Abstract
Morphine is one of the analgesics used most to treat chronic pain, although its long-term administration produces tolerance and dependence through neuronal plasticity. The ability of morphine to regulate neuron differentiation in vivo has been reported. However, the detailed mechanisms have not yet been elucidated because of the inability to separate maternal influences from embryonic events. Using zebrafish embryos as the model, we demonstrate that morphine decreases miR-133b expression, hence increasing the expression of its target, Pitx3, a transcription factor that activates tyrosine hydroxylase and dopamine transporter. Using a specific morpholino to knock down the zebrafish μ-opioid receptor (zfMOR) in the embryos and selective mitogen-activated protein kinase inhibitors, we demonstrate that the morphine-induced miR-133b decrease in zebrafish embryos is mediated by zfMOR activation of extracellular signal-regulated kinase 1/2. A parallel morphine-induced down-regulation of miR-133b was observed in the immature but not in mature rat hippocampal neurons. Our results indicate for the first time that zebrafish embryos express a functional μ-opioid receptor and that zebrafish serves as an excellent model to investigate the roles of microRNA in neuronal development affected by long-term morphine exposure.
Introduction
Opioids are the most potent compounds known to control pain and are also among the most common drugs of abuse (Corbett et al., 2006). They bind to the classic μ- (MOR), δ- (DOR), and κ-opioid receptors. Although great efforts have been made on the study of the different mechanisms that are activated by the opioid system, using mammalian models, many issues regarding opioid regulation remain unknown. The zebrafish (Danio rerio) has been used as an experimental model to study not only genetics and development but also disease-related pathways, given its easy in vivo manipulation. In this sense, the zebrafish can be an important tool to analyze in vivo the molecular mechanisms related to the activity and function of the opioid system that cannot be fully established in other models. For instance, in contrast to mammalian embryos, which develop in the uterus and are influenced by the maternal biochemical processes, zebrafish embryos develop externally, avoiding the maternal effect on these embryos. This is essential when dealing with drug exposure, because the effects observed in mammalian embryos might be due to the susceptibility of the mother and not the embryo per se. The study of the direct effects of morphine in the embryos will provide a better understanding on the molecular mechanisms that underlie the physical and neurobehavioral defects shown in fetuses and offspring after maternal morphine consumption (Nasiraei-Moghadam et al., 2010). In addition, the endogenous opioid system has been characterized in the zebrafish, which has a MOR (zfMOR), two DOR duplicates (zfDOR1 and zfDOR2), a κ-opioid receptor, and an opioid receptor-like (Barrallo et al., 2000; Rodriguez et al., 2000; Alvarez et al., 2006; Pinal-Seoane et al., 2006). The opioid-induced drug addiction pathway has been suggested to involve the midbrain dopaminergic neurons located within ventral tegmental area and the nucleus accumbens (NAc). The alteration of dopamine levels in this region produces neuronal sensitization or desensitization, depending on the drug used. It has also been established that morphine increases dopamine level through the MOR in the NAc, which may mediate the reinforcing effects of morphine (Gianoulakis, 2009). In relation to these observations, the endogenous opioid peptides, such as enkephalins or dynorphin, are up-regulated in the NAc after exposure to morphine and modulate dopamine release in the midbrain (Gieryk et al.). Therefore, the studies on the probable opioid regulation of dopaminergic activities in zebrafish could provide insights on the mammalian embryonic development during chronic exposure to the drug.
MicroRNAs (miRNAs), a class of ∼22-nucleotide RNA molecules, are known to bind to their mRNA targets to inhibit the transcripts translation and/or destabilize them (Valencia-Sanchez et al., 2006). They have been shown to regulate the expression of many genes, including those in the central nervous system (CNS). For example, miR-134 regulates dendritic spine morphology by controlling actin filament dynamics (Schratt et al., 2006), whereas miR-190 regulates NeuroD level, a transcription factor that is known to regulate the differentiation and maturation of neurons (Zheng et al., 2010). Another class of miRNA, miR-133b, regulates the differentiation, maturation, and function of dopaminergic neurons by down-regulating the transcription of its target, the homeobox gene pitx3 (Hébert and De Strooper, 2009). Pitx3 activates the transcription of genes directly involved in the differentiation of dopaminergic neurons, genes such as the tyrosine hydroxylase (th) and the dopamine transporter (dat) (Kim et al., 2007).
In the current study, we analyze the effect of morphine on the miR-133b regulatory pathway using zebrafish embryos, which have been widely used to study the role of miR on development, as a model (Schier and Giraldez, 2006). At 24 h after fertilization (hpf), the dopaminergic system begins its differentiation, and the first TH-positive neurons begin to be detected at this particular developmental stage (Filippi et al., 2007). Our previous studies also indicated that at 24 hfp, there is a robust expression of zfMOR, the putative target of morphine (de Velasco et al., 2009). Therefore, the use of 24 hpf zebrafish embryos not only will provide information on the implication of the opioid system in the maturation and differentiation of dopaminergic neurons compared with any other stages of development but also will demonstrate that the μ-opioid receptor is functional in the zebrafish and has a specific role in the development of the CNS and a possible pathway that leads to addiction.
Materials and Methods
Zebrafish.
