Abstract
Purpose
The aims of this study were to assess the trans-scleral delivery of dexamethasone phosphate (DexP) with a prototype lens device and a formulation comprising a vasoconstrictor and to determine the efficacy of this delivery system in treating experimentally induced uveitis in a rabbit model.
Methods
Passive trans-scleral delivery was performed on New Zealand white rabbits in vivo, using the lens device and a formulation of 0.034 M oxymetazoline (OMZ, the vasoconstrictor) and 0.5 M of dexamethasone sodium phosphate (DexNaP). Trans-scleral delivery of DexP without OMZ was the control. The amounts of DexP delivered into the eye and its distributions in the eye were determined by dissection of the eye and high-performance liquid chromatography assay in the pharmacokinetics study. The efficacy of the DexP delivery system in treating lipopolysaccharide-induced uveitis was also evaluated in the rabbit model in vivo. The effect of OMZ upon DexP delivery and its treatment efficacy was studied by comparing the DexP results with and without OMZ.
Results
In the pharmacokinetics study, the amounts of DexP delivered into the eye using the lens system with OMZ were significantly higher than those without OMZ. The results in the efficacy study showed a better treatment outcome with OMZ to relieve the symptoms of endotoxin-induced uveitis in rabbits.
Conclusions
The potential of vasoconstrictors to enhance eye disease treatments in passive trans-scleral drug delivery was demonstrated. The higher DexP level in the eye and the improvement of the outcome in the efficacy study in the presence of the vasoconstrictor are consistent with the hypothesis that the vasoconstrictor enhances drug delivery by decreasing clearance.
Introduction
The most common form of drug administration to the eye is topical administration. In topical applications, only a small fraction of the topically applied drug, typically less than 5%, is absorbed into the eye,1,2 and only a small fraction of the drug that has penetrated into the anterior segment of the eye reaches the posterior segment due to the fluid dynamics in the anterior chamber and the barriers of the lens, iris, and ciliary body. Several reports have investigated the use of a sustained-release drug-delivery device or formulation to increase the residence time of the medication on the eye surface to enhance absorption.3–6 It has also been suggested that drugs can be delivered to the back of the eye through the trans-scleral route. Geroski and Edelhauser1 found that the permeability of rabbit and human sclera is relatively high. Despite the relatively high permeability and large surface area of the sclera, topical ocular drug delivery to the posterior segment of the eye by the trans-scleral route has not been very effective. This suggests that the major barrier of trans-scleral drug delivery is not the sclera. Some researchers believe that the clearance of the drug by the episcleral and choroidal blood flow precludes topically applied drugs from reaching the posterior ocular structures in therapeutic concentrations.1,2,7
Ocular clearance does not only reduce the residence time of the drug in contact with the eye for absorption, but can also be an absorption barrier, thereby limiting trans-scleral drug delivery.8,9 The importance of preretinal drug clearance by the conjunctival and choroidal circulation has been previously suggested. For example, Urtti10 has stated that the primary source of drug loss in topical administration is diffusion into the blood circulation. A major portion of the instilled dose is absorbed systemically by way of the conjunctiva, through the highly vascular conjunctival stroma, and through the lid margin vessels.1 Ogura11 stated that even if a compound was able to diffuse through the sclera, the compound would not be able to exert a therapeutic effect because it would be swept away by the choroidal blood flow. Choroidal clearance is evidenced by a decrease in the efficiency of iontophoresis drug delivery when the choroidal blood flow was increased after cryotherapy.12 Under a disease state, such as in uveitis, where the blood vessels are inflamed, have a higher blood flow, and are more permeable than in a noninflammatory state,13,14 the clearance from these vessels is expected to be even greater. In addition to hindering trans-scleral absorption, clearance through the conjunctival and scleral vasculature also pose the risk of inducing systemic toxicity when drugs enter the systemic circulation.15 Topically applied vasoconstrictors can reduce ocular clearance, such as the conjunctival and choroidal clearance. In a previous study, when the vasoconstrictor, phenylephrine, was coadministered with topical timolol, the peak systemic plasma concentration after transcorneal administration decreased by 70–80%.16 Four- to five-fold higher timolol concentrations were also observed in the aqueous humor and the iris/ciliary body in this study. However, the effects of a vasoconstrictor upon trans-scleral drug delivery have not been investigated.
Uveitis is an inflammation of the uveal tract and may occur as a result of a diverse series of stimuli, including infectious, traumatic, and idiopathic causes. The inflammation associated with uveitis may be acute, subacute, or chronic. In recent decades, uveitis was responsible for about 10% of all visual impairment in the United States17 and was the third leading cause of blindness worldwide. Uveitis may be classified anatomically into anterior, intermediate, posterior, and panuveitis. For anterior uveitis, treatments include the instillation of topical corticosteroid drops, such as 1% prednisolone acetate, 3–4 times or more daily or combined with a cycloplegic, such as 1 or 2% cyclopentolate 3–4 times daily.7 For intermediate and posterior uveitis, due to inaccessibility of the posterior eye to topically applied medications, systemic drug administration is commonly used, but the large systemic dose of corticosteroid in these treatments often induces systemic toxicities.18 For more persistent, serious intermediate and posterior uveitis conditions, the periocular and intravitreal injections of corticosteroid are used. Surgical implants are also available for the chronic treatment of uveitis.19,20 However, these methods have their drawbacks, such as poor patient compliance, high health care cost, and adverse effects.
