Abstract
Purpose
Most eye drops contain preservatives; benzalkonium chloride (BAK) is most common. Recent data demonstrated BAK adding to toxicity. BAK is degraded into hydrogen peroxide (H2O2), which in even small amounts is known to be an irritant. Increased toxicity should cause localized inflammation with increased elaboration of inflammatory biomarkers. To evaluate the inflammation BAK causes to the ocular surface, enzyme linked immunosorbant assays (ELISAs) were utilized to quantify the levels of inflammatory biomarkers in response to BAK and/or H2O2.
Methods
Immortalized human conjunctival and corneal epithelial cells were exposed to: BAK (0.001%–0.1%), hydrogen peroxide (H2O2) (0.01%–0.1%), and cell media for 1 h. Cytokine quantification was performed via enzyme-linked immunosorbent assays [ELISAs]). Additional experimentation was performed in which testing solutions were replaced with media after 1 h and the resulting supernatants quantified after 24 h.
Results
BAK induced significant amounts of interleukin (IL-) 1 and tumor necrosis factor (TNF), but only moderate amounts of C-reactive protein (CRP), IL- 10 and 12, and H2O2. Lower concentrations of BAK induced proportionally less elaboration. Replacing the test solutions with media and providing 23 h for cytokine elaboration significantly increased TNF, but not IL-1. Lipopolysaccharide (LPS) positive controls induced substantial elaboration/release of both IL-1 and TNF as did in increasing the exposure to the full 24 h.
Conclusions
After 1 h of exposure, BAK increased quantities of all biomarkers. The biomarkers in decreasing order of induction/upregulation were: TNF ≥ IL-1 ≥ IL-12 ≥ IL-10 ≥ CRP. Even low concentrations caused some degree of inflammation. Replacing the testing solution with media and providing 23 h for cytokine elaboration, significantly increased the elaboration/release of TNF, but not IL-1, as compared to the 1-h BAK exposure. Whereas increasing the exposure to the full 24 h by not removing the testing solution at the 1-h time point significantly increased the elaboration/release of both IL-1 and TNF.
Introduction
Most eye drops contain preservatives that provide a level of antimicrobial activity in multiuse bottles, limiting secondary bacterial, mycotic, and amoebal ocular infections caused by contaminated solutions and prolong the shelf life of the drug by preventing biodegradation and maintaining drug potency.1 Preservatives can be classified into four main categories: detergents, oxidants, chelating agents, and metabolic inhibitors.2,3 Metabolic inhibitors have been further subdivided into three subcategories: pentavalent antimonials (SbV), quartenary ammoniums, and organomercurials.2,3 The most common preservative currently used in topical ophthalmic medications is BAK.4 It is typically used in concentrations varying from 0.01% to 0.05%.4 Recently, multiple authors have reported BAK as adding to the toxicity of eye drops and as potentially causing ocular surface disease.1,2,5,6 Although it (BAK) stabilizes drugs in solution, and prevents spoilage by microbial growth; it also has the potential of initiating ocular surface damage and subconjunctival inflammation.1,4 Although BAK is classified as a quaternary ammonium compound composed of a mixture of alkylbenzyl-dimethylammonium chloride homologues,7 it exhibits many detergent-like actions. Among them, it can affect cell membrane permeability, interrupt the metabolic processes of the cell, cause lysis of cell contents, and allow vital substances to escape, eventually causing death of the microorganism.8 BAK is also a cationic surfactant, reducing surface tension at interfaces. As such it also is attracted to negatively charged surfaces, including those of microorganisms having the ability to lyze cytoplasmic membranes and denature intracellular proteins.8
BAK, is known to be degraded into hydrogen peroxide (H2O2),8 which, in even small amounts as low as 30 parts per million (0.003%), is known to be an ophthalmic irritant.9,10
C-reactive protein (CRP),11 fibrinogen,12 and multiple cytokines,13,14 specifically interleukin (IL-)1α and β, IL-2, IL-4, IL-6, IL-10, IL-12, IL-23, and tumor necrosis factor (TNF-)α, have long been described as conventional biomarkers of inflammation. Increased toxicity should cause a localized inflammatory response with concomitant increased elaboration of these inflammatory biomarkers. To evaluate the potential toxicity of BAK to the ocular surface and the secondary resultant inflammatory response, enzyme linked immunosorbant assays (ELISAs) were utilized to quantify the resultant levels of the above-mentioned biomarkers of inflammation in response to varying concentrations of BAK and/or H2O2 on a corneal and conjunctival epithelial cell models.
Methods
Conjunctival epithelial cell line
Wong-Kilbourne-derived conjunctival cells, an established cell line15,16 [Wong-Kilbourne derivative of conjunctiva, clone 1-5c-4, American Type Culture Collection (ATCC, Manasas, VA)] were cultured under standard conditions (humidified atmosphere of 5% CO2 at 37°C) in Medium 199 in Hank's balanced salt solution (Gibco, Grand Island, NY) supplemented with 10% fetal calf serum (Hyclone, Logan, UT), 1% l-glutamine (Sigma Aldrich, St. Louis, MO), and 1% penicillin-streptomycin (Sigma Aldrich, St. Louis, MO). Cells from passages 5 through 20 were used for the experiments. Normal culture cell development was assessed daily by phase-contrast microscopy. Confluent cultures were dissociated with cell dissociation solution (Sigma Aldrich, St. Louis, MO) for 23 min and subcultured in ratios from 1:5 to 1:10 in 75 cm2 tissue culture flasks with media renewal every 2 days. They were then seeded into 96-well culture plates to the equal ratios used in the tissue culture flasks, kept at 37°C for 24 h. After approximately 75%–80% of confluency was attained they were used for experimentation.
Corneal epithelial cell line
Immortalized human corneal epithelial cells, an established cell line17,18 [10.014 pRSV-T, American Type Culture Collection (ATCC Manasas, VA)] were cultured under standard conditions (humidified atmosphere of 5% CO2 at 37°C) in precoated 25 cm2 tissue culture flasks. The precoating solution contains a mixture of 0.01 mg/mL bovine fibronectin (Sigma Aldrich, St. Louis, MO), 0.03 mg/mL Collagen I (Pure-Col, Palo Alto, CA), and 0.01 mg/mL bovine serum albumin (Sigma Aldrich, St. Louis, MO) diluted in balanced salt solution. The growth medium used was keratinocyte serum-free medium (Gibco, Grand Island, NY) supplemented with 0.05 mg/mL bovine pituitary extract (Gibco, Grand Island, NY), 5 ng/mL epidermal growth factor (Gibco, Grand Island, NY), 0.005 mg/mL human insulin (Sigma Aldrich, St. Louis, MO), and 500 ng/mL hydrocortisone (Sigma Aldrich, St. Louis, MO). Cells from passages 12 through 19 were used for the experiments. Normal culture cell development was assessed daily by phase-contrast microscopy. Eighty percent preconfluent cultures were dissociated with 0.025% trypsin-EDTA (Sigma Aldrich, St. Louis, MO) for 2.45 min, then centrifuged 125g for 10 min and, after discarding supernatant and resuspended cells in fresh medium, seeded in a ratio of 1:2 with media renewal every 2 days. They were then seeded into 96-well culture plates at the equivalent dilution ratios used in the tissue culture flasks and incubated (37°C) for 24 h. After subconfluence was attained (approximately 75%–80%) they were used for experimentation.
