Abstract
Introduction
The topical application of prostaglandin F2α (FP)-receptor agonists has been shown to significantly lower intraocular pressure (IOP) in humans and is now considered the first-line treatment for ocular hypertension. Despite the prominent role FP-receptor agonists play in the treatment of glaucoma, our understanding of how these agents lower IOP remains incomplete. The present study was designed to evaluate the role of matrix metalloproteinase (MMP) activation and the cytokine, tumor necrosis factor alpha (TNF-α), in latanoprost-induced changes in IOP.
Methods
Changes in IOP following an acute topical administration of latanoprost (60 ng) in normotensive Brown Norway rats were evaluated by means of a commercially available rebound tonometer. To examine the role of MMPs and TNF-α in this response, the rats were pretreated with a broad-spectrum MMP inhibitor, GM-6001 (100 μg), or the TNF-α inhibitor, thalidomide (25 μg).
Results
The topical administration of latanoprost (60 ng) alone produced a biphasic change in ipsilateral IOP: an initial hypertension (4.21 ± 0.52), followed by a prolonged hypotension (−4.79 ± 0.65). In rats, pretreatment with GM-6001 blocked the latanoprost-induced reduction in IOP but did not prevent the initial rise in IOP. Pretreatment with thalidomide also blocked the ocular hypotension induced by latanoprost; however, thalidomide pretreatment enhanced the duration of the initial hypertension.
Conclusions
These results provide evidence that the secretion and activation of MMPs and the release of TNF-α play a central role in the ocular hypotension induced by FP-agonists. The administration of FP-agonists appears to lower IOP directly by inducing the activation of MMPs within the ciliary body, leading to improved uveoscleral outflow and indirectly through the release of TNF-α within the ciliary body. Secreted TNF-α may then activate TNF-receptors in the uvea and trabecular meshwork, increasing both uveoscleral and conventional outflow.
Introduction
The primary risk factor for the development of glaucoma is elevated intraocular pressure (IOP). Topical application of prostaglandin F2α (FP) or its analogs have been shown to significantly reduce IOP in humans1,2 and primates.3,4 However, early animal studies demonstrated that this response is unusual, in that the response to FP-receptor agonists improves with multiple dosing over time.5–7 This enhanced functional response supports the idea that the chronic use of FP-receptor agonists induces a structural and/or molecular change within the outflow pathway that acts synergistically with a later administration of an FP-receptor agonist to lower IOP.
Topical treatment with prostaglandin F2α analogs alters the extracellular matrix (ECM) in the tissues of the uveoscleral outflow pathway8 and increases the production of matrix metalloproteinases (MMPs) in the ciliary muscle cells.8–15 Although studies have shown that MMPs can alter conventional outflow facility in human and animal perfused eyes,16,17 direct evidence that MMPs participate in the FP-agonist-induced modulation of IOP remains to be determined.
In other systems, the activation of FP-receptors has been shown to induce the expression of the cytokines.18–20 In the eye, tumor necrosis factor alpha (TNF-α) induces the secretion of MMPs and ECM remodeling in a number of tissues, including the trabecular meshwork, cornea, and optic nerve head.21–25 Although TNF-α has been shown to increase conventional outflow facility, the participation of this cytokine in modulating uveoscleral outflow has not been investigated.
To evaluate the roles of TNF-α and MMPs in the latanoprost-induced changes in IOP, Brown Norway rats were pretreated with an inhibitor of TNF-α production, thalidomide, or the MMP inhibitor, GM-6001. Rats were selected for this study because they share similar anatomic and developmental characteristics in the anterior segment with those of the human.26–29 Therefore, aqueous-humor-dynamic studies in rats may be more predictive of the response in humans than those from other nonprimate laboratory animals. Results from these studies support the idea that both TNF-α and MMPs play a role in prostaglandin-induced changes in IOP.
