Abstract
Pancreatic cancer (PC) has the poorest overall survival rate among all human cancers because of late diagnosis and absence of screening tools. We compared the expression profile of microRNAs (miRNAs) in the plasma of patients diagnosed with PC (n=50) with healthy volunteers (n=10). Data was further validated by quantitative realtime PCR and cell-based assays. Thirty-seven miRNAs were down-regulated and 54 were up-regulated in plasma from patients with PC. The expression of miR-21 was significantly higher, and the expression of let-7 family (especially let-7d) and miR-146a was significantly lower in PC. Most interestingly, the expression of miR-21 was correlated with worse survival, and the expression of let-7 was inversely correlated with survival in this pilot study with mixed patient population. Moreover, we found that miR-21 family was markedly over-expressed in chemo-resistant PC cell lines, which was consistent with the plasma data from PC patients. Our previous studies have shown increased expression of miR-21 with concomitant loss of PTEN expression in PC cells, which is consistent with our current findings showing the loss of three additional targets of miR-21 (PDCD4, Maspin and TPM1). These results suggest that identifying and validating the expression of miRNAs in newly diagnosed patients could serve as potential biomarker for tumor aggressiveness, and such miRNAs could be useful for the screening of high-risk patients, and may also serve as targets for future drug development.
Keywords: Pancreatic cancer, miR-21, miR-221, Drug Resistance, PTEN, let-7d
Introduction
The overall survival of patients diagnosed with pancreatic cancer (PC) is the worst among human malignancies because of lack of tools for its early detection and the aggressive nature of PC compared to other malignancies. PC remains the fourth leading cause of cancer-related deaths in the United States with an estimated 43,140 new cases and 36,800 deaths expected in 2010 [1]. The discovery of effective tools for diagnosing PC in its early stages may significantly improve the survival rates. One strategy would be the development of novel sensitive and non-invasive biomarkers by using plasma samples. Recent gene expression studies identified a set of small number of genes that are differentially expressed in PC [2] but no information is available for assessing their impact on disease prognosis. In recent years there has been a dramatic increase in the discovery of microRNAs (miRNAs) that are associated with cancer aggressiveness as summarized in our recent review articles [3,4,5]. The miRNAs are naturally occurring small non-coding molecules found in human that regulate gene expression and consequently have a potential functional role in a wide array of cellular processes, including differentiation, proliferation, and apoptosis [3,5]. The miRNAs are classified into different families based on location and function in various biological processes. Novel molecular technologies, like miRNAs profiling, have recognized more than 1000 miRNA sequences in the human genome as documented in the miRBase database (release 15; miRBase) [6]. Profiling of miRNAs from human tumors identified a number of miRNAs as either tumor suppressors or oncogenes depending on whether they specifically target tumor suppressor genes or oncogenes, respectively.
Recent studies confirmed that miRNAs are present in remarkably stable form in blood and as biomarkers could distinguish cancer vs. non-cancer bearing subjects [7,8,9,10]. miRNAs that are dysregulated are referred to as oncomiRs and one such oncomiR is the miR-21 family that is over-expressed in nine non-hematological cancers including PC [11,12,13,14,15]. We and others have shown a significant correlation between miR-21 expression, tumor growth, and resistance to cytotoxic agents in a variety of malignancies [11,10,15]. Conversely, tumor suppressive miRNAs such as let-7, and miR-146a are usually under-expressed as documented in human lung and PC cell lines [16,17]. A recent study suggested that over-expression of miR-21 is linked to advanced tumor stage and poor survival of breast cancer patients [18]. There is also evidence that miR-21 may have anti-apoptotic properties [13].
Over-expression of miR-21 has been shown to enhance tumor growth in a xenograft model and was found to be regulated by activated andro-gen receptor in prostate cancer [19]. Moreover, it is believed that over-expression of miR-21 could lead to chemo-resistance in PC [20,21], and these findings are consistent with our published report showing that the down-regulation of miR-21 in PC cells may reverse drug resistance [11]. The findings suggest that the miRNAs in plasma may be useful prognostic or predictive biomarkers that could be useful especially in PC patients that may facilitate the development of novel strategies by which miRNAs could be down-regulated or up-regulated to improve the overall survival.
