Structure and general properties
In 1992, two groups independently reported a new doubly spliced mRNA which potentially encodes for two viral proteins1;2. The first one shares the Env/Tax AUG and encodes for the nuclear/nucleolar p303. The second potentially initiates in frame through an internal methionine codon. This shorter protein, p13, represents the carboxy-terminal 87 amino acids of p30 and localizes in the mitochondria4;5. Whether p30 and p13 are indeed translated from the same doubly spliced mRNA is uncertain. To date, only indirect evidence of p30 protein expression in patients infected with HTLV-I exists. However, this is also true for most other viral proteins, including Tax and Rex, and this possibly reflects the latent state of the virus in vivo. p30-encoded mRNAs can be readily detected in HTLV-I-infected cell lines in vitro and in some freshly isolated samples from HTLV-1-infected subjects, and ATL and HAM/TSP patients6;7. Antibodies directed to p30 and cytotoxic T cells that recognize peptides from p30 have been found in HAM/TSP patients and HTLV-1 carriers8;9. p30 is dispensable for T-cell immortalization in vitro but is required for efficient viral propagation in vivo10–14. p30 is a 241 amino acid protein (Figure 1). The protein does not share any significant homology with known human proteins, but its composition is unusual with 23% Ser and 12% Arg residues. p30 is exceptionally basic with a theoretical isoelectric point (pI) of 11.71 and the net positive charge may support tight interactions between p30 and nucleic acids. Heterokaryon assays demonstrated that in the absence of other viral components, p30 is a non-shuttling protein. Using GFP-tagged p30 mutants, two Arg-rich domains were identified as nucleolar localization and/or retention signals (NoRS)15. These signals can also serve as nuclear localization signals (NLS). However, two additional NLS were identified in the N-terminal and C-terminal regions 16 (Figure 1). Live cell imaging technologies, fluorescence recovery after photo bleaching (FRAP) and inverse FRAP (iFRAP), indicated a high mobility of GFP-p30 in the nucleus. In contrast, a much slower kinetic was consistently detected in the nucleolus, suggesting that p30 is strongly retained in the nucleolus17. More detailed confocal analyses revealed that p30 is localized mainly to the granular component (GC) of the nucleolus, which corresponds to the site of pre-ribosome assembly. In fact, p30 was found to interact with the large ribosomal subunit L18a18. These interactions were functionally relevant and played a role in p30 nucleolar retention along with nascent mRNA. Several studies have shown that the ribosomal protein L18a interacts with eukaryotic initiation factor-3 and facilitates internal re-initiation of translation. Whether this is or is not the case for HTLV-I remains to be investigated.