Zebrafish from the AB strain were bred and raised in the Fish Facilities of the University of Salamanca and the University of Minnesota following standard protocols (Westerfield, 1995). Embryos were separated into two experimental groups for the miRNA microarray: control (untreated) group and embryos exposed to 10 nM morphine. For the analysis of the effects of morphine on the expression of miRNA-133b, Pitx3, TH, and DAT, zebrafish embryos were divided into four experimental groups: control, exposed to 10 nM morphine, exposed to 1 nM morphine, and exposed to 1 μM naloxone.
miRNA Microarray.
Custom miRNA microarray experiments and data analyses were performed as described previously (Kalscheuer et al., 2008). Microarray data have been deposited into the Gene Expression Omnibus database of the National Center for Biotechnology Information (NCBI) under the series GSE18847. Zebrafish embryo samples were prepared in triplicate. The data were normalized against internal control in each chip. The expression of miRNAs in morphine-exposed embryos was compared with those in control embryos. The miRNAs with significant expressional change (>125 or <80%; p < 0.225 by Student's t test) were identified, and the miRNA-133b was chosen for this study, given its implication in addiction.
RNA Extraction and qRT-PCR.
Total RNA, including miRNA, was extracted using Tri-Reagent (Molecular Research Center, Cincinnati, OH), following the manufacturer's protocol. NCode miRNA First-Strand cDNA Synthesis (Invitrogen, Carlsbad, CA) was used to synthesize cDNA from miRNA and mRNA. cDNA concentration was determined by measuring the absorbance at 260 nm with a spectrophotometer (SmartSpec Plus; Bio-Rad Laboratories, Hercules, CA). The absolute quantification of the PCR products was accomplished with a standard curve using the SYBR-Green method. The SYBR-Green was included in a 2× Master Mix (QuantiTect SYBR Green PCR Kit; QIAGEN, Valencia, CA). The oligonucleotides used to amplify the different genes studied in this work were as follws: EF1α: forward, GTACTTCTCAGGCTGACTGTG; reverse, ACGATCAGCTGTTTCACTCC. dre-miRNA-133b: TTTGGTCCCCTTCAACCAGCTA. zfPitx3: forward, GACAACAGTGACACAGAGAAGT; reverse, TGTCGGGATAACGGTTTCTC. zfTH: forward, TTTGAAGAGAAGTGCAGAGGAT; reverse, TCAGTAAATCCTGGGTGATCC. zfDAT: forward, AGACATCTGGGAAGGTGGTG; reverse, ACCTGAGCAT CATACAGGCG. The final volume of each reaction was 20:10 μl of Master Mix, 0.8 μl of each oligonucleotide, 7.4 μl of distilled water, and 1 μl of cDNA in a concentration of 25 ng/μl. A standard curve was constructed for each experiment by serial dilutions of cDNA: 0.1 ng/μl, 0.01 ng/μl, 0.001 ng/μl, and 0.0001 ng/μl. The amplification reaction took place in an iCycler System (Bio-Rad Laboratories), with the following conditions: 15 min at 95°C followed by 35 cycles of 15 s at 95°C, 30 s at 57°C, and 30 s at 70°C. PCR was performed three times for each sample per plate, and each experiment was repeated with two different samples. We have used EF1α as an endogenous control.
Pitx3 3′UTR Cloning and Microinjection.
QuantiTect SYBR Green PCR Kit from QIAGEN was used to amplify the 3′UTR from Pitx3 using primers based on the sequence of the full-length cDNA from Ensembl (accession number ENSDARG00000070069). The following primers were used: zfPitx3 3′UTR: forward, CGGTATGAAAGCGATGCGTCTA; reverse, AGACAAAGCAGGCTACACCAGGA. The program used for the amplification was as follows: 15 min at 95°C followed by 35 cycles of 15 s at 95°C, 30 s at 57°C, and 1 min at 70°C. At the end of the cycles, a final extension temperature of 70°C was added for 10 min. The PCR product was purified and cloned into a TOPO-TA 2.1 vector (Invitrogen). TOP 10′F cells (Invitrogen) were transformed with the construct, and a maxi-prep was performed to obtain high quantities of the construct. This construct was digested with EcoRI for 1 h at 37°C and sent for sequencing. The digested product was injected at a concentration of 0.1 ng/μl into one-cell zebrafish embryos with a micromanipulator-microinjector system from Eppendorf AG (Hamburg, Germany).
Morpholino Microinjection.
The morpholino antisense (MO) oligomer used to knock down zfMOR was purchased from Gene Tools, LLC (Philomath, OR), and its sequence was AATGTTGCCAGTG TTTTCCATCATG. The MO was diluted in sterilized water to a stock concentration of 0.3 mM. In addition to the three MO experimental groups (untreated, 10 nM morphine, and 10 nM morphine plus 1 μM naloxone), each experiment included a control MO group injected with morpholino that exhibits no binding target or biological activity, as well as a control group (uninjected) for each experimental group (untreated, 10 nM morphine and 10 nM morphine plus 1 μM naloxone). Zebrafish embryos were injected into the yolk at the one-to-four-cell stage with the morpholino oligonucleotide according to the published protocols (Nasevicius and Ekker, 2000). Several MO concentrations were used to establish the concentration that produced the greatest effect on the expression level of the studied genes and the lowest embryonic death. To calibrate the amount of solution injected, 10-ms pulses are injected into a 1-l microcapillary (Drummond Scientific, Broomall, PA). The amount of solution in the capillary is measured using a millimeter ruler. These capillaries have 1 μl of total capacity and are 33 mm long; thus, 1 mm represents 30 nl of solution. The concentrations of zfMOR MO and control MO used were 0.2 and 1 μM, respectively (3 nl were injected into each embryo). Embryos were maintained in E3 medium at 28.5°C until sacrificed at 24 hpf.