Testing solutions
All testing solutions were prepared hours prior to each experiment and then pre-equilibrated to (37°C, 5% CO2). These solutions contained various concentrations of the previously mentioned representatives of the categories of the ophthalmic preservatives and stabilizing/buffering agents, as well as the pure cell media control (see Table 1). The ratios were 100 μL of solution to be tested (equivalent to two drops) per 17 μL of growth media [equivalent to twice the normal volume of tear film (7–10 μL)].
Table 1.
Groups: Initial and Follow-Up Experimentation
|
Groups in corneal and conjunctival cell lines | |
|---|---|
| Initial experimentation | Follow-up experimentation |
| 1. Benzalkonium chloride (0.100%–0.001) + media (14.5%); | 1. Benzalkonium chloride (0.1%) + media (14.5%); |
| 2. Hydrogen peroxide (0.100%–0.010) + media (14.5%); | 2. Benzalkonium chloride (0.02%) + media (14.5%); |
| 3. Media (100%: total viable control). | 3. Benzalkonium chloride (0.002%) + media (14.5%); |
| 4. Lypopolysaccharide (100 ng/mL) + media (14.5%); | |
| 5. Lypopolysaccharide (10 ng/mL) + media (14.5%); | |
| 6. Media (100%: total viable control). | |
Experimental testing solutions used in tissue culture with immortalized human corneal (HCE: 10.014 prsv-t) and conjunctival (CCC: Wong-Kilbourne derivative of conjunctiva) epithelial cells in the initial and follow-up phases of the experimentation.
Experimental procedure
Initial experimentation
Each cell line was divided into the individual groups listed in Table 1/Initial experimentation (12 wells each): (1–9) BAK 0.10%–0.001%; (10–20) H2O2: 0.10%–0.01%; (21) appropriate medium. Ten standards of varying known concentrations for each of the enzyme-linked immunosorbent assays (ELISAs) were also run.
When the cells reached 75%–80% of confluency in the 96-well plates, the medium was removed and 117 μL of the appropriate pre-equilibrated (37°C, 5% CO2) testing solution (see Table 1/Initial experimentation) was added to each well. After a 1-h incubation at 37°C, the testing solutions were removed for CRP (American Diagnostica, Inc., Stamford, CT), IL-1α/IL-β (Becton, Dickinson and Company Biosciences, San Jose, CA), IL-10 (Becton, Dickinson and Company Biosciences), IL12 (Becton, Dickinson and Company Biosciences), TNFα (Becton, Dickinson and Company Biosciences), and H2O2 (Ozone Services, Yanco Ltd, Burton, B.C., Canada) quantification via ELISA. After following the staining procedure as per the manufacturers' instructions, the resulting solutions were spectrophotometrically measured at a wavelength of 450 nm (with 570 nm used as a background) utilizing a Quart Reader (BioTek Instruments, Inc., Winooski, VT) and the results displayed with Kineticalc for Windows version # 2.6, rev # 3 software (Bio Tek Instruments, Inc.). CRP and cytokine ELISAs give results in pg/mL to four significant figures (three decimal places). H2O2 ELISA gives results in mg/mL to 2 significant figures (one decimal place).
Follow-up experimentation
As a 1-h incubation is sufficient for cytokine release but insufficient for cytokine elaboration, additional experiments were run in which each cell line was divided into the individual groups listed in Table 1/Follow-up (three wells each): (1) BAK: 0.1%; (2) BAK: 0.02%; (3) BAK: 0.002%; (4) lypopolysaccharide (LPS: 100 ng/mL); (5) lypopolysaccharide (LPS: 10 ng/mL); and (6) appropriate cell medium. As in the initial experimentation, 10 standards of varying known concentrations for each of the enzyme-linked immunosorbent assays (ELISAs) were also run. In these experiments, after the cells reached 75%–80% of confluency in the 96-well plates, the medium was removed and the 117 μL of the appropriate pre-equilibrated (37°C, 5% CO2) testing solutions (Table 1/Follow-up) were incubated for 1 h, the testing solutions were discarded and replaced with the appropriate cell media, which was then allowed to incubate for an additional 23 h. The resulting cell supernatants after the 23-h incubation were then removed for IL-1 and TNFα (cytokines most markedly increased following 1-h incubation) quantification via enzyme-linked immunosorbent assays [ELISAs; CRP (American Diagnostica, Inc., Stamford, CT), IL-1α/IL-β (Becton, Dickinson and Company Biosciences, San Jose, CA), IL-10 (Becton, Dickinson and Company Biosciences), IL-12 (Becton, Dickinson and Company Biosciences), TNFα (Becton, Dickinson and Company Biosciences), and H2O2 (Ozone Services, Yanco Ltd, Burton, B.C., Canada)] and subsequent spectrophotometrical measurement at 450 nm (and 570 nm for background), as described above. Each cell line was divided into the individual groups listed in Table 1/Initial experimentation (12 wells each): (1–9) BAK 0.10%–0.001%; (10–20) H2O2: 0.10%–0.01%; (21) appropriate medium. Ten standards of varying known concentrations for each of the enzyme-linked immunosorbent assays (ELISAs) were also run.
Data analysis
Parametric statistics
Utilizing the standards, the optical density to CRP/cytokine concentration conversions were calculated for each ELISA via linear regression of power (log-log) plots. The concentrations of CRP (pg/mL), IL-1α/IL-β (pg/mL), IL-10 (pg/mL), IL12 (pg/mL), TNFα (pg/mL), and H2O2 (mg/mL) of each test solution were then calculated. The resulting concentrations were averaged first between samples within an experiment, then between experiments. The individual groups were analyzed for statistical differences, against the control (appropriate medium), utilizing the mean ± standard deviation for each of the various groups in computer-generated two-tailed bivariant Student's t-tests19,20 (GB-STAT, New England Software, Inc., College Station, TX, USA; SAS, SAS Institute Inc., Cary, NC, USA; and SPSS, SPSS Inc., Chicago, IL, USA). The parametric data was also analyzed for statistical differences, utilizing the mean ± standard deviation for each of the various study groups utilizing computer generated contingency tables with the Monte Carlo randomization test (SPSS)19–21 as well as individual Fisher exact tests (SPSS),19–21 overall chi-square analysis (SPSS),19,20 Bonferroni Post-Hoc Comparisons (SPSS),19–21 correlation coefficients,19,20 and a One Way Analysis of Variance (One Way ANOVA: SPSS).21,22 Normality and group independence/equivalency were confirmed with Shapiro-Wilk W (SPSS)21,22 and Skewness/Kurtosis (SPSS)21,22 tests for normality. Two-tailed significance was established at a confidence level of 0.05 > P > 0.95.
