Abstract
Anergy is a state of long term hyporesponsiveness in T cells that is characterized by an active repression of TCR signaling and IL-2 expression [1]. Several forms of anergy have been described and the last few years have brought to light an increasing number of “anergic factors” involved in the induction and the active maintenance of the state in lymphocytes. The role of mTOR and other related metabolic sensors and regulators has recently emerged as of particular importance in broadening our view of anergy-inducing signals. We will discuss the role of these molecules in regulating the choice between anergy and activation, a decision faced by all T cells undergoing TCR stimulation. We will then explore the relationship between the induction of anergy and the induction of regulatory T cells as well as the potential crosstalk responsible for the phenomenon of infectious tolerance.
Introduction: TCR and CD28, the two signal paradigm of anergy
The state of T cell clonal anergy was initially described in vitro as the result of T cell activation through its antigen-specific receptor, referred to as signal 1, in the absence of a second costimulatory signal mediated by CD28 ligation [2–4]. This hyporesponsive state is characterized by a block in the Ras/MAP kinase pathway [5], mediated by an elevation in diacylglycerol kinase alpha (DGKα) [6], which could be reversed by IL-2 or OX40 signaling (reviewed in [1]). Careful subsequent molecular studies have characterized the need for a joint TCR/CD28 signaling to fully recruit the transcription factors involved in IL-2 gene transcription: NFAT, AP1, and NF- B. In the presence of both signals 1 and 2, IL-2 is then transcribed and its subsequent signaling through the IL2R complex serves to fully activate the downstream PI3K/AKT-mTOR pathway leading to p27kip1 degradation and entry into the cell cycle (reviewed in [7•]).
On the other hand, T cell activation involving only signal 1 leads to deficient IL-2 transcription and induction, via the Calcineurin (CaN)/NFAT pathway, of an anergic gene expression profile characterized by the augmentation of several key E3 ubiquitin ligases, such as Cbl-b, GRAIL, Itch and the more recently described, Deltex 1 [8]. These molecules impair TCR signaling in anergic T cells by negative feedback. For example, GRAIL has recently been shown to promote CD3 ζ ubiquitinylation and subsequent targeting of the TCR/CD3 complex to the proteasome for degradation following T cell activation [9•]. In addition, transcription factors such as, Ikaros, Egr2/3 and cAMP response element modulator (CREM), are induced, which are involved in the maintenance of the anergic state and the active repression of the IL-2 gene locus (reviewed in [7•]).
Environmental cues that regulate T cell activation versus anergy induction: extending the signal 2 paradigm
Activation of the AKT-mTOR pathway is also critically required to induce a switch in the T cell metabolic machinery from catabolism to anabolism, through stimulation of glycolysis and up-regulation of key nutrient transporters [10]. This establishes the basis for the optimal activation, proliferation and differentiation of the T cell (reviewed in [10–12]). Anergic T cells have been shown to be blocked at the G1/S checkpoint of the cell cycle and, as recently coined by Powell and colleagues, are in a metabolically anergic state characterized by a failure to up-regulate nutrient transporters, such as CD71, CD98 and Glut1, and to switch to an anabolic state of metabolism [13••].
mTOR and AMPK: integrating environmental cues beyond CD28 and IL2
Initial studies using the mTOR inhibitor rapamycin had shown that blocking mTOR activation was sufficient to induce anergy in T cells following full activation with anti-CD3 and anti-CD28 [14]. This effect was subsequently shown to go beyond the simple inhibition of cell proliferation. Drugs like Cyclosporine A (CSA) and FK506, which inhibit the CaN/NFAT pathway, or Sanglifehrin (SFA), which like rapamycin induces an arrest at the G1/S checkpoint without affecting Ca2+ responses, did not induce subsequent anergy in the T cells [15,16]. Full anergy induction required the activation of the CaN/NFAT pathway with concomitant repression of mTOR activation [17]. Thus, mTOR, downstream of the IL-2R pathway, appeared as a major regulator of anergy versus activation.
Interestingly, not only is mTOR downstream of CD28 and IL-2 signaling pathways but it also serves downstream of several energy and nutrient-sensing pathways in eukaryotic cells [18]. Based on this, recent studies have extended the signal 1 + 2 model by pointing out the essential role of such environmental cues in the anergy induction process. For example, activation in the presence of a leucine or glucose antagonist could actively induce anergy in T cells, even in the presence of the classical signal 1 and 2, as well as normal IL-2 production at the time of initial activation [13••]. In the same study, mTOR regulation was also shown to extend to energy sensing via the heterotrimeric kinase complex AMPK. AMPK is activated by a decrease in ATP in the cell leading to an increase in AMP that specifically binds to the subunit of the AMPK complex, leading it to inhibit mTOR activation [12] [18]. Thus, AMPK serves as a direct sensor of ATP deprivation and hypoxia upstream of mTOR and, similar to nutrient deprivation, direct activation of AMPK using aminoimidazole carboxamide ribonucleotide (AICAR) readily induces anergy in T cells [13••].
