanthamoebae expresses a trypsin-sensitive determinant that stimulates cytokine production by macrophages. TNF and IL-6 production by BMDMs exposed to heat-inactivated P. acanthamoebae insoluble material treated or not with trypsin or DNase is shown. Data are means ± SD for triplicates samples from one experiment. *, P < 0.05.
Altogether, these data suggested that heat inactivation of P. acanthamoebae exposed a trypsin-sensitive microbial molecular pattern that stimulated cytokine release by macrophages.
Roles of MyD88, TLR2, and TLR4 in the host response to Parachlamydia acanthamoebae pneumonia.
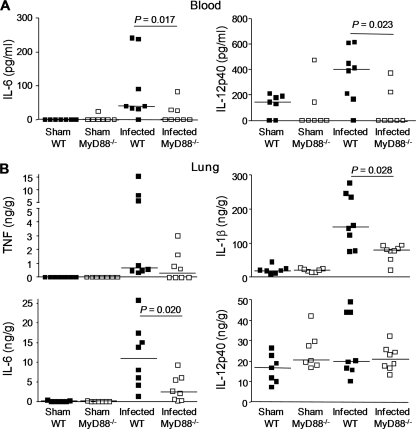
To analyze in vivo the roles of the TLR pathways in modulating the innate immune response to P. acanthamoebae, we established a model of pneumonia following the intratracheal instillation of 2.5 × 108 bacteria in wild-type and MyD88−/− animals. Lungs and blood were collected from wild-type and MyD88−/− animals at 24 h after infection with 2.5 × 108 P. acanthamoebae bacteria to quantify cytokines. In line with our in vitro observations, larger amounts of IL-1β and IL-6 were detected in the lungs and larger amounts of IL-6 and IL-12p40 were detected in the circulation of wild-type mice, confirming in vivo that MyD88 is involved in the host response to P. acanthamoebae (Fig. 6A and B).
FIG. 6.
Reduced cytokine levels in the blood and the lungs of MyD88−/− mice infected with Parachlamydia acanthamoebae. Wild-type (WT) (black symbols) and MyD88−/− (white symbols) C57BL/6 mice (n = 8 mice per group) were either sham operated or infected with 2.5 × 108 P. acanthamoebae. Blood (A) and lungs (B) were collected after 24 h to quantify TNF, IL-6, IL-1β, and IL-12p40. The horizontal line represents the median value. Of note, TNF and IL-1β were not detected in the blood.
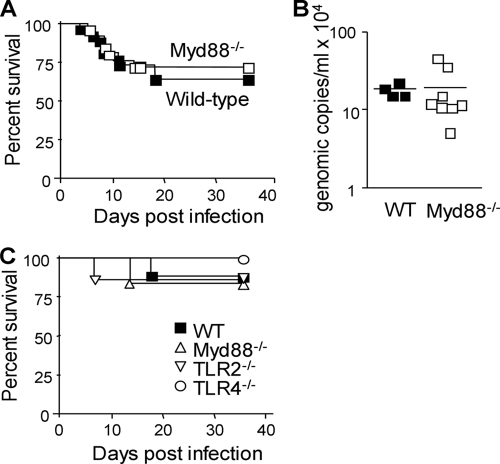
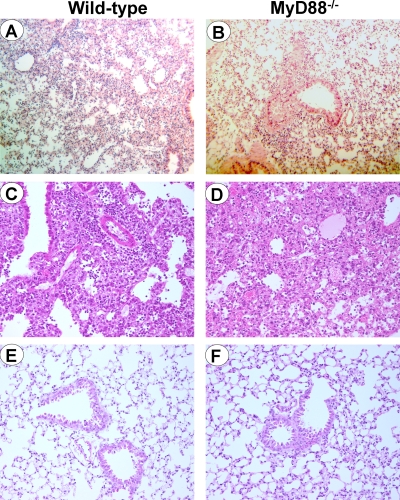
To analyze whether the reduced inflammatory response impaired host defenses, we then compared the susceptibilities of wild-type and MyD88−/− animals to infection with 2.5 × 108 bacteria (Fig. 7A). Interestingly, MyD88−/− mice were as resistant as wild-type mice to infection with P. acanthamoebae (64% survival and 72% survival in wild-type and MyD88−/− mice, respectively; n = 25 or 26 [P > 0.5]). In agreement with these findings, at 8 days after the onset of infection, the copy numbers of the P. acanthamoebae genome in the lungs of wild-type and MyD88−/− mice were similar (Fig. 7B). Confirming these observations, deficiency in TLR2 or TLR4 did not increase the susceptibility of mice to infection with P. acanthamoebae (1.25 × 108 bacteria) (Fig. 7C). Moreover, histological analyses showed that the natural history was similar in wild-type and MyD88−/− mice infected with P. acanthamoebae. Thus, at 24 h postinfection, one mouse in each group (three mice per group) presented an inflammatory infiltrate, which was composed mainly of neutrophils (Fig. 8A and B). Moreover, wild-type and MyD88−/− mice that died at about 7 to 10 days postinfection exhibited signs of severe pneumonia, with multifocal to confluent lympho-monocytic infiltrates composed primarily of macrophages (Fig. 8C and D).