Results
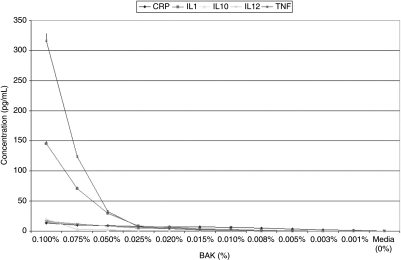
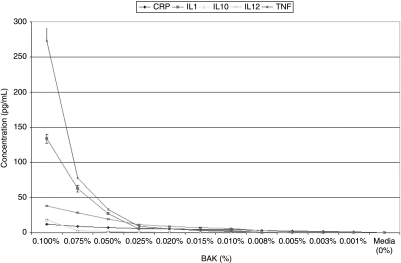
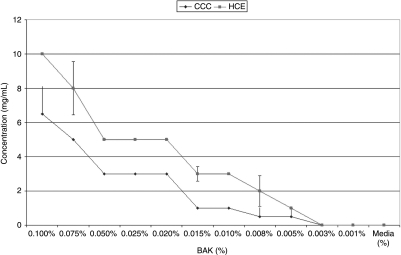
The optical densities of a total of 456 wells were measured for each study group in each of the two lines (5,472 total wells or 57 96-well plates). Media alone induced very small amounts of CRP (CCC: 0.256 ± 0.060 pg/mL; HCE: 0.275 ± 0.088 pg/mL), while levels of IL-1 (CCC: 0.006 ± 0.001 pg/mL; HCE: 0.013 ± 0.0007 pg/ml), IL-10 (CCC: 0.000 ± 0.000 pg/mL; HCE: 0.000 ± 0.000 pg/mL), IL-12 (CCC: 0.001 ± 0.001 pg/mL; HCE: 0.003 ± 0.001 pg/mL), TNF-α (CCC: 0.000 ± 0.000 pg/mL; HCE: 0.000 ± 0.001 pg/mL), and H2O2 (CCC: 0.00 ± 0.00 mg/mL; HCE: 0.00 ± 0.00 mg/mL) remained imperceptible in both corneal and conjunctival epithelial cells [see: Table 2 and Table 3, Fig. 1 (BAK-induced cytokines in CCC) and Fig. 2 (BAK-induced cytokines in HCE), Fig. 3 (BAK-induced H2O2 in CCC and HCE)].
Table 2.
Conjunctival Cells
| |
CRP (pg/mL) |
IL-1 (pg/mL) |
IL-10 (pg/mL) |
IL-12 (pg/mL) |
TNF-α (pg/mL) |
H2O2 (mg/mL) |
|---|---|---|---|---|---|---|
| Average ± σ | Average ± σ | Average ± σ | Average ± σ | Average ± σ | Average ± σ | |
| Media | 0.26 ± 0.06 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| BAK 0.1000% | 13.15 ± 0.77 | 145.33 ± 5.69 | 19.90 ± 0.41 | 16.29 ± 0.71 | 316.44 ± 12.30 | 6.5 ± 1.64 |
| BAK 0.0750% | 10.16 ± 0.72 | 70.63 ± 3.27 | 3.14 ± 0.17 | 11.78 ± 0.54 | 123.23 ± 3.73 | 5 ± 0.00 |
| BAK 0.0500% | 9.31 ± 0.52 | 29.90 ± 1.90 | 2.28 ± 0.26 | 8.25 ± 0.43 | 32.19 ± 3.06 | 3 ± 0.00 |
| BAK 0.0250% | 7.65 ± 0.62 | 8.27 ± 0.61 | 1.05 ± 0.11 | 4.87 ± 0.30 | 6.80 ± 0.36 | 3 ± 0.00 |
| BAK 0.0200% | 6.32 ± 1.32 | 6.13 ± 0.79 | 0.94 ± 0.10 | 3.95 ± 0.31 | 3.93 ± 0.14 | 3 ± 0.00 |
| BAK 0.0150% | 7.11 ± 0.38 | 3.62 ± 0.26 | 0.70 ± 0.10 | 3.16 ± 0.21 | 2.07 ± 0.15 | 1 ± 0.00 |
| BAK 0.0100% | 6.83 ± 0.32 | 2.59 ± 0.33 | 0.60 ± 0.11 | 2.49 ± 0.17 | 1.15 ± 0.09 | 1 ± 0.00 |
| BAK 0.0075% | 4.98 ± 0.31 | 0.70 ± 0.03 | 0.21 ± 0.03 | 1.22 ± 0.22 | 0.12 ± 0.01 | 0.5 ± 0.55 |
| BAK 0.0050% | 3.49 ± 0.21 | 0.09 ± 0.01 | 0.07 ± 0.02 | 0.44 ± 0.07 | 0.01 ± 0.00 | 0.05 ± 0.55 |
| BAK 0.0025% | 2.26 ± 0.11 | 0.04 ± 0.00 | 0.01 ± 0.00 | 0.29 ± 0.05 | 0.00 ± 0.00 | 0 ± 0.00 |
| BAK 0.0010% | 1.74 ± 0.12 | 0.02 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0 ± 0.00 |
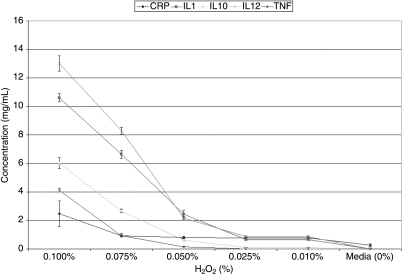
| H2O2 0.1000% | 3.08 ± 0.14 | 9.28 ± 0.89 | 7.07 ± 0.39 | 4.59 ± 0.26 | 1.50 ± 0.07 | 80 ± 0.00 |
| H2O2 0.0750% | 1.12 ± 0.08 | 5.84 ± 0.51 | 3.01 ± 0.18 | 2.53 ± 0.21 | 0.30 ± 0.03 | 70 ± 5.16 |
| H2O2 0.0500% | 1.01 ± 0.07 | 2.65 ± 0.36 | 0.67 ± 0.12 | 0.72 ± 0.12 | 0.03 ± 0.01 | 50 ± 5.16 |
| H2O2 0.0250% | 0.94 ± 0.04 | 0.68 ± 0.08 | 0.12 ± 0.01 | 0.40 ± 0.05 | 0.00 ± 0.00 | 15 ± 5.89 |
| H2O2 0.0100% | 0.79 ± 0.03 | 0.26 ± 0.03 | 0.00 ± 0.00 | 0.16 ± 0.03 | 0.00 ± 0.00 | 5 ± 0.00 |
Concentrations of inflammatory biomarkers [C reactive protein (CRP), interleukin (IL)-1, interleukin (IL)-10, interleukin (IL)-12, and tumor necrosis factor (TNF)] elaborated by conjunctival epithelial cells (CCC: Wong-Kilbourne derivative of conjunctiva) after being put in contact with varying concentrations of each of the initial experimental testing solutions [media alone, benzalkonium chloride (BAK: 0.001%–0.1%), and hydrogen peroxide (H2O2: 0.01%–0.1%) for 1 h.
Table 3.