These experiments demonstrate the ability of the AKT/mTOR pathway to integrate various extracellular and intracellular signals to control T cell responses and dictate T cell choice between activation and tolerance. In addition to its direct role in regulating the metabolic switch induced by T cell activation, recent studies have demonstrated that mTOR can also act as a facilitator of T cell activation by modulating the expression of anergy maintaining genes through direct transcriptional repression [19] or indirect regulation of their degradation [20]. For example, activated mTOR directly induces the epistatic regulator of GRAIL, Otub1, whose expression results in endogenous GRAIL degradation and removal of an active block in T cell proliferation [20].
GCN2 and A2AR: Extending anergy regulation to mTOR-independent pathways
The capacity of extracellular signals to actively regulate T cell activation versus anergy induction extends beyond the simple competition with CD28 and IL2 signaling at the level of mTOR activation and involves other nutrients and energy sensing pathways. The first hint of this came from the work of Munn and colleagues in trying to decipher the molecular mechanism of proliferation arrest induced by tryptophan catabolism by Indoleamine 2,3-Dioxygenase (IDO)-expressing APCs. This effect could not be fully recapitulated by the action of rapamycin and mTOR inhibition [21]. This led them to focus their attention on the second known amino acid sensing pathway involving the GCN2 kinase. GCN2 specifically binds and becomes activated by uncharged forms of transfer RNA (tRNA), thus acting as a sensor of amino acid deprivation for the T cell. Its activation leads to a downstream stress response program known as the integrated stress response (ISR), which regulates entry into the S phase of the cell cycle [22]. Careful examination of this process in CD8 T cells in vitro, as well as in vivo, showed that GCN2−/− T cells are fully resistant to IDO+ DC-induced tolerance, and that triggering this pathway in the cell induces a clonal anergy like phenotype reversible by IL-2 treatment [21]. The role of this pathway in CD4 T cells, however, is less clear. Similar studies with GCN2−/− T cells responding in the context of amino-acid starvation, revealed a more prominent action on the overall survival of activated CD4 T cells and induction of regulatory T cells [23,24••]. Nonetheless, the CD8 study provides a good example of a TCR/CD28/mTOR-independent pathway that can control anergy induction in T cells upon otherwise full activation.
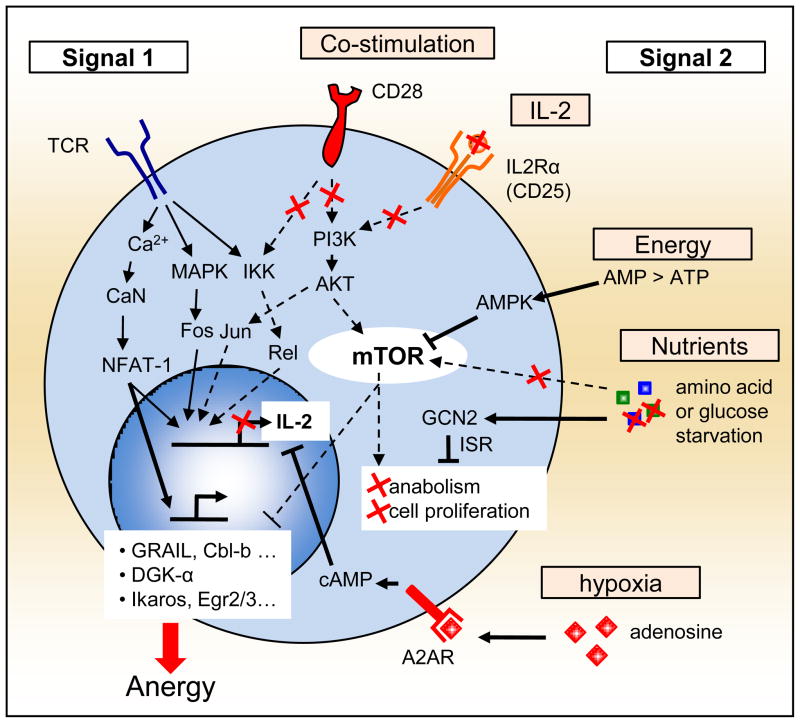
In addition to amino acid sensing pathways, the role of hypoxia and adenosine, the metabolite of ATP, should also be mentioned for their ability to create an active local immunosuppressive environment (reviewed in [25] and [26]). For T cells, adenosine signals through the A2A receptor (A2AR), and stimulates adenyl cyclase and increases intracellular cAMP. Mimicking this effect using the A2AR agonist, CGS-21680 (CGS), Zarek and colleagues demonstrated that this results in decreased signaling through the Ras-MAP-Kinase pathway as well as reduced recruitment of active AP-1 to the nucleus. This ultimately led to the promotion of T cell anergy both in vitro and in vivo [27•]. Such results further extend the set of extracellular cues responsible for signal 2, beyond the initial CD28/IL2 pathway and its regulation of mTOR activation (Figure 1).