Methods
Animals
Normotensive, male Brown Norway (200–300 g) rats were used in this study. All animals were housed under standard laboratory conditions of a 12-h light and 12-h dark cycle. Animal handling was in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, and study protocols were approved by the Animal Care and Use Committee at the Medical University of South Carolina (Charleston, SC).
Drug treatment
Latanoprost (0.005%; Pfizer, San Diego, CA) was diluted in saline to a final concentration of 0.002% and administered topically (3 μL). Thalidomide (0.25%; Sigma Chemical Company, St Louis, MO) or GM-6001 (0.05%; Calbiochem, La Jolla, CA) was formulated in saline containing 10% dimethyl sulfoxide (DMSO). For thalidomide pretreatment, the agent was topically administered as a 5 μL-drop, twice, 15 min apart. For GM-6001 pretreatment, the agent was topically administered as a 10-μL drop, twice, 15 min apart.
IOP measurement in rats
IOP was measured in conscious rats by using a calibrated TonoLab (Colonial Medical Supply Co. Inc., Franconia, NH) rebound tonometer, as previously described.30 To minimize any discomfort to the animals during tonometry, corneas were lightly anesthetized by the topical application of 0.1% proparacaine (3 μL). Baseline IOPs of both eyes were determined (t =−1 h). Rats were then pretreated bilaterally with GM-6001, thalidomide, or vehicle. After 1 h, IOPs were again determined (t = 0 h) and latanoprost applied topically to 1 eye. Ipsilateral and contralateral IOPs were then measured at 1, 2, 3, 4, 5, 6, and 24 h postlatanoprost treatment. All IOP measurements were recorded in a masked fashion. In separate studies, unilateral treatment with GM-6001 or thalidomide alone was evaluated to determine if these agents produced any acute change in IOP.
Data analysis
Values are presented as the mean ± standard error of the mean. Ipsilateral IOPs from latanoprost-treated animals were compared with corresponding IOPs from animals pretreated with thalidomide or GM-6001 by means of the Student t-test for nonpaired data. A P-value of ≤0.05 was considered significant.
Results
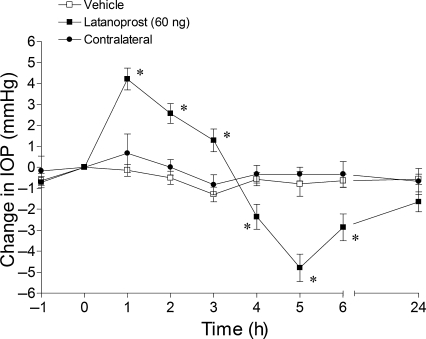
In conscious Brown Norway rats, the mean IOP measured in this study was 18.6 ± 1.2 mmHg. The topical administration of 60 ng of latanoprost alone produced a biphasic change in IOP: an initial rise in pressure peaking at 1 h (4.21 ± 0.52 mmHg), followed by a prolonged hypotensive response from 4–6 h, with a maximum reduction in IOP (−4.79 ± 0.65 mmHg) at 5 h (Fig. 1). No significant change in contralateral IOP was noted in these animals.
FIG. 1.
Effect of latanoprost-induced intraocular pressure changes in Brown Norway rats. Rats were treated topically with 60 ng of latanoprost or vehicle (t = 0 h). Values are recorded as the mean ± standard error of the mean. Asterisks denote a significant (P < 0.05) difference between eyes treated with latanoprost alone and eyes receiving vehicle (n = 6).
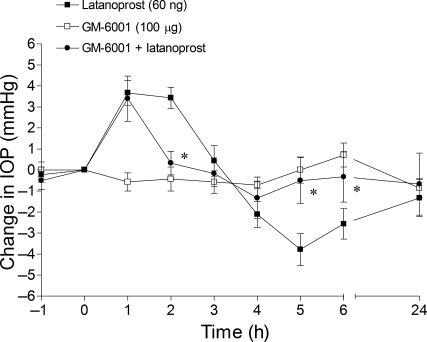
The administration of GM-6001 (100 μg) alone did not significantly alter IOP (Fig. 2). In eyes pretreated with GM-6001, the administration of latanoprost induced an initial increase in pressure at 1 h. However, the latanaprost-induced hypertension at 2 h and the subsequent hypotension from 4 to 6 h were completely blocked by pretreatment with GM-6001.