Human Corneal Epithelial Cells
| |
CRP (pg/mL) |
IL-1 (pg/mL) |
IL-10 (pg/mL) |
IL-12 (pg/mL) |
TNF-α (pg/mL) |
H2O2 (mg/mL) |
|---|---|---|---|---|---|---|
| Average ± σ | Average ± σ | Average ± σ | Average ± σ | Average ± σ | Median ± “σ” | |
| Media | 0.28 ± 0.09 | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0 ± 0.00 |
| BAK 0.1000% | 11.84 ± 0.62 | 133.73 ± 6.49 | 17.95 ± 1.36 | 38.02 ± 1.19 | 272.79 ± 18.82 | 10 ± 0.00 |
| BAK 0.0750% | 9.28 ± 0.45 | 62.79 ± 4.76 | 3.05 ± 0.18 | 28.59 ± 0.94 | 77.75 ± 1.92 | 8 ± 1.55 |
| BAK 0.0500% | 7.45 ± 0.42 | 27.37 ± 0.91 | 0.63 ± 0.13 | 19.12 ± 0.60 | 33.33 ± 1.62 | 5 ± 0.00 |
| BAK 0.0250% | 5.94 ± 0.37 | 5.68 ± 0.41 | 0.92 ± 0.07 | 11.47 ± 0.33 | 9.21 ± 1.50 | 5 ± 0.00 |
| BAK 0.0200% | 5.49 ± 0.27 | 5.37 ± 0.20 | 0.81 ± 0.10 | 9.25 ± 0.29 | 5.18 ± 0.69 | 5 ± 0.00 |
| BAK 0.0150% | 5.09 ± 0.18 | 4.48 ± 0.13 | 0.69 ± 0.05 | 7.40 ± 0.30 | 2.83 ± 0.28 | 3 ± 0.41 |
| BAK 0.0100% | 4.73 ± 0.34 | 3.31 ± 0.17 | 0.63 ± 0.04 | 5.78 ± 0.25 | 1.52 ± 0.11 | 3 ± 0.00 |
| BAK 0.0075% | 3.15 ± 0.21 | 0.58 ± 0.03 | 0.19 ± 0.02 | 3.20 ± 0.13 | 0.19 ± 0.05 | 2 ± 0.89 |
| BAK 0.0050% | 2.33 ± 0.24 | 0.08 ± 0.01 | 0.10 ± 0.01 | 1.23 ± 0.07 | 0.01 ± 0.00 | 1 ± 0.00 |
| BAK 0.0025% | 1.63 ± 0.12 | 0.04 ± 0.01 | 0.01 ± 0.00 | 0.54 ± 0.00 | 0.00 ± 0.00 | 0 ± 0.00 |
| BAK 0.0010% | 1.02 ± 0.12 | 0.02 ± 0.00 | 0.00 ± 0.00 | 0.02 ± 0.00 | 0.00 ± 0.00 | 0 ± 0.00 |
| H2O2 0.1000% | 2.48 ± 0.90 | 10.61 ± 0.29 | 6.03 ± 0.39 | 13.01 ± 0.54 | 4.11 ± 0.15 | 90 ± 0.00 |
| H2O2 0.0750% | 0.92 ± 0.08 | 6.65 ± 0.29 | 2.67 ± 0.13 | 8.29 ± 0.25 | 0.92 ± 0.17 | 70 ± 0.00 |
| H2O2 0.0500% | 0.82 ± 0.06 | 2.45 ± 0.26 | 0.64 ± 0.07 | 2.18 ± 0.11 | 0.15 ± 0.03 | 50 ± 5.16 |
| H2O2 0.0250% | 0.78 ± 0.06 | 0.65 ± 0.04 | 0.10 ± 0.01 | 0.87 ± 0.06 | 0.00 ± 0.00 | 25 ± 2.58 |
| H2O2 0.0100% | 0.72 ± 0.12 | 0.29 ± 0.03 | 0.00 ± 0.00 | 0.28 ± 0.04 | 0.00 ± 0.00 | 7.5 ± 2.74 |
Concentrations of inflammatory biomarkers [C reactive protein (CRP), interleukin (IL)-1, interleukin (IL)-10, interleukin (IL)-12, and tumor necrosis factor (TNF)] elaborated by human corneal epithelial cells (HCE; 10.014 pRSV-T) after being put in contact with varying concentrations of each of the initial experimental testing solutions [media alone, benzalkonium chloride (BAK: 0.001%–0.1%), and hydrogen peroxide (H2O2: 0.01%–0.1%) for 1 h].
FIG. 1.
Graphic representation of concentrations of the various inflammatory cytokines studied [C reactive protein (CRP), interleukin (IL)-1, interleukin (IL)-10, interleukin (IL)-12, and tumor necrosis factor (TNF)] elaborated by conjunctival cells (Wong-Kilbourne derivative of conjunctiva, clone 1–5c) after being put into contact for 1 h with varying concentrations of benzalkonium chloride (BAK) as compared to media alone. All depicted values are statistically significant as compared to controls.
FIG. 2.
Graphic representation of concentrations of the various inflammatory cytokines studied [C reactive protein (CRP), interleukin (IL)-1, interleukin (IL)-10, interleukin (IL)-12, and tumor necrosis factor (TNF)] elaborated by human corneal epithelial cells (HCE; 10.014 pRSV-T) after being put into contact for 1 h with varying concentrations of benzalkonium chloride (BAK) as compared to media alone. All depicted values are statistically significant as compared to control.
FIG. 3.
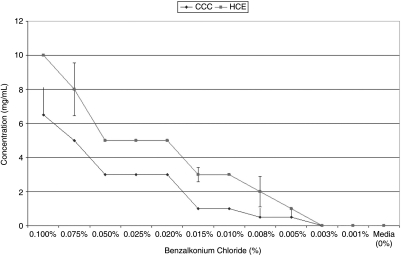
Graphic representation of concentrations of hydrogen peroxide (H2O2) as produced by conjunctival (CCC; Wong-Kilbourne derivative of conjunctiva, clone 1–5c) and human corneal (HCE; 10.014 pRSVT) epithelial cells after being put into contact for 1 h with varying concentrations of benzalkonium chloride (BAK) as compared to media alone. Probability (P) values of statistical comparisons of BAK concentrations from 0.01 to 0.005 are significantly different, lower concentrations are not.
In both cell lines, BAK: 0.1% induced, in a dose-dependent fashion, markedly increased quantities of: CRP (50-fold increase: ≤13.147 ± 0.768 pg/mL), IL-1 (104-fold increase: ≤145.329 ± 5.687 pg/mL), and TNF-α (104-fold increase: ≤316.442 ± 12.304 pg/mL) over media (CRP: 0.256 ± 0.001 pg/mL; IL-1: 0.006 ± 0.001 pg/mL; TNF: 0.001 ± 0.001 pg/mL). Elaborations of IL-10 and 12 were only marginally increased (IL-10: ≤19.903 ± 0.413 pg/mL; IL-12: ≤16.286 ± 0.712 pg/mL) from that of media (IL-10: ≤0.001 ± 0.001 pg/mL; IL-12: ≤0.001 ± 0.001 pg/mL; see: Tables 2 and 3, Figs. 1 and 2). Production of H2O2 was induced by BAK (≤6.5 ± 0.01 mg/mL) as compared to media alone (0.01 ± 0.01 mg/mL; see: Tables 2 and 3, Fig. 3). Lower concentrations of BAK induced the elaboration of proportionally lower amounts of all studied biomarkers in a dose-dependent fashion (see: Tables 2 and 3, Fig. 1 (BAK-induced cytokines in CCC) and Fig. 2 (BAK-induced cytokines in HCE), Fig. 3 (BAK-induced H2O2 in CCC and HCE)].