Embryonic Treatment with MAPK Inhibitors.
Zebrafish embryos were first divided into five experimental groups: control (untreated), exposed to 0.6 μM JNKII inhibitor, exposed to 60 μM 4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)1H-imidazole (SB203580; p38 inhibitor), exposed to 50 μM 2′-amino-3′-methoxyflavone (PD98059, ERK 1/2 inhibitor), and exposed to 50 μM 1,4-diamino-2,3-dicyano-1,4-bis(methylthio)butadiene (U0126; ERK 1/2 inhibitor). Exposure to the MAPK inhibitors was done after 5 hpf. Because only the ERK 1/2 inhibitors produced the desired effect, different zebrafish embryos were then divided into other experimental groups: control, exposed to PD98059 from 5 to 24 hpf, exposed to PD98059 from 16 to 24 hpf (the exact period in which the CNS is developing and differentiating), exposed to U016 from 5 to 24 hpf, and exposed to U0126 from 16 to 24 hpf. All embryos were sacrificed at 24 hpf.
Primary Neuronal Culture and RNA Extraction.
Hippocampal neurons from P1 rats were isolated and cultured in six-well plates according to previously described protocols (Liao et al., 2007). The 1-week-cultured neurons were divided into four experimental groups: untreated, treated with 100 nM morphine, treated with 1 μM naloxone, and treated simultaneously with morphine and naloxone. Total RNA (including miRNAs) was isolated using the QIAGEN RNA-easy purification system based on columns. qRT-PCR was performed as described under RNA Extraction and qRT-PCR using the same miRNA-133b oligonucleotide that was used with the zebrafish samples, because both species share the same sequence.
Results
Morphine Modulates the Expression of miR-133b.
Using a miRNA array, we observed a decrease in the expression of several miRNAs after embryonic exposure to morphine at three developmental stages: 16, 24, and 48 hpf [array data were deposited in the NCBI Gene Expression Omnibus database (USA) under the series GSE18847]. Considering the pathways in which each miRNA could be involved, we focused on miR-133b in our studies because of its reported effect on dopaminergic neurons, an essential component in drug addiction process. Our studies were carried out with the 24-hpf embryos, when the differentiation of the zebrafish CNS begins.
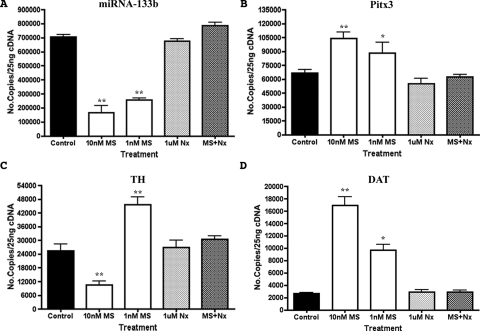
To validate our microarray results, qRT-PCR assays were carried out. As shown in Fig. 1A, miR-133b level was decreased in 24-hpf embryos exposed to morphine at two different concentrations, 10 and 1 nM. The antagonist naloxone did not significantly change the expression of this miR, but it could block the morphine effect. Although more selective agonists such as [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin or antagonists such as Cys2-Tyr3-Orn5-Pen7-amide were not used to define the receptor involved because of the lack of affinity of such ligands for zfMOR (de Velasco et al., 2009), such morphine effect of miR-133b level was probably mediated by the activation of zfMOR.
Fig. 1.
Effect of morphine on the expression of miRNA-133b, Pitx3, TH and DAT. A, qRT-PCR analyses of miRNA-133b levels in 24-hpf zebrafish embryos at two different morphine concentrations, 10 and 1 nM. B, the Pitx3 levels measured at both concentrations of morphine with qRT-PCR. C, the level of TH transcript measured with qRT-PCR in morphine-exposed embryos. D, the level of DAT transcript measured with qRT-PCR in embryos exposed to morphine. In all cases, the levels of miR-133b and all three transcripts were measured by qRT-PCR in control embryos (no drug exposure), embryos exposed to naloxone only, or embryos exposed to morphine and naloxone. *, P ≤ 0.05; **, P ≤ 0.005 (unpaired Student's t test with Welch correction), n = 3.
Morphine Modulates the Expression of miR-133b Targets.
One of the miR-133b targets is the transcription factor Pitx3. Pitx3 in turn has been shown to regulate the transcription of th and dat. Because microRNAs normally regulate the stability or the translation of the transcripts, morphine, by decreasing the miR-133b, should either increase the levels or the activities of these transcripts. As shown in Fig. 1, B to D, treatment of zebrafish embryos with 1 and 10 nM morphine increases the mRNA levels of pitx3 and dat, whereas morphine treatment decreases miR-133b level. However, the level of th increases only after treatment with 1 nM morphine, whereas higher concentrations induce a decrease, producing a biphasic effect.