The aims of the present study were to (1) examine a passive trans-scleral delivery lens device prototype for sustained topical drug delivery, (2) assess the effect of a vasoconstrictor upon passive trans-scleral drug delivery, and (3) determine the effectiveness of this delivery system in treating experimentally induced uveitis in a rabbit model. Oxymetazoline (OMZ, an active ingredient in eye drops for allergic conjunctivitis) was the vasoconstrictor tested in the present study. Dexamethasone phosphate (DexP, the phosphate pro-drug of dexamethasone) was the model corticosteroid. It was expected that the codelivery of the vasoconstrictor with the drug would exert a pharmacologic effect, both in the conjunctival circulatory bed and in the choroid plexus, because of its small molecular size and high permeability. The effect of the vasoconstrictor upon the passive trans-scleral delivery of DexP was first evaluated in rabbits in vivo. The efficacy of the passive trans-scleral system in treating endotoxin-induced uveitis was then evaluated by direct ophthalmologic examination in rabbits. The effect of the vasoconstrictor upon treatment efficacy was assessed by comparing the outcomes of the treatments with and without the vasoconstrictor.
Methods
Materials and animals
Ketamine hydrochloride injectable USP (100 mg/mL) was purchased from Hospira, Inc. (Lake Forest, IL). Sodium chloride 0.9% USP (pH between 5 and 7) was from Baxter Healthcare (Deerfield, IL). Lipopolysaccharide (LPS) from Escherichia coli serotype and oxymetazoline hydrochloride (OMZ) were from Sigma-Alrich (St. Louis, MO). Dexamethasone sodium phosphate (DexNaP) and dexamethasone (Dex) were from Letco Products (Decatur, AL) and Sigma-Aldrich (St. Louis, MO). DexNaP and OMZ solutions were prepared in distilled deionized water and adjusted to a pH of 7.
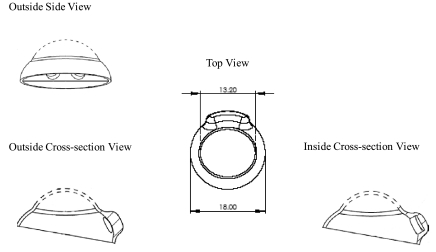
The prototype drug-delivery device was made of silicone and had a lens shape designed for rabbit applications (Fig. 1A). The lens was about 0.5 mm thick. The spherical dome-shaped design (13.2 mm in diameter) of the lens (total, 18 mm in diameter and 7.6 mm in height) allowed the lens to fit conveniently on the cornea of the rabbit. The lens had two drug-delivery chambers (about 2 mm apart) in direct contact with the conjunctiva adjacent to the limbus. The Chambers were circular in shape, 3 mm in diameter and 2 mm in depth. Each drug-delivery chamber contained a hydroxylated polyvinyl acetal (PVAc) surgical sponge (Ivalon; Fabco, Inc., Old Mystic, CT) of the same diameter as the chamber. The sponge matrix could swell and hold approximately 20 μL of solution.
FIG. 1A.
Schematic diagram of the lens device.
New Zealand white rabbits, male, weighing 3–4 kg, were purchased from the Western Oregon Rabbit Co. (Philomath, OR) and were used under the approval of the Institutional Animal Care and Use Committee at the University of Utah (Salt Lake City, UT) and in compliance with the Association for Research in Vision and Ophthalmology statement for the use of animals in Ophthalmic and Vision Research. All procedures were performed when the rabbits were anesthetized by an intramuscular injection of 25 mg/kg of ketamine and 15 mg/kg of xylazine. Figure 1B shows a picture of a rabbit with the lens device.
FIG. 1B.
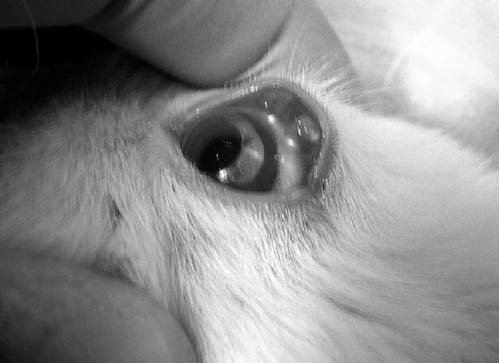
Picture of the lens device on a rabbit.