The addition of H2O2 (0.1%) induced increased quantities of all cytokines studied in both conjunctival (CRP: ≤3.084 ± 0.140 pg/mL; IL-1: ≤9.261 ± 0.889 pg/mL; IL-10: ≤7.071 ± 0.389 pg/mL; IL-12: ≤4.590 ± 0.262 pg/mL; TNFα: ≤1.504 ± 0.070 pg/mL) and corneal (CRP: ≤2.481 ± 0.897 pg/mL; IL-1: ≤10.608 ± 0.287 pg/mL; IL-10: ≤6.033 ± 0.389 pg/mL; IL-12: ≤13.014 ± 0.535 pg/mL; TNFα: ≤4.112 ± 0.150 pg/mL) cells over media (CCC: CRP: 0.256 ± 0.001 pg/mL; IL-1: 0.006 ± 0.001 pg/mL; IL-10: ≤0.001 ± 0.001 pg/mL; IL-12: ≤0.001 ± 0.001 pg/mL; TNF: 0.001 ± 0.001 pg/mL; HCE: CRP: 0.275 ± 0.088 pg/mL; IL-1: 0.013 ± 0.007 pg/mL; IL-10: ≤0.001 ± 0.001 pg/mL; IL-12: ≤0.003 ± 0.001 pg/mL; TNF: 0.000 ± 0.001 pg/mL; see: Tables 2 and 3, Figs. 4 and 5). Lower concentrations of H2O2 induced the elaboration of proportionally lower amounts of all studied biomarkers in a dose-dependent fashion (see: Tables 2 and 3, Figs. 4 and 5).
FIG. 4.
Graphic representation of concentrations of the various inflammatory cytokines studied [C reactive protein (CRP), interleukin (IL)-1, interleukin (IL)-10, interleukin (IL)-12, and tumor necrosis factor (TNF)] elaborated by human corneal epithelial cells (HCE; 10.014 pRSV-T) after being put into contact for 1 h with varying concentrations of hydrogen peroxide (H2O2) as compared to media alone. Probability (P) values of statistical comparisons of quantities of IL-10 and TNF elaborated in response to exposure to 0.01% H2O2 are not statistically significant as compared to controls. All other depicted values are statistically significant as compared to controls.
FIG. 5.
Graphic representation of concentrations of the various inflammatory cytokines studied [C reactive protein (CRP), interleukin (IL)-1, interleukin (IL)-10, interleukin (IL)-12, and tumor necrosis factor (TNF)] elaborated by conjunctival cells (Wong-Kilbourne derivative of conjunctiva, clone 1–5c) after being put into contact for 1 h with varying concentrations of hydrogen peroxide (H2O2) as compared to media alone. Probability (P) values of statistical comparisons of quantities of IL-10 and TNF elaborated in response to exposure to 0.01% H2O2 are not statistically significant as compared to controls. All other depicted values are statistically significant as compared to controls.
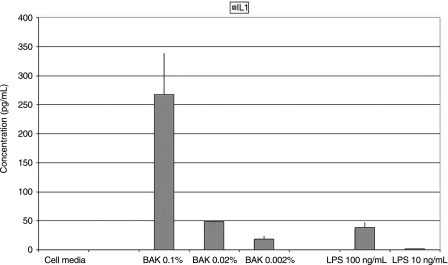
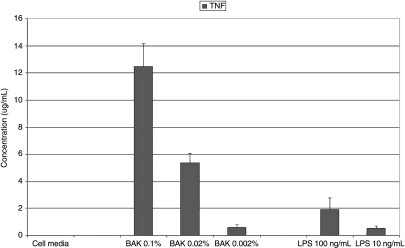
Replacing the testing solution with media and providing 23 h for cytokine elaboration, significantly increased the elaboration/release of TNF (12.456 ± 1.701 ug/mL), but not IL-1 (267.639 ± 71.502 pg/mL) as compared to the 1-h BAK exposure (see: Table 4, Figs. 6 and 7). Of course, both IL-1 and TNF were significantly increased as compared to the negative controls (IL-1: 267.639 ± 71.502 pg/mL; TNF: 2.909 ± 2.454 pg/mL; see: Table 4, Figs. 6 and 7). Lipopolysaccharide (LPS) positive controls at 100 and 10 ng/mL, respectively, induced substantial elaboration/release of both IL-1 (100 ng/mL: 38.541 ± 9.112 pg/mL; 10 ng/mL: 1.449 ± 0.339 pg/mL; see: Table 4, Figs. 6 and 7) and TNF (100 ng/mL: 1.925 ± 0.859 ug/mL; 10 ng/mL: 12.456 ± 1.701 ug/mL; see: Table 4, Figs. 6 and 7). Increasing the exposure to the full 24 h by not removing the testing solution at the 1-h time point significantly increased the elaboration/release of both IL-1 (100 ng/mL: 617.985 ± 100.057 pg/mL; 10 ng/mL: 274.115 ± 99.892 pg/mL) and TNF (100 ng/mL: 12.655 ± 2.400 ug/mL; 10 ng/mL: 7.516 ± 1.343 ug/mL).
Table 4.
Follow-Up Experimentation
| |
IL-1 (pg/mL) |
TNF-α pg/mL) |
|---|---|---|
| Average ± σ | Average ± σ | |
| Media | 0.007 ± 0.01 | 0.00 ± 0.00 |
| BAK 0.100% | 145.33 ± 5.69 | 316.44 ± 12.30 |
| BAK 0.020% | 48.26 ± 2.87 | 5.37 ± 0.71 |
| BAK 0.002% | 18.59 ± 5.31 | 0.59 ± 0.19 |
| LPS 100 ng/mL | 38.54 ± 9.11 | 1.93 ± 1.86 |
| LPS 10 ng/mL | 1.45 ± 0.34 | 0.51 ± 0.17 |
Concentrations of inflammatory biomarkers [interleukin (IL)-1 and tumor necrosis factor (TNF)] elaborated by conjunctival epithelial cells (CCC: Wong-Kilbourne derivative of conjunctiva) after being put in contact with varying concentrations of each of the follow-up experimental testing solutions [media alone, benzalkonium chloride (BAK: 0.1%, 0.02%, 0.002%), and lypopolysaccharide (LPS: 100 ng/mL, 10 ng/mL) for 1 h, the solutions replaced with media and then incubated for 23 h.
FIG. 6.
Graphic representation of concentrations of the inflammatory biomarker interleukin (IL)-1 elaborated by conjunctival epithelial cells (CCC: Wong-Kilbourne derivative of conjunctiva) after being put in contact with varying concentrations of each of the follow-up experimental testing solutions [media alone, benzalkonium chloride (BAK: 0.1%, 0.02%, 0.002%), and lypopolysaccharide (LPS: 100 ng/mL, 10 ng/mL) for 1 h, the solutions replaced with media and then incubated for 23 h. All depicted values are statistically significant as compared to control.
FIG. 7.
Graphic representation of concentrations of the inflammatory biomarker tumor necrosis factor (TNF) elaborated by conjunctival epithelial cells (CCC: Wong-Kilbourne derivative of conjunctiva) after being put in contact with varying concentrations of each of the follow-up experimental testing solutions [media alone, benzalkonium chloride (BAK: 0.1%, 0.02%, 0.002%), and lypopolysaccharide (LPS: 100 ng/mL, 10 ng/mL) for 1 h, the solutions replaced with media and then incubated for 23 h. All depicted values are statistically significant as compared to control.