FIG. 7.
MyD88−/−, TLR2−/−, and TLR4−/− mice are resistant to Parachlamydia infection. Survival (A and C) and P. acanthamoebae genomic copy number in the lungs (day 8) (B) of mice infected with 2.5 × 108 (A and B) and 1.25 × 108 (C) P. acanthamoebae bacteria is shown (n = 25 or 26 mice per group for panel A and n = 6 to 8 mice per group for panel C); P > 0.05 for all conditions.
FIG. 8.
Histology of lungs from C57BL/6 wild-type (A, C, and E) and MyD88−/− (B, D, and F) mice either infected with 2.5 × 108 P. acanthamoebae bacteria (A to D) or sham operated (E and F). Lungs were collected after 24 h (A, B, E, and F) or at the time of death (C and D). Note the presence of focal inflammation with the presence of neutrophils at 24 h postinfection of both wild-type (A) and MyD88−/− (B) mice and the presence of multifocal lympho-monocytic infiltrates, with a predominance of macrophages, in both wild-type mice (C) and MyD88−/− (D) mice at time of death. No inflammatory infiltrates were observed in sham operated wild-type (E) and MyD88−/− (F) mice. Hematoxylin-eosin staining was used; magnification, ×200 (A to F).
DISCUSSION
Clinical and experimental data suggest that P. acanthamoebae represents a new agent of lower respiratory tract infection (18). The characterization of the innate immune receptors engaged by P. acanthamoebae is essential for improving our understanding of the pathogenesis of P. acanthamoebae infection. Analyses of the response of macrophages to P. acanthamoebae revealed a critical role for the MyD88/TLR4 pathway, and to a lesser extent TLR2, in the production of proinflammatory cytokines. Yet, heat-inactivated bacteria were much more immunogenic than live bacteria. In line with these results, mice deficient in either MyD88, TLR4, or TLR2 were resistant to lung infection with P. acanthamoebae.
P. acanthamoebae quickly entered BMDMs. P. acanthamoebae internalization was not affected in macrophages deficient in either TLR2 or TLR4, corroborating the notion that TLRs do not function primarily as phagocytic receptors (46). Of note, the initial rate of infection of BMDMs (around 10 bacteria per BMDM) was similar to that of human macrophages, A549 pneumocytes, and HEL lung fibroblasts (two to four bacteria per infected cell [7, 24]). Nonetheless, upon internalization, P. acanthamoebae did not multiply inside BMDMs, whereas it grows poorly in human macrophages, A549, and HEL cells (7, 24). Overall, these data suggest that P. acanthamoebae has a rather limited replication capacity in innate immune cells compared to amoebae, where an exponential growth of 2 log units was observed (22).
Unexpectedly, heat-inactivated P. acanthamoebae induced the production of substantial levels of cytokines by macrophages, whereas living bacteria only weakly stimulated cytokine production. Most likely, heat inactivation exposed an immunogenic determinant, probably a protein given its partial susceptibility to trypsin, hidden in living bacteria. An obvious candidate is a heat shock protein (HSP). Indeed, the genome of P. acanthamoebae contains several genes encoding putative HSPs (21), and HSP60 from Chlamydia pneumoniae and Chlamydia trachomatis have been reported to strongly stimulate innate immune cells (4, 13, 31). Further investigations will be required to characterize the structure of the microbially associated molecular pattern(s) of P. acanthamoebae that stimulates innate immune cells.