In this study, we initially determined the expression profiles of miRNAs in pooled plasma samples of 50 PC patients and compared to those from 10 normal healthy volunteers by miRNA microarray profiling technology and further validated by quantitative real-time PCR (RT-PCR) in individual plasma samples. We found 91 differentially expressed miRNAs in the plasma of PC patients compared to healthy controls. Further analysis by quantitative real-time PCR (qRT-PCR) focused on several miRNAs based on our previously published reports showing the biological significance of miR-21, let-7 family, miR-200 family and miR-146a with respect to tumor aggressiveness [11,16,3,5]. Consistent with published literature, we found higher expression of miR-21 and miR-221, and lower expression of let-7b, let-7d, miR-200b, miR-200c, and miR-146a in the plasma of PC patients compared to normal controls, which is consistent with the signature of tumor aggressiveness. The expression of miR-21 was correlated with worse survival, suggesting that over-expression of miR-21 could define biologic tumor behavior. We further tested this hypothesis experimentally by assessing differential expression of miRNAs in two parental and four chemo-resistant human PC cell lines by miRNA profiling and qRT-PCR. We found that miR-21 was significantly up-regulated in chemo-resistant cell lines compared to parental cell lines and that the over-expression of miR-21 was negatively correlated with the expression of its target genes such as PTEN, PDCD4, Maspin and TPM1. Based on these results, we conclude that miR-21 expression may predict tumor aggressiveness and may be exploited for the development of novel strategies by which miR-21 could be down-regulated for the successful treatment of patients diagnosed with PC.
Materials and methods
Collection of plasma
The study subjects consisted of newly diagnosed patients with pancreatic cancer (PC) and for whom we had the survival data. Patients who are newly diagnosed, undergoing surgery, or receiving treatment for PC at the Karmanos Cancer Center were considered eligible for this study. All blood samples were collected prior to any therapeutic procedures, including surgery. We enrolled 76 patients for the current study, and we collected eight milliliters of venous blood in CPT tubes (BD Vacutainer). The plasma was isolated within an hour by centrifugation at 1,500 × g at room temperature for 20 minutes and stored as multiple aliquots in fresh tubes at −80°C. Blood from controls often normal healthy individuals with no evidence of any disease were also collected for comparison. Study was approved by the institutional human investigation review board and each subject provided signed informed consent.
Sample preparation and RNA isolation
Fifty PC patients were randomly selected from the total 76 patients, and their plasma was pooled together along with 10 controls (normal subjects) into two separate groups and was subjected to miRNA microarray profiling to select miRNAs whose expression was differentially expressed in PC patients compared to the normal control subjects. Subsequently, we selected seven miRNAs for further validation in the individual plasma samples using TaqMan probe based qRT-PCR for only those PC patients (n=32) who were treated at Karmanos Cancer Center and for whom we had the survival data. Total RNA containing small RNA was isolated from plasma using Trizol LS reagent (Invitrogen Life Technologies) according to the manufacturer's protocol with the following modifications. The Trizol LS reagent was mixed with 3:1 ratio with plasma and incubated for 5 minutes. After the addition of chloroform, tubes were shaken well and centrifuged to separate the upper aqueous phase which was carefully transferred to a fresh tube. Isopropanol was then added to the aqueous phase for 30 minutes followed by centrifugation at 12,000 × g for 10 minutes. The RNA precipitate was then washed with 75% ethanol and centrifuged at 7,500 for 5 minutes. The RNA pellet was then purified using mirVana miRNA isolation kit (Ambion, Inc.) according to the manufacturer's protocol. Briefly, the pellet was lysed in lysis solution and incubated with 1/10 volume of miRNA homogenate additive for 10 minutes. Equal volume of Acid-Phenol:Chloroform was added to the mixture and vortexed for a minute before centrifugation to separate aqueous phase. About 1.25 volume of 100% ethanol was added to the aqueous phase before being applied directly to the filter cartridge. The RNA was washed with the buffers provided with the kit to remove impurities and eluted in a final volume of 100 μl.
Similarly total RNA was also isolated from human pancreatic cancer cell lines using Trizol as described above. The purified RNA samples from both plasma (normal and patient samples) and cell lines were analyzed by LC Sciences for miRNA microarray profiling (LC Sciences Houston, Tx).