Discussion
Our results showed that even at low concentrations BAK induced markedly increased quantities of IL-1 and TNFα (see: Tables 2 and 3, Figs. 1 and 2). Elaboration of CRP, IL-10 and −12 was only marginally increased (see: Tables 2 and 3, Figs. 1 and 2). The addition of H2O2 induced increased quantities of all cytokines studied in both conjunctival and corneal epithelial cells (see: Tables 2 and 3, Figs. 1 and 2). Production of H2O2 was induced by BAK as well (see: Tables 2 and 3, Figs. 1 and 2). Lower concentrations of BAK and/or H2O2 induced the elaboration of proportionally lower amounts of all studied biomarkers in a dose-dependent fashion (see: Tables 2 and 3, Figs. 1 and 2). Media alone induced very small amounts of CRP, while levels of IL-1, IL-10, IL-12, TNF-α, and H2O2 remained imperceptible in both corneal and conjunctival epithelial cells (see: Tables 2 and 3, Figs. 1 and 2). In general, we found that even low-concentrations of BAK and/or H2O2 caused some degree of inflammation/inflammatory response by corneal and conjunctival cells in tissue culture.
Our results also showed that with all agents, there was an increased elaboration of cytokines and/or H2O2 with increasing concentration. We found the biomarkers in decreasing order of induction/upregulation in response to BAK to be: TNF-α ≥ IL-1 ≥ IL-12 ≥ IL-10 ≥ CRP, while in response to H2O2 the order was: IL1 ≥ IL-12 ≥ IL-10 ≥ TNF-α ≥ CRP. The only discrepancy is with respect to TNF-α, which was induced in the highest amounts after BAK stimulation, while the second to lowest in response to H2O2, seeming to indicate that the primary mechanism of BAK-induced toxicity is not secondary to H2O2.
Although this does not correlate with the findings of authors studying the levels of inflammatory cytokines in the tear film of humans and animal models of Dry Eye Disease,23–31 this is not surprising as the induction of inflammation is believed to be primarily mechanical in dry eye (secondary to lack of lubrication), as compared to chemical (BAK, H2O2) induction, here.
As mentioned, most eye drops contain preservatives that provide a level of antimicrobial activity in the bottle, limiting secondary bacterial, mycotic, and amoebal-ocular infections caused by contaminated solutions and prolong the shelf life of the drug by preventing biodegradation and maintaining drug potency.1 As previously discussed, preservatives can be classified in four main categories: detergents, oxidants, chelating agents, and metabolic inhibitors, which are further subdivided into three subgroups: pentavalent antimonials (SbV), quartenary ammoniums, and ethyl mercurials.2,3 Preservatives added to eye drops can add to the toxicity of the pharmaceuticals and can cause ocular surface disease with a resulting inflammatory response.1,32
By far, the most common of the topical ophthalmic medication preservatives, and the best studied, is BAK, typically used in concentrations varying from 0.015% to 0.05%,4 although the American College of Toxicology has concluded that BAK can be safely used as an antimicrobial agent at concentrations up to 0.1%.33 In addition, BAK stabilizes drugs in solution and prevents spoilage by microbial growth; but has a markedly low-pH in aqueous solutions (ie, is highly acidic)34–36 and can initiate ocular surface damage and subconjunctival inflammation.1,4 BAK comes from the quaternary ammoniums and is a detergent preservative, which can affect cell membrane permeability, interrupt the metabolic processes of the cell, cause lysis of cell contents, and allow vital substances to escape, eventually causing death of the microorganism.8 The detergent properties of BAK have also been shown to interfere with the integrity of the external lipid layer of the precorneal tear film, reduce tear film breakup times, and exacerbate dry eye symptoms.1,37 As previously mentioned, BAK is also a cationic surfactant, which reduces surface tension at interfaces and attracts to negatively charged surfaces, including those of microorganisms. However, cationic surfactants have the ability to lyze cytoplasmic membranes and denature intracellular proteins.8
A variety of authors have raised the possibility of preservatives adding to the formulations imparting toxic effects to the ocular surface1,3,6,38,39 and recent data have demonstrated BAK as adding to the toxicity of eye drops and as potentially causing ocular surface disease.1,2,5,6 Such toxicity of preservatives such as BAK may cause ocular discomfort, changes in vision, and may interfere with patient compliance with recommended dosages.6 Dose-dependency has been previously reported with BAK2,3,5; at low concentrations (0.0001%–0.01%) BAK has been reported as having the potential to cause growth arrest or apoptotic mechanisms,38,40,41 while at higher concentrations (0.05%–0.2%) it has been reported as being able to cause cell death by necrosis.40–42
Mammalian cells are unable to neutralize preservatives BAK, and most of the detergent preservatives, in general, as the human ocular surface cannot break them down into more basic components, which the eye can better tolerate.1,42,43 Such preservatives can thus accumulate in ocular tissue and remain there for extended periods of time,1,42,43 prolonging adverse reactions and explaining “chronic-like” appearances to the ocular surface (ie, an inflammatory response).44–47
The detergents/surfactant preservatives such as BAK generate chloride dioxide free radicals that oxidize unsaturated lipids and glutathione in the cell, and H2O2.1,37,42 These preservatives all attribute their antimicrobial activity to their production of H2O2,1,37,42 an efficient antimicrobial,48 but also a known ophthalmic irritant.9,10 However, there has yet to be shown a link between the production of H2O2 and ocular inflammation and/or ocular surface disease following BAK administration.
There is evidence, however, that ocular surface disease is an immunopathological state with increased release of inflammatory cytokines and increased elaboration of inflammatory markers in tear film.13,14 In fact, tear samples from glaucoma patients have been shown to have greater amounts of inflammatory cytokines compared to their normal counterparts.49 Although fibrinogen is suspected as being upregulated indirectly (secondary to the inflammatory cytokines),50 it (ie, fibrinogen),12 CRP,11 and multiple cytokines,13,14 specifically IL-1α and β, IL-2, IL-4, IL-6, IL-10, IL-12, IL-23, and TNFα, have long been described as conventional markers of inflammation.
Yet, despite BAK being the most common, and the best studied, of the topical ophthalmic medication preservatives,4 there have been no studies published regarding BAK and inflammation or quantification of inflammatory cytokines specifically in response to BAK. As previously mentioned, a variety of authors have studied the levels of inflammatory cytokines (IL-1, IL-6, IL-8, and tumor necrosis factor TNF-α) in the tear film and conjunctival epithelium in humans as well as animal models, mostly in relation to Dry Eye Disease.23–31 In addition, a few similar studies comparing the concentrations of inflammatory cytokines in tears of patients with Sjögren syndrome, in particular, to normal patients have also been reported.28,30,31 However, nothing directly dealing with inflammatory cytokine levels with respect to BAK appear to have been published.