The recognition of heat-inactivated P. acanthamoebae by macrophages occurred mainly through MyD88/TLR4. Yet, TNF and IL-6 production by TLR2−/− BMDMs was also reduced to some extent. Interestingly, the MyD88/TLR4 pathway has previously been reported to be involved in the sensing of C. pneumoniae and C. pneumoniae HSP60 (4, 42, 43) and to mediate the acute pulmonary inflammation in mice following the intratracheal instillation of C. pneumoniae HSP60 (5). Furthermore, the TLR4 pathway was implicated in C. trachomatis HSP60-induced trophoblast apoptosis (16). Besides TLR4, TLR2 was shown to play a primary role in the activation of dendritic cells by C. pneumoniae and C. pneumoniae HSP60 (13, 38) and to mediate C. pneumoniae-induced macrophage foam cell formation (6). Moreover, TLR2 was implicated in oviduct pathology resulting from genital tract infection with C. trachomatis (14) and triggered the early immune response to C. pneumoniae pulmonary infection (39). Further substantiating a role for TLRs in the sensing of Chlamydiaceae, C. pneumoniae-infected MyD88−/− mice showed impaired production of cytokines and chemokines and delayed recruitment of T cells, and they ultimately died from severe chronic lung infection (34). Altogether, these data point toward a fundamental role of MyD88, TLR2, and/or TLR4 in detecting Chlamydia spp., in particular Chlamydia HSPs. In the future, it might be interesting to study the role of intracellular sensors of bacteria such as nucleotide-binding and oligomerization domain-like receptors (NLRs), which have been shown to mediate a cell response to C. pneumoniae, C. trachomatis, and Chlamydia muridarum (3, 36, 44, 47).
Models of lung infection have underscored the vital role of the early inflammatory response in mediating bacterial clearance and conditioning survival. As mentioned above, mice deficient in MyD88, TLR4, and TLR2 ultimately developed a severe and chronic lung inflammation involved in morbidity and mortality after C. pneumoniae infection (34, 39, 44). These observations demonstrate that TLRs exert a two-sided role in Chlamydia infections, acting on the one hand as key initiators of the host anti-Chlamydia defense responses and on the other hand as important contributors to tissue damage and Chlamydia pathogenicity. Astonishingly at first sight, MyD88, TLR4, and TLR2 deficient mice were fully resistant to infection with P. acanthamoebae. Whereas these data could argue for a minor pathogenic role of P. acanthamoebae in healthy individuals, it is worthwhile to remember that most documented cases of probable infection with P. acanthamoebae were reported in immunocompromised HIV-infected or grafted patients (19, 33). Thus, it would be of interest to further analyze the pathogenicity of P. acanthamoebae in severely immunocompromised mice. Taking into account that P. acanthamoebae modestly replicated in infected mammalian cells in vitro (7, 24) and that the bacterial load decreased with time (10-fold in 2 days and 1,000-fold in 10 days) in mice infected with P. acanthamoebae (9), P. acanthamoebae may compensate for its low replication capacity by escaping innate immune surveillance. Importantly, there are significant differences in the replication capacities of Parachlamydia in murine and human macrophages (no versus limited replication capacity) as well as in cytokine production by these cells following exposure to heat-inactivated bacteria (presence and absence of proinflammatory cytokine secretion). Thus, the mouse model may partially reflect what occurs in human infections.
In summary, we report the first insight into the mechanisms of recognition of P. acanthamoebae by the innate immune system. MyD88 or TLR4 deficiencies do not impair mouse survival after P. acanthamoebae infection, whereas they did so after C. pneumoniae infection, indicating that this Chlamydia-related bacterium has evolved a different strategy to interact with the host. It will be interesting to investigate the innate immune response to other Chlamydia-related bacteria such as Waddlia chondrophila, a potential agent of miscarriage and lower respiratory tract infection which was shown to replicate in human macrophages (17). Finally, further work will be devoted to the identification of the molecular pattern(s) of P. acanthamoebae that stimulates innate immune cells, as this may help in development of improved serologic diagnostic tests for infection and better understanding of the pathogenesis of Parachlamydia infection.
