Abstract
Studies were designed to examine the roles of individual protein kinase C (PKC) isoforms in the prostaglandin F2α (PGF2α)-induced matrix metalloproteinase-2 (MMP-2) secretion from human ciliary muscle cells.
Studies utilized primary cultures of human ciliary muscle cells. Individual PKC isoforms were detected by Western blotting, using PKC-isoform-specific antibodies. To evaluate MMP-2 secretion, cells were serum-starved overnight, treated with PGF2α (1 μmol/L) for 4 h and the media analyzed for MMP-2 by Western blotting. To assess ERK1/2 activation, cells were serum-starved overnight, treated with PGF2α (1 μmol/L) for 5 min and cell lysates analyzed for ERK1/2 phosphorylation by Western blot analysis. To evaluate the roles of individual PKC isoforms, cells were pretreated with PKC inhibitors or siRNAs prior to the addition of PGF2α.
In cultured human ciliary muscle cells, the PKC isoforms exhibiting the highest level of expression were PKC α, ɛ, í, and λ. The δ and η isoforms exhibited moderate levels of expression and β, γ, and φ were not detected. The administration of PGF2α (1 μmol/L) primarily induced the translocation of PKCɛ from cytosol to the membrane fraction, as well as increased MMP-2 secretion and ERK1/2 phosphorylation. The secretion of MMP-2 was inhibited by pretreatment with the broad-range PKC inhibitor, chelerythrine chloride; however, this response was not blocked by Go-6976, an inhibitor of conventional PKC isoforms. The PGF2α-induced secretion of MMP-2 was also blocked by pretreatment with the PKCɛ-selective peptide translocation inhibitor, EAVSLKPT, or the transfection of siRNA-targeting PKCɛ. The activation of ERK1/2 was inhibited by chelerythrine and the PKCɛ translocation inhibitor.
Human ciliary muscle cells express the α, ɛ, í, and λ PKC isoforms. Stimulation of FP receptors in these cells activates PKCɛ, resulting in ERK1/2 activation and an eventual increase in MMP-2 secretion. These data support the idea that the activation of FP receptors in vivo modulate uveoscleral outflow through the PKCɛ-dependent secretion of MMPs.
Introduction
Prostaglandin (PG) F2α is one of the biologically active prostanoids formed from the cyclo-oxygenase-catalyzed metabolism of arachidonic acid. PGF2α exerts a broad range of actions by binding to the FP receptor. The FP receptor is a member of the superfamily of G-protein-coupled receptors and has been cloned from a number of species, including human,1 mouse,2 and bovine.3 Response to FP receptors are mediated by a variety of second messenger generations, including phosphoinositides, intracellular calcium, protein kinase C (PKC), and MAP kinases.4–6 Physiologically, PGF2α is known to play key roles in regulating smooth-muscle contraction, DNA synthesis, cell proliferation, and extracellular matrix remodeling.7–10
Analogs of PGF2α, such as travoprost11 and latanoprost,12,13 are now considered first-line therapies in treating ocular hypertension. The effects of PGF2α analogs on intraocular pressure (IOP) reduction principally involved an increase in uveoscleral outflow.14–20 This increase in uveoscleral outflow is thought to involve the secretion and activation of various matrix metalloproteinases (MMPs) and the turnover of extracellular matrix.17,18,21–23 In the anterior segment tissues, a number of different ligands, such as growth factors, cytokines, prostaglandins, and phorbol esters, have been shown to regulate MMP secretion.21,24–27 Although these studies have provided evidence that multiple receptors regulate the secretion of MMPs from cells within the outflow pathway, the signal-transduction systems coupled to these receptors in ciliary muscle cells is not fully understood.
In the ciliary muscle, we have shown that the activation of PKC plays an important role in the secretion of MMP-2.28 However, the expression pattern and physiologic role of individual PKC isoform in this tissue has not been evaluated. The aim of this study was to elucidate the roles of individual PKC isoform(s) in the PGF2α-induced increase in MMP-2 secretion from human ciliary muscle cells. In this paper, we provide evidence that PGF2α primarily activates PKCɛ in ciliary muscle cells, and this activation of PKCɛ is an early signaling event regulating the secretion of MMP-2 from these cells.
Methods
Reagents
Prostaglandin F2α was obtained from Cayman (Ann Arbor, MI). Monoclonal antibodies to PKC isoforms (α, β, γ, δ, ɛ, η, μ, í, and λ) were purchased from BD Transduction laboratories (San Diego, CA) and monoclonal MMP-2 antibodies were obtained from Calbiochem (San Diego, CA). Transfection reagent (Silent Fect) was obtained from Bio-Rad (Hercules, CA) and siRNA duplexes targeting PKCɛ and Lamin were obtained from Dharmacon RNA Technologies (Dharmacon Inc., Lafayette, CO). Fetal bovine serum (FBS) was obtained from Hyclone (Logan, UT), and all other cell culture supplies were obtained from Cell Gro (Herndon, VA).