Pharmacokinetic study
Twelve (12) rabbits were divided into two groups of 6 animals. Only 1 eye from each rabbit was used. In the first group, the passive-delivery lens device (see Fig. 1) was loaded with 20 μL of 0.5 M DexNaP in one chamber and 0.034 M (or 1%) OMZ in the other chamber and placed on the eye of the anesthetized rabbit, giving a dose of 5 mg of DexNaP and 0.2 mg of OMZ. A passive-delivery lens device containing only DexNaP without OMZ was placed on the eye of the second group of rabbits as the control. The position of the device was checked to make sure that the drug matrix was in immediate contact with the conjunctiva/sclera under the superior cul-de-sac. A plastic collar was placed on the rabbit to prevent it from scratching the eye. The rabbit was then returned to a cage, where it was monitored closely. When the rabbit became agitated or the lens did not stay in the eye due to eye movement, further sedation, using ketamine alone, was administered. The duration of the device application was 4 h. The 4-h period was chosen because more than 80% of DexNaP in the delivery device was delivered in this time period, as determined by the amount of DexP left in the device (data not shown). Immediately after the 4-h treatment, the device on the treated eye was removed and the rabbits were sacrificed. The eyes were enucleated and the amounts of DexP in the eye tissues were determined by HPLC assay.
In the tissue assay, the eyes were enucleated, dissected, weighed, and separated into seven tissue sections: anterior chamber, lens, retina-choroid, cornea, vitreous, conjunctiva, and sclera for the extraction of DexP and Dex. DexP and Dex was extracted from the tissue with an extraction solvent of 1 M HCl in methanol (5:95, v/v). Each tissue was mixed with 5 mL of the extraction solvent. The tissue was then equilibrated with the solvent overnight. After that, the tissue was separated from the extracted solution. The extracted solution was concentrated by evaporation of the solvent in a water bath at 50°C, using nitrogen gas, and reconstituted in 1 mL of the extraction solvent. The samples were analyzed by HPLC. The concentration of the drugs in the tissue was calculated by comparing the area under the curve (AUC) of the tissue sample against the AUC of known standards. The extraction efficiency was determined in DexP and Dex recovery experiments. Recovery of DexP and Dex extracted from the tissues ranged from 48 to 100%, depending on the tissues. The HPLC DexP and Dex results were corrected with these extraction-efficiency values. The amounts of the pro-drug DexP and the drug Dex were combined in the analysis in the present study.
HPLC assay
The samples were assayed by using a Dionex HPLC system (Dionex Corp., Sunnyvale, CA): BioLC AS 50 AutoSelect with thermal compartment, Dionex ultraviolet (UV) detector AD25, BioLC GP50 gradient pump with vacuum degasser, PeakNet 6 computer software or an Agilent 1100 series HPLC system (Agilent Technologies, Santa Clara, CA) equipped with UV detector (Hewlett Packard 1050, Palo Alto, CA) with an integrator (Hewlett Packard 3396 series III). DexP and Dex were detected by a single UV wavelength absorbance, at λ = 242 nm. The mobile phase was 30% by volume of acetonitrile (HPLC grade) and 0.1% by volume of trifluoroacetic acid (99%) in distilled deionized water, and the HPLC column was a Phenomenex Hyperclone BDS C8ˇcolumn (3 microns, 150 mm × 4.6 mm). The HPLC method was isocratic with a 1-mL/min flow rate. The injection volume was 10 μL. Typical retention times for DexP and Dex were 4 and 7 min, respectively. DexP and Dex standard curves of 0.0002 to 0.2 mg/mL were generated.
Efficacy study
The animals were divided into four groups: control without any treatment, control with OMZ, control with DexNaP, and treatment with both OMZ and DexNaP. Only 1 eye from each animal was used. Uveitis was induced in each eye21 at the beginning of the experiment with a 250-ng intravitreal injection of LPS in 25 μL. Immediately after the LPS injection, all animals received the passive-drug-delivery device in the superior cul-de-sac. The control group (group 1) did not receive any DexNaP treatments. The OMZ control group (group 2) received a passive-delivery lens with 1% OMZ without DexNaP. Then, 1% OMZ was dissolved in deionized, distilled water and loaded in the surgical sponge in the drug chamber. The DexNaP treatment group (group 3) received a passive-delivery lens with 20 μL of 0.5 M DexNaP in the sponge. The DexNaP+OMZ group (group 4) received a passive-delivery lens with 20 μL of 1% OMZ and 0.5 M DexNaP in the sponges. After 4 h of the lens treatment, the lens device was removed from the sedated rabbit and the eyes were photographed. Then, the animals received a complete ophthalmologic examination at predetermined time points up to 7 days after the treatment. The animals were judged on a scale of 0–4+ based on the criterion in Table 1. Assessment of the anterior chamber was not reported, because the anterior of the rabbit eye was generally clear. Symptoms of anterior uveitis were observed but uncommon with the endotoxin-induced uveitis model in the present study. In the histopathology evaluation of the rabbit eyes 24 h after the uveitis induction, inflammatory reactions were found posterior to the iris, extending into the anterior vitreous in the area of the pars plicata and the choroid underlying the retina to the area of approximately the equator. Inflammatory cells were found in the vitreous adjacent to the retina and in the anterior chamber.
Table 1.