Addition of naloxone effectively abolished the morphine-induced changes in the expression levels of miRNA-133b, pitx3, th, and dat (Fig. 1). These data suggest that morphine regulates the level of the dopaminergic genes via the control of miR-133b by activating zfMOR.
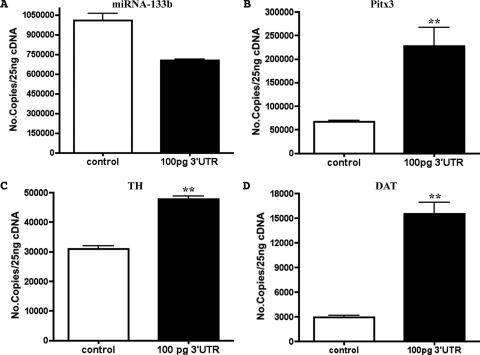
Although treatment of embryos with morphine clearly decreases the miR-133b level and increases the Pitx3 and its subsequent targets (i.e., TH and DAT levels), whether miR-133b indeed interacts with Pitx3 thereby destabilizing the transcript has not been demonstrated in zebrafish. Hence, to demonstrate that miR-133b does indeed interact with Pitx3 and regulates Pitx3's target levels in zebrafish, the 3′UTR sequence of Pitx3 was amplified and cloned into TOPO-TA pCR 2.1 vector for the injection into one-cell embryos. As shown in Fig. 2A, the embryos injected with the Pitx3 3′UTR sequence displayed a decrease in the miR-133b level compared with control embryos (noninjected embryos). At the same time, an increase in the Pitx3, TH, and DAT transcript levels were observed in embryos injected with the 3′UTR sequence (Fig. 2, B–D). Thus, the presence of the 3′UTR sequence of Pitx3, the putative target of miR-133b, decreased the free miR-133b level in zebrafish embryos resulting in an increase in the expression of Pitx3 and its subsequent targets.
Fig. 2.
Effect of the injection of Pitx3 3′UTR on the expression of miRNA-133b, Pitx3, TH, and DAT. Analyses of the level of miR-133b (A), Pitx3 (B), TH (C), and DAT (D) by qRT-PCR in 24-hpf zebrafish embryos injected with 100 pg of Pitx3 3′UTR. *, P ≤ 0.05; **, P ≤ 0.005 (unpaired Student's t test with Welch correction), n = 3.
The Role of zfMOR in Morphine-Induced Regulation of miR-133b Pathway.
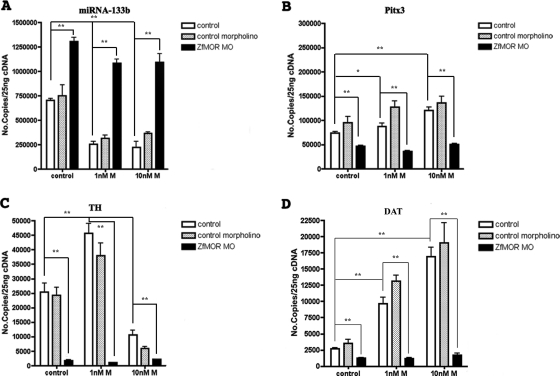
The effects of morphine in the embryos are probably mediated by zfMOR, the opioid receptor that exhibits highest affinity toward morphine (de Velasco et al., 2009). To establish the role of zfMOR in regulating miR-133b without the availability of a zfMOR-selective antagonist, we decided to silence zfMOR by morpholino oligonucleotide injection. The efficiency of the morpholino oligonucleotide to decrease the zfMOR level was determined with qRT-PCR. Injection of 0.2 μM morpholino oligonucleotide per embryo reduced the zfMOR transcription level by 95% (the injection of zfMOR decreased the expression of both zfDOR1 and zfDOR2 by approximately 2,5%, which is not statistically significant, showing the specificity of the zfMOR MO; data not shown).
The absence of zfMOR increases the number of miR-133b molecules within the embryos (Fig. 3A). Such an increase was not observed after the injection of a control morpholino. Furthermore, 1 or 10 nM morphine exposure did not alter the miR-133b level in embryos injected with zfMOR morpholino, whereas the same concentrations of morphine treatment resulted in a decrease of miR-133b level in embryos injected with control morpholino (Fig. 3A). The increased expression in miR-133b detected in the zfMOR knockdown embryos also led to a decrease of the subsequent miR-133b targets (i.e., Pitx3, TH, and DAT) (Fig. 3, B–D). The morpholino and the opioid antagonist naloxone studies clearly indicate that zfMOR is the mediator for the morphine-induced regulation of miR-133b and its targets.
Fig. 3.
Effect of MOR knockdown on the expression of miRNA-133b, Pitx3, TH, and DAT. The morpholino oligonucleotide technique was used to knock down the μ-opioid receptor from zebrafish as described under Materials and Methods. The consequence of MOR knockdown on the levels of miR-133b (A), Pitx3 (B), TH (C), and DAT (D) in control and 1 or 10 nM morphine-treated embryos were determined by qRT-PCR as described under Materials and Methods. Data were analyzed by two-way analysis of variance with post hoc Bonferroni test for comparisons. Error bars, S.E.M.. *, P ≤ 0.05; **, P ≤ 0.005, n = 3.
