Cyclosporine (CsA) has proven to be an effective immunosuppressant. There is wide variability in the pharmacokinetics of CsA in transplant patients.1 Variability in the elimination of CsA appears to be a major contributing factor for such an observation. CsA is eliminated primarily by hepatic metabolism, which involves N-demethylation and hydroxylation of various amino acid residues.2 Carboxylation of the novel amino acid 1 has also been reported in humans and rabbits.3 Less than 1% of the administered dose is excreted as unchanged CsA in the bile and the urine obtained from dogs or transplant patients.4
Animal models are commonly used in transplant research. However, the pharmacokinetics of CsA are not completely characterized in all the animal species. Available information indicates large variation in absorption, plasma protein binding, and elimination of CsA between several animals.5–7 However, very little is known about the metabolic fate of CsA in various animals. The objective of this study is to investigate the metabolic profile of CsA in rats, rabbits, dogs, and cats as it relates to CsA metabolism in transplant patients.
METHODS
The animals studied include four male beagle dogs (body weight of 15 kg) with choledochoureterostomy, four male AC Lewis rats each weighing about 250 g, four male random source domestic short hair cats each weighing about 1.9 kg, and two male New Zealand rabbits weighing 3 kg each. The animals received CsA (Sandimune, Sandoz Inc, Basel, Switzerland) diluted in normal saline intravenously (IV) (3 to 5 mg/kg) over two minutes. Blood samples were obtained from the femoral vein (dogs and cats), tail vein (rats), or marginal ear vein (rabbits) at times corresponding to approximately one to two half-lives after CsA administration from each group of animals (Table 1). Blood was stored in heparinized glass tubes at 5°C and extracted within 1 week. Cumulative urine was also collected over a time period corresponding to four half-lives of CsA in rats, cats, and rabbits. In dogs combined urine and bile were collected for 12 hours.
Table 1.
Cyclosporine Metabolism in Different Species: Study Protocol
| Species | Route of Administration | IV Dose (mg/kg) | Time of Blood Sample (h) | Urine Collection (h) |
|---|---|---|---|---|
| Mice | IV | 3 | 4 | — |
| Rats | IV | 5 | 16 | 0–72 |
| Cats | IV | 3 | 6 | 0–48 |
| Rabbits | IV | 3 | 3, 4 | 0–24 |
| Dogs | IV | 4 | 12 | 0–12* |
| Liver transplant patients | IV | — | 12 | — |
| Kidney transplant patients | PO | — | — | 0–24 |
Abbreviation: PO, orally.
Mixture of bile and urine was collected.
For comparative purposes, 12-hour trough blood samples were obtained from four liver transplant patients receiving IV CsA in doses ranging from 100 to 240 mg. Bile was collected from the T tube in three additional patients. Cumulative urine samples also collected over 24 hours from four kidney transplant patients receiving 150 to 2,000 mg of CsA orally.
CsA and several of its metabolites were analyzed in blood, urine, and bile by a gradient elution high-pressure liquid chromatographic method developed in our laboratory.8 In brief, this method consists of extraction of CsA and its metabolites into ether, subsequent purification using hexane, and re-extraction into ether. Cyclosporine D was used as the internal standard. The residue was injected onto a Resolve C-18 column (Waters, Milford, MA) maintained at 70°C. Separation of different CsA metabolites M-17, M-1, M-18, M-21 (nomenclature as per Maurer et al2) from CsA and other endogenous compounds was achieved using a mobile phase of acetonitrite and water. The composition of the mobile phase was changed from 47% acetonitrile at time 0 to 73% acetonitrile at 55 minutes. Pure metabolites (M-17, M-18, M-1, and M-21) isolated from human bile were used for construction of the standard curve.
Analysis of variance was used to determine the presence of any significant differences in the ratio of M-17 to CsA, M-21 to CsA, and M-1 to CsA in all the species studied.