Cell culture
Human eyes (donor ages between 25 and 71 years) without any ocular history were obtained from Life-Point Ocular Tissue Division (Charleston, SC). Human ciliary smooth muscle cells were prepared from normal human eyes by using a procedure previously described.4 Briefly, ciliary muscles were dissected with the aid of a dissecting microscope under sterile conditions, cleaned, and cut into 1–2-mm pieces. The explants were placed in Dulbecco's modified Eagle's medium (DMEM) containing 2 mg/mL collagenase type IA, 10% FBS, and 50 μg/mL of gentamicin and was then incubated for 1–2 h at 37°C with occasional shaking. When a major part of the explant was dispersed into single cells or groups of cells, the cell suspension was centrifuged at 200g for 10 min and resuspended in DMEM199 supplemented with 10% FBS, 100 U/mL of penicillin G, 100 μg/mL of streptomycin, and 0.25 μg/mL of amphotericin B and maintained in a 5% CO2-humidified atmosphere. The confluent cells were subcultured at a split-ratio of 1:4, using 0.05% trypsin and 0.02% ethylenediaminetetraacetic acid (EDTA). Cultured cells from two to six passages were used in this study. Ciliary muscle cells in culture were identified by their pattern of growth, morphology, and immunocytochemical staining to smooth-muscle actin. These cells formed a confluent monolayer of spindle-shaped cells organized into a “hill and valley” distribution, which is characteristically typical of ciliary smooth muscle cells, as described earlier.4
Protein kinase C analysis
PKC isoforms were detected in cytosolic and membrane fractions, using the procedure described earlier,29 by Western blotting. Briefly, cells were lysed in Tris-HCl buffer (pH 7.5) containing 1 mmol/L ethylene glycol tetraacetic acid (EGTA), 2.5 mmol/L of EDTA, 5 mmol/L of DTT, 0.3 mol/L of sucrose, 1 mmol/L Na3VO4, 20 mmol/L NaF, and a protease cocktail inhibitor, using G-20 syringes, followed by centrifugation at 600g for 10 min at 4°C. The supernatant was again centrifuged at 100,000g for 45 min at 4°C. The supernatant was saved as the cytosolic fraction. The pellet was resuspended in lysis buffer containing 0.1% Triton X-100 and served as the membrane fraction. Equal amounts of protein were separated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes for Western blot analysis, using anti-PKC isoform antibodies (1:1000) and secondary antibodies (horseradish peroxidase [HRP]-conjugated IgG at 1:3000). Rat brain extract, Jurkat, and WI-38 were run as the positive control for the immunodetection of PKC isoforms (as directed by the manufacturer). Band densities were quantified by means of a Bio-Rad Versa Doc Imaging System (Bio-Rad Laboratories).
MMP-2 assay
Human ciliary muscle cells were starved in serum-free medium for 16 hours. Cells were treated with vehicle or PGF2α (1 μmol/L) for 4 h, and the media were collected and concentrated tenfold, using Amicon Ultra-10 centrifugal filter concentrators (Millipore, Billerica, MA). Equivalent volumes of media (40 μL) were loaded onto 10% SDS-PAGs followed by transfer to a nitrocellulose membrane. The membranes were then probed with anti-MMP-2 antibodies overnight at 4°C. Bands were visualized by the addition of secondary antibodies (HRP-conjugated at 1:3000) and enhanced chemiluminescence (ECL) reagents. The band intensities were quantified by densitometry by using a Bio-Rad Versa Doc Imaging System (Bio-Rad Laboratories). Purified MMP-2 was run in parallel to confirm the identity of the MMP-2 band.
Extracellular signal-regulated kinase (ERK1/2) assay
Cells were cultured in serum-free medium for 16 h before the addition of any agent. Cells were treated with PGF2α (1 μM) for 5 min. In experiments evaluating chelerythrine chloride or the PKC translocation inhibitor, cells were pretreated prior to the addition of PGF2α for 60 min. At the end of the incubation period, cells were rinsed with ice-cold phosphate-buffered saline (PBS) and lysed by the addition of lysis buffer (50 mM of Tris-HCl buffer, pH 8.0, containing 100 mM NaCl, 1 mM of EDTA, 1% Nonidet P-40, 0.1% SDS, 0.5% deoxycholate, 50 mM NaF, 1 mM/L Na3VO4, 5 mM of phenylmethylsulfonyl fluoride, 10 μg/mL of leupeptin, and 50 μg/mL of aprotinin) for 20 min on ice. To determine the level of ERK1/2 activation (phosphorylation), equivalent amounts of protein (15 μg) were loaded onto 10% SDS-PAGs, and proteins were separated according to molecular weight by using standard SDS-PAGE protocols and transferred to a nitrocellulose membrane. The membranes were then probed with anti-phospho-ERK1/2 antibodies for 2 h at room temperature. Bands were visualized by the addition of antirabbit HRP-conjugated secondary antibodies (at 1:3000) and ECL reagents. Blots were then stripped by incubation in stripping buffer (62.5 mM of Tris-HCl, pH 6.7, 100 mM of β-mercaptoethanol, and 2% SDS) for 30 min at 50°C, and total extracellular-signal-regulated kinase (ERK) levels (phosphorylated and nonphosphorylated forms) were determined by immunoblot techniques, using polyclonal anti-ERK1/2 antibodies. Band densities were quantified with a Bio-Rad Versa Doc Imaging System (Bio-Rad Laboratories). The level of phosphorylated ERK1/2 isoforms was normalized for differences in loading, using the total ERK protein band intensities.
PKC translocation inhibitor and siRNA treatment
The translocation peptide inhibitor, EAVSLKPT, selective for PKCɛ was delivered into human ciliary muscle cells by using Chariot (Active Motif, Carlsbad, CA) as a carrier. Chariot is a reagent capable of efficiently delivering proteins, peptides, and antibodies into cultured mammalian cells.30 A complex of chariot-peptide (50:50) was added to serum-starved human ciliary muscle cells for 60 minutes. Cells were treated with PGF2α (1 μmol/L) for 4 h, and the media were collected and stored at −80°C until analyzed by Western blotting for MMP-2.