Morphine-Induced Regulation of the miR-133b Pathway Depends on ERK 1/2 Activity.
Morphine via the mammalian MOR regulates multiple signaling pathways. In the rat hippocampus, morphine activates ERK1/2 and decreases the expression level of miR-190 (Zheng et al.). Whether similar signaling mechanism is involved in morphine-induced regulation of the miR-133b pathway in zebrafish is unknown. Hence, several MAPK inhibitors (such as JNK inhibitor II for JNK, SB203580 for p38, and PD98059 and U0126 for ERK1/2 pathways) were used to identify the signals involved in morphine-induced miR-133a regulation. The inhibition of JNK and p38 did produce a significant decrease in the miR-133b level and, hence, an increase in the level of Pitx3, TH, and DAT (Table 1). In contrast, the inhibition of MEK1/2 by either U0126 or PD98059 enhanced miR-133b expression; as a consequence, it decreased the level of Pitx3, TH, and DAT transcripts (Fig. 4, A–D). Simultaneous exposure to morphine and one of the MEK1/2 inhibitors did not alter the miR-133b level or its related genes' transcript levels compared with embryos that were exposed only to the MEK1/2 inhibitors. Parallel treatment of embryos with morphine in the presence of either JNK or p38 inhibitor did not eliminate the morphine-induced decrease in the miR-133b level (Table 1). Morphine, by activating zfMOR morphine via the ERK1/2 pathway, probably regulates the miR-133b level in the zebrafish embryos.
TABLE 1.
Effect of the inhibition of different MAPK on the level of miRNA-133b, Pitx3, TH, and DAT in zebrafish embryos
Summary of the results obtained by qRT-PCR analysis of miR-133b, Pitx3, TH, and DAT levels in 24-hpf zebrafish embryos exposed to different MAPK inhibitors. The values represent average ± S.E.M. of the miR and mRNA copy numbers determined as described under Materials and Methods.
| MAPK Inhibitors | miR-133b | Pitx3 | TH | DAT |
|---|---|---|---|---|
| Control | 7.10 × 105 ± 10,260 | 66,684 ± 3537 | 25,465 ± 3006 | 2730 ± 508 |
| JNKII-inhibitor (0.6 μM) | 1.73 × 105 ± 9229** | 1.33 × 105 ± 5786** | 50,799 ± 3031** | 11,777 ± 1120** |
| SB203580 (60 μM) | 2.97 × 105 ± 15,142** | 1.51 × 105 ± 7494** | 52,061 ± 2575** | 18,492 ± 2311** |
| PD98059 (50 μM) | 1.04 × 106 ± 19,805* | 40,060 ± 2797* | 17,546 ± 771* | 1231 ± 250* |
P ≤ 0.05 (unpaired Student's t test with Welch correction).
P ≤ 0.005 (unpaired Student's t test with Welch correction).
Fig. 4.
Effect of the inhibition of ERK 1/2 on the expression of miRNA-133b, Pitx3, TH, and DAT. A, qRT-PCR analyses of miRNA-133b expression levels in 24-hpf zebrafish embryos exposed to the ERK 1/2 inhibitors PD98059 (PD) and U0126 (U) added at 5 or t 16 hpf. B, the Pitx3 gene expression levels are also measured in the same embryos, as well as TH (C) and DAT (D). qRT-PCR analyses were also carried out in 24-hpf zebrafish embryos treated simultaneously with 10 nM morphine and ERK 1/2 inhibitors. Data were analyzed by two-way analysis of variance with post hoc Bonferroni test for comparisons. Error bars, S.E.M. *, P ≤ 0.05; **, P ≤ 0.005, n = 3.
Morphine Regulates miR-133b Expression in Hippocampal Neurons.
To determine whether the observed regulation of miR-133b by zfMOR in the zebrafish embryos has any mammalian counterparts, hippocampal neurons obtained from P1 rats were treated with 100 nM morphine. Similar to reports from previous studies that used mature hippocampal neuron cultures from mice receiving long-term treatment with morphine, in which microRNA array and qRT-PCR studies revealed no effect on the miR-133b level (10), our current studies with neurons that were mature and differentiated (3-week culture) revealed the absence of morphine or morphine and naloxone effect on the expression of miR-133b (Fig. 5A). In contrast, the level of miR-133b was decreased in 1-week-old neurons treated with morphine but not with naloxone, an effect that was abolished by the coadministration of morphine and naloxone (Fig. 5B). Thus, similar to what we have observed in the zebrafish embryos, only the miR-133b level within the immature neurons could be affected by the addition of morphine.
Fig. 5.
Effect of morphine on the expression of miRNA-133b in mammalian neurons. The level of miRNA-133b in 1-week (A) and 3-week (B) hippocampal neuron culture treated with 100 nM morphine, 1 μM naloxone, or morphine and naloxone simultaneously were determined by qRT-PCR. *, P ≤ 0.05; **, P ≤ 0.005 (unpaired Student's t test with Welch correction), n = 4.