FIG. 2.
Effect of the matrix metalloproteinase inhibitor, GM-6001, on latanoprost-induced intraocular pressure changes in Brown Norway rats. Rats were treated topically with: GM-6001 (100 μg) bilaterally 1 h prior to the topical, unilateral administration of 60 ng of latanoprost (t = 0 h). Values are recorded as the mean ± standard error of the mean. Asterisks denote a significant (P < 0.05) difference between eyes treated with latanoprost alone and eyes receiving both GM-6001 and latanoprost (n = 6).
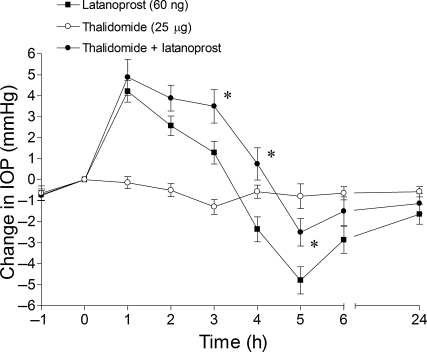
The administration of thalidomide (25 μg) alone did not induce any significant change in IOP (Fig. 3). In eyes pretreated with thalidomide, the duration of the early hypertensive response was enhanced, with pressures remaining elevated above baseline for up to 4 h. At times beyond 4 h, the ocular hypotensive response was significantly inhibited, when compared to eyes treated with latanoprost alone.
FIG. 3.
Effect of the tumor necrosis factor alpha inhibitor, thalidomide, on latanoprost-induced intraocular pressure changes in Brown Norway rats. Rats were treated bilaterally with thalidomide (25 μg) 1 h prior to the unilateral administration of 60 ng of latanoprost (t = 0 h). Values are recorded as the mean ± standard error of the mean. Asterisks denote a significant (P < 0.05) difference between eyes treated with latanoprost alone and eyes receiving both thalidomide and latanoprost (n = 8).
No changes in conjunctival hyperemia or abnormal animal behavior were observed during the course of these experiments.
Discussion
Topically administered prostaglandin F2α and its analogs lower IOP in human and nonhuman primates.1,3,4,31,32 Although these drugs are now considered first-line therapies for the treatment of glaucoma, the cellular mechanisms that mediate this response are poorly understood. The ocular hypotensive response to FP-agonists is unusual, in that it improves over time with multiple dosing.5–7 This enhanced response supports the idea that the chronic use of FP-agonists induces a structural and/or molecular change within the outflow pathway that acts synergistically with the subsequent administration of FP-agonists to lower IOP. Morphologic, molecular, and biochemical studies have demonstrated that FP-agonists lead to a loss of connective tissue within the ciliary body and increase the expression and/or secretion of MMPs.8,13,15,33 However, studies have not provided direct evidence that MMPs are involved in the ocular hypotensive response to FP-agonists.
In vitro studies have shown that exposure to prostaglandins induces the secretion of MMPs from human ciliary muscle cells.9,11 In addition, a reduction in collagen types I, III, and IV in PGF2α-treated human ciliary muscle cultures have been reported.14 Further, a reduction in these collagens was observed in the ciliary muscle of inflamed monkey eyes previously treated with PGF2α-IE, in which uveoscleral outflow was also increased.8,34 Thus, increased degradation of ciliary muscle ECM by MMP is thought to decrease the hydraulic resistance to uveoscleral outflow, thereby lowering the IOP in the eye. To provide direct evidence that the secretion and activation of MMPs was involved in the IOP responses to latanoprost, rats were pretreated with the broad-spectrum MMP inhibitor, GM-6001. GM-6001 is a hydroxamic acid derivative originally synthesized as an inhibitor of human skin collagenase35 and has been shown also to block MMP-1, −2, −3, and −9 activities.36,37 Pretreatment with GM-6001 blocked the ocular hypotensive responses induced by latanoprost. These results provide evidence, for the first time, that the secretion and activation of MMPs are required steps in the latanoprost-induced reduction in rat IOP.
