Abstract
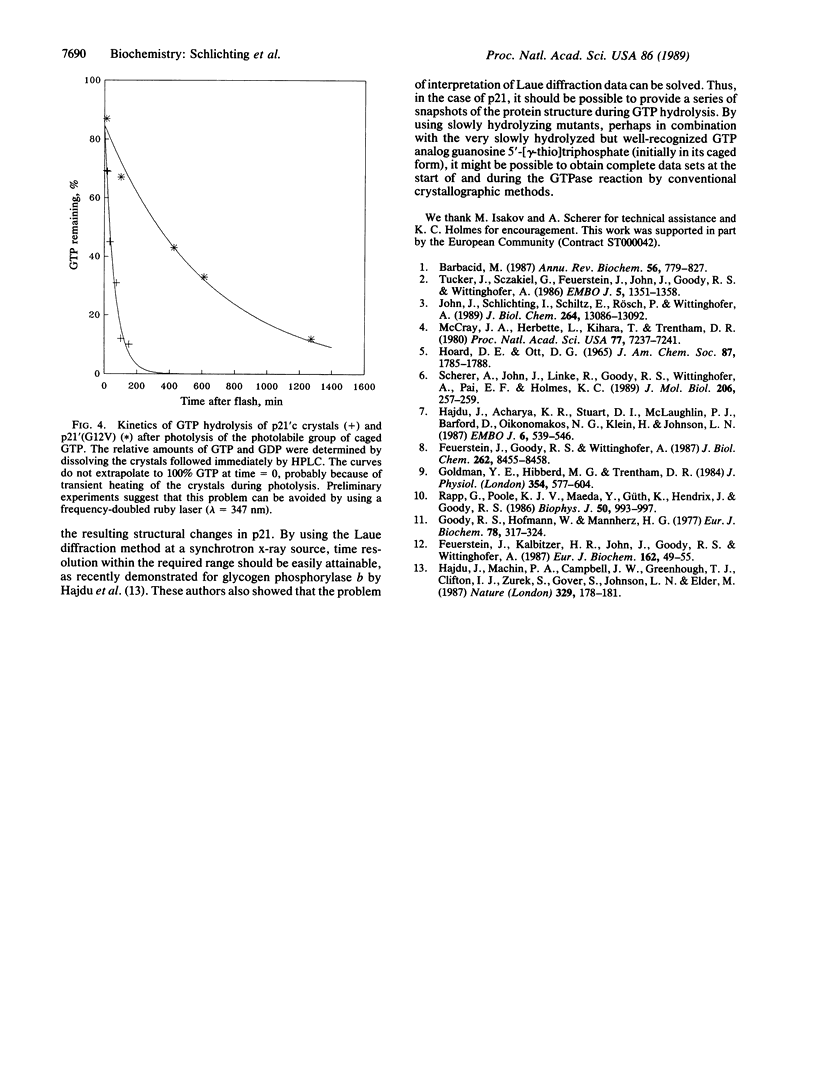
The GTP binding domain of the c-Ha-ras protooncogene product (p21'c) and the corresponding region from an oncogenic mutant form of the protein in which glycine at position 12 has been replaced by valine [p21'(G12V)] have been crystallized with P3-1-(2-nitro)phenylethylguanosine 5'-O-triphosphate (caged GTP) at their active sites. The crystals give x-ray diffraction patterns to a resolution of better than 0.3 nm. Photolysis can be achieved in the crystal, after which GTP hydrolysis takes place at the rate expected from solution studies. Complete x-ray data sets have been obtained for the starting caged-GTP state and the final GDP state after photolysis and hydrolysis, demonstrating the feasibility of time-resolved structural investigations of the process of GTP hydrolysis.