Discussion
Opioid receptors are involved not only in endogenous analgesia and development of tolerance, dependence, and addiction but also in certain aspects of the maturation of the CNS, such as neurogenesis or differentiation of neuronal stem cells (Kim et al., 2006). The molecular signals for such opioid activity are being described, but many issues concerning how opioids regulate the maturation of the CNS remain widely unknown, including the interaction between opioids and miRNAs. miRNAs are one of the most important regulatory systems in the organism (Giraldez et al., 2006; Bonauer et al., 2009; Zeng et al., 2009). Their main function is to activate cell proliferation by targeting and therefore promoting the down-regulation of genes involved in differentiation or genes that inhibit proliferation-inducer genes. One of these miRNAs, miR-133b, targets the transcription factor Pitx3, which activates dopaminergic differentiation by up-regulating the expression of genes that are dopaminergic neuron-specific, such as th and dat, among others (Kim et al., 2007). Similar to the observations in mammals, miR-133b controls pitx3 transcription by targeting its 3′UTR (Fig. 2) in zebrafish. Taking into consideration the implication of the dopaminergic system in addictive disorders, including addiction to opioid drugs (Flores et al., 2004; Leggio et al., 2009), we have established in this work a pathway that could account for the observed morphine-induced increase in dopamine production (Gianoulakis, 2009). By the modulation of miR-133b regulatory pathways and, hence, dopaminergic differentiation, zfMOR has a specific role in the CNS and is capable of regulating transcription through miRNAs.
In our miRNA arrays screen, morphine regulates multiple miRs (the identities of the miRs regulated have been deposited in the NCBI Gene Expression Omnibus database). The dose-dependent regulation of these miRs is unknown. Furthermore, the agonist concentrations to regulate various signaling pathways are not identical. An excellent example for apparent opposite effect of the drug is reported with neurite growth or regeneration. At lower concentrations, ∼10−14 M, morphine promotes neurite outgrowth and has a neurotrophic role in rat spinal and cortical neurons, but at higher doses, ∼10−6 M, it inhibits axon regeneration (Sinatra and Ford, 1979; Brailoiu et al., 2004). Both actions are antagonized by naloxone, indicative of the involvement of opioid receptor. Although the difference in the morphine doses used in our study is not as obvious, the changes in the transcription of TH induced by 1 and 10 nM morphine could be antagonized by naloxone and so could be blocked by reducing the receptor concentration with specific morpholinos. The TH biphasic responses to these morphine concentrations probably reflect the interactions between myriad of signals and miRs regulated by the opioid receptor expressed in the embryos.
The mammalian μ-opioid receptor activates ERK 1/2 (Zheng et al., 2008) and subsequently activates different sets of transcriptional factors leading to the many functions displayed by the receptor, such as proliferation (Persson et al., 2003). In addition, long-term exposure to morphine disrupts ERK 1/2 signaling, which is thought to enhance the development of tolerance. (Macey et al., 2009). Although in zebrafish there is no evidence yet that zfMOR induces phosphorylation of ERK 1/2, given the close molecular, pharmacological, and functional homology between mammalian and the zebrafish MOR, we use different MAPK inhibitors to demonstrate that only ERK 1/2 activity was involved in the zfMOR regulation of miR-133b and its targets' expression (Fig. 4). As observed in Table 1, the inhibition of other MAPK, such as p38 or JNK, decreased the level of miR-133b, opposite to the inhibition of ERK 1/2, which induced an increase in miR-133b copies. Considering that the absence of the μ-opioid receptor produced an effect similar to that observed after the inhibition of ERK 1/2, these results point out that it is through ERK 1/2 and not the other MAPK that zfMOR regulates the differentiation of dopaminergic neurons. Apart from its implication in MOR function (Zhang et al., 2009), phosphorylation by ERK 1/2 is involved in TH activation to synthesize dopamine (Haycock et al., 1992), which is the main step in the differentiation of neuronal precursors to the dopaminergic phenotype. Hence, by inhibiting ERK1/2, dopaminergic differentiation is reduced in two different ways: 1) by attenuating MOR regulation of miR-133b, which leads to a decrease in pitx3 expression, and 2) by preventing TH phosphorylation. ERK1/2 is also known to be involved in embryogenesis (Krens et al., 2008a,b). Hence, the consequences of maternal morphine intake on the fetus could take place through the intracellular pathways of miR133b and Pitx3. As our results suggest, the neonate abstinence syndrome might be caused by the alteration in dopaminergic differentiation induced by morphine. In addition, the inhibition of ERK 1/2 at two different developmental stages (5 and 16 hpf) have shown that the closer the treatment to the beginning of the differentiation of the CNS, the greater the effect of this inhibition in the expression levels of the genes involved in the maturation and differentiation of dopaminergic neurons. These data point out the importance of the developmental stage at which embryos are exposed to drugs, because exposure at different stages varies the impact of such drugs on embryo development. Thus, the control of miR-133b level could be a possible mechanism responsible for the development of morphine addiction or other drugs of abuse that also increase dopamine levels in the extracellular space. These results show for the first time that the miRNA-133b is a possible new target for the design of new treatments against addictive disorders.