The initial hypertensive response seen at 1 h following a latanoprost administration was not altered. These results support the idea that the early hypertensive response is independent of MMP secretion and activation within the anterior segment of the eye. This response is likely mediated by the vasodilation of uveal vessels producing a rapid increase in uveal volume and IOP.
TNF-α is a proinflammatory cytokine produced by a variety of cells, including lymphocytes, astrocytes, and smooth muscle cells. In other systems, the activation of FP-receptors has been shown to induce the expression of the cytokine TNF-α.18–20 In the anterior segment, elevation in TNF-α is thought to contribute to the increase in conventional outflow facility produced by argon laser trabeculoplasty and the breakdown of the blood-aqueous barrier.38,39 Thalidomide is a potent inhibitor of TNF-α production.40,41 This inhibition is due to the increased degradation of TNF-α mRNA and reduced expression by stabilizing NFκB within the cytosol.42 However, it should be noted that thalidomide can also suppress the levels of interleukin (IL)-1β, IL-6, and granulocyte macrophage-colony stimulating factor and enhance the levels of IL-10.43 In the current study, pretreatment with thalidomide suppressed the ocular hypotension induced by the addition of latanoprost. These data support the idea that the secretion of TNF-α, or other cytokines, is, in part, responsible for the ocular hypotensive response to FP-agonists. This release of TNF-α might also provide an explanation for observations that FP-agonists can also increase conventional outflow facility.44,45 Although FP-receptor expression is only a minor fraction of the total prostanoid-receptor expression in the trabecular meshwork,46 the latanoprost-induced secretion of TNF-α from the ciliary muscle may act in a paracrine fashion to stimulate MMP secretion from the trabecular meshwork, thus increasing conventional outflow indirectly.
Rats pretreated with thalidomide also exhibited an enhanced early hypertensive response. These data indicate that the full expression of the ocular hypertension induced by FP-agonists is masked by the hypotensive action of TNF-α released into the intraocular environment.
Conclusions
In summary, we have shown that the topical application of latanoprost in rats produced a biphasic change in IOP: an initial rise in pressure, followed by a prolonged hypotensive response. The prolonged hypotension could be blocked by pretreatment with the general MMP inhibitor, GM-6001, or the TNF-α inhibitor, thalidomide. However, neither agent suppressed the initial hypertension induced in these animals. Taken together, these results provide evidence that the secretion and activation of MMP and the release of TNF-α play a role in the ocular hypotension induced by FP-agonists. Based on the results from this study and others, Figure 4 provides a putative working model for the ocular hypotensive actions of FP-agonists. In this model, FP-agonists lower IOP directly by inducing the activation of MMPs within the ciliary body, leading to improved uveoscleral outflow. They also lower IOP indirectly through the release of TNF-α within the ciliary body. Secreted TNF-α may then activate TNF-receptors in the ciliary body, and possibly, the trabecular meshwork to increase both conventional and uveoscleral outflow.
FIG. 4.