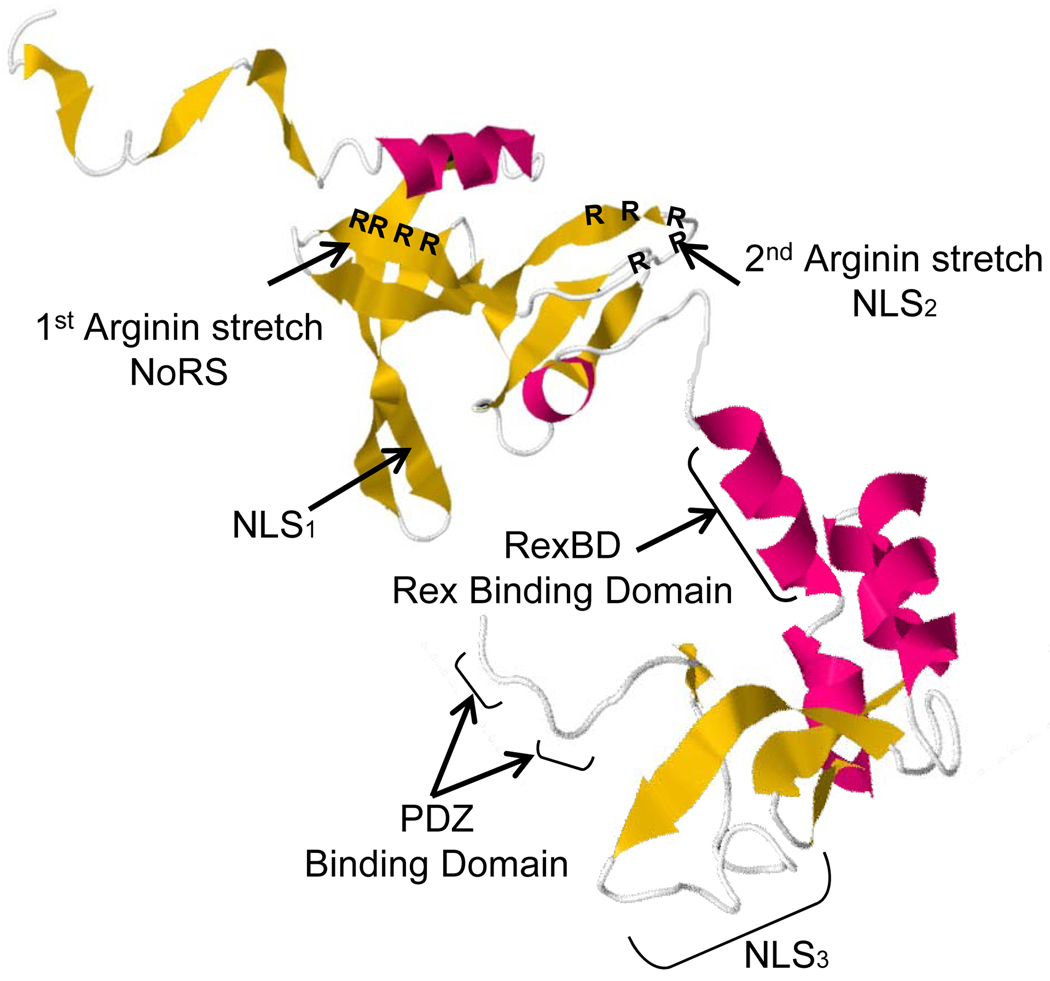
Figure 1.
Tertiary structure prediction of p30 protein. Protein structure prediction on the web: http://www.sbg.bio.ic.ac.uk/phyre a case study using the Phyre server. Kelley LA and Sternberg MJE Nature Protocols 4, 363–371 (2009). The first Arginin strech domain (73aa–78aa) is required for the Nucleolar Retention of the protein (NoRS); Three other domains serve as Nuclear Localization Signal. NLS1 (66–73), NLS2 (91–98) and NLS3 (200–241). The domain mapped between 131aa and 164aa is involved in interaction with HTLV-1 Rex protein. Two small domain at the C-terminal end of p30 have been predicted to be PDZ domain.
Because HTLV-I is very immunogenic and has a low variability, reducing expression of viral antigens is key to virus maintenance in vivo. We reported previously that p30 is able to potently suppress virus expression and thus may be involved in silencing in vivo19. The mechanism by which p30 prevents HTLV-I expression is not fully understood, but it may result from inhibition of Tax-mediated LTR activation20;21 along with interaction and nuclear retention of the tax/rex mRNA22.
The role of p30 in the virus life cycle: Rex and p30 interplay
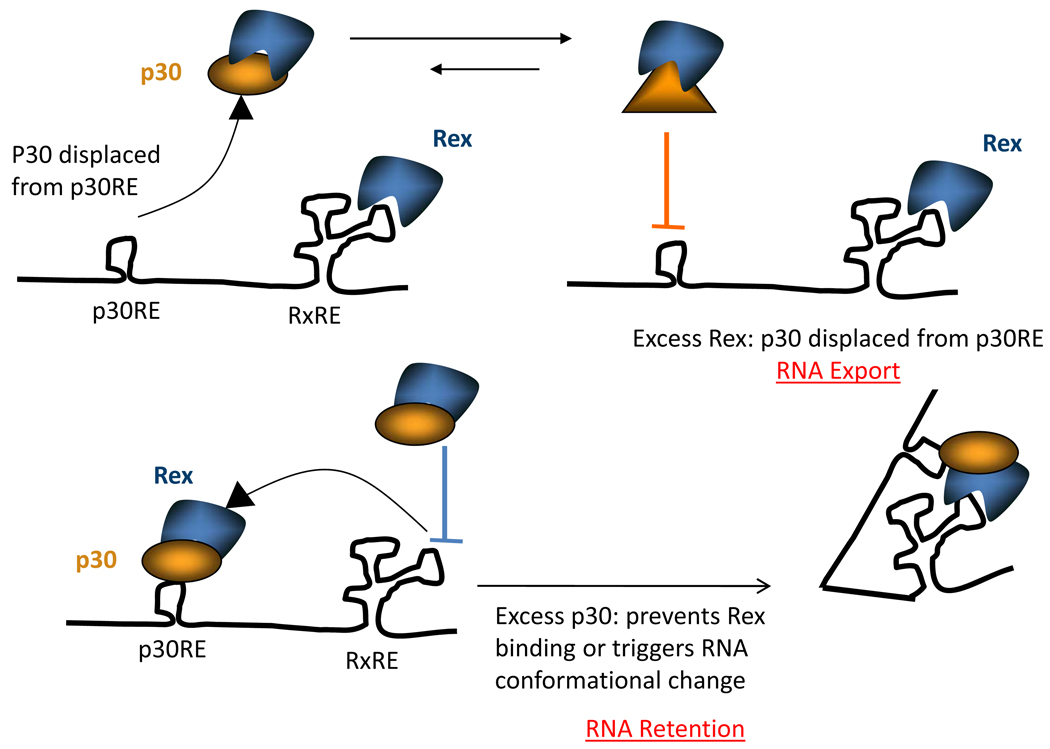
HTLV-I replication relies on the viral Tax and Rex proteins. Tax stimulates transcription through three 21-bp repeat elements localized in the U3 region of the provirus long terminal repeats (LTRs)23;24. Rex is an RNA-binding post-transcriptional regulator that binds specifically to the Rex-response element (RxRE) present at the 3′-end of all viral mRNA. Rex possesses a nuclear export signal (NES), which allows it to selectively export the unspliced (gag/pol) and incompletely spliced (env) viral mRNA from the nucleus to the cytoplasm. In contrast, expression of p30 blocks the nuclear export of tax/rex mRNA to the cytoplasm, resulting in down-regulation of both positive regulators Tax and Rex and suppression of virus replication25. These results were subsequently confirmed using the HTLV-II homolog p28 protein26. Interestingly, p28 was also found to be required for HTLV-II infection in vivo27. The fact that the inhibitory function of p28 and p30 is conserved in both HTLV-I and HTLV-II emphasizes its importance and suggests a common pathway for modulation of replication by these two distinct but related retroviruses. Little is known about the molecular mechanisms used by p30 or p28 to suppress nuclear export of the tax/rex mRNA. Recent studies have shown that nucleolar expression of p30 is not required, inasmuch as p30-9RA, a p30 mutant lacking both nucleolar retention signals28, retains post-transcriptional inhibition comparable to that of the wild type p3029. Additional studies have shed light on the mechanism used by the virus to discriminate