TaqMan miRNA Real-Time Reverse Transcrip-tase-Polymerase Chain Reaction (RT-PCR)
Quantitative RT-PCR (qRT-PCR) is the standard method for validation of microarray profiling data which provides quantitative analysis of microRNA expression in real time for the miRNAs of interest from the samples analyzed by microarray profiling. To determine the expression of miRNA-21 family (miRNA-21, miR-210, and miR-214) and other oncogenes in six pancreatic cancer cell lines and plasma from normal individuals and PC patient, we used TaqMan MicroRNA Assay kit (Applied Biosys-tems) following manufacturer's protocol. About 10 ng of RNA from plasma or cells were reverse transcribed using 7 μl of master mix containing dNTPs, reverse transcriptase and RNase inhibi tor and 3 μl of respective primer. The mixture was incubated at 16°C for 30 min, 42°C for 60 min, followed by 85°C for 5 minutes. Real-time PCR reactions were then carried out in a total volume of 25 μl reaction mixture containing 1.66 μl of RT product mixed with 1.25 μl Taqman primers, 12.5 μl of 2X Taqman universal PCR master mix, 9.58 μl of water and 1.25 μl of probe. All reactions, including controls were performed in triplicate using Smart Cycler II (Cepheid). Relative expression of miRNAs was analyzed using Ct method and was normalized by miRNA-16 expression for plasma samples and RNU6B expression for cell lines. RT-PCR is a sensitive and reproducible gene expression quantitative technique which is now being used to profile miRNA expression in cells and tissues. With rapid development of technology, detection of miRNA has become more easy, sensitive and credible.
Cells culture, drugs and reagents
Human PC cell lines MIAPaCa-2, and AsPc-1, were chosen for this study. Both the cell lines were exposed to gemcitabine, oxaliplatin or tarceva every other week for six months to create the resistant cell lines. We named these cell lines as MIAPaCa-GR (gemcitabine resistant), AsPc-1OR (oxaliplatin resistant), MIAPaCa-GTR, AsPc-1GTR (gemcitabine and tarceva resistant) based on their exposure to chemo-drugs. The parental cell lines MIAPaCa-2 and AsPc-1 have been tested and authenticated through our core facility; Applied Genomics Technology Center at Wayne State University on March 13, 2009. The method used for testing was short tandem repeat (STR) profiling using the PowerPlex® 16 System from Promega (Madison, WI). The cells were frozen in liquid nitrogen in multiple ali-quots and the cells in cultures were used for six months after which new aliquot of frozen cells was used to initiate new culture.
Protein extraction and Western blot analysis
BxPC-3, MIAPaCa-2, MIAPaCa-GR, MIAPaCa-GTR, AsPc-1, AsPc-1OR, AsPc-1GTR cells were used to evaluate the basal level of E-cadherin, vimentin, FEN-1, PTEN, PDCD4, Tropomyosin 1, Maspin, and β-actin expression. Western blot analysis was performed as described previously [22] and the signal intensity was measured using chemiluminescent detection system (Pierce Rockford, IL).
Statistical methods
Expression levels of the seven miRNAs in PC patients and normal subjects were stratified by the median value, and the correlation between survival outcome and miRNA levels was determined by the Kaplan-Meier survival analysis. The correlations among the seven biomarkers were calculated using the Pearson's correlation coefficient. The survival was defined as time from diagnosis to any cause of death. The Regression Tree method was used to find the best threshold for each biomarker's expression with respect to its prognostic value. The Log-rank test was used to evaluate the survival difference among the subgroups defined by marker expression (low vs high) and tumor stage. The Cox model was fit to estimate the prognostic value of each marker stratified on tumor stage with different baseline hazards. We assumed that all patients received best care. The performance status (ps) was 0 or 1 for all the patients. The statistical significance level was adjusted to 0.01 for multiple testing.
Results
Description of patient study
All 76 patients enrolled in the study were both clinically and pathologically diagnosed for pancreatic cancer (PC). Among the 32 patients who had survival data, one patient (patient ID 62) with ps=3 was removed from the survival analysis. The median age was 57 and gender count was 51.6% male and 48.4% female. Five (16%) patients had ps=0 and 25(84%) had ps=l. Nine had (29%) locally advanced tumor and 22 (71%) had metastatic disease. Only one out of 31 patients was censored. The remaining patients were all followed up to the event of death. The median survival for overall group of 31 patients was 7 months (Cl 5.65, 12.3).