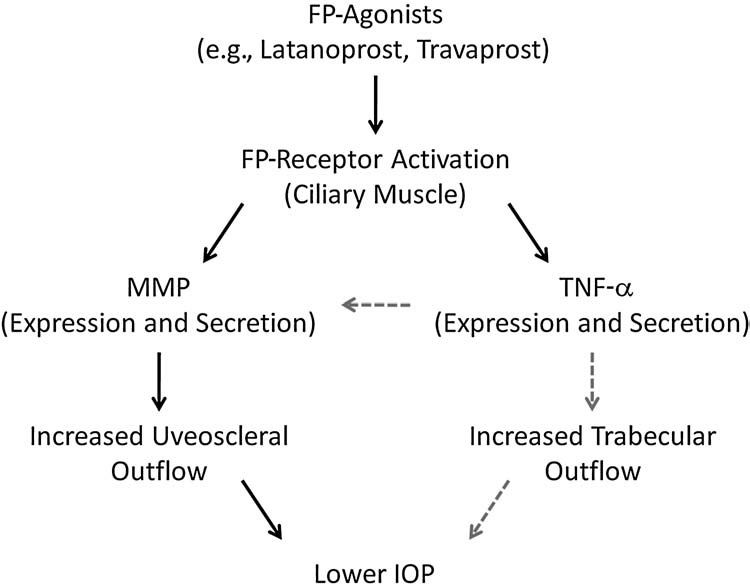
Putative model for the ocular mechanisms responsible for intraocular pressure lowering by prostaglandin F2α (FP)-receptor agonists. Solid arrows show direct FP actions, whereas gray, dashed arrows indicate indirect actions.
Acknowledgments
This study was supported, in part, by NIH/NEI grants EY-09741 (C.E.C.) and EY-14793 (C.E.C.); the American Health Assistance Foundation (S.H.), NIH grant C-06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources, and an unrestricted grant to the Storm Eye Institute from Research to Prevent Blindness (New York, NY). The authors acknowledge the critical review of the original manuscript by L. Bartholomew, Ph.D.
Disclosure
The authors have no commercial relationships in the products or equipment mentioned in this paper.
References
- 1.Camras CB. Comparison of latanoprost and timolol in patients with ocular hypertension and glaucoma: A six-month masked, multicenter trial in the United States. The United States Latanoprost Study Group. Ophthalmology. 1996;103:138–147. doi: 10.1016/s0161-6420(96)30749-5. [DOI] [PubMed] [Google Scholar]
- 2.Alm A. Comparative phase III clinical trial of latanoprost and timolol in patients with elevated intraocular pressure. Adv. Prostagland. Thrombox. Leukot. Res. 1995;23:527–532. [PubMed] [Google Scholar]
- 3.Gabelt B.T. Kaufman P.L. The effect of prostaglandin F2alpha on trabecular outflow facility in cynomolgus monkeys. Exp. Eye Res. 1990;51:87–91. doi: 10.1016/0014-4835(90)90174-s. [DOI] [PubMed] [Google Scholar]
- 4.Camras C.B. Bito L.Z. Reduction of intraocular pressure in normal and glaucomatous primate (Aotus trivirgatus) eyes by topically applied prostaglandin F2alpha. Curr. Eye Res. 1981;1:205–209. doi: 10.3109/02713688109001850. [DOI] [PubMed] [Google Scholar]
- 5.Wang R.F. Camras C.B. Lee P.Y., et al. Effects of prostaglandins F2alpha, A2, and their esters in glaucomatous monkey eyes. Invest. Ophthalmol. Vis. Sci. 1990;31:2466–2470. [PubMed] [Google Scholar]
- 6.Crawford K.S. Kaufman P.L. Dose-related effects of prostaglandin F2alpha isopropylester on intraocular pressure, refraction, and pupil diameter in monkeys. Invest. Ophthalmol. Vis. Sci. 1991;32:510–519. [PubMed] [Google Scholar]
- 7.Gabelt B.T. Seeman J.L. Podos S.M., et al. Aqueous humor dynamics in monkeys after topical 8-iso PGE(2) Invest. Ophthalmol. Vis. Sci. 2004;45:892–899. doi: 10.1167/iovs.03-0911. [DOI] [PubMed] [Google Scholar]
- 8.Sagara T. Gaton D.D. Lindsey J.D., et al. Topical prostaglandin F2alpha treatment reduces collagen types I, III, and IV in the monkey uveoscleral outflow pathway. Arch. Ophthalmol. 1999;117:794–801. doi: 10.1001/archopht.117.6.794. [DOI] [PubMed] [Google Scholar]
- 9.Husain S. Jafri F. Crosson C.E. Acute effects of PGF2alpha on MMP-2 secretion from human ciliary muscle cells, a PKC- and ERK-dependent process. Invest. Ophthalmol. Vis. Sci. 2005;46:1706–1713. doi: 10.1167/iovs.04-0993. [DOI] [PubMed] [Google Scholar]