Figure 1. Extending the signal 2 paradigm.
induction of anergy in T cells was initially described as the result of TCR (signal 1) without concomitant CD28 and IL-2R signaling (signal 2). Recent studies have further demonstrated that the T cell actively sense its microenvironment, through mTOR dependent and independent mechanisms, for available energy and nutrients, as well as additional negative cues such as adenosine. This regulates the T cell commitment to switch its metabolic machinery and enter the S phase of the cell cycle. Interestingly, failing to positively commit to full T cell activation in such cases induces anergy and long-term tolerance in the T cell. Full lines represent active pathways and dashed lines represent blunted pathways during the induction of T cell anergy in an anergic environment.
From anergy to suppression: a role for TGF-β and dedicated APCs in regulating anergy versus regulatory T cell commitment?
In addition to long term hyporesponsiveness of the T cell, various models of anergy have described the acquisition of suppressive functions by a subpopulation of those T cells. This extends an initially cell intrinsic decision to the level of regulating a population in a process initially coined by Gershon and Kondo as “Infectious Tolerance” [28]. As such, the two most studied regulatory T cell populations, Tr1 and Foxp3+CD4+ T cells, were initially described as having all of the characteristics of an anergic T cell population in vitro (reviewed in [1]). One question that still hasn’t been answered, however, is whether differentiation to a regulatory T cell is a linear process in which anergy induction is the first step and acquisition of suppressive function follows subsequently or if those two events represent two independent choices the cell can make, putting Tregs on the same level as other T helper populations in the choice between anergy versus activation and differentiation.
The AKT/mTOR pathway and environmental cues regulating Foxp3+ T cell peripheral development
In the case of Foxp3+ regulatory T cells, peripheral induction from mature naïve T cells has now been documented both in vivo and in vitro [29,30]. These cells are currently referred to as iTregs. Initial observations on their in vivo peripheral induction emphasized the importance of a low level of TCR stimulation in the absence of strong co-stimulation [29,30]. Mimicking observations done in clonal anergy models, recently published data have demonstrated an essential role for classical anergy signature genes such as the E3 ligases Cbl/b [31] or Itch [32] in regulating Foxp3+ T cell induction, or GRAIL [9,33] in regulating the acquisition and/or maintenance of the suppressive properties of such cells.
Even more interesting, in the context of this review, are recent studies demonstrating the key role played by the AKT/mTOR pathway in regulating effector versus regulatory T cell lineage commitment in the periphery [34–36]. In this regard, T cells expressing a constitutively active AKT fail to up-regulate Foxp3 [34•]. mTORC1-deficient T cells also exhibit blunted responses to IL-12, IFN-γ, IL-4 or IL-6, revealing a role for mTORC1 in regulating T helper lineage differentiation, potentially through direct regulation of the various STAT pathways [36••]. In addition, both GCN2, in response to IDO-mediated tryptophan degradation [23,37], and A2AR signaling pathways have been implicated in positive regulation of Foxp3 expression [27•]. This further emphasizes an important role for what we describe as an “anergy-inducing” environment in the regulation of Foxp3+ iTreg differentiation, and highlights the similarity in the induction of the two states.
TGF-β and a major role for dedicated APC populations?
From pioneering in vitro experiments, the key molecule responsible for Foxp3 expression has been postulated to be TGF-β [38]. On a molecular level, TGF-β seems to divert the cell from the classical pathway of anergy induction by activating Smad3, which will then cooperate with NFAT to induce Foxp3 [39,40]. Subsequently, Foxp3 has been shown to form a complex with NFAT, and other transcription factors, to induce the Treg-signature [41,42].