between viral mRNAs that are p30 responsive (tax/rex) and those that are Rex responsive (gag/pol and env). p30 specifically forms complexes with Rex and p30-Rex interactions are markedly increased by the presence of viral mRNAs. RNAse treatment destabilizes p30-Rex complexes, suggesting that RNA is part of the complex and is required to maintain a strong interaction between p30 and Rex30. p30 was found to interact with the RNA-binding domain of Rex and binding of p30 to Rex prevents the latter from interacting with its RxRE and stimulating nuclear export. In addition, p30 efficiently interacts with Rex only when p30 is bound onto RNA. Since p30 could interact with tax/rex but not gag/pol or env mRNA, these data explain that p30 is able to prevent tax/rex nuclear export but has little or no effect on other viral mRNAs31 (Figure 2). Although it is critical for HTLV-I to reduce its expression to evade immune detection and clearance, complete or “true” latency would not benefit the virus, which in early stages needs some Tax expression to alter cell cycle checkpoints, alter DNA repair, and extend the lifespan of infected cells, thus facilitating transformation. In agreement with such a model, studies show that Rex permits export of residual p30-bound tax/rex mRNA and authorizes a low level of virus expression32. Although it is unclear how Rex may oppose p30 function, Rex may recruit a limiting cellular factor and induce a conformational change in p30 or in the local RNA structure releasing p30 from its p30RE and allowing export of some tax/rex mRNA to the cytoplasm.
Figure 2.
p30 affects transcription and nuclear export of cellular genes
In addition to its post-transcriptional functions, p30 has been reported to have transcriptional activities. When fused to the Gal4 DNA-binding domain, p30 shows strong transcriptional activation and the transcriptional activation domain was found to reside between amino acids 62 and 220. Further study suggested that CBP/p300 was indispensable for p30-mediated transactivation. Additional studies showed that p30 repressed the cellular CREB-responsive element-driven reporter gene activity in a dose-dependent manner. When expressed at low levels, p30 was shown to activate the HTLV-I LTR reporter gene. However, at high levels, p30 repressed the LTR reporter activity. Glutathione-S-transferase pull-down assays demonstrated that in vitro translated p30 interacts with amino-acid residues 1–595 of GST-p300 and residues 451–682 of GST-CBP, which comprise the kinase-inducible exchange domain of CBP also known to bind to CREB and Tax. Furthermore, p30 can also disrupt the assembly of the CREB-Tax-p300 complex on TRE probes in the DNA pull-down assay. Taken together, these data suggest that p30 might decrease transcription of the viral genome, thereby facilitating viral latency.