Expression profiling of plasma miRNAs characterized 91 miRNAs that were differentially expressed in PC patients
In this study, plasma samples (about 100 μl each) from 50 PC patients and 10 normal (about 500 μl each) healthy individuals were pooled separately. Total RNA was then extracted from both the pooled samples. Plasma miRNA expression profiling revealed 91 miRNAs that were differentially expressed of which 54 were up-regulated and 37 were down-regulated compared to control subjects (Table 1). The molecular mechanisms identified with the seven selected miRNAs for subsequent analysis and their corresponding target genes are shown in Figure 1. Analysis of miRNA microarray profiles showed that the miR-21 family was significantly up-regulated (Figure 2A) whereas let-7 family and miR-146a were significantly down-regulated (Figure 2B and 2C). Real-time qRT-PCR was conducted on 32 patient plasma samples and compared to 10 healthy controls individually to validate the miRNA profiling results by TaqMan miRNA based assay as shown below.
Table 1.
Statistical and clustering analysis of miRNA data between the two groups
| Group 1 | Group 2 | ||||
|---|---|---|---|---|---|
| Normal | Patient | Log2 (G2/G1) | |||
| No. | Reporter Name | p-value | Mean | Mean | |
| 706 | hsa-miR-574-5p | 1.93E-07 | 215 | 10,953 | 5.67 |
| 431 | hsa-miR-32* | 2.36E-07 | 25 | 961 | 5.24 |
| 67 | hsa-miR-1228* | 7.71E-07 | 113 | 3,535 | 4.97 |
| 4 | hsa-let-7b | 8.84E-07 | 827 | 104 | −2.99 |
| 254 | hsa-miR-188-5p | 9.05E-07 | 348 | 1,249 | 1.84 |
| 60 | hsa-miR-1224-5p | 1.26E-06 | 301 | 882 | 1.55 |
| 814 | hsa-miR-671-5p | 1.78E-06 | 282 | 1,565 | 2.47 |
| 280 | hsa-miR-193b* | 1.94E-06 | 742 | 37 | −4.32 |
| 295 | hsa-miR-1977 | 2.35E-06 | 5,647 | 1,831 | −1.62 |
| 833 | hsa-miR-765 | 1.04E-05 | 425 | 1,282 | 1.59 |
| 520 | hsa-miR-423-5p | 1.05E-05 | 3,260 | 336 | −3.28 |
| 6 | hsa-let-7c | 1.07E-05 | 599 | 60 | −3.31 |
| 110 | hsa-miR-1268 | 1.23E-05 | 867 | 3,409 | 1.97 |
| 435 | hsa-miR-320d | 1.27E-05 | 2,070 | 258 | −3.01 |
| 55 | hsa-miR-1207-5p | 2.33E-05 | 137 | 778 | 2.51 |
| 242 | hsa-miR-1826 | 3.13E-05 | 2,406 | 793 | −1.60 |