When delivered in an excess amount to T cells in vitro, TGF-β is sufficient to drive Foxp3 expression in the presence of strong TCR stimulation and this process is further enhanced by costimulation and IL-2 signaling [43]. Such in vitro data clearly differ from the classical anergy induction conditions and the initial in vivo generated data [29,30]. However, as levels of TGF-β are probably limiting in vivo, Treg induction might be more tightly regulated and dependent on the actual environment in which the T cell is being triggered. This was nicely demonstrated by recent studies on the role of mTOR in iTreg differentiation. In a general context of transient TCR stimulation, and thus low mTOR activation, TGF-β seemed to be dispensable for Foxp3 induction [35•]. Alternatively, as revealed by hyperactive Smad3 in mTOR deficient T cells, minute amounts of TGF-β might be sufficient to drive a default differentiation to the Treg lineage under such conditions [36••].
In addition to this, TGF-β levels can be regulated locally; a role that seems to be mostly undertaken by the professional antigen-presenting cells ([44] and reviewed by [45]). Using DEC-205 antibody-coupled peptide administration in vivo, regulatory T cell induction by foreign antigen was initially postulated to require low antigenic doses directly targeted to DEC-205+ dendritic cells (DCs) in the spleen under steady state conditions [30]. Such results were further confirmed in vitro where CD8+ DEC-205+ DCs could induce Foxp3 expression in the presence of low doses of antigen and this process was further shown to be entirely dependent on their endogenous secretion of TGF-β [46]. This property was then extended to lamina propria CD103+ DCs in a model of oral tolerance induction, with again an essential role for TGF-β in combination with the metabolite of vitamin A, retinoic acid (RA) [47,48]. A non-redundant role of RA in promoting Foxp3+ T cell differentiation has also recently been described for a specific population of migrating dermal DCs [49•].
The exact role of RA is still a matter of debate [50–52]. RA has been postulated to act on nearby pre-activated/memory T cells, preventing expression and secretion of IFN- γ or other negative regulators of Foxp3 induction [51]. It has also been reported to directly act on naïve T cells, mediating enhanced TGF-β-induced Foxp3 expression [52]. In this manner, RA might act similarly to mTOR inhibition [53], amino-acid deprivation [37] or adenosine [27], illustrating the potential synergistic action of an “anergy-inducing” environment and TGF-β in the regulation of iTreg differentiation and the generation of a dominant form of tolerance.
Crosstalk between Treg-mediated suppression and anergy induction: Tregs as regulators of environmental cues?
Numerous mechanisms have been proposed for regulatory T cell-mediated suppression such as TGF-β and IL-10 secretion, modulation of DC maturation and function, IL-2 consumption from the extracellular environment or even direct killing of target cells through granzyme B dependent mechanisms (reviewed in [54]). It is interesting, however, to note that several knockout models, shown to be resistant to anergy induction, are also resistant to Treg-mediated suppression. Examples include E3 ubiquitin ligases, Cbl-b [55,56] and TRAF6 [57,58], and the molecular inducer of most anergic factor genes, NFAT1, in combination with NFAT4 [59]. Data are currently missing for molecules such as GRAIL or Itch as well as for the various transcriptional repressors involved in anergy, but it is nonetheless striking that classical anergy markers seem to be playing a non-redundant role in regulating the subsequent activation of T cells undergoing Treg-mediated suppression. It is thus possible that Tregs work, at least in part, as inducers of T cell anergy. Two recently described and/or revived molecular mechanisms, CTLA-4/IDO and CD39/CD73/adenosine, might be playing an important role in this task.
CTLA-4 and IDO: targeting DCs and inducing infectious tolerance through the consumption of essential amino acids
CTLA-4 was noted in early studies as being expressed on Tregs and was thus postulated to have an important role in their function in vitro [60]. This has now been clearly demonstrated by various groups using targeted deletion in Foxp3+ T cells resulting in the impairment of both in vitro and in vivo suppressive function of the Tregs [61–64]. CTLA-4 expressed on Tregs has been shown to directly target B7 ligands expressed on activated Foxp3− T cells and to induce CREM expression ending in attenuated IL-2 transcription [65]. However, the major role of CTLA-4 in Treg-mediated suppression is regulating DC maturation and expression of CD80, CD86 [61•] as well as inducing their expression of IDO [66]. This would lead to decrease co-stimulation as well as tryptophan deprivation, resulting in down-regulation of T cell responses, as discussed earlier.
Again, this extends far beyond simply regulating tryptophan levels in the extracellular milieu. In a very interesting study, Cobbold and colleagues describe in vivo and in vitro evidence that both Foxp3+ iTregs and Tr1 cells can induce expression in DCs of a large variety of amino acid-consuming enzymes including arginase 1 (Arg1), iNOS, Histidine decarboxylase (Hdc), L-amino acid oxidase (IL4i1), Branched chain aminotransferase (Bcat1), Threonine dehydrogenase (Tdh), Tryptophan hydroxylase (Tph) and of course IDO (Indo) [24••]. Each of these enzymes seems to be differentially regulated by the action of CTLA-4, TGF-β or IL-10 expressed by the various Treg populations studied. Specific depletion of any one of the targeted amino acids was sufficient to suppress naïve T cell activation.