Affymetrix microarray gene expression analyses of Jurkat cells stably transduced with a lentiviral vector expressing p30 indicate that p30 represses many cellular genes, particularly adhesion molecules, such as integrins and cadherins, but increases the expression of certain target genes involved in T-cell activation and apoptosis33. Whether these effects are transcriptional or post-transcriptional were unclear from these studies. Recently, genome wide affymetrix microarray gene expression analyses of transduced human T-cells confirmed that p30 affects a number of cellular genes at the transcriptional and post transcriptional levels34. p30-dependent transcription resulted in the 2.5 fold up-regulation of 15 genes and the down-regulation of 65 human genes. In differential analyses of mRNA expression in the cytoplasmic fraction versus total RNA, p30 expression was found to result in a 2.5 fold post-transcriptional down-regulation of 90 genes and the up-regulation of 33 genes34. These data indicate a global effect of p30 on numerous genes in infected cells, suggesting that p30 is involved in more than just virus replication control. Until now, no consistent DNA response element has been reported, so the function of p30 in transcription of cellular genes remains to be determined.
p30 as a modulator of host innate immune responses
The host immune system detects and responds to microbial infection mainly through a family of pattern recognition receptors called Toll-like receptors (TLRs). TLR4 is essential for dendritic cell maturation and links the innate and adaptive immune responses. HTLV-I-infected ATL patients have pronounced immunodeficiency associated with frequent opportunistic infections. Importantly, concurrent infections of HTLV-I with bacteria and/or parasites are known to increase the risk of progression to ATL. Interaction between p30 and PU.1 was initially found in a Yeast Two-hybrid Screen35. Further studies confirmed interactions in human cells. p30 interacts with the ets-domain of PU. 1 and inhibits the DNA binding and transcription activity of PU.1, resulting in the down-regulation of TLR4 expression from the cell surface36. In addition, since PU.1 autoregulates expression from its own promoter, decreased PU.1 transcriptional activity effectively reduces endogenous PU.1 expression. Finally, p30-mediated inhibition of GSK3-β potently suppresses the production of pro-inflammatory cytokines (MCP-1, TNF-α, and IL-8), while concurrently augmenting production of the anti-inflammatory cytokine IL-10 following stimulation of TLR4 in human macrophages37. This finding is of interest because 80% of ATL patients have high levels of IL-10 in serum. Interference of TLR4 signaling by p30 and reduction of released amounts of pro-inflammatory cytokine IL-10 may impair the ability of dendritic cells to activate adaptive immunity in ATL patients and thereby explain the limited proliferation of virus-specific CTL reported in ATL patients.
Functions of p30 in HTLV-I-associated leukemia
p30 has been reported to recruit the co-activator Tat-interacting protein 60 (TIP60) and promote the formation of the Myc/TIP60 transcription complex on the Myc-response E-box element and transactivate transcription38. Due to the importance of Myc as a proto-oncogene in many solid tumors and hematological malignancies, especially in leukemia/lymphoma, p30 may contribute to the transformation of the HTLV-1- infected cell. In fact, p30 has been shown to modulate expression of cellular genes involved in the cell cycles and apoptosis33;34;39, two pathways often linked to cancer. Two recent studies have shown that p30 expression results in alteration of the cell cycle events that would promote early viral spread and T cell survival. Indeed, it has been shown that p30 activates the G2-M cell cycle checkpoints by increasing or decreasing the phosphorylation states of the G2/M checkpoints.
Recent evidence indicates that graded reduction in the expression of PU.1 in CD34+ derived bone marrow stem cells led to an intermediate stage of a poorly differentiated pre-leukemic cell population, which, with the accumulation of additional genetic mutations, resulted in an aggressive form of acute myeloid leukemia (AML)40. Along these lines, it has been shown that HTLV-I can infect and replicate in bone marrow derived undifferentiated CD34+ stem cells41. Using microRNA profiling, a recent study suggests that ATL cells may derive from a population of undifferentiated hematopoeitic stem cells (HSC)42. It is possible that p30 expression in HSC progenitors favors the development of pre-leukemic clones that may reinitiate their differentiation program as they acquire additional mutations and culminate to CD4+CD25+ leukemic clones. Future studies will likely reveal more surprises about this intriguing protein.