Acknowledgments
This study was supported by Swiss National Science Foundation grants 310000-1140 (T.R.), 310000-118266 (T.C.), 310030-124843 (G.G.), and 3200BO-116445 (G.G.) and by the Leenaards Foundation (T.R., T.C., and G.G.). T.C. is supported by the Bristol-Myers Squibb and the Santos-Suarez Foundations. Gilbert Greub is supported by the Leenaards Foundation through a career award (Bourse Leenaards pour la Relève Académique en Médecine Clinique à Lausanne).
None of the authors has a conflict of interest.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 13 September 2010.
REFERENCES
- 1.Beutler, B., and E. T. Rietschel. 2003. Innate immune sensing and its roots: the story of endotoxin. Nat. Rev. Immunol. 3:169-176. [DOI] [PubMed] [Google Scholar]
- 2.Birtles, R. J., T. J. Rowbotham, C. Storey, T. J. Marrie, and D. Raoult. 1997. Chlamydia-like obligate parasite of free-living amoebae. Lancet 349:925-926. [DOI] [PubMed] [Google Scholar]
- 3.Buchholz, K. R., and R. S. Stephens. 2006. Activation of the host cell proinflammatory interleukin-8 response by Chlamydia trachomatis. Cell. Microbiol. 8:1768-1779. [DOI] [PubMed] [Google Scholar]
- 4.Bulut, Y., E. Faure, L. Thomas, H. Karahashi, K. S. Michelsen, O. Equils, S. G. Morrison, R. P. Morrison, and M. Arditi. 2002. Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88-dependent pathway. J. Immunol. 168:1435-1440. [DOI] [PubMed] [Google Scholar]
- 5.Bulut, Y., K. Shimada, M. H. Wong, S. Chen, P. Gray, R. Alsabeh, T. M. Doherty, T. R. Crother, and M. Arditi. 2009. Chlamydial heat shock protein 60 induces acute pulmonary inflammation in mice via the Toll-like receptor 4- and MyD88-dependent pathway. Infect. Immun. 77:2683-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao, F., A. Castrillo, P. Tontonoz, F. Re, and G. I. Byrne. 2007. Chlamydia pneumoniae-induced macrophage foam cell formation is mediated by Toll-like receptor 2. Infect. Immun. 75:753-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casson, N., N. Medico, J. Bille, and G. Greub. 2006. Parachlamydia acanthamoebae enters and multiplies within pneumocytes and lung fibroblasts. Microbes Infect. 8:1294-1300. [DOI] [PubMed] [Google Scholar]
- 8.Casson, N., K. M. Posfay-Barbe, A. Gervaix, and G. Greub. 2008. New diagnostic real-time PCR for specific detection of Parachlamydia acanthamoebae DNA in clinical samples. J. Clin. Microbiol. 46:1491-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casson, N., J. M. Entenza, N. Borel, A. Pospischil, and G. Greub. 2008. Murine model of pneumonia caused by Parachlamydia acanthamoebae. Microb. Pathog. 45:92-97. [DOI] [PubMed] [Google Scholar]
- 10.Casson, N., K. Sommer, A. Klos, J. C. Stehle, M. Pusztaszeri, and G. Greub. 2008. Intranasal murine model of infection by Parachlamydia acanthamoebae, p. 17. Proc. Annu. Meet. Swiss Soc. Infect. Dis. [DOI] [PubMed]
- 11.Corsaro, D., D. Venditti, A. Le Faou, P. Guglielmetti, and M. Valassina. 2001. A new chlamydia-like 16S rDNA sequence from a clinical sample. Microbiology 147:515-516. [DOI] [PubMed] [Google Scholar]
- 12.Corsaro, D., D. Venditti, and M. Valassina. 2002. New parachlamydial 16S rDNA phylotypes detected in human clinical samples. Res. Microbiol. 153:563-567. [DOI] [PubMed] [Google Scholar]
- 13.Costa, C. P., C. J. Kirschning, D. Busch, S. Durr, L. Jennen, U. Heinzmann, S. Prebeck, H. Wagner, and T. Miethke. 2002. Role of chlamydial heat shock protein 60 in the stimulation of innate immune cells by Chlamydia pneumoniae. Eur. J. Immunol. 32:2460-2470. [DOI] [PubMed] [Google Scholar]
- 14.Darville, T., J. M. O'Neill, C. W. Andrews, Jr., U. M. Nagarajan, L. Stahl, and D. M. Ojcius. 2003. Toll-like receptor-2, but not Toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J. Immunol. 171:6187-6197. [DOI] [PubMed] [Google Scholar]