Scoring System for Uveitis Assessment in the Rabbits
| Score designation | Posterior chamber interpretation |
|---|---|
| 0 | Vitreous totally clear |
| 1 | Mild haziness in vitreous |
| 2 | Moderate haziness in vitreous |
| 3 | Structures of the posterior retina faintly visible, detailed examination of retina limited |
| 4 | Vitreous totally opaque; optic disc and vasculature not observable |
Results
Pharmacokinetic study
Although DexP was the drug in the passive-delivery system, Dex was found in the extraction samples from the eye in the HPLC assay. It is believed that the Dex prodrug, DexP, was converted to Dex through the cleavage of the phosphate group in the presence of phosphatase that was readily available in the eye tissues. Since it is difficult to control the enzymatic activities in the ocular tissues, the DexP and Dex data were combined and the total amounts of drugs (DexP and Dex) found in the eye tissues are reported in the present study.
Table 2 summarizes the total amounts of drugs in different parts of the eye after the passive application of the lens device for 4 h with and without OMZ. Comparison was made between the total amounts of DexP and Dex in the cornea, aqueous humor, vitreous, conjunctiva directly under the treatment site, sclera under the treatment site, choroid and retina under the treatment site, the rest of the sclera excluding the area under the treatment site, and the retina and choroid excluding the treatment site with and without OMZ. The total amounts of drugs in the eye were 19 nmol without OMZ, which corresponds to approximately 9 μg. With OMZ, the amounts increased to 42 nmol, or 17 μg. The total amounts of drugs found in the eye in the presence of OMZ were significantly larger than those without (P = 0.005; Student t-test). Particularly, larger amounts of drug were found in the conjunctiva, sclera that were the treatment or non-treatment sites, aqueous humor, and cornea in the presence of OMZ than those without OMZ (all P < 0.05; Student t-test). The prototype passive-lens-delivery system and OMZ provided tissue drug concentrations of around 0.02 and 0.2 nmol/mg in the posterior and anterior segments of the eye, respectively. The aqueous and vitreous concentrations were lower, at approximately 0.02 and 0.0003 nmol/μL, respectively. These concentrations are generally higher than those after periocular injections and topical eye-drop administrations in rabbits and humans, and are suggested to be effective to suppress inflammation in vitro.4,15,22–28
Table 2.
Amount of DexP and Dex in the Eye After 4 h Passive Delivery of DexNaP with the Lens Device
| Tissue | Total amount DexP and Dex, nmol, without OMZa | Total amount DexP and Dex, nmol, with OMZa | Total concentration, nmol/mg wet tissue without OMZ | Total concentration, nmol/mg wet tissue with OMZ |
|---|---|---|---|---|
| Corneab | 8 ± 5 | 17 ± 7 | 0.1 | 0.2 |
| Aqueous humorb | 2 ± 1 | 6 ± 3 | 0.007e | 0.02e |
| Vitreous | 0.3 ± 0.1 | 0.4 ± 0.3 | 0.0003e | 0.0003e |
| Conjunctivac | 2 ± 1 | 8 ± 6 | 0.006 | 0.02 |
| Sclera treatment siteb | 2 ± 1 | 5 ± 3 | 0.06 | 0.1 |
| Sclera otherb | 2 ± 1 | 4 ± 1d | 0.01 | 0.02 |
| Choroid/retina | 0.6 ± 0.7 | 0.8 ± 0.3 | 0.06 | 0.07 |
| Treatment site | ||||
| Choroid/retina other | 1.2 ± 0.5d | 1.2 ± 1.3 | 0.02 | 0.02 |
| Totalb | 19 ± 9 | 42 ± 11 | ||
Mean ± standard deviation; n = 6 unless otherwise stated.
Data with and without OMZ are statistically different (P < 0.05, t-tests).
Difference between data with and without OMZ is not quite significant (P = 0.08, t-test).
n = 5, an outlier was removed using the outlier Q-test.
Concentration in nmol/μL.
Efficacy study
Table 3 shows the results in the present in vivo efficacy study. The endotoxin-induced uveitis model of a 250-ng LPS intravitreal injection showed reproducible uveitis, with the posterior symptoms lasting for more than 3 days (group 1). At around 5–7 days, the symptoms began to disappear. Treatment with OMZ alone topically with the lens device slowed the emergence of the symptoms (group 2). DexNaP alone in the passive-device treatment reduced the symptoms of endotoxin-induced uveitis in this animal model (group 3). The coadministration of OMZ enhanced the outcome of the DexNaP treatments (group 4). The efficacy results suggest that trans-scleral passive delivery with the lens device, which allows the prolonged release of the drug to the eye through good surface contact, is effective in relieving the symptoms of endotoxin-induced uveitis. Table 4 summarizes the statistical analyses comparing the different groups in the present study. According to the analyses, OMZ reduced the symptoms of uveitis in the first 2 days. However, the effect of OMZ did not last more than 2 days (group 2 vs. 1); the uveitis score of the OMZ control became, essentially, the same as that of the uveitis no-drug control after the 2nd day. Passive delivery of DexNaP without OMZ improved the conditions of uveitis over the no-drug control (group 3 vs. 1). The score of passive DexNaP treatment with OMZ was lower than those of the uveitis no-drug control and OMZ control (group 4 vs. groups 1 and 2). Because of the OMZ effects in the first 2 days, comparison of the DexNaP treatment with and without OMZ was focused on the 3rd day. The combination of DexNaP and OMZ showed better efficacy than that of either OMZ or DexP alone on the 3rd day (group 4 vs. groups 2 and 3). The enhanced efficacy on day 3, when the OMZ therapeutic effect had worn off, suggests that OMZ increased the concentration of DexP at its site of action in the eye. Although the interplay of OMZ and DexNaP in the uveitis rabbit model is unclear, the efficacy study results support the hypothesis that OMZ enhances the delivery of DexP to the site of action in the eye, this eradicating the experimentally induced uveitis in the rabbit model.