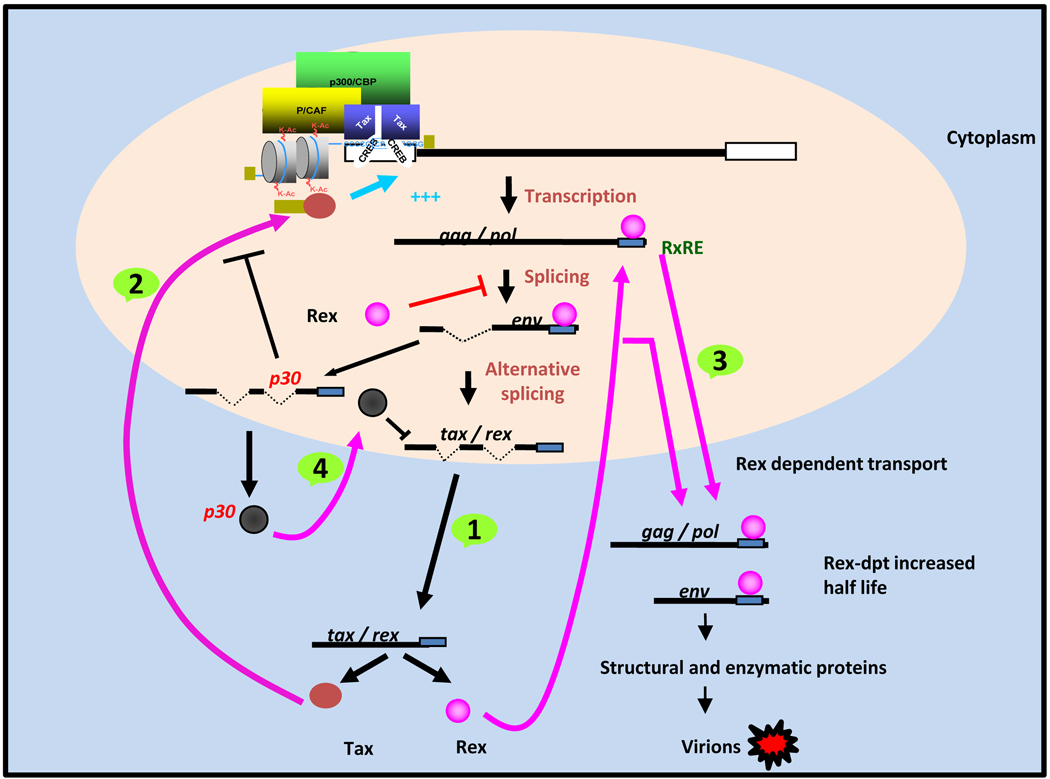
Fig 3.
Life cycle of HTLV-1 virus and related regulatory proteins. Four regulatory phases control the life cycle of HTLV-1 virus: 1) Tax and Rex are produced first from completely spliced mRNA, no other viral proteins are co-produced at this time. 2) Tax trans-activates LTR promoter to generate viral RNAs which are completely spliced. 3) Sufficient amount of Rex protein is accumulated, inhibit splicing and export non-spliced mRNA to the cytoplasm to form the viral particles. 4) p30 is expressed from an alternatively spliced mRNA; p30 interferes with Tax function and binds to and retains tax/rex mRNA in the nucleus leading to viral repression.
Acknowledgement
This work was supported by grant AI058944 to C.N. The authors thank Elizabeth Jenkins for editorial assistance.