| 434 | hsa-miR-320c | 3.16E-05 | 3,324 | 749 | −2.15 |
| 153 | hsa-miR-1305 | 3.26E-05 | 8,440 | 2,878 | −1.55 |
| 433 | hsa-miR-320b | 3.45E-05 | 3,353 | 746 | −2.17 |
| 169 | hsa-miR-134 | 4.76E-05 | 494 | 1,028 | 1.06 |
| 209 | hsa-miR-149* | 5.12E-05 | 854 | 1,848 | 1.11 |
| 80 | hsa-miR-1246 | 5.42E-05 | 383 | 658 | 0.78 |
| 812 | hsa-miR-670 | 1.44E-04 | 24 | 454 | 4.23 |
| 211 | hsa-miR-150* | 2.19E-04 | 309 | 609 | 0.98 |
| 731 | hsa-miR-595 | 2.73E-04 | 23 | 629 | 4.75 |
| 432 | hsa-miR-320a | 3.09E-04 | 2,610 | 576 | −2.18 |
| 780 | hsa-miR-638 | 3.51E-04 | 3,979 | 8,649 | 1.12 |
| 821 | hsa-miR-711 | 6.67E-04 | 1,803 | 162 | −3.48 |
| 679 | hsa-miR-550 | 9.78E-04 | 587 | 58 | −3.33 |
| 549 | hsa-miR-483-5p | 1.15E-03 | 6,038 | 10,071 | 0.74 |
| 43 | hsa-miR-1183 | 1.18E-03 | 834 | 403 | −1.05 |
| 486 | hsa-miR-371-5p | 1.41E-03 | 282 | 477 | 0.76 |
| 297 | hsa-miR-1979 | 1.50E-03 | 15,069 | 10,529 | −0.52 |
| 831 | hsa-miR-762 | 1.66E-03 | 480 | 814 | 0.76 |
| 273 | hsa-miR-1915 | 4.54E-03 | 996 | 1,472 | 0.56 |
| 195 | hsa-miR-1469 | 7.94E-03 | 306 | 452 | 0.56 |
| Following transcripts are statistically significant but have low signals (signal < 500) | |||||
| 746 | hsa-miR-610 | 2.43E-07 | 14 | 194 | 3.75 |
| 1 | hsa-let-7a | 5.71E-07 | 343 | 37 | −3.20 |
| 648 | hsa-miR-539 | 1.30E-06 | 12 | 161 | 3.71 |
| 772 | hsa-miR-630 | 2.51E-06 | 99 | 370 | 1.91 |
| 761 | hsa-miR-623 | 4.11E-06 | 22 | 108 | 2.29 |
| 154 | hsa-miR-1306 | 4.53E-06 | 21 | 289 | 3.77 |
| 767 | hsa-miR-627 | 6.60E-06 | 199 | 40 | −2.33 |
| 322 | hsa-miR-206 | 1.10E-05 | 24 | 270 | 3.48 |
| 372 | hsa-miR-23a* | 1.11E-05 | 57 | 148 | 1.36 |
| 755 | hsa-miR-617 | 4.28E-05 | 27 | 158 | 2.57 |
| 868 | hsa-miR-92a | 5.32E-05 | 436 | 163 | −1.42 |
| 737 | hsa-miR-601 | 6.43E-05 | 229 | 346 | 0.59 |
| 699 | hsa-miR-568 | 1.01E-04 | 73 | 21 | −1.76 |
| 393 | hsa-miR-297 | 3.82E-04 | 13 | 90 | 2.75 |
| 174 | hsa-miR-136 | 4.13E-04 | 6 | 47 | 2.90 |
| 40 | hsa-miR-1180 | 4.97E-04 | 53 | 185 | 1.81 |
| 592 | hsa-miR-510 | 5.08E-04 | 43 | 26 | −0.75 |
| 371 | hsa-miR-23a | 5.11E-04 | 164 | 60 | −1.44 |