For siRNA transfections, cells were grown to 70–80% confluency, and transfection with duplex siRNA was performed by using methods similar to Elbashir and colleagues.31 Briefly, human ciliary muscle cells were incubated in serum-free medium containing transfection reagent and siRNA cocktail for 4 h at 37°C in humidified air. After incubation, the serum-free medium was removed and complete medium, containing 10% FBS, was added and incubation continued for 48 h. Prior to the addition of the agonist, cells were starved overnight in serum-free medium. PKCɛ and Lamin A/C siRNAs were obtained from Dharmacon RNA Technologies. Twenty-one-nucleotide siRNA for Lamin A/C (Catalogue no. D-001000-02-05) and smart pool upgrade siRNA of PKCɛ (catalogue no. MU-004653-00-0002) were used in this study. These siRNAs were obtained as annealed, desalted, and in the 2′-hydroxyl form, as prepared by the manufacturer. Preliminary studies utilizing siRNA concentrations ranging from 1 to 100 nmol/L demonstrated that cells exposed to 10 nmol/L produced a maximal knockdown of PKCɛ with little or no toxicity. All subsequent studies for the knockdown of proteins were performed by using 10 nmol/L of siRNA.
Results
Previous studies from our laboratory have demonstrated that the PGF2α-induced secretion of MMP-2 from human ciliary muscle cells is mediated by the activation of FP receptors.28 To initially investigate the involvement of PKC in PGF2α-induced MMP-2 secretion, cells were treated with the broad-spectrum inhibitor, chelerythrine chloride (1 μmol/L). As shown in Figure 1, the PGF2α-induced increase in MMP-2 secretion was blocked by the administration of chelerythrine chloride. In contrast, the addition of Go-6976 (1 μmol/L), a conventional PKC-isoform-selective inhibitor, did not significantly alter MMP-2 secretion (Fig. 1). Whereas these studies support the idea that FP receptors in human ciliary muscle are linked to one of the novel or atypical PKC isoforms, they are complicated by the fact that the PKC inhibitor, chelerythrine, has been shown to influence the activities of multiple kinases and receptors.32–35
FIG. 1.
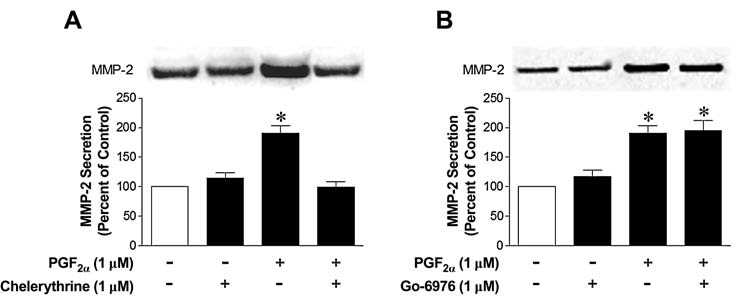
Effects of protein kinase C inhibitors on matrix metalloproteinase-2 (MMP-2) secretion from human ciliary muscle cells. Serum-deprived human ciliary muscle cells were pretreated with vehicle, 1.0 μmol/L of chelerythrine chloride (A), or 1.0 μmol/L of Go-6976 (B) for 60 min, followed by 1.0 μmol/L of prostaglandin F2-alpha treatment for 4 h. The media were then collected, concentrated, and analyzed for MMP-2 by Western blotting, using anti-MMP-2 antibodies. Data are the mean ± standard error of densitometry measurements (n = 3–7; *P < 0.05 when compared to the control levels).
To investigate the involvement of individual PKC isoform in PGF2α-mediated MMP-2 secretion from human ciliary muscle, we first evaluated the expression pattern of PKC isoforms in these cells. The Western blot analyses of PKC isoforms in cytosolic and membrane fractions are shown in Figure 2. The PKCα isoform exhibited the highest level of immunoreactivity in these cells, when compared to other PKC isoforms. The PKC ɛ, í, and λ isoforms exhibited moderate levels of expression, whereas PKC η and δ isoforms exhibited very low levels of expression. The PKC β, γ, and φ isoforms were not detected in human ciliary muscle cells. All the isoforms were detected in positive controls (rat brain extract, Jurkat, and WI-38), suggesting that the antibodies had sufficient titer and binding affinity for visualization (data not shown).
FIG. 2.
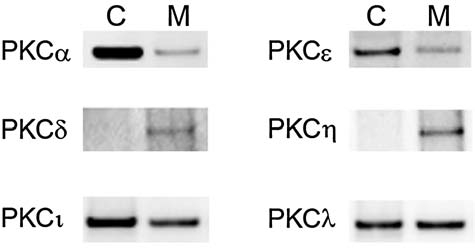
Western blot analyses for individual protein kinase C (PKC) isoforms in cytosolic (C) and membrane (M) fractions of nonstimulated human ciliary muscle cells. Equal amounts of protein (15 μg) from cytosolic or membrane fractions were subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis. Proteins were transferred to nitrocellulose membranes and probed with primary antibodies selective for each PKC isoform, as described in the Methods section.
To determine if PGF2α activates a single or multiple PKC isoform(s), the translocation of individual PKC isoforms from cytosol to the membrane fraction were measured in the presence or absence of PGF2α. These responses were then compared to the translocation induced by PMA, an activator of both conventional and novel PKC isoforms. As shown in Figure 3, the addition of PGF2α (1 μmol/L) increased the association of PKCɛ with the membrane fraction by 535 ± 146%, when compared to control preparations. The addition of PGF2α produced a relatively small increase (70%) in the translocation of PKCα isoform, and no significant changes in the distribution of PKC δ, η, í, and λ isoforms in the membrane fractions (data not shown). In contrast, the addition of PMA, increased the translocation of PKCα and PKCɛ isoforms to the membrane fraction by 730 ± 159% and 256 ± 46%, respectively. The addition of PMA did not alter the membrane association of atypical PKC isoforms í and λ (data not shown).
FIG. 3.