Table 3.
Uveitis Scores on 1, 2, 3, and 5–7 Days in the In Vivo Efficacy Studya
| Group # | Protocol | 24 h | 2 days | 3 days | 5–7 days |
|---|---|---|---|---|---|
| 1 | No drug | 3.9 ± 0.4 | 3.9 ± 0.4 | 3.9 ± 0.3 | 2.6 ± 1.0 |
| 2 | OMZ control | 2.0 ± 1.3 | 2.5 ± 0.6 | 3.5 ± 0.5 | 3.2 ± 0.4 |
| 3 | DexNaP passive | 2.2 ± 1.6 | 2.0 ± 0.6 | 2.2 ± 1.2 | 1.2 ± 1.0 |
| 4 | DexNaP + OMZ passive | 1.3 ± 1.7 | 1.3 ± 1.3 | 1.0 ± 0.6 | 0.5 ± 0.8 |
Mean ± standard deviation; n ≥ 6.
Table 4.
Comparison Statistics of the Uveitis Efficacy Resultsa
| Comparison | 24 h | 2 days | 3 days | 5–7 days |
|---|---|---|---|---|
| Group 1 versus 2 | x | x | ns | ns |
| Group 1 versus 3 | ns | xx | xx | x |
| Group 1 versus 4 | xx | xx | xx | xx |
| Group 2 versus 4 | ns | ns | xx | xx |
| Group 3 versus 4 | ns | ns | x | ns |
x: statistically different, P < 0.05; xx, very statistically different, P < 0.01; ns, not statistically different, P > 0.05.
Discussion
OMZ-enhanced drug delivery
In the pharmacokinetics study, the data at the end of the 4-h lens application show that OMZ-enhanced delivery was mainly at the outer tissue layers, such as the conjunctiva, sclera, cornea, and anterior chamber, and the levels of drugs in the retina and vitreous humor of the eye were not significantly different with and without OMZ. The large amounts of drugs in the sclera and conjunctiva and the enhanced drug levels in these tissues with OMZ are consistent with enhanced ocular penetration through the conjunctiva and sclera. Another possible delivery route is through the cornea into the anterior segment, as suggested by the high concentration of drugs in the cornea. This route is unlikely because the drug-delivery chamber of the lens device was in direct contact with the conjunctival/sclera surface next to the limbus, although drug leakage from the chamber to the cornea surface during device application cannot be ruled out. Trans-scleral delivery can also deliver the drugs into the anterior segment through the ciliary body. Previous studies have shown that periocular injections mainly deliver drugs to the anterior segment, such as the anterior chamber.29,30 OMZ-enhanced trans-scleral drug delivery can result in ocular distribution mainly to the anterior chamber similar to those in periocular injections. The effect of OMZ on drug delivery to the posterior segment of the eye was less conclusive. The pharmacokinetic data do not show a significant increase in drug delivery to the vitreous and choroid/retina in the presence of OMZ. On the other hand, the efficacy study results are consistent with higher levels of the drug at the sites for the treatment of uveitis, which presumably include the posterior segment of the eye, according to the symptoms observed. It is also possible that the symptoms, such as vitreous haze, observed in the present endotoxin-induced uveitis model were related to inflammation at the anterior uvea.
Possible mechanisms of enhanced topical ocular drug delivery with OMZ
OMZ provides fast onset effects within 1–5 min after topical application in the treatment of hyperemia and is slowly absorbed into the eye.31 The results in the present pharmacokinetic and efficacy studies suggest that OMZ “assisted” the passive transport of DexP into the eye. The mechanisms of enhanced DexP delivery are believed to be related to the increase of drug residence time on the eye, lowering of intraocular pressure (IOP), decrease of aqueous-humor turnover and flow rate, and/or reduction of the clearance barrier, such as blood vasculature clearance. For example, OMZ has been shown to decrease tear flow and tear volume up to 6 h after instillation32 and thus can reduce clearance related to the tear. OMZ also reduces aqueous flow rate.33 This enhances ocular drug delivery by decreasing the clearance in the anterior chamber. OMZ also lowers IOP.34 As IOP has been suggested to affect trans-scleral transport,35 it is possible that OMZ can enhance trans-scleral transport through the IOP-lowering effect. In addition, alpha-adrenergic agonists similar to OMZ have been shown to decrease choroidal blood flow,36,37 and this can be a mechanism for enhancing DexP absorption.