CD39/CD73 and the regulation of T cell activation by Treg-induced adenosine
Finally, Treg-mediated suppression might extend beyond simply inducing tolerogenic DCs. Tregs have recently been postulated to promote a hypoxic environment via their expression of both the 5′-ectonucleotidase CD73 and the ATPase/ADPase CD39 [67•]. We have already mentioned the role of the adenosine receptor in inducing anergy [25,27]. Here, CD39, by hydrolyzing ATP to AMP, and CD73, by converting AMP into adenosine, are postulated to work in tandem to induce an immunosuppressive environment by targeting the A2AR [68]. The genes coding for CD39 and CD73 are both targets of direct transcriptional activation by Foxp3 [69,70] and the proteins are expressed at high frequency on Foxp3+ T cells in both mouse and human [68,70]. Interestingly, CD39 consumption of ATP in vitro is only detected on TCR-activated Tregs, adding a level of antigen specificity to the mechanism [70].
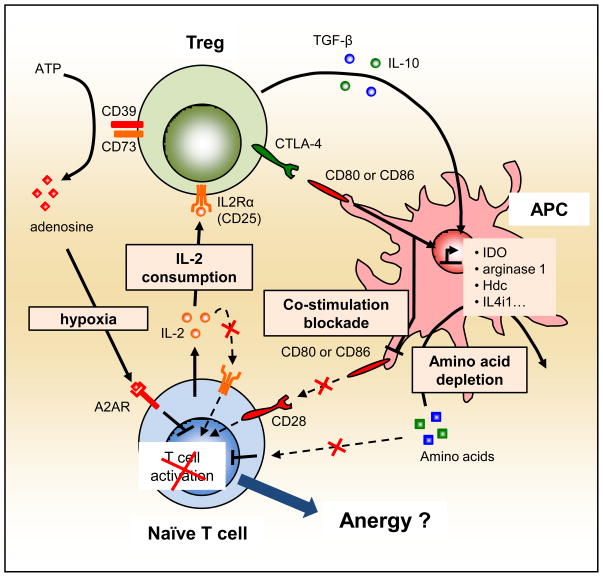
In both examples described in this section, it is interesting to note that the regulation of the environment, i.e. amino acid deprivation and adenosine generation, should directly induce anergy in T cells undergoing activation, provided that Tregs do not induce at the same time a block in the CaN/NFAT pathway (Figure 2).
Figure 2. Infectious tolerance by creating an “anergy-inducing” environment.
It has recently been shown that Tregs can influence the microenvironment in which a naïve T cell gets activated. First, Tregs can, via CTLA-4 expression and IL-10 and TGF-β secretion, induce various amino acid-consuming enzymes, as well as down-regulate CD80 and CD86 expression, in DCs. Second, Tregs, via their constitutive expression of the high affinity chain of the IL2R (CD25), can actively consume IL-2 in the extracellular milieu. Finally, Tregs express the 5′-ectonucleotidase CD73 and the ATPase/ADPase CD39 and activated Tregs have the potency to hydrolyze extracellular ATP into adenosine, thus favoring an hypoxic environment. Full lines represent active pathways and dashed lines represent blunted pathways during active suppression of naïve T cell activation by Tregs.
Conclusion
Overall, it has become increasingly clear that signal 2 in the classical anergy model extends far beyond simple CD28/IL-2 signaling and is likely the result of active integration of multiple environmental cues received by the T cell undergoing activation (Figure 1). Although the CD28 and IL-2 pathways, being specific to the immune system, remain central in explaining how a T cell makes the choice between activation or tolerance in a favorable environment, the role of an extended set of extracellular cues could help to explain various observations of T cell tolerance in vivo under what were thought to be optimal activation conditions. In addition, it seems that, when triggered by a similar set of extracellular cues in the presence of TGF-β, the classical “anergy inducing” program can be diverted to induce the differentiation of iTregs with additional suppressive functions. These Tregs in turn act in various ways to modulate the T cell environment and favor tolerance/anergy induction rather than full activation (Figure 2). Additional studies on the fate of T cells undergoing Treg- mediated suppression could help link the fields of anergy and regulatory T cells and would further enhance our global understanding of T cell tolerance.
Acknowledgments
The authors thank Dr. Nevil Singh for critical reading and helpful discussion of this review. The authors were supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Pascal Chappert, Email: chappertp@mail.nih.gov.