- 10.Ocklind A. Effect of latanoprost on the extracellular matrix of the ciliary muscle. A study on cultured cells and tissue sections. Exp. Eye Res. 1998;67:179–191. doi: 10.1006/exer.1998.0508. [DOI] [PubMed] [Google Scholar]
- 11.Lindsey J.D. Kashiwagi K. Boyle D., et al. Prostaglandins increase pro-MMP-1 and pro-MMP-3 secretion by human ciliary smooth muscle cells. Curr. Eye Res. 1996;15:869–875. doi: 10.3109/02713689609017628. [DOI] [PubMed] [Google Scholar]
- 12.Weinreb R.N. Kashiwagi K. Kashiwagi F., et al. Prostaglandins increase matrix metalloproteinase release from human ciliary smooth muscle cells. Invest. Ophthalmol. Vis. Sci. 1997;38:2772–2780. [PubMed] [Google Scholar]
- 13.Gaton D.D. Sagara T. Lindsey J.D., et al. Increased matric metalloproteinases 1, 2, and 3 in the monkey uveoscleral outflow pathway after topical prostaglandin F2alpha-isopropyl ester treatment. Arch. Ophthalmol. 2001;119:1165–1170. doi: 10.1001/archopht.119.8.1165. [DOI] [PubMed] [Google Scholar]
- 14.Lindsey J.D. Kashiwagi K. Kashiwagi F., et al. Prostaglandin action on ciliary smooth muscle extracellular matrix metabolism: Implications for uveoscleral outflow. Surv. Ophthalmol. 1997;41(Suppl 2):S53–S59. doi: 10.1016/s0039-6257(97)80008-2. [DOI] [PubMed] [Google Scholar]
- 15.Lutjen-Drecoll E. Tamm E. Morphological study of the anterior segment of cynomolgus monkey eyes following treatment with prostaglandin F2alpha. Exp. Eye Res. 1988;47:761–769. doi: 10.1016/0014-4835(88)90043-7. [DOI] [PubMed] [Google Scholar]
- 16.Crosson C.E. Sloan C.F. Yates P.W. Modulation of conventional outflow facility by the adenosine A1 agonist N6-cyclohexyladenosine. Invest. Ophthalmol. Vis. Sci. 2005;46:3795–3799. doi: 10.1167/iovs.05-0421. [DOI] [PubMed] [Google Scholar]
- 17.Bradley J.M. Vranka J. Colvis C.M., et al. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest. Ophthalmol. Vis. Sci. 1998;39:2649–2658. [PubMed] [Google Scholar]
- 18.Fujino H. Regan J.W. Prostaglandin F2alpha amplifies tumor necrosis factor-alpha promoter activity by the FPB prostanoid receptor. Biochem. Biophys. Res. Commun. 2004;317:1114–1120. doi: 10.1016/j.bbrc.2004.03.167. [DOI] [PubMed] [Google Scholar]
- 19.Timmermann M. Hogger P. Oxidative stress and 8-iso-prostaglandin F2a induce ectodomain shedding of CD163 and release of tumor necrosis factor-alpha from human monocytes. Free Radic. Biol. Med. 2005;39:98–107. doi: 10.1016/j.freeradbiomed.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 20.Noguchi K. Endo H. Kondo H., et al. Prostaglandin F2a upregulates interleukin-6 production in human gingival fibroblasts. J. Endodontal. Res. 2001;36:80–87. doi: 10.1034/j.1600-0765.2001.360203.x. [DOI] [PubMed] [Google Scholar]
- 21.Alexander J.P. Samples J.R. Acott T.S. Growth factor and cytokine modulation of trabecular meshwork matrix metalloproteinase and TIMP expression. Curr. Eye Res. 1998;17:276–285. doi: 10.1076/ceyr.17.3.276.5219. [DOI] [PubMed] [Google Scholar]