Reference List
- 1.Ciminale V, Pavlakis GN, Derse D, Cunningham CP, Felber BK. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: novel mRNAs and proteins produced by HTLV type I. J. Virol. 1992;66:1737–1745. doi: 10.1128/jvi.66.3.1737-1745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koralnik IJ, Gessain A, Klotman ME, Lo MA, Berneman ZN, Franchini G. Protein isoforms encoded by the pX region of human T-cell leukemia/lymphotropic virus type I. Proc.Natl.Acad.Sci.U.S.A. 1992;89:8813–8817. doi: 10.1073/pnas.89.18.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koralnik IJ, Fullen J, Franchini G. The p12I, p13II, and p30II proteins encoded by human T-cell leukemia/lymphotropic virus type I open reading frames I and II are localized in three different cellular compartments. J.Virol. 1993;67:2360–2366. doi: 10.1128/jvi.67.4.2360-2366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koralnik IJ, Fullen J, Franchini G. The p12I, p13II, and p30II proteins encoded by human T-cell leukemia/lymphotropic virus type I open reading frames I and II are localized in three different cellular compartments. J.Virol. 1993;67:2360–2366. doi: 10.1128/jvi.67.4.2360-2366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Agostino DM, Silic-Benussi M, Hiraragi H, Lairmore MD, Ciminale V. The human T-cell leukemia virus type 1 p13II protein: effects on mitochondrial function and cell growth. Cell Death.Differ. 2005;12 Suppl 1:905–915. doi: 10.1038/sj.cdd.4401576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciminale V, Pavlakis GN, Derse D, Cunningham CP, Felber BK. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: novel mRNAs and proteins produced by HTLV type I. J.Virol. 1992;66:1737–1745. doi: 10.1128/jvi.66.3.1737-1745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koralnik IJ, Gessain A, Klotman ME, Lo MA, Berneman ZN, Franchini G. Protein isoforms encoded by the pX region of human T-cell leukemia/lymphotropic virus type I. Proc.Natl.Acad.Sci.U.S.A. 1992;89:8813–8817. doi: 10.1073/pnas.89.18.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pique C, Dokhelar MC. In vivo production of Rof and Tof proteins of HTLV type 1: evidence from cytotoxic T lymphocytes. AIDS Res.Hum.Retroviruses. 2000;16:1783–1786. doi: 10.1089/08892220050193317. [DOI] [PubMed] [Google Scholar]
- 9.Pique C, Ureta-Vidal A, Gessain A, Chancerel B, Gout O, Tamouza R, Agis F, Dokhelar MC. Evidence for the chronic in vivo production of human T cell leukemia virus type I Rof and Tof proteins from cytotoxic T lymphocytes directed against viral peptides. J.Exp.Med. 2000;191:567–572. doi: 10.1084/jem.191.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lairmore MD, Albrecht B, D'Souza C, Nisbet JW, Ding W, Bartoe JT, Green PL, Zhang W. In vitro and in vivo functional analysis of human T cell lymphotropic virus type 1 pX open reading frames I and II. AIDS Res.Hum.Retroviruses. 2000;16:1757–1764. doi: 10.1089/08892220050193272. [DOI] [PubMed] [Google Scholar]
- 11.Robek MD, Wong FH, Ratner L. Human T-cell leukemia virus type 1 pX-I and pX-II open reading frames are dispensable for the immortalization of primary lymphocytes. J.Virol. 1998;72:4458–4462. doi: 10.1128/jvi.72.5.4458-4462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derse D, Mikovits J, Ruscetti F. X-I and X-II open reading frames of HTLV-I are not required for virus replication or for immortalization of primary T-cells in vitro. Virology. 1997;237:123–128. doi: 10.1006/viro.1997.8781. [DOI] [PubMed] [Google Scholar]
- 13.Albrecht B, Lairmore MD. Critical role of human T-lymphotropic virus type 1 accessory proteins in viral replication and pathogenesis. Microbiol.Mol.Biol.Rev. 2002;66:396–406. doi: 10.1128/MMBR.66.3.396-406.2002. table. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicot C, Harrod RL, Ciminale V, Franchini G. Human T-cell leukemia/lymphoma virus type 1 nonstructural genes and their functions. Oncogene. 2005;24:6026–6034. doi: 10.1038/sj.onc.1208977. [DOI] [PubMed] [Google Scholar]
- 15.Ghorbel S, Sinha-Datta U, Dundr M, Brown M, Franchini G, Nicot C. Human T-cell leukemia virus type I p30 nuclear/nucleolar retention is mediated through interactions with RNA and a constituent of the 60 S ribosomal subunit. J.Biol.Chem. 2006;281:37150–37158. doi: 10.1074/jbc.M603981200. [DOI] [PubMed] [Google Scholar]