Ronald H. Schwartz, Email: rs34r@nih.gov.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
••of special interest
••of outstanding interest
- 1.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quill H, Schwartz RH. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987;138:3704–3712. [PubMed] [Google Scholar]
- 4.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Whaley CD, Mondino A, Mueller DL. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science. 1996;271:1272–1276. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 6.Zha Y, Marks R, Ho AW, Peterson AC, Janardhan S, Brown I, Praveen K, Stang S, Stone JC, Gajewski TF. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat Immunol. 2006;7:1166–1173. doi: 10.1038/ni1394. [DOI] [PubMed] [Google Scholar]
- •7.Wells AD. New insights into the molecular basis of T cell anergy: anergy factors, avoidance sensors, and epigenetic imprinting. J Immunol. 2009;182:7331–7341. doi: 10.4049/jimmunol.0803917. An updated and comprehensive review on the molecular mechanisms of Anergy. [DOI] [PubMed] [Google Scholar]
- 8.Hsiao H-W, Liu W-H, Wang C-J, Lo Y-H, Wu Y-H, Jiang S-T, Lai M-Z. Deltex1 is a target of the transcription factor NFAT that promotes T cell anergy. Immunity. 2009;31:72–83. doi: 10.1016/j.immuni.2009.04.017. [DOI] [PubMed] [Google Scholar]
- •9.Nurieva RI, Zheng S, Jin W, Chung Y, Zhang Y, Martinez GJ, Reynolds JM, Wang S-L, Lin X, Sun S-C, et al. The E3 Ubiquitin Ligase GRAIL Regulates T Cell Tolerance and Regulatory T Cell Function by Mediating T Cell Receptor-CD3 Degradation. Immunity. 2010;32(5):670–80. doi: 10.1016/j.immuni.2010.05.002. This study nicely shows the mechanism of the E3 Ubitiquitin Ligase GRAIL. The authors have generated a GRAIL deficient mouse and describe GRAIL’s role in enhancing CD3 ubiquitination and degradation of the whole TCR/CD3 complex. Of interest, GRAIL seems to also be required for the suppressive functions of Tregs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones RG, Thompson CB. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007;27:173–178. doi: 10.1016/j.immuni.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Pearce EL. Metabolism in T cell activation and differentiation. Current Opinion in Immunology. 2010;22(3):314–20. doi: 10.1016/j.coi.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- ••13.Zheng Y, Delgoffe GM, Meyer CF, Chan W, Powell JD. Anergic T cells are metabolically anergic. J Immunol. 2009;183:6095–6101. doi: 10.4049/jimmunol.0803510. This study describes for the first time the metabolic state of clonally anergic T cells and coins the term “metabolic anergy”. Of great interest, the authors also extend the set of environmental cues regulating the induction of anergy, via mTOR, by studying the impact of leucine and glucose antagonists, NALA and 2DG, as well as the AMPK activator AICAR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162:2775–2784. [PubMed] [Google Scholar]
- 15.Powell JD, Bruniquel D, Schwartz RH. TCR engagement in the absence of cell cycle progression leads to T cell anergy independent of p27(Kip1) Eur J Immunol. 2001;31:3737–3746. doi: 10.1002/1521-4141(200112)31:12<3737::aid-immu3737>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 16.Allen A, Zheng Y, Gardner L, Safford M, Horton MR, Powell JD. The novel cyclophilin binding compound, sanglifehrin A, disassociates G1 cell cycle arrest from tolerance induction. J Immunol. 2004;172:4797–4803. doi: 10.4049/jimmunol.172.8.4797. [DOI] [PubMed] [Google Scholar]
- 17.Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, Powell JD. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- 18.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duré M, Macian F. IL-2 signaling prevents T cell anergy by inhibiting the expression of anergy-inducing genes. Mol Immunol. 2009;46:999–1006. doi: 10.1016/j.molimm.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin JT, Lineberry NB, Kattah MG, Su LL, Utz PJ, Fathman CG, Wu L. Naive CD4 t cell proliferation is controlled by mammalian target of rapamycin regulation of GRAIL expression. J Immunol. 2009;182:5919–5928. doi: 10.4049/jimmunol.0803986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Grallert B, Boye E. The Gcn2 kinase as a cell cycle regulator. Cell Cycle. 2007;6:2768–2772. doi: 10.4161/cc.6.22.4933. [DOI] [PubMed] [Google Scholar]