- 22.Samples J.R. Alexander J.P. Acott T.S. Regulation of the levels of human trabecular matrix metalloproteinases and inhibitor by interleukin-1 and dexamethasone. Invest. Ophthalmol. Vis. Sci. 1993;34:3386–3395. [PubMed] [Google Scholar]
- 23.Li D.Q. Lokeshwar B.L. Solomon A., et al. Regulation of MMP-9 production by human corneal epithelial cells. Exp. Eye Res. 2001;73:449–459. doi: 10.1006/exer.2001.1054. [DOI] [PubMed] [Google Scholar]
- 24.Yan X. Tezel G. Wax M.B., et al. Matrix metalloproteinases and tumor necrosis factor alpha in glaucomatous optic nerve head. Arch. Ophthalmol. 2000;118:666–673. doi: 10.1001/archopht.118.5.666. [DOI] [PubMed] [Google Scholar]
- 25.Yuan L. Neufeld A.H. Activated microglia in the human glaucomatous optic nerve head. J. Neurosci. Res. 2001;64:523–532. doi: 10.1002/jnr.1104. [DOI] [PubMed] [Google Scholar]
- 26.Daimon T. Kazama M. Miyajima Y., et al. Immunocyto-chemical localization of thrombomodulin in the aqueous humor passage of the rat eye. Histochem. Cell Biol. 1997;108:121–131. doi: 10.1007/s004180050153. [DOI] [PubMed] [Google Scholar]
- 27.van der Zypen E. Experimental morphological study on structure and function of the filtration angel of the rat eye. Ophthalmologica. 1997;174:285–298. doi: 10.1159/000308617. [DOI] [PubMed] [Google Scholar]
- 28.Reme C. Urner U. Aeberhard B. The development of the chamber angle in the rat eye. Morphological characteristics of developmental stages. Graefe's Arch. Clin. Exp. Ophthalmol. 1983;220:139–153. doi: 10.1007/BF02175946. [DOI] [PubMed] [Google Scholar]
- 29.Nucci P. Tredici G. Manitto M.P., et al. Neuron-specific enolase and embryology of the trabecular meshwork of the rat eye: An immunohistochemical study. Int. J. Biol. Markers. 1992;7:253–255. doi: 10.1177/172460089200700410. [DOI] [PubMed] [Google Scholar]
- 30.Husain S. Whitlock N.A. Rice D.S., et al. Effects of latanoprost on rodent intraocular pressure. Exp. Eye Res. 2006;83:1453–1458. doi: 10.1016/j.exer.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Giuffre G. The effects of prostaglandin F2alpha in the human eye. Graefe's Arch. Clin. Exp. Ophthalmol. 1985;222:139–141. doi: 10.1007/BF02173538. [DOI] [PubMed] [Google Scholar]
- 32.Toris C.B. Camras C.B. Yablonski M.E. Effects of PhXA41, a new prostaglandin F2alpha analog, on aqueous humor dynamics in human eyes. Ophthalmology. 1993;100:1297–1304. doi: 10.1016/s0161-6420(93)31484-3. [DOI] [PubMed] [Google Scholar]
- 33.Richter M. Krauss A.H. Morphological changes in the anterior eye segment after long-term treatment with different receptor selective prostaglandin agonists and a prostamide. Invest. Ophthalmol. Vis. Sci. 2003;44:4419–4426. doi: 10.1167/iovs.02-1281. [DOI] [PubMed] [Google Scholar]