Here, we have demonstrated that cell media alone induces release of minimal to no amounts of inflammatory cytokines or H2O2. BAK in concentrations from 0.1% to 0.001%. induces release of high to moderate amounts of CRP and IL-12, release of very high to minimal amounts of IL-1 and TNF, responses are dose-dependent, but in an irritational, rather than truly “toxic,” fashion (quickly drops off). BAK induces the release of IL-10 and the production of H2O2, at higher concentrations (through 0.005%), but not at lower concentrations (0 pg/cell). H2O2 in concentrations from 0.1% to 0.01% induces release of small amounts of CRP and IL-10, moderate to small amounts of IL-1 and IL-12, and small to minimal amounts of Tumor Necrosis Factor. H2O2 also induces the production of high amounts of H2O2 (∼700 μg/cell). All responses are dose-dependent, although many are not truly “toxic,” fashion (quickly drops off). Thus, based on the data presented here, if a link between the production of H2O2 and ocular inflammation and/or ocular surface disease following BAK administration does exist, it does not seem to be of a direct “cause and effect” etiology.
Recently, a great deal of attention has been made regarding the potential contamination of the Wong-Kilbourne-derived human conjunctival cells utilized in these experiments with the HeLa cells utilized for the immortalization of the line.51 The degree of “loss” of the epithelial nature of this line has not been determined and, as a result, the potential exists for their response to not completely mimic conjunctival epithelium. In addition, while epithelial cells are a major inhabitant of the ocular surface, small numbers of inflammatory cells may also inhabit it and will certainly become involved in the ocular surface response to BAK toxicity through cell-cell interactions or cytokines.
While a multitude of authors have published regarding the potential toxicity of BAK used in ophthalmic preparations as a preservative, our tissue culture model of toxicity looking at corneal and conjunctival epithelial cells demonstrates that, regardless of the preservative, chronic, long-term use of any preserved pharmaceutical agent can negatively affect the ocular surface. Even using a low-concentration preservative for a long period of time can cause adverse reactions and high concentration of some preservatives can cause immediate damage and irritation to the ocular tissue. The inflammatory responses observed with pharmaceutical agents could be related to the preservative used in the different formulations (BAK) and might be decreased with the use of a different, less toxic, preservative. Use of a BAK-preserved pharmaceutical agent can negatively affect the ocular surface, although whether or not this reflects upon in vivo immunological responses remains to be determined.
Acknowledgments
Supported by grants from Fight for Sight, New York, NY; Research to Prevent Blindness, Inc., New York, NY; and The Martin and Toni Sosnoff Foundation; as well as EY01867 from the National Eye Institute, National Institutes of Health, Bethesda, MD.
References
- 1.Noecker R. Effects of common ophthalmic preservatives on ocular health. Adv. Ther. 2001;18:205–215. doi: 10.1007/BF02853166. [DOI] [PubMed] [Google Scholar]
- 2.Spickett C. Studies of Cellular Responses to Purite and Other Preservatives. Strthclyde, UK: University of Strathclyde; 2001. [Google Scholar]
- 3.Asbell P.A. Tear Substitutes: Past, Present and Future. Fifth 5th International Symposium on Ocular Pharmacology and Therapeutics (ISOPT); Monte Carlo, Monaco. Mar 1;; 2004. pp. 1–14. [Google Scholar]
- 4.Rhee D.J. Rapuano C.J. Belzer T.L., et al. Physicians' Desk Reference for Ophthalmic Medicines. Montvale, NJ: Thomson PDR; 2009. Pharmaceuticals in ophthalmology: agents for treatment of glaucoma; pp. 11–13. [Google Scholar]
- 5.Epstein S.P. Ahdoot M. Marcus E. Asbell P.A. Comparative toxicity of preservatives on immortalized corneal and conjunctival epithelial cells. J Ocul Pharmacol Ther. 2009;25(2):113–119. doi: 10.1089/jop.2008.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asbell P.A. Potapova N. Effects of topical antiglaucoma medications on the ocular surface. Ocul. Surf. 2005;3(1):27–40. doi: 10.1016/s1542-0124(12)70120-9. [DOI] [PubMed] [Google Scholar]
- 7.United States Pharmacopeial Convention. United States Pharmacopeia-National Formulary. Rockville, MD: U.S. Pharmacopeia; 2009. General information; pp. 1173–1175. [Google Scholar]
- 8.Ingram P.R. Pitt A.R. Wilson C.G., et al. A comparison of the effects of ocular preservatives on mammalian and microbial ATP and glutoathione levels. Free Radic Res. 2004;38(7):739–750. doi: 10.1080/10715760410001712773. [DOI] [PubMed] [Google Scholar]
- 9.Ohia S.E. Opere C.A. Leday A.M. Pharmacological consequences of oxidative stress in ocular tissues. Mutat. Res. 2005;579(1–2):22–36. doi: 10.1016/j.mrfmmm.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Watt B.E. Proudfoot A.T. Vale J.A. Hydrogen peroxide poisoning. Toxicol. Rev. 2004;23(1):51–57. doi: 10.2165/00139709-200423010-00006. [DOI] [PubMed] [Google Scholar]
- 11.Okamura J.M. Miyagi J.M. Terada K., et al. Potential applications of C-reactive protein. J. Clin. Lab. Anal. 1990;4(3):231–235. doi: 10.1002/jcla.1860040316. [DOI] [PubMed] [Google Scholar]
- 12.Senior R.M. Skogen W.F. Friffin G.L., et al. Effects of fibrinogen and its derivatives upon the inflammatory response. J. Clin. Invest. 1986;77(3):1014–1019. doi: 10.1172/JCI112353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang X. Moudgil K.D. The unveiling of hidden T-cell determinants of a native antigen by defined mediators of inflammation: implications for the pathogenesis of autoimmunity. Scand. J. Immunol. 2006;63(5):338–346. doi: 10.1111/j.1365-3083.2006.01748.x. [DOI] [PubMed] [Google Scholar]
- 14.Cohen S. Winkler S. Cellular immunity and the inflammatory response. J. Periodontol. 1974;45(5):348–350. doi: 10.1902/jop.1974.45.5.2.348. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi N. Quantitative cytotoxicity of preservatives evaluated in cell culture with Chang's human conjunctival cells—effect of temperature on cytotoxicity. Jpn. J. Ophthalmol. 1982;26(2):234–238. [PubMed] [Google Scholar]
- 16.Takahashi N. Cytotoxicity of mercurial preservatives in cell culture. Ophthalmic. Res. 1982;14(1):63–69. doi: 10.1159/000265175. [DOI] [PubMed] [Google Scholar]
- 17.Offord E.A. Sharif N.A. Mace K., et al. Immortalized human corneal epithelial cells for ocular toxicity and inflammation studies. Invest. Ophthalmol. Vis. Sci. 1999;40(6):1091–1101. [PubMed] [Google Scholar]
- 18.Walker T.L. Kahn C.R. Human Corneal Epithelial Cells with Extended Lifespan. U. S. Patent. 5,672,498 Sept 30, 1997.