- 16.Ghorbel S, Sinha-Datta U, Dundr M, Brown M, Franchini G, Nicot C. Human T-cell leukemia virus type I p30 nuclear/nucleolar retention is mediated through interactions with RNA and a constituent of the 60 S ribosomal subunit. J.Biol.Chem. 2006;281:37150–37158. doi: 10.1074/jbc.M603981200. [DOI] [PubMed] [Google Scholar]
- 17.Ghorbel S, Sinha-Datta U, Dundr M, Brown M, Franchini G, Nicot C. Human T-cell leukemia virus type I p30 nuclear/nucleolar retention is mediated through interactions with RNA and a constituent of the 60 S ribosomal subunit. J.Biol.Chem. 2006;281:37150–37158. doi: 10.1074/jbc.M603981200. [DOI] [PubMed] [Google Scholar]
- 18.Ghorbel S, Sinha-Datta U, Dundr M, Brown M, Franchini G, Nicot C. Human T-cell leukemia virus type I p30 nuclear/nucleolar retention is mediated through interactions with RNA and a constituent of the 60 S ribosomal subunit. J.Biol.Chem. 2006;281:37150–37158. doi: 10.1074/jbc.M603981200. [DOI] [PubMed] [Google Scholar]
- 19.Nicot C, Dundr M, Johnson JM, Fullen JR, Alonzo N, Fukumoto R, Princler GL, Derse D, Misteli T, Franchini G. HTLV-1-encoded p30II is a post-transcriptional negative regulator of viral replication. Nat.Med. 2004;10:197–201. doi: 10.1038/nm984. [DOI] [PubMed] [Google Scholar]
- 20.Michael B, Nair AM, Datta A, Hiraragi H, Ratner L, Lairmore MD. Histone acetyltransferase (HAT) activity of p300 modulates human T lymphotropic virus type 1 p30II-mediated repression of LTR transcriptional activity. Virology. 2006;(354):225–239. doi: 10.1016/j.virol.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W, Nisbet JW, Albrecht B, Ding W, Kashanchi F, Bartoe JT, Lairmore MD. Human T-lymphotropic virus type 1 p30(II) regulates gene transcription by binding CREB binding protein/p300. J.Virol. 2001;75:9885–9895. doi: 10.1128/JVI.75.20.9885-9895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicot C, Dundr M, Johnson JM, Fullen JR, Alonzo N, Fukumoto R, Princler GL, Derse D, Misteli T, Franchini G. HTLV-1-encoded p30II is a post-transcriptional negative regulator of viral replication. Nat.Med. 2004;10:197–201. doi: 10.1038/nm984. [DOI] [PubMed] [Google Scholar]
- 23.Michael B, Nair AM, Datta A, Hiraragi H, Ratner L, Lairmore MD. Histone acetyltransferase (HAT) activity of p300 modulates human T lymphotropic virus type 1 p30II-mediated repression of LTR transcriptional activity. Virology. 2006;354:225–239. doi: 10.1016/j.virol.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Nisbet JW, Albrecht B, Ding W, Kashanchi F, Bartoe JT, Lairmore MD. Human T-lymphotropic virus type 1 p30(II) regulates gene transcription by binding CREB binding protein/p300. J.Virol. 2001;75:9885–9895. doi: 10.1128/JVI.75.20.9885-9895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicot C, Dundr M, Johnson JM, Fullen JR, Alonzo N, Fukumoto R, Princler GL, Derse D, Misteli T, Franchini G. HTLV-1-encoded p30II is a post-transcriptional negative regulator of viral replication. Nat.Med. 2004;10:197–201. doi: 10.1038/nm984. [DOI] [PubMed] [Google Scholar]
- 26.Younis I, Khair L, Dundr M, Lairmore MD, Franchini G, Green PL. Repression of human T-cell leukemia virus type 1 and type 2 replication by a viral mRNA-encoded posttranscriptional regulator. J.Virol. 2004;78:11077–11083. doi: 10.1128/JVI.78.20.11077-11083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto B, Li M, Kesic M, Younis I, Lairmore MD, Green PL. Human T-cell leukemia virus type 2 post-transcriptional control protein p28 is required for viral infectivity and persistence in vivo. Retrovirology. 2008;5:38. doi: 10.1186/1742-4690-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghorbel S, Sinha-Datta U, Dundr M, Brown M, Franchini G, Nicot C. Human T-cell leukemia virus type I p30 nuclear/nucleolar retention is mediated through interactions with RNA and a constituent of the 60 S ribosomal subunit. J.Biol.Chem. 2006;281:37150–37158. doi: 10.1074/jbc.M603981200. [DOI] [PubMed] [Google Scholar]