- 15.Delaloye, J., T. Roger, Q. G. Steiner-Tardivel, D. Le Roy, M. Knaup Reymond, S. Akira, V. Petrilli, C. E. Gomez, B. Perdiguero, J. Tschopp, G. Pantaleo, M. Esteban, and T. Calandra. 2009. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 5:e1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Equils, O., D. Lu, M. Gatter, S. S. Witkin, C. Bertolotto, M. Arditi, J. A. McGregor, C. F. Simmons, and C. J. Hobel. 2006. Chlamydia heat shock protein 60 induces trophoblast apoptosis through TLR4. J. Immunol. 177:1257-1263. [DOI] [PubMed] [Google Scholar]
- 17.Goy, G., A. Croxatto, and G. Greub. 2008. Waddlia chondrophila enters and multiplies within human macrophages. Microbes Infect. 10:556-562. [DOI] [PubMed] [Google Scholar]
- 18.Greub, G. 2009. Parachlamydia acanthamoebae, an emerging agent of pneumonia. Clin. Microbiol. Infect. 15:18-28. [DOI] [PubMed] [Google Scholar]
- 19.Greub, G., P. Berger, L. Papazian, and D. Raoult. 2003. Parachlamydiaceae as rare agents of pneumonia. Emerg. Infect. Dis. 9:755-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greub, G., I. Boyadjiev, B. La Scola, D. Raoult, and C. Martin. 2003. Serological hint suggesting that Parachlamydiaceae are agents of pneumonia in polytraumatized intensive care patients. Ann. N. Y. Acad. Sci. 990:311-319. [DOI] [PubMed] [Google Scholar]
- 21.Greub, G., C. Kebbi-Beghdadi, C. Bertelli, F. Collyn, B. M. Riederer, C. Yersin, A. Croxatto, and D. Raoult. 2009. High throughput sequencing and proteomics to identify immunogenic proteins of a new pathogen: the dirty genome approach. PLoS One 4:e8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greub, G., B. La Scola, and D. Raoult. 2003. Parachlamydia acanthamoeba is endosymbiotic or lytic for Acanthamoeba polyphaga depending on the incubation temperature. Ann. N. Y. Acad. Sci. 990:628-634. [DOI] [PubMed] [Google Scholar]
- 23.Greub, G., J. L. Mege, J. P. Gorvel, D. Raoult, and S. Meresse. 2005. Intracellular trafficking of Parachlamydia acanthamoebae. Cell. Microbiol. 7:581-589. [DOI] [PubMed] [Google Scholar]
- 24.Greub, G., J. L. Mege, and D. Raoult. 2003. Parachlamydia acanthamoebae enters and multiplies within human macrophages and induces their apoptosis Infect. Immun. 71:5979-5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greub, G., and D. Raoult. 2002. Crescent bodies of Parachlamydia acanthamoeba and its life cycle within Acanthamoeba polyphaga: an electron micrograph study. Appl. Environ. Microbiol. 68:3076-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greub, G., and D. Raoult. 2002. Parachlamydiaceae: potential emerging pathogens. Emerg. Infect. Dis. 8:625-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horn, M., A. Collingro, S. Schmitz-Esser, C. L. Beier, U. Purkhold, B. Fartmann, P. Brandt, G. J. Nyakatura, M. Droege, D. Frishman, T. Rattei, H. W. Mewes, and M. Wagner. 2004. Illuminating the evolutionary history of chlamydiae. Science 304:728-730. [DOI] [PubMed] [Google Scholar]
- 28.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 29.Ishii, K. J., S. Koyama, A. Nakagawa, C. Coban, and S. Akira. 2008. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe 3:352-363. [DOI] [PubMed] [Google Scholar]
- 30.Kawai, T., O. Adachi, T. Ogawa, K. Takeda, and S. Akira. 1999. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11:115-122. [DOI] [PubMed] [Google Scholar]
- 31.Kol, A., T. Bourcier, A. H. Lichtman, and P. Libby. 1999. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J. Clin. Invest. 103:571-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamoth, F., and G. Greub. 2010. Amoebal pathogens as emerging causal agents of pneumonia. FEMS Microbiol. Rev. 34:260-280. [DOI] [PubMed] [Google Scholar]