Potential side effects of OMZ as an absorption enhancer
No adverse effect was observed after 4 h of device wearing without OMZ. The eye and cornea were generally unremarkable and showed no sign of toxicity. In a few occasions, a slight conjunctiva injection was observed at the site of application but returned to normal within a day. In the application of the lens device with OMZ, the main adverse effect observed was pupil dilation of the rabbit eyes. OMZ is a sympathomimetic amine, classified as an alpha-adrenergic agonist, and is a potent vasoconstrictor with a prolonged effect.38,39 It is a common ingredient in eye care and other products. OMZ is administered to patients as eye drops for allergic conjunctivitis and as a decongestant in the form of nasal spray or nasal drops. The regular dose of the OMZ nasal drop (e.g., Nezeril) is 2–3 drops at 0.05% every 12 h. As an eye drop, such as OcuClear or Visine LR, 0.025% OMZ is applied to the eye every 6 h. When used in the treatment of allergic or environmental conjunctivitis, OMZ shows systemic safety40,41 and mostly remained on the external surface. In a rabbit study, only 0.006% OMZ of the original drug concentration was found in the aqueous humor.31 Assuming each drop provides 0.1 mL of the medication, the dose in the eye and nasal applications is equivalent to approximately 0.2 mg of OMZ per day. The dose of OMZ used in the present study was 0.2 mg (20 μL of 1%), which is comparable to the daily dose of these over-the-counter OMZ medications. Although OMZ decreases tear flow and IOP, these effects are not expected to be harmful. Potential effects of OMZ upon the choroidal blood flow are also not expected to injure ocular tissues due to the ocular mechanisms of autoregulating blood flow, such as choroidal blood flow,42 that will supply enough oxygen and nutrients to these tissues. However, OMZ dilates the pupil of the eye, resulting in mydriasis,34 which can cause patient discomfort.
Conclusions
The prototype lens device used in this study provided an effective means of passive trans-scleral drug delivery. The amounts of drugs into the eye in the presence of OMZ were greater than those delivered without OMZ. The results in the efficacy study suggest the feasibility of the passive delivery of DexP for the treatment of uveitis by using the trans-scleral-delivery lens system. The improvement of the treatment outcome in the presence of the vasoconstrictor is consistent with our hypothesis that the vasoconstrictor enhances trans-scleral drug delivery by reducing clearance, such as blood vasculature clearance.
Acknowledgments
This study was supported by NIH Grant EY014772, awarded to Aciont, Inc. (Salt Lake City, UT). All the authors were employees or consultants receiving financial support from the company. The authors thank Matthew S. Hastings, Dalynn Berglund, and Guang Yan, PhD., for their help, Nick Mamalis, MD, and the Moran Eye Center (University of Utah) for performing the eye examination in the uveitis assessment, and Paul S. Bernstein, MD, PhD and Albert T. Vitale, MD for their helpful discussion.
References
- 1.Geroski D.H. Edelhauser H.F. Transscleral drug delivery for posterior segment disease. Adv. Drug Deliv. Rev. 2001;52:37–48. doi: 10.1016/s0169-409x(01)00193-4. [DOI] [PubMed] [Google Scholar]
- 2.Lee V.H. Robinson J.R. Topical ocular drug delivery: Recent developments and future challenges. J. Ocul. Pharmacol. Ther. 1986;2:67–108. doi: 10.1089/jop.1986.2.67. [DOI] [PubMed] [Google Scholar]
- 3.Davies N.M. Biopharmaceutical considerations in topical ocular drug delivery. Clin. Exp. Pharmacol. Physiol. 2000;27:558–562. doi: 10.1046/j.1440-1681.2000.03288.x. [DOI] [PubMed] [Google Scholar]
- 4.Hwang D.G. Stern W.H. Hwang P.H., et al. Collagen shield enhancement of topical dexamethasone penetration. Arch. Ophthalmol. 1989;107:1375–1380. doi: 10.1001/archopht.1989.01070020445052. [DOI] [PubMed] [Google Scholar]
- 5.Loftsson T. Stefansson E. Cyclodextrins in eye drop formulations: Enhanced topical delivery of corticosteroids to the eye. Acta. Ophthalmol. Scand. 2002;80:144–150. doi: 10.1034/j.1600-0420.2002.800205.x. [DOI] [PubMed] [Google Scholar]