RESULTS
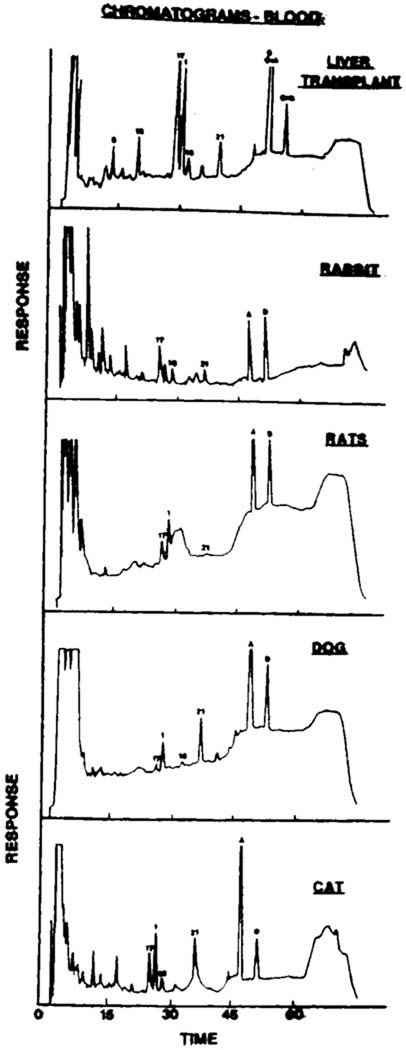
The analytic method used in this study produced optimal separation of different CsA metabolites in blood, urine, and bile. Figure 1 illustrates the chromatogram of the extract of blood from a liver transplant patient, a rabbit, a rat, a cat, and a dog. The retention times were approximately 25 minutes (M-17), 26.3 minutes (M-1), 27.9 minutes (M-18), 35 minutes (M-21) 45.8 minutes (CsA), and 49.7 minutes (cyclosporine D). The coefficient of variation of the method ranged from 1.8% for M-17 and CsA to 9.3% for M-18. The minimum detectable concentration of CsA and all the metabolites was 20 ng/mL. The standard curve was linear over a concentration range of 50 to 2,000 ng/mL.
Fig 1.
Chromatogram of the extract of blood obtained from a liver transplant patient, rabbit, rat, cat, and dog. The numbers 17, 1, 18, 21 and the letters A and D correspond to M-17, M-1, M-18, M-21, CsA and cyclosporine D.
The mean (± SD) blood concentrations of CsA, M-17, M-1, M-18, and M-21 in liver transplant patients, rabbits, rats, cats, and dogs are listed in Table 2. In liver transplant patients, the concentration of M-17 was higher as compared to CsA and the other metabolites. In rabbits, even though the concentration of CsA was higher than that of any other metabolites, M-17 appeared to be the major metabolite. In rats, M-1 is the major metabolite while similar concentrations of M-1, M-21, and M-17 were observed in cats.
Table 2.
Blood Cyclosporine Metabolites in Different Species
| Species | CsA | M-17 | M-1 | M-18 | M-21 |
|---|---|---|---|---|---|
| Liver transplant patients | 338 ± 160 | 714 ± 358 | 278 ± 147 | 79 ± 60 | 56 ± 82 |
| Rabbits* | 421 | 367 | 213 | 151 | 157 |
| Rat | 879 ± 270 | 163 ± 83 | 365 ± 151 | INT | 41 ± 26 |
| Cats | 493 ± 380 | 301 ± 73 | 429 ± 140 | 136 ± 27 | 549 ± 278 |
| Dogs | 192 ± 126 | 75 ± 64 | 169 ± 77 | — | 113 ± 81 |
Abbreviation: INT, interference.
Mean of two values.