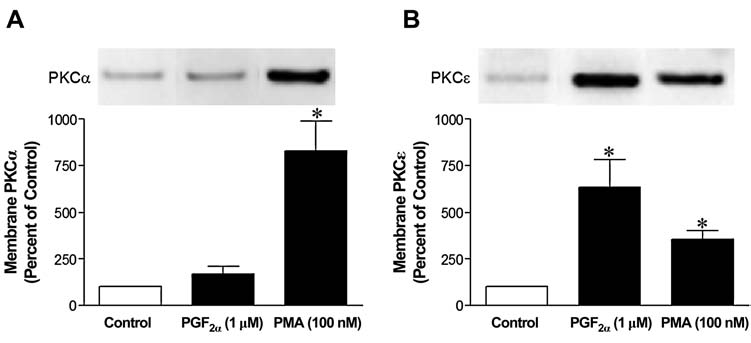
Effects of prostaglandin F2-alpha (PGF2α) on the translocation of protein kinase C (PKC) isoforms in human ciliary muscle cells. Serum-deprived human ciliary muscle cells were treated with vehicle or 1 μmol/L PGF2α for 5 min, followed by the isolation of the membrane fraction and Western blot analysis. The upper panel sections are representative immunoblots of PKCα (A) and PKCɛ (B) translocations to the membrane fraction. Summary data (lower panel sections) are the mean ± standard error of densitometry (n = 3–7; *P < 0.05).
To investigate if the PGF2α-induced increase in MMP-2 secretion is mediated through the activation of PKCɛ, human ciliary muscle cells were pretreated with the PKCɛ translocation inhibitor, EAVSLKPT, or siRNA targeting PKCɛ. As shown in Figure 4, the PGF2α-induced increase in MMP-2 secretion was blocked by a pretreatment with the PKCɛ translocation inhibitor. However, administration of the PKCɛ translocation inhibitor alone did not significantly alter basal MMP-2 secretion.
FIG. 4.
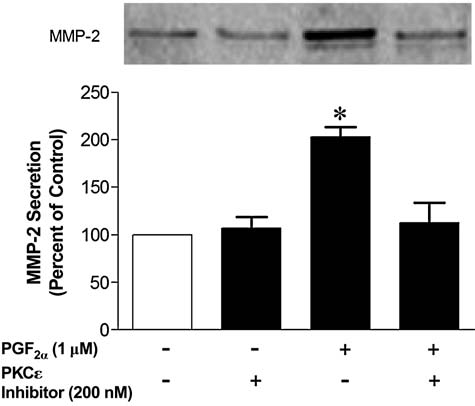
Effects of protein kinase C (PKC)ɛ translocation inhibitors on matrix metalloproteinase-2 (MMP-2) secretion from human ciliary muscle cells. Serum-deprived human ciliary muscle cells were treated with vehicle or 200 nmol/L of PKCɛ translocation inhibitor, EAVSLKPT, for 60 min followed by 1 μmol/L PGF2α treatment for 4 h. The media were then collected, concentrated, and analyzed for MMP-2 by Western blotting, using anti-MMP-2 antibodies. The upper panel section shows representative immunoblots of MMP-2. Summary data (lower panel section) are the mean ± standard error of densitometry measurements (n = 4; *P < 0.05, compared to control levels).
The cellular responses to siRNA targeting of the endogenous PKCɛ isoform are shown in Figure 5. Transfection of human PKCɛ siRNA (10 nmol/L) resulted in an over 80% knockdown of PKCɛ for up to 48 h. However, PKCα expression was not affected by this treatment. Similarly, expressions of other PKC isoforms were not altered by this treatment with siRNA targeting PKCɛ (data not shown). In cells treated with siRNA targeting PKCɛ, the PGF2α-induced secretion of MMP-2 was blocked. However, in cells transfected with siRNA targeting Lamin A/C, no change in the expression of PKCα or ɛ were measured, and the PGF2α-induced increase in MMP-2 secretion was not significantly altered.
FIG. 5.
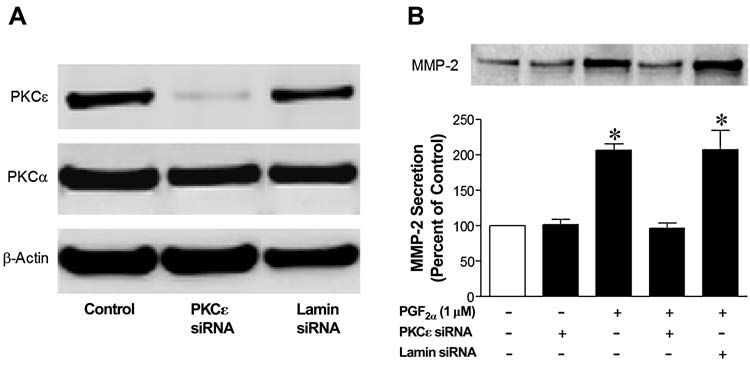
Inhibition of prostaglandin F2-alpha (PGF2α)-induced matrix metalloproteinase-2 (MMP-2) secretion by siRNA targeting endogenous protein kinase C (PKC)ɛ. Human ciliary muscle cells were transfected with 10 nmol/L of siRNA targeting PKCɛ or Lamin A/C for 4 h in serum-free media. Two (2) days after transfection, cells were harvested and immunoblotted with PKC isoform and β-actin antibodies. (A) Representative immunoblots of PKCɛ and PKCα from cells treated with PKCɛ siRNA, Lamin A/C siRNA, or tranfection reagent alone (control). Beta-actin was used as an internal control to insure that equal amounts of proteins were loaded in each lane. Lamin A/C siRNA was used as a positive control for the silencing of nonessential, abundantly expressed housekeeping genes in these cells. (B) Alteration in MMP-2 secretion induced by PGF2α (1 μmol/L) produced by siRNA transfection. The upper panel section is a representative immunoblot of MMP-2 from cells treated with PKCɛ siRNA, Lamin A/C siRNA, or tranfection reagent alone (control). Summary data (lower panel section) are the mean ± standard error of densitometry measurements (n = 3–7; *P < 0.05, compared to control levels).