| 17 | hsa-let-7i | 5.51E-04 | 102 | 49 | −1.06 |
| 318 | hsa-miR-205* | 5.64E-04 | 9 | 63 | 2.82 |
| 744 | hsa-miR-608 | 5.70E-04 | 22 | 68 | 1.64 |
| 64 | hsa-miR-1226* | 6.37E-04 | 24 | 123 | 2.34 |
| 270 | hsa-miR-1913 | 6.48E-04 | 178 | 123 | −0.53 |
| 247 | hsa-miR-185 | 1.15E-03 | 382 | 33 | −3.55 |
| 73 | hsa-miR-1237 | 1.18E-03 | 167 | 93 | −0.84 |
| 718 | hsa-miR-584 | 1.80E-03 | 51 | 120 | 1.23 |
| 380 | hsa-miR-26a | 1.98E-03 | 56 | 30 | −0.87 |
| 707 | hsa-miR-575 | 2.12E-03 | 154 | 351 | 1.19 |
| 696 | hsa-miR-564 | 2.17E-03 | 29 | 69 | 1.26 |
| 95 | hsa-miR-125a-3p | 2.17E-03 | 37 | 64 | 0.81 |
| 290 | hsa-miR-1972 | 2.32E-03 | 29 | 113 | 1.98 |
| 329 | hsa-miR-21 | 2.36E-03 | 60 | 127 | 1.08 |
| 404 | hsa-miR-300 | 2.76E-03 | 41 | 90 | 1.12 |
| 745 | hsa-miR-609 | 2.77E-03 | 32 | 73 | 1.18 |
| 298 | hsa-miR-198 | 3.77E-03 | 236 | 169 | −0.48 |
| 196 | hsa-miR-146a | 3.77E-03 | 98 | 24 | −2.04 |
| 871 | hsa-miR-92b | 3.96E-03 | 97 | 59 | −0.71 |
| 893 | hsa-miR-99b | 4.62E-03 | 39 | 22 | −0.80 |
| 518 | hsa-miR-422a | 4.66E-03 | 20 | 44 | 1.11 |
| 597 | hsa-miR-513a-5p | 5.08E-03 | 15 | 59 | 1.98 |
| 42 | hsa-miR-1182 | 6.56E-03 | 175 | 382 | 1.13 |
| 540 | hsa-miR-451 | 7.21E-03 | 61 | 39 | −0.65 |
| 8 | hsa-let-7d | 7.25E-03 | 129 | 29 | −2.17 |
| 90 | hsa-miR-1255b | 7.35E-03 | 11 | 27 | 1.32 |
| 705 | hsa-miR-574-3p | 7.40E-03 | 150 | 75 | −1.01 |
| 462 | hsa-miR-342-3p | 7.63E-03 | 38 | 19 | −0.95 |
| 364 | hsa-miR-223 | 8.08E-03 | 70 | 21 | −1.72 |
| 163 | hsa-miR-1321 | 8.32E-03 | 11 | 32 | 1.48 |
| 343 | hsa-miR-214 | 8.40E-03 | 34 | 169 | 2.30 |
| 392 | hsa-miR-296-5p | 8.53E-03 | 143 | 91 | −0.65 |
| 260 | hsa-miR-1908 | 8.68E-03 | 116 | 135 | 0.21 |
| 554 | hsa-miR-486-5p | 8.76E-03 | 229 | 160 | −0.52 |
| 178 | hsa-miR-138-1* | 8.82E-03 | 36 | 68 | 0.94 |
| 127 | hsa-miR-1281 | 9.17E-03 | 186 | 240 | 0.37 |
| 131 | hsa-miR-1285 | 9.64E-03 | 26 | 62 | 1.29 |
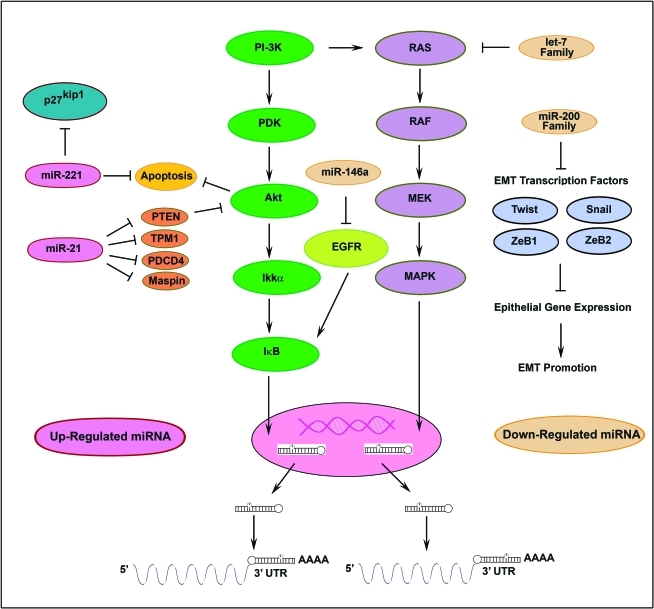
Figure 1.
A schematic presentation of microRNAs in pancreatic cancer (PC). Various families of microRNAs are shown as either up-regulated or down-regulated miRNAs with their corresponding targeted genes.