- 23.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- ••24.Cobbold SP, Adams E, Farquhar CA, Nolan KF, Howie D, Lui KO, Fairchild PJ, Mellor AL, Ron D, Waldmann H. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci USA. 2009;106:12055–12060. doi: 10.1073/pnas.0903919106. This comprehensive study extends the original concept of IDO induction in dendritic cells by Tregs to a large set of amino acid-consuming enzymes. Of particular interest, the authors also demonstrate the non-redundant and overlapping functions of CTLA-4, IL-10 and TGF-β in this process. Finally, using GCN2−/minus; T cells, they are able to dissociate the role of GCN2 and mTOR in mediating tolerance in naïve T cells following Treg-induced amino acid deprivation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zarek PE, Powell JD. Adenosine and anergy. Autoimmunity. 2007;40:425–432. doi: 10.1080/08916930701464939. [DOI] [PubMed] [Google Scholar]
- 26.Sitkovsky MV, Ohta A. The ‘danger’ sensors that STOP the immune response: the A2 adenosine receptors? Trends in Immunology. 2005;26:299–304. doi: 10.1016/j.it.2005.04.004. [DOI] [PubMed] [Google Scholar]
- •27.Zarek PE, Huang C-T, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. The first study showing active induction of clonal anergy in T cell by signaling through the adenosine receptor A2AR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gershon RK, Kondo K. Infectious immunological tolerance. Immunology. 1971;21:903–914. [PMC free article] [PubMed] [Google Scholar]
- 29.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 31.Wohlfert EA, Gorelik L, Mittler R, Flavell RA, Clark RB. Cutting edge: deficiency in the E3 ubiquitin ligase Cbl-b results in a multifunctional defect in T cell TGF-beta sensitivity in vitro and in vivo. J Immunol. 2006;176:1316–1320. doi: 10.4049/jimmunol.176.3.1316. [DOI] [PubMed] [Google Scholar]
- 32.Venuprasad K, Huang H, Harada Y, Elly C, Subramaniam M, Spelsberg T, Su J, Liu Y-C. The E3 ubiquitin ligase Itch regulates expression of transcription factor Foxp3 and airway inflammation by enhancing the function of transcription factor TIEG1. Nat Immunol. 2008;9:245–253. doi: 10.1038/niXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacKenzie DA, Schartner J, Lin J, Timmel A, Jennens-Clough M, Fathman CG, Seroogy CM. GRAIL is up-regulated in CD4+ CD25+ T regulatory cells and is sufficient for conversion of T cells to a regulatory phenotype. J Biol Chem. 2007;282:9696–9702. doi: 10.1074/jbc.M604192200. [DOI] [PubMed] [Google Scholar]
- •34.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •35.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••36.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. These three studies explore the role of mTOR in the induction of Foxp3 in naïve T cells. Haxhinasto et al. use retroviral transduction of a constitutively active form of AKT in naïve T cells and thymocytes to demonstrate the antagonistic role of the AKT/mTOR pathway in the promotion of Foxp3+ Tregs cells. Sauer et al. demonstrate the same effect of the AKT/mTOR pathway in the case of continued TCR signaling. Of great interest, Delgoffe et al., by using knock-out mouse models for both mTORC1 and mTORC2 protein complexes, provide us with the first in depth analysis of the role of mTOR in the regulation of Th17 and regulatory T cell differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, et al. Tryptophan catabolism generates autoimmune-preventive regulatory T cells. Transpl Immunol. 2006;17:58–60. doi: 10.1016/j.trim.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Chen W, Jin W, Hardegen N, Lei K-J, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 40.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 42.Marson A, Kretschmer K, Frampton GM, Jacobsen ES, Polansky JK, MacIsaac KD, Levine SS, Fraenkel E, von Boehmer H, Young RA. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–935. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 44.Yamazaki S, Bonito AJ, Spisek R, Dhodapkar M, Inaba K, Steinman RM. Dendritic cells are specialized accessory cells along with TGF- for the differentiation of Foxp3+ CD4+ regulatory T cells from peripheral Foxp3 precursors. Blood. 2007;110:4293–4302. doi: 10.1182/blood-2007-05-088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamazaki S, Steinman RM. Dendritic cells as controllers of antigen-specific Foxp3+ regulatory T cells. Journal of Dermatological Science. 2009;54:69–75. doi: 10.1016/j.jdermsci.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, Nussenzweig MC, Steinman RM. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun C-M, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, Hall J, Sun C-M, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •49.Guilliams M, Crozat K, Henri S, Tamoutounour S, Grenot P, Devilard E, de Bovis B, Alexopoulou L, Dalod M, Malissen B. Skin-draining lymph nodes contain dermis-derived CD103(-) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood. 2010;115:1958–1968. doi: 10.1182/blood-2009-09-245274. This study extends the work done previously by Sun et al. and Coombes et al. on CD103+ DCs in the gut to a subpopulation of dermal DCs in the skin. Interestingly, CD103 did not appear as a reliable marker to identify tissue-derived Foxp3-inducing DCs. RA expression, however, correlated nicely with such function. [DOI] [PubMed] [Google Scholar]