- 33.Marrie, T. J., D. Raoult, B. La Scola, R. J. Birtles, and E. de Carolis. 2001. Legionella-like and other amoebal pathogens as agents of community-acquired pneumonia. Emerg. Infect. Dis. 7:1026-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naiki, Y., K. S. Michelsen, N. W. Schroder, R. Alsabeh, A. Slepenkin, W. Zhang, S. Chen, B. Wei, Y. Bulut, M. H. Wong, E. M. Peterson, and M. Arditi. 2005. MyD88 is pivotal for the early inflammatory response and subsequent bacterial clearance and survival in a mouse model of Chlamydia pneumoniae pneumonia. J. Biol. Chem. 280:29242-29249. [DOI] [PubMed] [Google Scholar]
- 35.O'Neill, L. A., and A. G. Bowie. 2007. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 7:353-364. [DOI] [PubMed] [Google Scholar]
- 36.Opitz, B., S. Forster, A. C. Hocke, M. Maass, B. Schmeck, S. Hippenstiel, N. Suttorp, and M. Krull. 2005. Nod1-mediated endothelial cell activation by Chlamydophila pneumoniae. Circ. Res. 96:319-326. [DOI] [PubMed] [Google Scholar]
- 37.Ossewaarde, J. M., and A. Meijer. 1999. Molecular evidence for the existence of additional members of the order Chlamydiales. Microbiology 145:411-417. [DOI] [PubMed] [Google Scholar]
- 38.Prebeck, S., C. Kirschning, S. Durr, C. da Costa, B. Donath, K. Brand, V. Redecke, H. Wagner, and T. Miethke. 2001. Predominant role of Toll-like receptor 2 versus 4 in Chlamydia pneumoniae-induced activation of dendritic cells. J. Immunol. 167:3316-3323. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez, N., N. Wantia, F. Fend, S. Durr, H. Wagner, and T. Miethke. 2006. Differential involvement of TLR2 and TLR4 in host survival during pulmonary infection with Chlamydia pneumoniae. Eur. J. Immunol. 36:1145-1155. [DOI] [PubMed] [Google Scholar]
- 40.Roger, T., J. David, M. P. Glauser, and T. Calandra. 2001. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature 414:920-924. [DOI] [PubMed] [Google Scholar]
- 41.Roger, T., C. Froidevaux, D. Le Roy, M. K. Reymond, A. L. Chanson, D. Mauri, K. Burns, B. M. Riederer, S. Akira, and T. Calandra. 2009. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc. Natl. Acad. Sci. U. S. A. 106:2348-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romano Carratelli, C., N. Mazzola, R. Paolillo, S. Sorrentino, and A. Rizzo. 2009. Toll-like receptor-4 (TLR4) mediates human beta-defensin-2 (HBD-2) induction in response to Chlamydia pneumoniae in mononuclear cells. FEMS Immunol. Med. Microbiol. 57:116-124. [DOI] [PubMed] [Google Scholar]
- 43.Sasu, S., D. LaVerda, N. Qureshi, D. T. Golenbock, and D. Beasley. 2001. Chlamydia pneumoniae and chlamydial heat shock protein 60 stimulate proliferation of human vascular smooth muscle cells via Toll-like receptor 4 and p44/p42 mitogen-activated protein kinase activation. Circ. Res. 89:244-250. [DOI] [PubMed] [Google Scholar]
- 44.Shimada, K., S. Chen, P. W. Dempsey, R. Sorrentino, R. Alsabeh, A. V. Slepenkin, E. Peterson, T. M. Doherty, D. Underhill, T. R. Crother, and M. Arditi. 2009. The NOD/RIP2 pathway is essential for host defenses against Chlamydophila pneumoniae lung infection. PLoS Pathog. 5:e1000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 46.Underhill, D. M., and B. Gantner. 2004. Integration of Toll-like receptor and phagocytic signaling for tailored immunity. Microbes Infect. 6:1368-1373. [DOI] [PubMed] [Google Scholar]
- 47.Welter-Stahl, L., D. M. Ojcius, J. Viala, S. Girardin, W. Liu, C. Delarbre, D. Philpott, K. A. Kelly, and T. Darville. 2006. Stimulation of the cytosolic receptor for peptidoglycan, Nod1, by infection with Chlamydia trachomatis or Chlamydia muridarum. Cell. Microbiol. 8:1047-1057. [DOI] [PubMed] [Google Scholar]