- 6.Mainardes R.M. Urban M.C. Cinto P.O., et al. Colloidal carriers for ophthalmic drug delivery. Curr. Drug Targ. 2005;6:363–371. doi: 10.2174/1389450053765914. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett J.D. Jaanus S.D. Blaho K.E. Clinical Ocular Pharmacology. Boston: Butterworth Heinemann; 2001. [Google Scholar]
- 8.Lee T.W. Robinson J.R. Drug delivery to the posterior segment of the eye II: Development and validation of a simple pharmacokinetic model for subconjunctival injection. J. Ocul. Pharmacol. Ther. 2004;20:43–53. doi: 10.1089/108076804772745455. [DOI] [PubMed] [Google Scholar]
- 9.Robinson M.R. Lee S.S. Kim H., et al. A rabbit model for assessing the ocular barriers to the trans-scleral delivery of triamcinolone acetonide. Exp. Eye Res. 2006;82:479–487. doi: 10.1016/j.exer.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Urtti A. Salminen L. Minimizing systemic absorption of topically administered ophthalmic drugs. Surv. Ophthalmol. 1993;37:435–456. doi: 10.1016/0039-6257(93)90141-s. [DOI] [PubMed] [Google Scholar]
- 11.Ogura Y. Preface: Drug delivery to the posterior segment of the eye. Adv. Drug Deliv. Rev. 2001;52:1–3. doi: 10.1016/s0169-409x(01)00199-5. [DOI] [PubMed] [Google Scholar]
- 12.Lam T.T. Edward D.P. Zhu X.A., et al. Trans-scleral iontophoresis of dexamethasone. Arch. Ophthalmol. 1989;107:1368–1371. doi: 10.1001/archopht.1989.01070020438050. [DOI] [PubMed] [Google Scholar]
- 13.Greenwood J. The blood-retinal barrier in experimental autoimmune uveoretinitis (EAU): A review. Curr. Eye Res. 1992;11(Suppl):25–32. doi: 10.3109/02713689208999508. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni P.S. Steroidal and nonsteroidal drugs in endotoxin-induced uveitis. J. Ocul. Pharmacol. Ther. 1994;10:329–334. doi: 10.1089/jop.1994.10.329. [DOI] [PubMed] [Google Scholar]
- 15.Weijtens O. van der Sluijs F.A. Schoemaker R.C., et al. Peribulbar corticosteroid injection: Vitreal and serum concentrations after dexamethasone disodium phosphate injection. Am. J. Ophthalmol. 1997;123:358–363. doi: 10.1016/s0002-9394(14)70131-x. [DOI] [PubMed] [Google Scholar]
- 16.Kyyronen K. Urtti A. Improved ocular: Systemic absorption ratio of timolol by viscous vehicle and phenylephrine. Invest. Ophthalmol. Vis. Sci. 1990;31:1827–1833. [PubMed] [Google Scholar]
- 17.Stein J. Coogan M. NEI Press Release; 1997. New treatment for eye disease reduces need for strong drugs. [Google Scholar]
- 18.Goodman L.S. Gilman A. Hardman J.G., et al. New York: McGrawHill, Health Professions Division; 1996. Goodman & Gilman's The Pharmacological Basis of Therapeutics. [Google Scholar]
- 19.Jaffe G.J. McCallum R.M. Branchaud B., et al. Long-term follow-up results of a pilot trial of a fluocinolone acetonide implant to treat posterior uveitis. Ophthalmology. 2005;112:1192–1198. doi: 10.1016/j.ophtha.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Kodama M. Numaga J. Yoshida A., et al. Effects of a new dexamethasone-delivery system (Surodex) on experimental intraocular inflammation models. Graefe's Arch. Clin. Exp. Ophthalmol. 2003;241:927–933. doi: 10.1007/s00417-003-0753-2. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni P.S. Paterson C.A. Diclofenac and prednisolone inhibit endotoxin-induced uveitis in rabbits. Exp. Eye Res. 1995;61:767–768. doi: 10.1016/s0014-4835(05)80029-6. [DOI] [PubMed] [Google Scholar]
- 22.Bodker F.S. Ticho B.H. Feist R.M., et al. Intraocular dexamethasone penetration via subconjunctival or retrobulbar injections in rabbits. Ophthalmic Surg. 1993;24:453–457. [PubMed] [Google Scholar]
- 23.Cheng C.K. Berger A.S. Pearson P.A., et al. Intravitreal sustained-release dexamethasone device in the treatment of experimental uveitis. Invest. Ophthalmol. Vis. Sci. 1995;36:442–453. [PubMed] [Google Scholar]
- 24.Fialho S.L. da Silva-Cunha A. New vehicle based on a microemulsion for topical ocular administration of dexamethasone. Clin. Exp. Ophthalmol. 2004;32:626–632. doi: 10.1111/j.1442-9071.2004.00914.x. [DOI] [PubMed] [Google Scholar]