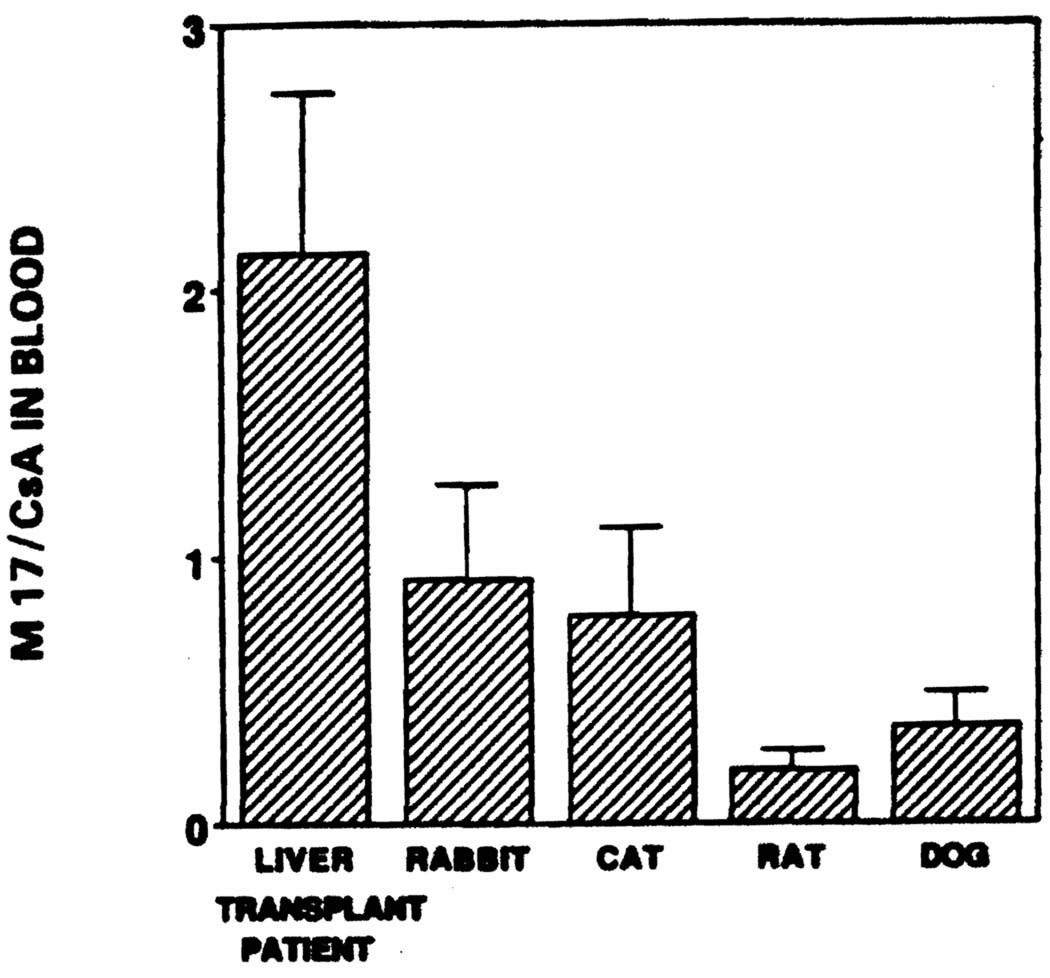
The mean (± SD) ratio of M-17 to CsA blood concentration in liver transplant patients, rabbits, rats, cats, and dogs were 2.14 (±0.6), 0.92 (±0.35), 0.78 (±0.33), 0.2 (±0.07), and 0.36 (±0.13), respectively (Fig 2). There was a significant (P < .05) interspecies difference in the M-17 to CsA ratio. The M-1 to CsA ratio of 0.82,0.52, 1.1, 0.46, and 1.0 in liver transplant patients, rabbits, cats, rats, and dogs, respectively was not significantly different from each other. There was, however, a significant (P < .05) difference in the M-21 to CsA ratio between liver transplant patients (0.14), rabbits (0.4), rats (0.048), cats (0.96), and dogs (0.63).
Fig 2.
Cyclosporine metabolism: the ratio of M-17 to cyclosporine concentration in blood from liver transplant patients, rabbits, rats, cats, and dogs.
In all the animals tested less than 1% of the CsA dose was excreted in the urine as the parent drug. Significantly (P < .05) different amounts of CsA and its metabolites were excreted in the urine of rats and in the urine and bile mixture obtained from dogs (Table 3). In rats most of the CsA derived material excreted in the urine was M-1 (3% of the dose) and M-17 (1.2%). In the dog bile and urine mixture, M-1 and M-21 were the major metabolites. Large amounts of M-17 and M-18 were also present in the bile, urine mixture. Cats primarily excreted M-1 and M-21 while rabbit’s urine contained M-1, M-18, and M-17. In human bile, the major metabolite is M-17. The concentrations of M-17 and M-1 were higher than M-21, M-18, and CsA in the bile.
Table 3.