- 29.Sinha-Datta U, Datta A, Ghorbel S, Dodon MD, Nicot C. Human T-cell lymphotrophic virus type I rex and p30 interactions govern the switch between virus latency and replication. J.Biol.Chem. 2007;282:14608–14615. doi: 10.1074/jbc.M611219200. [DOI] [PubMed] [Google Scholar]
- 30.Sinha-Datta U, Datta A, Ghorbel S, Dodon MD, Nicot C. Human T-cell lymphotrophic virus type I rex and p30 interactions govern the switch between virus latency and replication. J.Biol.Chem. 2007;282:14608–14615. doi: 10.1074/jbc.M611219200. [DOI] [PubMed] [Google Scholar]
- 31.Sinha-Datta U, Datta A, Ghorbel S, Dodon MD, Nicot C. Human T-cell lymphotrophic virus type I rex and p30 interactions govern the switch between virus latency and replication. J.Biol.Chem. 2007;282:14608–14615. doi: 10.1074/jbc.M611219200. [DOI] [PubMed] [Google Scholar]
- 32.Sinha-Datta U, Datta A, Ghorbel S, Dodon MD, Nicot C. Human T-cell lymphotrophic virus type I rex and p30 interactions govern the switch between virus latency and replication. J.Biol.Chem. 2007;282:14608–14615. doi: 10.1074/jbc.M611219200. [DOI] [PubMed] [Google Scholar]
- 33.Michael B, Nair AM, Hiraragi H, Shen L, Feuer G, Boris-Lawrie K, Lairmore MD. Human T lymphotropic virus type-1 p30II alters cellular gene expression to selectively enhance signaling pathways that activate T lymphocytes. Retrovirology. 2004;1:39. doi: 10.1186/1742-4690-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor JM, Ghorbel S, Nicot C. Genome wide analysis of human genes transcriptionally and post-transcriptionally regulated by the HTLV-I protein p30. BMC.Genomics. 2009;10:311. doi: 10.1186/1471-2164-10-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Datta A, Sinha-Datta U, Dhillon NK, Buch S, Nicot C. The HTLV-I p30 interferes with TLR4 signaling and modulates the release of pro- and anti-inflammatory cytokines from human macrophages. J.Biol.Chem. 2006;281:23414–23424. doi: 10.1074/jbc.M600684200. [DOI] [PubMed] [Google Scholar]
- 36.Datta A, Sinha-Datta U, Dhillon NK, Buch S, Nicot C. The HTLV-I p30 interferes with TLR4 signaling and modulates the release of pro- and anti-inflammatory cytokines from human macrophages. J.Biol.Chem. 2006;281:23414–23424. doi: 10.1074/jbc.M600684200. [DOI] [PubMed] [Google Scholar]
- 37.Datta A, Sinha-Datta U, Dhillon NK, Buch S, Nicot C. The HTLV-I p30 interferes with TLR4 signaling and modulates the release of pro- and anti-inflammatory cytokines from human macrophages. J.Biol.Chem. 2006;281:23414–23424. doi: 10.1074/jbc.M600684200. [DOI] [PubMed] [Google Scholar]
- 38.Awasthi S, Sharma A, Wong K, Zhang J, Matlock EF, Rogers L, Motloch P, Takemoto S, Taguchi H, Cole MD, Luscher B, Dittrich O, Tagami H, Nakatani Y, McGee M, Girard AM, Gaughan L, Robson CN, Monnat RJ, Jr., Harrod R. A human T-cell lymphotropic virus type 1 enhancer of Myc transforming potential stabilizes Myc-TIP60 transcriptional interactions. Mol.Cell Biol. 2005;25:6178–6198. doi: 10.1128/MCB.25.14.6178-6198.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Datta A, Silverman L, Phipps AJ, Hiraragi H, Ratner L, Lairmore MD. Human T-lymphotropic virus type-1 p30 alters cell cycle G2 regulation of T lymphocytes to enhance cell survival. Retrovirology. 2007;4:49. doi: 10.1186/1742-4690-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenbauer F, Owens BM, Yu L, Tumang JR, Steidl U, Kutok JL, Clayton LK, Wagner K, Scheller M, Iwasaki H, Liu C, Hackanson B, Akashi K, Leutz A, Rothstein TL, Plass C, Tenen DG. Lymphoid cell growth and transformation are suppressed by a key regulatory element of the gene encoding PU.1. Nat.Genet. 2006;38:27–37. doi: 10.1038/ng1679. [DOI] [PubMed] [Google Scholar]
- 41.Feuer G, Fraser JK, Zack JA, Lee F, Feuer R, Chen IS. Human T-cell leukemia virus infection of human hematopoietic progenitor cells: maintenance of virus infection during differentiation in vitro and in vivo. J.Virol. 1996;70:4038–4044. doi: 10.1128/jvi.70.6.4038-4044.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bellon M, Lepelletier Y, Hermine O, Nicot C. Deregulation of microRNA involved in hematopoiesis and the immune response in HTLV-I adult T-cell leukemia. Blood. 2009;113:4914–4917. doi: 10.1182/blood-2008-11-189845. [DOI] [PMC free article] [PubMed] [Google Scholar]