The differences observed on the effect of morphine on the expression of miR-133b in 1-and 3-week-old neurons (Fig. 5) demonstrate that morphine induces differentiation by decreasing the expression of this particular miRNA only in the immature neurons. These results point out that the effects of morphine consumption during pregnancy could reflect the alteration in neuronal differentiation by changes in miR-133b expression. These data also point out that in mammals, morphine has the same effect as in the zebrafish in neuronal differentiation through miR-133b, therefore proving the validity of the zebrafish model to study neurological development during drug treatment.
Acknowledgments
We thank Angel Yuet Fong Kam and Dr. Juan Carlos Arevalo for helpful contribution to the miRNA-133b analysis in mammalian primary neuronal culture. We also acknowledge Dr. Lisa Schimenti and the staff of the University of Minnesota Fish Facility for invaluable help in obtaining zebrafish embryos.
This work was funded by the Spanish Ministry of Education and Science [Grant SAF2007-61581]; the Regional Government of Castilla y Leon [Grant SA0800A5]; and by the National Institutes of Health National Institute on Drug Abuse [Grants DA007339, DA011806].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.066837.
- MOR
- μ-opioid receptor
- DOR
- δ-opioid receptor
- zfMOR
- zebrafish μ-opioid receptor
- zfDOR
- zebrafish δ-opioid receptor
- NAc
- nucleus accumbens
- miRNA
- microRNA
- miR
- microRNA
- CNS
- central nervous system
- hpf
- hours after fertilization
- TH
- tyrosine hydroxylase
- DAT
- dopamine transporter
- UTR
- untranslated region
- MO
- morpholino
- MAPK
- mitogen-activated protein kinase
- JNK
- Jun N-terminal kinase
- ERK
- extracellular signal-regulated kinase
- MEK
- mitogen-activated protein kinase kinase
- Pitx3
- paired-like homeodomain transcription factor 3
- SB203580
- 4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)1H-imidazole
- PD98059
- 2′-amino-3′-methoxyflavone
- U0126
- 1,4-diamino-2,3-dicyano-1,4-bis(methylthio)butadiene
- qRT-PCR
- quantitative real-time-polymerase chain reaction.
References
- Alvarez FA, Rodriguez-Martin I, Gonzalez-Nuñez V, de Velasco EM, Gonzalez Sarmiento R, Rodríguez RE. (2006) New kappa opioid receptor from zebrafish Danio rerio. Neurosci Lett 405:94–99 [DOI] [PubMed] [Google Scholar]
- Barrallo A, González-Sarmiento R, Alvar F, Rodríguez RE. (2000) ZFOR2, a new opioid receptor-like gene from the teleost zebrafish (Danio rerio). Brain Res Mol Brain Res 84:1–6 [DOI] [PubMed] [Google Scholar]
- Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, et al. (2009) MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 324:1710–1713 [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Hoard J, Brailoiu GC, Chi M, Godbolde R, Dun NJ. (2004) Ultra low concentrations of morphine increase neurite outgrowth in cultured rat spinal cord and cerebral cortical neurons. Neurosci Lett 365:10–13 [DOI] [PubMed] [Google Scholar]
- Corbett AD, Henderson G, McKnight AT, Paterson SJ. (2006) 75 years of opioid research: the exciting but vain quest for the Holy Grail. Br J Pharmacol 147 (Suppl 1):S153–S162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Velasco EM, Law PY, Rodríguez RE. (2009) Mu opioid receptor from the zebrafish exhibits functional characteristics as those of mammalian mu opioid receptor. Zebrafish 6:259–268 [DOI] [PubMed] [Google Scholar]
- Filippi A, Dürr K, Ryu S, Willaredt M, Holzschuh J, Driever W. (2007) Expression and function of nr4a2, lmx1b, and pitx3 in zebrafish dopaminergic and noradrenergic neuronal development. BMC Dev Biol 7:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores JA, El Banoua F, Galán-Rodríguez B, Fernandez-Espejo E. (2004) Opiate anti-nociception is attenuated following lesion of large dopamine neurons of the periaqueductal grey: critical role for D1 (not D2) dopamine receptors. Pain 110:205–214 [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. (2009) Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem 9:999–1015 [DOI] [PubMed] [Google Scholar]
- Gieryk A, Ziolkowska B, Solecki W, Kubik J, Przewlocki R. (2010) Forebrain PENK and PDYN gene expression levels in three inbred strains of mice and their relationship to genotype-dependent morphine reward sensitivity. Psychopharmacology (Berl) 208:291–300 [DOI] [PubMed] [Google Scholar]
- Giraldez AJ, Mishima Y, Rihel J, Grocock RJ, Van Dongen S, Inoue K, Enright AJ, Schier AF. (2006) Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science 312:75–79 [DOI] [PubMed] [Google Scholar]
- Haycock JW, Ahn NG, Cobb MH, Krebs EG. (1992) ERK1 and ERK2, two microtubule-associated protein 2 kinases, mediate the phosphorylation of tyrosine hydroxylase at serine-31 in situ. Proc Natl Acad Sci USA 89:2365–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert SS, De Strooper B. (2009) Alterations of the microRNA network cause neurodegenerative disease. Trends Neurosci 32:199–206 [DOI] [PubMed] [Google Scholar]