Previous studies have shown that ERK1/2 is the converging pathway for PGF2α-induced MMP-2 secretion from ciliary muscle cells. To investigate if the activation PKCɛ is a prerequisite for the PGF2α-induced ERK1/2 activation, cells were treated with the nonselective PKC inhibitor, chelerythrine chloride, or the PKCɛ translocation inhibitor, EAVSLKPT (Fig. 6). Pretreatment with either chelerythrine, or the PKCɛ translocation inhibitor, blocked the PGF2α-induced activation of ERK1/2.
FIG. 6.
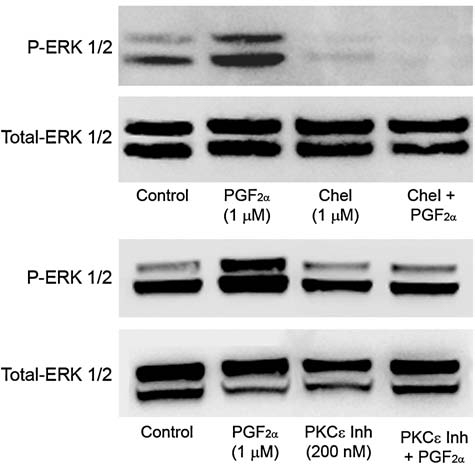
Effects of protein kinase C (PKC) inhibitors on prostaglandin F2-alpha (PGF2α)-induced ERK1/2 activation. Serum-deprived human ciliary muscle cells were pretreated with vehicle, 1 μmol/L of chelerythrine chloride or the PKCɛ translocation inhibitor, EAVSLKPT, for 60 min followed by 1 μmol/L of PGF2α treatment for 5 min. The cell lysates were analyzed for ERK1/2 phosphorylation by Western blot, using anti-phospho-ERK1/2 and anti-ERK1/2 antibodies.
Discussion
PKC is a family of serine-threonine kinases that regulate cellular differentiation, growth, tumor promotion, and muscle contraction. The PKC isoforms are activated in response to a large variety of stimuli, such as growth factors, hormones, and neurotransmitters36,37; however; our understanding of the role of PKC in aqueous-humor dynamics remains incomplete. Studies have shown that PKC can regulate trabecular-meshwork contractility and alter cytoskeletal organization.38,39 In addition, pharmacologic modulators of PKC have been shown to modulate the outflow facility in perfused porcine eyes.38,40 Although prostaglandin FP agonists have been shown to lower IOP by increasing uveoscleral outflow and the activation of PKC is often linked to the activation of prostanoid receptors, a definitive role for PKC in the regulation of uveoscleral outflow remains an open question. Recently, we have shown that PGF2α regulates the secretion of MMP-2 from human ciliary muscle.28 This study investigates the expression of PKC isoforms and their roles in the modulation of MMP-2 secretion by PGF2α in human ciliary muscle cells.
As shown in Figure 1, PGF2α-induced secretion of MMP-2 was completely inhibited by pretreatment with a broad range PKC inhibitor, chelerythrine chloride. In contrast, this response was not blocked by pretreatment with the conventional PKC (α, βI, βII, and γ) isoform inhibitor, Go-6976, indicating that PKC isoforms other than conventional PKC isoforms are involved in this process (Fig. 1). Whereas these studies support the idea that FP receptors in human ciliary muscle are linked to one of the novel or atypical PKC isoforms, they are complicated by the fact that chelerythrine has been shown to influence the activities of multiple kinases and receptors.32–35 To confirm that PGF22α induces MMP-2 secretion from these cells by a PKC-dependent pathway, we characterized the expression of individual PKC isoforms, the activation (translocation) of these isoforms by PGF2α, and the use of isoform-specific inhibitors to prevent PGF2α-induced secretion of MMP-2.
Using isoform-specific antibodies, we examined the presence and subcellular distribution of PKC α, δ, ɛ, í, λ, and η isoforms in human ciliary muscle cells. Under basal conditions, we found that PKC α, ɛ, and í isoforms were predominantly located to the cytosolic fraction, PKCδ and η were primarily located to the membrane fraction, and PKCλ was almost equally distributed between cytosol and membrane fractions. We found no evidence that PKC β, γ, or φ were expressed in cultured ciliary muscle cells. Our results for PKCα and ɛ isoforms are similar to previous reports in human ciliary muscle cells.39 As most inactive PKC isoforms reside within the cytosol and translocate to the membrane (or other subcellular sites) upon stimulation, the cytosolic and membrane-associated levels of each PKC isoform were determined in the presence and absence of PGF2α or the PKC activator, PMA. Treatment of human ciliary muscle cells with PGF2α induced almost a complete translocation of PKCɛ isoform from the cytosol to the membrane fraction. In contrast, PGF2α produced only a small increase in membrane-associated PKCα and no change in the distribution of other PKC isoforms. In contrast, PMA treatment produced a rapid increase of both PKCα and PKCɛ association within the membrane fraction. As expected, PMA had no significant effects on atypical PKC isoforms in these cells. Overall, these data support the idea that the activation of FP receptors in these cells is primarily coupled to the activation of PKCɛ.