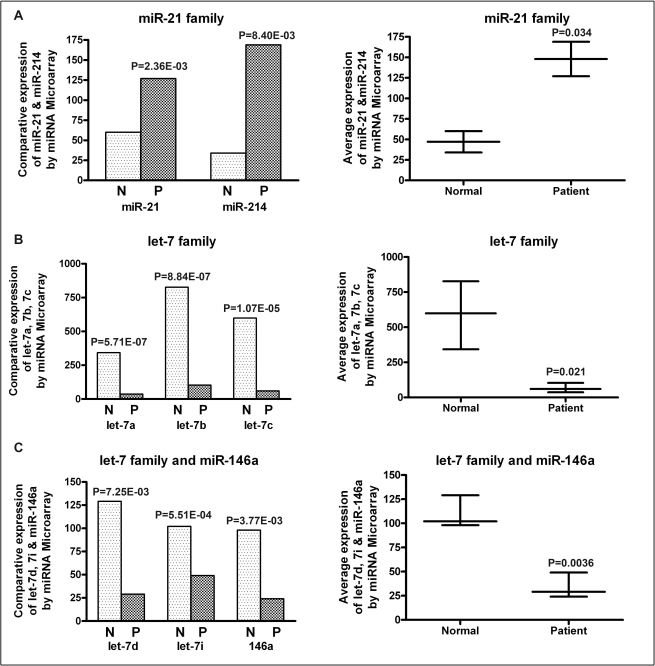
Figure 2.
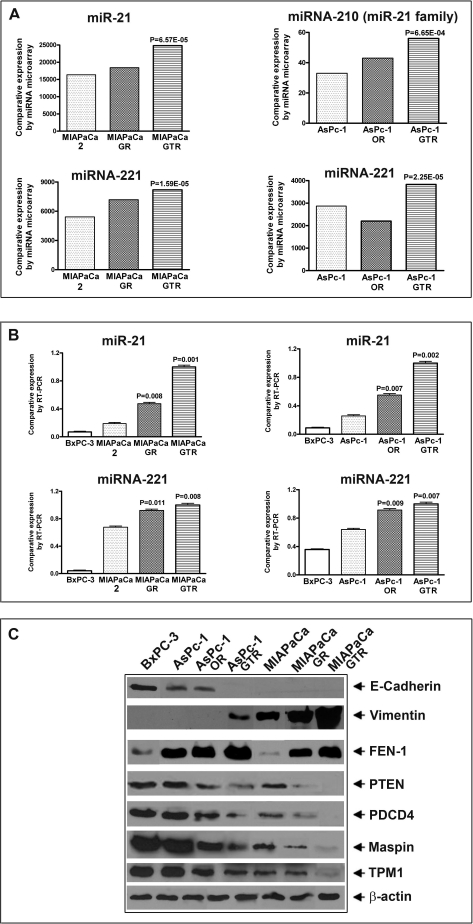
Comparative expression analysis of differentially expressed miRNAs by microarray profiling in plasma from PC patients and healthy controls. (A) The expression of miR-21 and miR-214 (left panel) and the miR-21 family (right panel), (B) The expression of let-7a, let-7b and let-7c (left panel) and the let-7 family (right panel), (C) The expression of let-7d, let-7i, miR-146a (left panel) and combined let-7 family and miR-146a (right panel). There was a significant up-regulation of miR-21 family in PC patients compared to normal subjects. Conversely, let-7 family and miR-146a expression showed a significant down-regulation compared to normal subjects.
Real-time RT-PCR of seven miRNAs of 32 PC patients and 10 normal controls
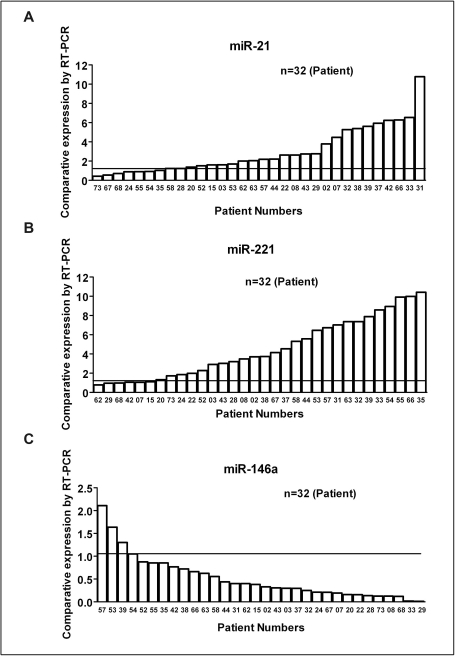
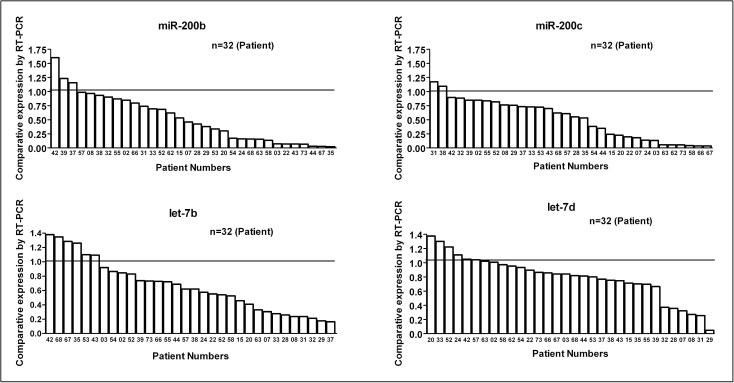
Of the seven miRNAs chosen for further analysis, two were oncogenes (miR-21 and miR-221) and five functioned as tumor suppressors (miR-200b, miR-200c, miR-146a, let-7b, and let-7d). The RT-PCR analysis was blinded to the source of the samples. The samples from control and PC patients were tested in parallel to avoid batch effects. The reproducibility of the RT-PCR assay showed that miRNAs can be efficiently extracted from plasma and could be compared across multiple samples. Compared to miRNA levels from controls, the expression levels of miR-21 and miR-221 were increased to more than 10 folds in some patients (Figure 3A and 3B) whereas the expression levels of tumor suppressor miRNA such as miR-146a were significantly reduced in most PC patients (Figure 3C). Moreover, analysis clearly indicated that the expression levels of miR-200b, miR-200c, let-7b and let-7d were significantly down-regulated in more than 80% of the PC patients compared to normal controls whose expression was set at 1.0 (Figure 4). We subsequently assessed the value of miR-21 and miR-221 in defining tumor aggressiveness using drug-resistant PC cell lines compared to parental cells as shown below.
Figure 3.