Full text
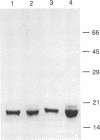
PDF
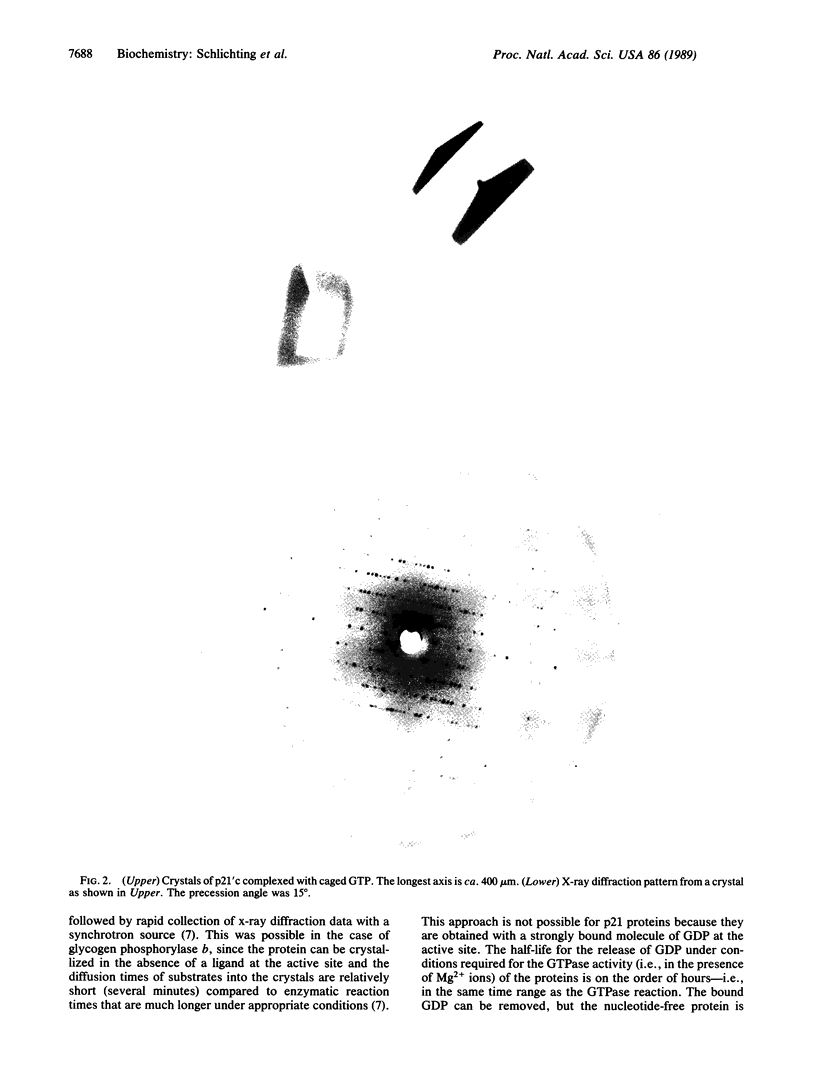
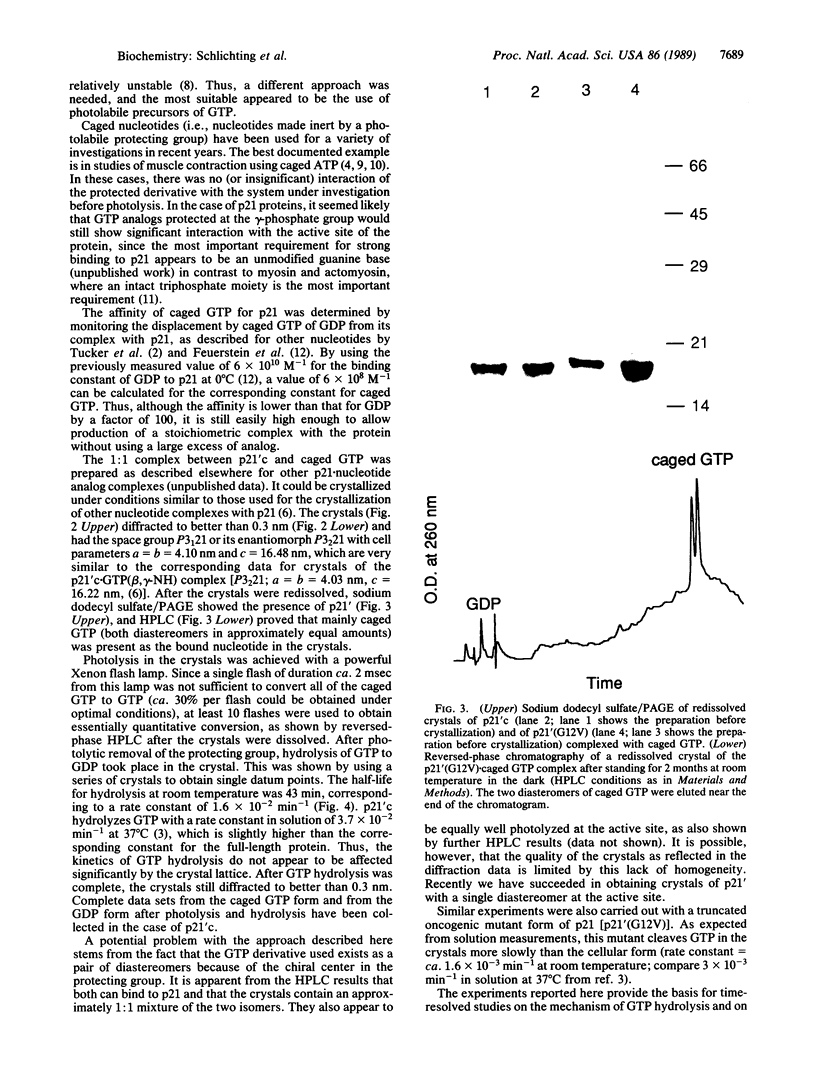
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Feuerstein J., Goody R. S., Wittinghofer A. Preparation and characterization of nucleotide-free and metal ion-free p21 "apoprotein". J Biol Chem. 1987 Jun 25;262(18):8455–8458. [PubMed] [Google Scholar]
- Feuerstein J., Kalbitzer H. R., John J., Goody R. S., Wittinghofer A. Characterisation of the metal-ion-GDP complex at the active sites of transforming and nontransforming p21 proteins by observation of the 17O-Mn superhyperfine coupling and by kinetic methods. Eur J Biochem. 1987 Jan 2;162(1):49–55. doi: 10.1111/j.1432-1033.1987.tb10540.x. [DOI] [PubMed] [Google Scholar]
- Goldman Y. E., Hibberd M. G., Trentham D. R. Relaxation of rabbit psoas muscle fibres from rigor by photochemical generation of adenosine-5'-triphosphate. J Physiol. 1984 Sep;354:577–604. doi: 10.1113/jphysiol.1984.sp015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goody R. S., Hofmann W., Mannherz G. H. The binding constant of ATP to myosin S1 fragment. Eur J Biochem. 1977 Sep;78(2):317–324. doi: 10.1111/j.1432-1033.1977.tb11742.x. [DOI] [PubMed] [Google Scholar]
- HOARD D. E., OTT D. G. CONVERSION OF MONO- AND OLIGODEOXYRIBONUCLEOTIDES TO 5-TRIPHOSPHATES. J Am Chem Soc. 1965 Apr 20;87:1785–1788. doi: 10.1021/ja01086a031. [DOI] [PubMed] [Google Scholar]
- Hajdu J., Acharya K. R., Stuart D. I., McLaughlin P. J., Barford D., Oikonomakos N. G., Klein H., Johnson L. N. Catalysis in the crystal: synchrotron radiation studies with glycogen phosphorylase b. EMBO J. 1987 Feb;6(2):539–546. doi: 10.1002/j.1460-2075.1987.tb04786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdu J., Machin P. A., Campbell J. W., Greenhough T. J., Clifton I. J., Zurek S., Gover S., Johnson L. N., Elder M. Millisecond X-ray diffraction and the first electron density map from Laue photographs of a protein crystal. Nature. 