To determine whether the PGF2α-induced secretion of MMP-2 requires the activation of PKCɛ, human ciliary muscle cells were pretreated with the PKCɛ translocation inhibitor or siRNAs targeting PKCɛ prior to the addition of PGF2α. Pretreatment with PKCɛ translocation inhibitor blocked the secretion of MMP-2 induced by PGF2α. Previous studies in cardiomyocytes have shown that the PKCɛ translocation inhibitor peptide selectively inhibited the translocation of PKCɛ without affecting other PKC isoforms.41,42 To confirm these results, we utilized siRNA duplexes to the knockdown endogenous expression of PKCɛ isoform. Our results demonstrate that siRNA technologies can selectively reduce the expression of individual PKC isoforms in primary human ciliary muscle cells. In cells treated with siRNA-targeting PKCɛ, the PGF2α-induced increase in MMP-2 secretion was completely eliminated. In contrast, treatment of cells with Lamin siRNA did not block the PGF2α-induced increase in MMP-2 secretion from human ciliary muscle cells. Together, these results demonstrate that the activation PKCɛ is a required step in PGF2α-induced increase in MMP-2 secretion from human ciliary muscle cells.
Previous studies have shown that PGF2α is an effective activator of the ERK1/2 pathway in ciliary muscle cells, and this activation is also required for PGF2α to induce MMP-2 secretion.28 To begin to understand the relationship between PKCɛ and ERK1/2 in ciliary muscle, cells were pretreated with chelerythrine or the PKCɛ translocation inhibitor, and PGF2α-induced ERK1/2 activation was evaluated. These studies demonstrate that both chelerythrine and the PKCɛ translocation inhibitor blocked ERK1/2 activation. These results, along with earlier studies,28 provide the initial evidence that in ciliary muscle cells, the PGF2α-induced secretion of MMP-2 requires the sequential activation of PKCɛ > MEK1/2 > ERK1/2.
Several reports have shown the involvement of PKC in agonist-induced MMP secretion from nonocular cells.43,44 Further, studies have also shown that TNFα-induced MMP secretion45 and interleukin-1α stimulated upregulation of MMP-3 in trabecular meshwork cells,45 are both mediated through PKC-dependent pathways. The FP agonist and latanoprost-induced TIMP-1 secretion from human ciliary muscle cells have been shown to be mediated through PKC activation.47 However, the role of individual PKC isoforms in the PGF2α-induced increase in MMP secretion from human ciliary muscle cells has remained unexplored. This study presents the first evidence directly linking PKCɛ to MMP-2 secretion from human ciliary muscle cells. The cellular mechanism of ocular PG hypotensive action is believed to involve MMP secretion from the ciliary muscle to promote uveoscleral outflow.48 Our results demonstrate that FP-receptor activation leads to the acute secretion of MMP-2, and that this functional response is mediated by PKCɛ- and ERK1/2-signaling pathways.
Conclusions
In summary, human ciliary muscle cells express PKCα, δ, ɛ, η, ι, and λ isoforms. From the results presented in this paper, we conclude that the activation of FP receptors leads to a rapid secretion of MMP-2, which requires the sequential activation of PKCɛ and ERK1/2. Hence, FP agonists in vivo appear to modulate uveoscleral outflow and IOP, in part, through the PKCɛ-dependent secretion of MMP-2.
Footnotes
The authors of this paper have no commercial relationships to the products or equipment herein.
Acknowledgments
This work was supported, in part, by the American Health Assistance Foundation (SH), NEI grants EY-09741 and EY-14793 (CEC), and an unrestricted grant to SEI from Research to Prevent Blindness (New York, NY). The authors acknowledge the critical review of the manuscript by L. Bartholomew, Ph.D.
References
- 1.Abramovitz M. Boie Y. Nguyen T., et al. Cloning and expression of a cDNA for the human prostanoid FP receptor. J. Biol. Chem. 1994;269:2632–2636. [PubMed] [Google Scholar]
- 2.Sugimoto Y. Hasumoto K. Namba T., et al. Cloning and expression of a cDNA for mouse prostaglandin F receptor. J. Biol. Chem. 1994;269:1356–1360. [PubMed] [Google Scholar]
- 3.Sakamoto K. Ezashi T. Miwa K., et al. Molecular cloning and expression of a cDNA of the bovine prostaglandin F2-alpha receptor. J. Biol. Chem. 1994;269:3881–3886. [PubMed] [Google Scholar]
- 4.Husain S. Kaddour-Djebbar I. Abdel-Latif A.A. Alterations in arachidonic acid release and phospholipase C-beta(1) expression in glaucomatous human ciliary muscle cells. Invest. Ophthalmol. Vis. Sci. 2002;43:1127–1134. [PubMed] [Google Scholar]
- 5.Sharif N.A. Crider J.Y. Husain S., et al. Human ciliary muscle cell responses to FP-class prostaglandin analogs: Phosphoinositide hydrolysis, intracellular Ca(2+) mobilization, and MAP-kinase activation. J. Ocul. Pharmacol. Ther. 2003;19:437–455. doi: 10.1089/108076803322473006. [DOI] [PubMed] [Google Scholar]