Cyclosporine Metabolite Excretion in Urine
| Species | CsA (µg) | M-17 (µg) | M-1 (µg) | M-18 (µg) | M-21 (µg) |
|---|---|---|---|---|---|
| Humans | 1,246 ± 1,037 | 3,473 ± 3,680 | 2,502 ± 2,763 | 1,161 ± 1,359 | 620 ± 663 |
| Rats | 4.8 ± 1.3 | 15.3 ± 1.2 | 37.7 ± 3.6 | 5.8 ± 0.7 | 4.8 ± 0.6 |
| Rabbits* | 8.9 | 21.5 | 13.0 | 3.2 | — |
| Cats* | 6.7 | 23.2 | 42.2 | 2.3 | 29.7 |
| Dogs | 90 ± 24 | 378.8 ± 103 | 814.4 ± 118 | 246.9 ± 55 | 1,008.7 ± 172.7 |
Based on one animal.
DISCUSSION
Even though CsA has a unique and complex chemical structure its metabolic pathways involve simple hydroxylation or N-demethylation at various amino acid residues or carboxylation of the methyl group in amino acid 1. M-17 and M-1 are the primary hydroxy metabolites of CsA while M-21 is the primary N-demethylated CsA. A number of secondary metabolites have been identified in human urine and bile or urine from animals dosed with CsA. M-18 is a secondary metabolite of CsA and is a cyclic ether analog of M-17. A carboxy metabolite of CsA is believed to be the major CsA metabolite in human and rabbit bile. In the present study due to lack of pure standards this metabolite was not quantitated.
M-17 has been shown to possess significant in vitro immunosuppressive activity against biopsy-grown lymphocytes.9 M-1, M-18, and M-21 are much less active in in vitro tests.9 In kidney,10 heart,11 liver8 and bone marrow8 transplant patients M-17 trough concentrations are often higher than trough CsA concentrations and may contribute to the overall immunosuppressive activity.
The present study indicates that while M-17 is the major metabolite of CsA in liver transplant patients and rabbits, M-1 appears to be the major metabolite in rats and dogs and M-21, M-1, and M-17 are in similar concentrations in cats. Recently, Wagner et al12 have reported M-1 to be the major CsA metabolite in rat blood and urine. While the metabolic pathways of CsA are similar in all the animals tested the relative importance of the various pathways differ between different species. In view of the fact that M-17 is the only CsA metabolite with significant in vitro immunosuppressive activity, careful attention should be paid to the selection of animal models for pharmacokinetic and pharmacodynamic studies involving CsA.
Acknowledgments
Supported in part by Grant RO3 AM34475 from the National Institute of Arthritis, Diabetes, Digestive and Kidney Diseases, and by Sandoz Pharmaceuticals, East Hanover, NJ.
REFERENCES
- 1.Ptachcinski RJ, Venkataramanan R, Burckart GJ. Clin Pharmacokinet. 1986;11:107. doi: 10.2165/00003088-198611020-00002. [DOI] [PubMed] [Google Scholar]
- 2.Maurer G, Loosli HR, Schreier, et al. Drug Metab Dispos. 1984;12:120. [PubMed] [Google Scholar]
- 3.Hartman NR, Trimble LA, Vederas JC, et al. Biochem Biophys Res Commun. 1985;133:964. doi: 10.1016/0006-291x(85)91230-6. [DOI] [PubMed] [Google Scholar]
- 4.Venkataramanan R, Starzl TE, Yang S, et al. Transplant Proc. 1985;17:286. [PMC free article] [PubMed] [Google Scholar]
- 5.D’Souza M. PhD dissertation submitted in partial fulfillment of the degree requirements to the University of Pittsburgh. 1987. [Google Scholar]
- 6.Zaghloul I. PhD dissertation submitted in partial fulfillment of the degree requirements to the University of Pittsburgh. 1987. [Google Scholar]
- 7.Wassef R, Cohen Z, Langer B. Transplantation. 1985;40:489. doi: 10.1097/00007890-198511000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Wang CP, Burckart GJ, Ptachcinski R, et al. Transplant Proc. (this issue) [Google Scholar]
- 9.Zeevi A, Venkataramanan R, Burckart G, et al. Hum Immunol. doi: 10.1016/0198-8859(88)90089-4. (in press) [DOI] [PubMed] [Google Scholar]
- 10.Rosano TG, Freed BM, Cerilli J, et al. Transplantation. 1986;42:262. doi: 10.1097/00007890-198609000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Shah AK, Lake KD, Sawchuk RJ. Pharm Res. 1987;4:S-107. [Google Scholar]
- 12.Wagner O, Schreier E, Heitz F, et al. Drug Metab Dispos. 1987;15:377. [PubMed] [Google Scholar]