1987 Sep 10;329(6135):178–181. doi: 10.1038/329178a0. [DOI] [PubMed] [Google Scholar]
- John J., Schlichting I., Schiltz E., Rösch P., Wittinghofer A. C-terminal truncation of p21H preserves crucial kinetic and structural properties. J Biol Chem. 1989 Aug 5;264(22):13086–13092. [PubMed] [Google Scholar]
- McCray J. A., Herbette L., Kihara T., Trentham D. R. A new approach to time-resolved studies of ATP-requiring biological systems; laser flash photolysis of caged ATP. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7237–7241. doi: 10.1073/pnas.77.12.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp G., Poole K. J., Maeda Y., Güth K., Hendrix J., Goody R. S. Time-Resolved Structural Studies on Insect Flight Muscle after Photolysis of Caged-ATP. Biophys J. 1986 Nov;50(5):993–997. doi: 10.1016/S0006-3495(86)83540-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer A., John J., Linke R., Goody R. S., Wittinghofer A., Pai E. F., Homes K. C. Crystallization and preliminary X-ray analysis of the human c-H-ras-oncogene product p21 complexed with GTP analogues. J Mol Biol. 1989 Mar 5;206(1):257–259. doi: 10.1016/0022-2836(89)90540-8. [DOI] [PubMed] [Google Scholar]
- Tucker J., Sczakiel G., Feuerstein J., John J., Goody R. S., Wittinghofer A. Expression of p21 proteins in Escherichia coli and stereochemistry of the nucleotide-binding site. EMBO J. 1986 Jun;5(6):1351–1358. doi: 10.1002/j.1460-2075.1986.tb04366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]