- 6.Toh H. Ichikawa A. Narumiya S. Molecular evolution of receptors for eicosanoids. FEBS Lett. 1995;361:17–21. doi: 10.1016/0014-5793(95)00129-w. [DOI] [PubMed] [Google Scholar]
- 7.Adams J.W. Migita D.S. Yu M.K., et al. Prostaglandin F2 alpha stimulates hypertrophic growth of cultured neonatal rat ventricular myocytes. J. Biol. Chem. 1996;271:1179–1186. doi: 10.1074/jbc.271.2.1179. [DOI] [PubMed] [Google Scholar]
- 8.Giuffre G. The effects of prostaglandin F2 alpha in the human eye. Graefe's Arch. Clin. Exp. Ophthalmol. 1985;222:139–141. doi: 10.1007/BF02173538. [DOI] [PubMed] [Google Scholar]
- 9.Horton E.W. Poyser N.L. Uterine luteolytic hormone: A physiological role for prostaglandin F2-alpha. Physiol. Rev. 1976;56:595–651. doi: 10.1152/physrev.1976.56.4.595. [DOI] [PubMed] [Google Scholar]
- 10.McCracken J.A. Glew M.E. Scaramuzzi R.J. Corpus luteum regression induced by prostagland in F2-alpha. J. Clin. Endocrinol. Metab. 1970;30:544–546. doi: 10.1210/jcem-30-4-544. [DOI] [PubMed] [Google Scholar]
- 11.Hellberg M.R. Sallee V.L. McLaughlin M.A., et al. Preclinical efficacy of travoprost, a potent and selective FP-prostaglandin-receptor agonist. J. Ocul. Pharmacol. Ther. 2001;17:421–432. doi: 10.1089/108076801753266802. [DOI] [PubMed] [Google Scholar]
- 12.Alm A. Stjernschantz J. Effects on intraocular pressure and side effects of 0.005% latanoprost applied once-daily, evening, or morning. A comparison with timolol. The Scandinavian Latanoprost Study Group. Ophthalmology. 1995;102:1743–1752. doi: 10.1016/s0161-6420(95)30798-1. [DOI] [PubMed] [Google Scholar]
- 13.Stjernschantz J. Selen G. Sjoquist B., et al. Preclinical pharmacology of latanoprost, a phenyl-substituted PGF2-alpha analogue. Adv. Prostagl. Thrombox. Leukot. Res. 1995;23:513–518. [PubMed] [Google Scholar]
- 14.Bito L.Z. Stjernschantz J. Resul B., et al. The ocular effects of prostaglandins and the therapeutic potential of a new PGF2 alpha analog, PhXA41 (latanoprost), for glaucoma management. J. Lipid Mediat. 1993;6:535–543. [PubMed] [Google Scholar]
- 15.Crawford K. Kaufman P.L. Pilocarpine antagonizes prostaglandin F2-alpha-induced ocular hypotension in monkeys. Evidence for enhancement of uveoscleral outflow by prostaglandin F2 alpha. Arch. Ophthalmol. 1987;105:1112–1116. doi: 10.1001/archopht.1987.01060080114039. [DOI] [PubMed] [Google Scholar]
- 16.Gabelt B.T. Kaufman P.L. Prostaglandin F2 alpha increases uveoscleral outflow in the cynomolgus monkey. Exp. Eye Res. 1989;49:389–402. doi: 10.1016/0014-4835(89)90049-3. [DOI] [PubMed] [Google Scholar]
- 17.Lindsey J.D. Kashiwagi K. Kashiwagi F., et al. Prostaglandin action on ciliary smooth muscle extracellular matrix metabolism: Implications for uveoscleral outflow. Surv. Ophthalmol. 1997;41:S53–S59. doi: 10.1016/s0039-6257(97)80008-2. [DOI] [PubMed] [Google Scholar]
- 18.Lindsey J.D. Kashiwagi K. Kashiwagi F., et al. Prostaglandins alter extracellular matrix adjacent to human ciliary muscle cells in vitro. Invest. Ophthalmol. Vis. Sci. 1997;38:2214–2223. [PubMed] [Google Scholar]
- 19.Lutjen-Drecoll E. Tamm E. Morphological study of the anterior segment of cynomolgus monkey eyes following treatment with prostaglandin F2 alpha. Exp. Eye Res. 1988;47:761–769. doi: 10.1016/0014-4835(88)90043-7. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson S.F. Samuelsson M. Bill A., et al. Increased uveoscleral outflow as a possible mechanism of ocular hypotension caused by prostaglandin F2-alpha-1-isopropylester in the cynomolgus monkey. Exp. Eye Res. 1989;48:707–716. doi: 10.1016/0014-4835(89)90011-0. [DOI] [PubMed] [Google Scholar]
- 21.Bradley J.M. Vranka J. Colvis C.M., et al. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest. Ophthalmol. Vis. Sci. 1998;39:2649–2658. [PubMed] [Google Scholar]
- 22.Crosson C.E. Sloan C.F. Yates P.W. Modulation of conventional outflow facility by the adenosine A1 agonist N6-cyclohexyladenosine. Invest. Ophthalmol. Vis. Sci. 2005;46:3795–3799. doi: 10.1167/iovs.05-0421. [DOI] [PubMed] [Google Scholar]
- 23.Lindsey J.D. Kashiwagi K. Boyle D., et al. Prostaglandins increase pro-MMP-1 and pro-MMP-3 secretion by human ciliary smooth muscle cells. Curr. Eye Res. 1996;15:869–875. doi: 10.3109/02713689609017628. [DOI] [PubMed] [Google Scholar]
- 24.Alexander J.P. Samples J.R. Acott T.S. Growth-factor and cytokine modulation of trabecular meshwork matrix metalloproteinase and TIMP expression. Curr. Eye Res. 1998;17:276–285. doi: 10.1076/ceyr.17.3.276.5219. [DOI] [PubMed] [Google Scholar]
- 25.Alexander J.P. Samples J.R. Van Buskirk E.M., et al. Expression of matrix metalloproteinases and inhibitor by human trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 1991;32:172–180. [PubMed] [Google Scholar]