- Kalscheuer S, Zhang X, Zeng Y, Upadhyaya P. (2008) Differential expression of microRNAs in early-stage neoplastic transformation in the lungs of F344 rats chronically treated with the tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis 29:2394–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Clark AL, Kiss A, Hahn JW, Wesselschmidt R, Coscia CJ, Belcheva MM. (2006) Mu- and kappa-opioids induce the differentiation of embryonic stem cells to neural progenitors. J Biol Chem 281:33749–33760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich A. (2007) A MicroRNA feedback circuit in midbrain dopamine neurons. Science 317:1220–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krens SF, Corredor-Adámez M, He S, Snaar-Jagalska BE, Spaink HP. (2008a) ERK1 and ERK2 MAPK are key regulators of distinct gene sets in zebrafish embryogenesis. BMC Genomics 9:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krens SF, He S, Lamers GE, Meijer AH, Bakkers J, Schmidt T, Spaink HP, Snaar-Jagalska BE. (2008b) Distinct functions for ERK1 and ERK2 in cell migration processes during zebrafish gastrulation. Dev Biol 319:370–383 [DOI] [PubMed] [Google Scholar]
- Leggio GM, Cathala A, Neny M, Rouge-Pont F, Drago F, Piazza PV, Spampinato U. (2009) In vivo evidence that constitutive activity of serotonin2C receptors in the medial prefrontal cortex participates in the control of dopamine release in the rat nucleus accumbens: differential effects of inverse agonist versus antagonist. J Neurochem 111:614–623 [DOI] [PubMed] [Google Scholar]
- Liao D, Grigoriants OO, Wang W, Wiens K, Loh HH, Law PY. (2007) Distinct effects of individual opioids on the morphology of spines depend upon the internalization of mu opioid receptors. Mol Cell Neurosci 35:456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey TA, Bobeck EN, Hegarty DM, Aicher SA, Ingram SL, Morgan MM. (2009) Extracellular signal-regulated kinase 1/2 activation counteracts morphine tolerance in the periaqueductal gray of the rat. J Pharmacol Exp Ther 331:412–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. (2000) Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet 26:216–220 [DOI] [PubMed] [Google Scholar]
- Nasiraei-Moghadam S, Kazeminezhad B, Dargahi L, Ahmadiani A. (2010) Maternal oral consumption of morphine increases Bax/Bcl-2 ratio and caspase 3 activity during early neural system development in rat embryos. J Mol Neurosci 41:156–164 [DOI] [PubMed] [Google Scholar]
- Persson AI, Thorlin T, Bull C, Eriksson PS. (2003) Opioid-induced proliferation through the MAPK pathway in cultures of adult hippocampal progenitors. Mol Cell Neurosci 23:360–372 [DOI] [PubMed] [Google Scholar]
- Pinal-Seoane N, Martin IR, Gonzalez-Nuñez V, de Velasco EM, Alvarez FA, Sarmiento RG, Rodriguez RE. (2006) Characterization of a new duplicate delta-opioid receptor from zebrafish. J Mol Endocrinol 37:391–403 [DOI] [PubMed] [Google Scholar]
- Rodriguez RE, Barrallo A, Garcia-Malvar F, McFadyen IJ, Gonzalez-Sarmiento R, Traynor JR. (2000) Characterization of ZFOR1, a putative delta-opioid receptor from the teleost zebrafish (Danio rerio). Neurosci Lett 288:207–210 [DOI] [PubMed] [Google Scholar]
- Schier AF, Giraldez AJ. (2006) MicroRNA function and mechanism: insights from zebra fish. ♣Cold Spring Harb Symp Quant Biol 71:195–203 [DOI] [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. (2006) A brain-specific microRNA regulates dendritic spine development. Nature 439:283–289 [DOI] [PubMed] [Google Scholar]
- Sinatra RS, Ford DH. (1979) The effects of acute and chronic morphine treatment on the process of facial nerve regeneration. Brain Res 175:315–325 [DOI] [PubMed] [Google Scholar]
- Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. (2006) Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev 20:515–524 [DOI] [PubMed] [Google Scholar]
- Westerfield M. (1995) The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 3rd ed University of Oregon, Eugene, OR [Google Scholar]
- Zeng L, Carter AD, Childs SJ. (2009) miR-145 directs intestinal maturation in zebrafish. Proc Natl Acad Sci USA 106:17793–17798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xiong W, Lin X, Ma X, Yu LC. (2009) Receptor trafficking induced by mu-opioid-receptor phosphorylation. Neurosci Biobehav Rev 33:1192–1197 [DOI] [PubMed] [Google Scholar]
- Zheng H, Loh HH, Law PY. (2008) Beta-arrestin-dependent mu-opioid receptor-activated extracellular signal-regulated kinases (ERKs) Translocate to Nucleus in Contrast to G protein-dependent ERK activation. Mol Pharmacol 73:178–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Zeng Y, Zhang X, Chu J, Loh HH, Law PY. (2010) mu-Opioid receptor agonists differentially regulate the expression of miR-190 and NeuroD. Mol Pharmacol 77:102–109 [DOI] [PMC free article] [PubMed] [Google Scholar]