- 34.Sagara T. Gaton D.D. Lindsey J.D., et al. Reduction of collagen type I in the ciliary muscle of inflamed monkey eyes. Invest. Ophthalmol. Vis. Sci. 1999;40:2568–2576. [PubMed] [Google Scholar]
- 35.Grobelny D. Poncz L. Galardy R.E. Inhibition of human skin fibroblast collagenase, thermolysin, and Pseudomonas aeruginosa elastase by peptide hydroxamic acids. Biochemistry. 1992;31:7152–7154. doi: 10.1021/bi00146a017. [DOI] [PubMed] [Google Scholar]
- 36.Hao J.L. Nagano T. Nakamura M., et al. Effect of galardin on collagen degradation by Pseudomonas aeruginosa. Exp. Eye Res. 1999;69:595–601. doi: 10.1006/exer.1999.0755. [DOI] [PubMed] [Google Scholar]
- 37.Agren M.S. Mirastschijski U. Karlsmark T., et al. Topical synthetic inhibitor of matrix metalloproteinases delays epidermal regeneration of human wounds. Exp. Dermatol. 2001;10:337–348. doi: 10.1034/j.1600-0625.2001.100506.x. [DOI] [PubMed] [Google Scholar]
- 38.Bradley J.M. Anderssohn A.M. Colvis C.M., et al. Mediation of laser trabeculoplasty-induced matrix metalloproteinase expression by IL-1beta and TNFalpha. Invest. Ophthalmol. Vis. Sci. 2000;41:422–430. [PubMed] [Google Scholar]
- 39.Parshley D.E. Bradley J.M. Fisk A., et al. Laser trabeculoplasty induces stromelysin expression by trabecular juxtacanalicular cells. Invest. Ophthalmol. Vis. Sci. 1996;37:795–804. [PubMed] [Google Scholar]
- 40.Raje N. Anderson K. Thalidomide—a revival story. N. Engl. J. Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412110. [DOI] [PubMed] [Google Scholar]
- 41.Tseng S. Pak G. Washenik K., et al. Rediscovering thalidomide: A review of its mechanism of action, side effects, and potential uses. J. Am. Acad. Dermatol. 1996;35:969–979. doi: 10.1016/s0190-9622(96)90122-x. [DOI] [PubMed] [Google Scholar]
- 42.Moreira A.L. Sampaio E.P. Zmuidzinas A., et al. Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J. Exp. Med. 1993;177:1675–1680. doi: 10.1084/jem.177.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corral L.G. Haslett P.A. Muller G.W., et al. Differential cytokine modulation and T-cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-a. J. Immunol. 1999;163:380–386. [PubMed] [Google Scholar]
- 44.Dinslage S. Hueber A. Diestelhorst M., et al. The influence of latanoprost 0.005% on aqueous humor flow and outflow facility in glaucoma patients: A double-masked, placebo-controlled clinical study. Graefe's Arch. Clin. Exp. Ophthalmol. 2004;242:654–660. doi: 10.1007/s00417-003-0835-1. [DOI] [PubMed] [Google Scholar]
- 45.Toris C.B. Zhan G. Fan S., et al. Effects of travoprost on aqueous humor dynamics in patients with elevated intraocular pressure. J. Glaucoma. 2007;16:189–195. doi: 10.1097/IJG.0b013e31802fc6d3. [DOI] [PubMed] [Google Scholar]
- 46.Kamphuis W. Schneemann A. van Beek L.M., et al. Prostanoid receptor gene expression profile in human trabecular meshwork: A quantitative real-time PCR approach. Invest. Ophthalmol. Vis. Sci. 2001;42:3209–3215. [PubMed] [Google Scholar]