- 19.Dixon W.J.Z. Introduction to Statistical Analysis. 3rd. New York: McGraw-Hill; 1969. Massey; p. 223. [Google Scholar]
- 20.Hoel P.H. Port S.C. Stone C.J. Introduction to Statistical Theory. Vol. 2. Boston MA: Houghton Mifflin; 1971. Testing hypotheses; pp. 52–110. [Google Scholar]
- 21.Anderson P.K. Borgan O. Gill R.D., et al. Statistical Models Based on Counting Processes. New York: Springer-Verlag; 1996. [Google Scholar]
- 22.Follman D. Multivariate tests for multiple endpoints. Stat. Med. 1995;14:1163–1176. doi: 10.1002/sim.4780141103. [DOI] [PubMed] [Google Scholar]
- 23.Massingale ML. Li X. Vallabhajosyula M., et al. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009. in press. [DOI] [PubMed]
- 24.Jones D.T. Monroy D. Ji Z., et al. Sjögren's syndrome: cytokine and Epstein-Barr viral gene expression within the conjunctival epithelium. Invest. Ophthalmol. Vis. Sci. 1994;35:3493–3504. [PubMed] [Google Scholar]
- 25.Pflugfelder S.C. Jones D. Ji Z., et al. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren's syndrome keratoconjunctivitis sicca. Curr. Eye Res. 1999;19:201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 26.Stern M.E. Gao J. Schwalb T.A., et al. Conjunctival T-cell subpopulations in Sjögren's and non-Sjögren's patients with dry eye. Invest. Ophthalmol. Vis. Sci. 2002;43:2609–2614. [PubMed] [Google Scholar]
- 27.Rolando M. Barabino S. Mingari C., et al. Distribution of conjunctival HLA-DR expression and the pathogenesis of damage in early dry eye. Cornea. 2005;24:951–954. doi: 10.1097/01.ico.0000157421.93522.00. [DOI] [PubMed] [Google Scholar]
- 28.Solomon A. Dursun D. Liu Z., et al. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest. Ophthalmol. Vis. Sci. 2001;42:2283–2292. [PubMed] [Google Scholar]
- 29.Corrales R.M. Villarreal A. Farley W., et al. Strain-related cytokine profiles on the murine ocular surface in response to desiccating stress. Cornea. 2007;26(5):579–584. doi: 10.1097/ICO.0b013e318033a729. [DOI] [PubMed] [Google Scholar]
- 30.Tishler M. Yaron I. Geyer O., et al. Elevated tear interleukin-6 levels in patients with Sjögren's syndrome. Ophthalmology. 1998;105:2327–2329. doi: 10.1016/S0161-6420(98)91236-2. [DOI] [PubMed] [Google Scholar]
- 31.Oshida T. Iwata M. Sakimoto T., et al. Tumor necrosis factor-alpha in tears of patients with Sjögren's syndrome. Nippon Ganka Gakkai Zasshi. 2004;108:297–301. [PubMed] [Google Scholar]
- 32.Baudouin C. Side effects of antiglaucomatous drugs on the ocular surface. Curr. Opin. Ophthalmol. 1996;7(2):80–86. doi: 10.1097/00055735-199604000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Hazardous Substances Data Bank. Benzalkonium Chloride. Sep 10, 2004. http://toxnet.nlm.nih.gov. http://toxnet.nlm.nih.gov.
- 34.Badavori S. 22nd. Whitehouse Station, NJ: Merck & Co., Inc.; 2006. The Merck Index; p. 128. [Google Scholar]
- 35.Weast R.C., editor; Astle M.J., editor; Beyer W.H., editor. CRC Handbook of Chemistry and Physics. 86th. Boca Raton, FL: CRC Press, Inc; 2006. [Google Scholar]
- 36.Hazardous Substances Data Bank. Thimerosal. Sep 10, 2004. http://toxnet.nlm.nih.gov. http://toxnet.nlm.nih.gov.
- 37.Noecker R. Herrygers L. Anwaruddin R. Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea. 2004;23(5):490–496. doi: 10.1097/01.ico.0000116526.57227.82. [DOI] [PubMed] [Google Scholar]
- 38.Debbasch C. Brignole F. Pisella P.J., et al. Quaternary ammoniums and other preservatives' contribution in oxidative stress and apoptosis on Chang conjunctival cells. Invest. Ophthalmol. Vis. Sci. 2001;42(3):642–652. [PubMed] [Google Scholar]
- 39.Van Horn D.L. Edelhauser H.F. Prodanovich G., et al. Effect of the ophthalmic preservative thimerosal on rabbit and human corneal endothelium. Invest. Ophthalmol. Vis. Sci. 1977;16(4):273–280. [PubMed] [Google Scholar]
- 40.De Saint Jean M. Brignole F. Bringuier A.F., et al. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest. Ophthalmol. Vis. Sci. 1999;40(3):619–630. [PubMed] [Google Scholar]
- 41.Lazarus H.M. Imperia P.S. Botti R.E., et al. An in vitro method which assesses corneal epithelial toxicity due to antineoplastic, preservative and antimicrobial agents. Lens Eye Toxic. Res. 1989;6(1–2):59–85. [PubMed] [Google Scholar]
- 42.Noecker R. Protecting the ocular surface in glaucoma and keratoconjunctivitis sicca. A Supplement to Refractive Eyecare® for Ophthalmologists. 2004;8(3):1–10. [Google Scholar]
- 43.Noecker R. Rev Ophthalmol. Jobson Publishing L.L.C.; Jun, 2001. Ophthalmic preservatives: considerations for long-term use in patients with dry eye or glaucoma. [Google Scholar]
- 44.Cooper M.L. Boyce S.T. Hansbrough J.F., et al. Cytotoxicity to Cultured Human Keratinocytes of Topical Antimicrobial Agents. J. Surg. Res. 1990;48:190–195. doi: 10.1016/0022-4804(90)90212-k. [DOI] [PubMed] [Google Scholar]
- 45.Geronemus R.G. Mertz P.M. Eaglstein W.H. Wound Healing: the effects of topical antimicrobial agents. Arch. Dermatol. 1979;115:1311–1314. doi: 10.1001/archderm.115.11.1311. [DOI] [PubMed] [Google Scholar]
- 46.Leitch I.O. Kucukcelebi A. Robson M.C. Inhibition of wound contraction by topical antimicrobials. Aust. N. Z. J. Surg. 1993;63:289–293. doi: 10.1111/j.1445-2197.1993.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 47.Bennett L.L. Rosenblum R.S. Perlov C., et al. An in vivo Comparison of topical agents on wound repair. Plast. Reconstr. Surg. 2001;108:675–687. doi: 10.1097/00006534-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 48.Kraus A. Antimicrobial effect of hydrogen peroxide and lactic acid in the mouth. Lancet. 1962;2:1328. doi: 10.1016/s0140-6736(62)90878-4. [DOI] [PubMed] [Google Scholar]
- 49.Pissella P.J. Conjunctival proinflammatory and proapoptotic effects of latanoprost and preserved and unpreserved timolol: an ex vivo and in vitro study. Inv. Ophthalmol. Vis. Sci. 2004;45:1360–1368. doi: 10.1167/iovs.03-1067. [DOI] [PubMed] [Google Scholar]
- 50.Bertini R. Bianchi M. Villa P., et al. Depression of liver drug metabolism and increase in plasma fibrinogen by interleukin 1 and tumor necrosis factor: a comparison with lymphotoxin and interferon. Int. J. Immunopharmacol. 1988;10(5):525–530. doi: 10.1016/0192-0561(88)90069-0. [DOI] [PubMed] [Google Scholar]
- 51.Brasnu E. Brignole-Baudouin F. Riancho L., et al. Comparative study on the cytotoxic effects of benzalkonium chloride on the Wong-Kilbourne derivative of Chang conjunctival and IOBANHC cell lines. Mol. Vision. 2008;14:394–402. [PMC free article] [PubMed] [Google Scholar]