- 26.Sagara T. Gaton D.D. Lindsey J.D., et al. Reduction of collagen type I in the ciliary muscle of inflamed monkey eyes. Invest. Ophthalmol. Vis. Sci. 1999;40:2568–2576. [PubMed] [Google Scholar]
- 27.Shearer T.W. Crosson C.E. Adenosine A1 receptor modulation of MMP-2 secretion by trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 2002;43:3016–3020. [PubMed] [Google Scholar]
- 28.Husain S. Jafri F. Crosson C.E. Acute effects of PGF2-alpha on MMP-2 secretion from human ciliary muscle cells: A PKC- and ERK-dependent process. Invest. Ophthalmol. Vis. Sci. 2005;46:1706–1713. doi: 10.1167/iovs.04-0993. [DOI] [PubMed] [Google Scholar]
- 29.Husain S. Young D. Wingard C.J. Role of PKC-alpha and PKC-iota in phenylephrine-induced contraction of rat corpora cavernosa. Int. J. Impot. Res. 2004;16:325–333. doi: 10.1038/sj.ijir.3901164. [DOI] [PubMed] [Google Scholar]
- 30.Morris M.C. Depollier J. Mery J., et al. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat. Biotechnol. 2001;19:1173–1176. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- 31.Elbashir S.M. Harborth J. Lendeckel W., et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 32.Schulte G. Fredholm B.B. Diverse inhibitors of intracellular signalling act as adenosine receptor antagonists. Cell Signal. 2002;14:109–113. doi: 10.1016/s0898-6568(01)00228-5. [DOI] [PubMed] [Google Scholar]
- 33.Ko F.N. Chen I.S. Wu S.J., et al. Antiplatelet effects of chelerythrine chloride isolated from Zanthoxylum simulans. Biochim. Biophys. Acta. 1990;1052:360–365. doi: 10.1016/0167-4889(90)90144-3. [DOI] [PubMed] [Google Scholar]
- 34.Lenfeld J. Kroutil M. Marsalek E., et al. Anti-inflammatory activity of quaternary benzophenanthridine alkaloids from Chelidonium majus. Planta Med. 1981;43:161–165. doi: 10.1055/s-2007-971493. [DOI] [PubMed] [Google Scholar]
- 35.Yu R. Mandlekar S. Tan T.H., et al. Activation of p38 and c-Jun N-terminal kinase pathways and induction of apoptosis by chelerythrine do not require inhibition of protein kinase C. J. Biol. Chem. 2000;275:9612–9619. doi: 10.1074/jbc.275.13.9612. [DOI] [PubMed] [Google Scholar]
- 36.Hug H. Sarre T.F. Protein kinase C isoenzymes: Divergence in signal transduction? Biochem. J. 1993;291:329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 38.Khurana N. Deng P.F. Epstein D.L., et al. The role of protein kinase C in modulation of aqueous humor outflow facility. Exp. Eye Res. 2003;76:39–47. doi: 10.1016/s0014-4835(02)00255-5. [DOI] [PubMed] [Google Scholar]
- 39.Thieme H. Nass J.U. Nuskovski M., et al. The effects of protein kinase C on trabecular meshwork and ciliary muscle contractility. Invest. Ophthalmol. Vis. Sci. 1999;40:3254–3261. [PubMed] [Google Scholar]
- 40.Tian B. Brumback L.C. Kaufman P.L. ML-7, chelerythrine, and phorbol ester increase outflow facility in the monkey eye. Exp. Eye Res. 2000;71:551–566. doi: 10.1006/exer.2000.0919. [DOI] [PubMed] [Google Scholar]
- 41.Huang X. Walker J.W. Myofilament anchoring of protein kinase C-epsilon in cardiac myocytes. J. Cell Sci. 2004;117:1971–1978. doi: 10.1242/jcs.01044. [DOI] [PubMed] [Google Scholar]
- 42.Johnson J.A. Gray M.O. Chen C.H., et al. A protein kinase C translocation inhibitor as an isozyme-selective antagonist of cardiac function. J. Biol. Chem. 1996;271:24962–24966. doi: 10.1074/jbc.271.40.24962. [DOI] [PubMed] [Google Scholar]
- 43.Louis K. Guerineau N. Fromigue O., et al. Tumor-cell-mediated induction of the stromal factor stromelysin-3 requires heterotypic cell contact-dependent activation of specific protein kinase C isoforms. J. Biol. Chem. 2005;280:1272–1283. doi: 10.1074/jbc.M405482200. [DOI] [PubMed] [Google Scholar]
- 44.McDonnell S.E. Kerr L.D. Matrisian L.M. Epidermal growth factor stimulation of stromelysin mRNA in rat fibroblasts requires induction of protooncogenes c-fos and c-jun and activation of protein kinase C. Mol. Cell. Biol. 1990;10:4284–4293. doi: 10.1128/mcb.10.8.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alexander J.P. Acott T.S. Involvement of protein kinase C in TNFα regulation of trabecular matrix metalloproteinases TIMPs. Invest. Ophthalmol. Vis. Sci. 2001;42:2831–2838. [PubMed] [Google Scholar]
- 46.Fleenor D.L. Pang I.H. Clark A.F. Involvement of AP-1 in interleukin-1-alpha-stimulated MMP-3 expression in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 2003;44:3494–3501. doi: 10.1167/iovs.02-0757. [DOI] [PubMed] [Google Scholar]
- 47.Anthony T.L. Lindsey J.D. Weinreb R.N. Latanoprost's effects on TIMP-1 and TIMP-2 expression in human ciliary muscle cells. Invest. Ophthalmol. Vis. Sci. 2002;43:3705–3711. [PubMed] [Google Scholar]
- 48.Weinreb R.N. Kashiwagi K. Kashiwagi F., et al. Prostaglandins increase matrix metalloproteinase release from human ciliary smooth muscle cells. Invest. Ophthalmol. Vis. Sci. 1997;38:2772–2780. [PubMed] [Google Scholar]