- 50.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 51.Hill JA, Hall JA, Sun C-M, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nolting J, Daniel C, Reuter S, Stuelten C, Li P, Sucov H, Kim B-G, Letterio JJ, Kretschmer K, Kim H-J, et al. Retinoic acid can enhance conversion of naive into regulatory T cells independently of secreted cytokines. J Exp Med. 2009;206:2131–2139. doi: 10.1084/jem.20090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang J, Huddleston SJ, Fraser JM, Khoruts A. De novo induction of antigen-specific CD4+CD25+Foxp3+ regulatory T cells in vivo following systemic antigen administration accompanied by blockade of mTOR. Journal of Leukocyte Biology. 2008;83:1230–1239. doi: 10.1189/jlb.1207851. [DOI] [PubMed] [Google Scholar]
- 54.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wohlfert EA, Callahan MK, Clark RB. Resistance to CD4+CD25+ regulatory T cells and TGF-beta in Cbl-b−/− mice. J Immunol. 2004;173:1059–1065. doi: 10.4049/jimmunol.173.2.1059. [DOI] [PubMed] [Google Scholar]
- 56.Heissmeyer V, Macián F, Im S-H, Varma R, Feske S, Venuprasad K, Gu H, Liu Y-C, Dustin ML, Rao A. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 57.King CG, Buckler JL, Kobayashi T, Hannah JR, Bassett G, Kim T, Pearce EL, Kim GG, Turka LA, Choi Y. Cutting edge: requirement for TRAF6 in the induction of T cell anergy. J Immunol. 2008;180:34–38. doi: 10.4049/jimmunol.180.1.34. [DOI] [PubMed] [Google Scholar]
- 58.King CG, Kobayashi T, Cejas PJ, Kim T, Yoon K, Kim GK, Chiffoleau E, Hickman SP, Walsh PT, Turka LA, et al. TRAF6 is a T cell-intrinsic negative regulator required for the maintenance of immune homeostasis. Nat Med. 2006;12:1088–1092. doi: 10.1038/nm1449. [DOI] [PubMed] [Google Scholar]
- 59.Bopp T, Palmetshofer A, Serfling E, Heib V, Schmitt S, Richter C, Klein M, Schild H, Schmitt E, Stassen M. NFATc2 and NFATc3 transcription factors play a crucial role in suppression of CD4+ T lymphocytes by CD4+ CD25+ regulatory T cells. J Exp Med. 2005;201:181–187. doi: 10.1084/jem.20041538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •61.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. This study finally demonstrates the essential role of CTLA-4 expressed by Foxp3+ Tregs. By selectively targeting CTLA-4 deletion in such cells, the authors show the critical requirement for CTLA-4 during in vitro and in vivo suppressive function of Tregs. Friedline et al and Jain et al further confirmed such observations in their respective studies. [DOI] [PubMed] [Google Scholar]
- 62.Friedline RH, Brown DS, Nguyen H, Kornfeld H, Lee J, Zhang Y, Appleby M, Der SD, Kang J, Chambers CA. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J Exp Med. 2009;206:421–434. doi: 10.1084/jem.20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jain N, Nguyen H, Chambers C, Kang J. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc Natl Acad Sci USA. 2010;107:1524–1528. doi: 10.1073/pnas.0910341107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ise W, Kohyama M, Nutsch KM, Lee HM, Suri A, Unanue ER, Murphy TL, Murphy KM. CTLA-4 suppresses the pathogenicity of self antigen-specific T cells by cell-intrinsic and cell-extrinsic mechanisms. Nat Immunol. 2010;11:129–135. doi: 10.1038/ni.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bodor J, Fehervari Z, Diamond B, Sakaguchi S. ICER/CREM-mediated transcriptional attenuation of IL-2 and its role in suppression by regulatory T cells. Eur J Immunol. 2007;37:884–895. doi: 10.1002/eji.200636510. [DOI] [PubMed] [Google Scholar]
- 66.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- •67.Sitkovsky MV. T regulatory cells: hypoxia-adenosinergic suppression and redirection of the immune response. Trends in Immunology. 2009;30:102–108. doi: 10.1016/j.it.2008.12.002. An interesting review and point of view of the author on the role of hypoxia in mediating immune tolerance and the role of Tregs in regulating the induction of such an hypoxic environment. [DOI] [PubMed] [Google Scholar]
- 68.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen J-F, Enjyoji K, Linden J, Oukka M, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 70.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Höpner S, Centonze D, Bernardi G, Dell’Acqua ML, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]