- 25.Kozak I. Kayikcioglu O.R. Cheng L., et al. The effect of recombinant human hyaluronidase on dexamethasone penetration into the posterior segment of the eye after sub-Tenon's injection. J. Ocul. Pharmacol. Ther. 2006;22:362–369. doi: 10.1089/jop.2006.22.362. [DOI] [PubMed] [Google Scholar]
- 26.Loftsson T. Hreinsdottir D. Stefansson E. Cyclodextrin microparticles for drug delivery to the posterior segment of the eye: Aqueous dexamethasone eye drops. J. Pharm. Pharmacol. Ther. 2007;59:629–635. doi: 10.1211/jpp.59.5.0002. [DOI] [PubMed] [Google Scholar]
- 27.Weijtens O. Feron E.J. Schoemaker R.C., et al. High concentration of dexamethasone in aqueous and vitreous after subconjunctival injection. Am. J. Ophthalmol. 1999;128:192–197. doi: 10.1016/s0002-9394(99)00129-4. [DOI] [PubMed] [Google Scholar]
- 28.Weijtens O. Schoemaker R.C. Romijn F.P., et al. Intraocular penetration and systemic absorption after topical application of dexamethasone disodium phosphate. Ophthalmology. 2002;109:1887–1891. doi: 10.1016/s0161-6420(02)01176-4. [DOI] [PubMed] [Google Scholar]
- 29.Lee T.W. Robinson J.R. Drug delivery to the posterior segment of the eye: some insights on the penetration pathways after subconjunctival injection. J. Ocul. Pharmacol. Ther. 2001;17:565–572. doi: 10.1089/10807680152729257. [DOI] [PubMed] [Google Scholar]
- 30.Li S.K. Molokhia S.A. Jeong E.K. Assessment of subconjunctival delivery with model ionic permeants and magnetic resonance imaging. Pharm. Res. 2004;21:2175–2184. doi: 10.1007/s11095-004-7669-3. [DOI] [PubMed] [Google Scholar]
- 31.Duzman E. Anderson J. Vita J.B., et al. Topically applied oxymetazoline. Ocular vasoconstrictive activity, pharmacokinetics, and metabolism. Arch. Ophthalmol. 1983;101:1122–1126. doi: 10.1001/archopht.1983.01040020124022. [DOI] [PubMed] [Google Scholar]
- 32.Gobbels M.J. Achten C. Spitznas M. Effect of topically applied oxymetazoline on tear volume and tear flow in humans. Graefe's Arch. Clin. Exp. Ophthalmol. 1991;229:147–149. doi: 10.1007/BF00170547. [DOI] [PubMed] [Google Scholar]
- 33.Wang R.F. Lee P.Y. Taniguchi T., et al. Effect of oxymetazoline on aqueous humor dynamics and ocular blood flow in monkeys and rabbits. Arch. Ophthalmol. 1993;111:535–538. doi: 10.1001/archopht.1993.01090040127046. [DOI] [PubMed] [Google Scholar]
- 34.Chu T.C. Ogidigben M.J. Potter D.E. Oxymetazoline: Potential mechanisms of inhibitory effects on aqueous humor dynamics. Pharmacology. 1996;53:259–270. doi: 10.1159/000139438. [DOI] [PubMed] [Google Scholar]
- 35.Rudnick D.E. Noonan J.S. Geroski D.H., et al. The effect of intraocular pressure on human and rabbit scleral permeability. Invest. Ophthalmol. Vis. Sci. 1999;40:3054–3058. [PubMed] [Google Scholar]
- 36.Chou P.I. Lu D.W. Chen J.T. Adrenergic supersensitivity of rabbit choroidal blood vessels after sympathetic denervation. Curr. Eye Res. 2001;23:352–356. doi: 10.1076/ceyr.23.5.352.5447. [DOI] [PubMed] [Google Scholar]
- 37.Weigert G. Resch H. Garhofer G., et al. Effects of topical clonidine versus brimonidine on choroidal blood flow and intraocular pressure during squatting. Invest. Ophthalmol. Vis. Sci. 2007;48:4220–4225. doi: 10.1167/iovs.07-0178. [DOI] [PubMed] [Google Scholar]
- 38.Fox S.L. Samson C.R. Danzig M.R. Oxymetazoline in the treatment of allergic and noninfectious conjunctivitis. J. Int. Med. Res. 1979;7:528–530. doi: 10.1177/030006057900700609. [DOI] [PubMed] [Google Scholar]
- 39.Xuan B. Chiou G.C. Efficacy of oxymetazoline eye drops in noninfectious conjunctivitis, the most common cause of acute red eyes. J. Ocul. Pharmacol. Ther. 1997;13:363–367. doi: 10.1089/jop.1997.13.363. [DOI] [PubMed] [Google Scholar]
- 40.Duzman E. Warman A. Warman R. Efficacy and safety of topical oxymetazoline in treating allergic and environmental conjunctivitis. Ann. Ophthalmol. 1986;18:28–31. [PubMed] [Google Scholar]
- 41.Samson C.R. Danzig M.R. Sasovetz D., et al. Safety and toleration of oxymetazoline ophthalmic solution. Pharmatherapeutica. 1980;2:347–352. [PubMed] [Google Scholar]
- 42.Polska E. Simader C. Weigert G., et al. Regulation of choroidal blood flow during combined changes in intraocular pressure and arterial blood pressure. Invest. Ophthalmol. Vis. Sci. 2007;48:3768–3774. doi: 10.1167/iovs.07-0307. [DOI] [PubMed] [Google Scholar]