Comparative expression analysis of miRNAs (miR-21, miR-221 and miR-146a) in the plasma of 32 PC patients analyzed individually compared to plasma samples obtained from 10 normal subjects by using qRT-PCR. The line drawn at 1.0 represents average of normal subjects (n=10). The results showed a significant increase in the expression of miR-21 and miR-221 (oncogene) over the cutoff of 1.0 by almost all PC patients. In contrast, a significant down-regulation of miR-146a (tumor suppressor gene) was observed in PC patients compared to normal subjects.
Figure 4.
Comparative expression analysis of miRNAs (miR-200b, miR-200c, let-7b and let-7d) in the plasma of 32 PC patients analyzed individually compared to plasma obtained from 10 normal subjects by using qRT-PCR. The line drawn at 1.0 represents average of normal subjects (n=10). The results showed a significant decrease in the expression of miR-200b, miR-200c, let-7b, let-7d (tumor suppressor genes) in almost all PC patients compared to normal subjects.
Expression of miR-21 and miR-221 in resistant cell lines was altered by chronic exposure to conventional anti-cancer drugs
MIAPaCa-2 and AsPc-1 cells were continuously exposed to gemcitabine, oxaliplatin or tarceva for a period of six months to create MIAPaCa-GR, MIAPaCa-GTR, AsPc-1OR, AsPc-1GTR cell lines. Profiling of these cell lines revealed miRNAs that were significantly altered and are presented in Tables 2 and 3. We chose miR-21 and miR-221 and compared their expression with their drug treated cells versus the parental cells. The levels of miR-21 and miR-221 were significantly increased in the drug treated cells (Figure 5A). We further confirmed this finding with real-time qRT-PCR and included the BxPC-3 cell line that is sensitive to conventional drugs. We found higher expression of miR-21 in MIA-PaCa-2, and AsPc-1 cells compared to BxPC-3 cells. Interestingly, when these cells were exposed to chemo-therapeutic drugs described above, highly significant increase in the expression levels of miR-21 was observed in MIAPaCa-GR (2 fold), MIAPaCa-GTR (4 fold), AsPc-1OR (2 fold) and AsPc-1GTR (4 fold). Similarly, the expression of miR-221 in all drug-resistant cell lines was higher compared to drug-sensitive and the parental cell lines; however, the effect was more pronounced in MIAPaCa-GTR and AsPc-1GTR cell lines compared to MIAPaCa-GR and AsPc-1OR (Figure 5B). These results confirmed that drug resistance could be mechanistically linked with increased expression of miR-21 and may be explained on the basis of loss of PTEN, PDCD4, Maspin, and TPM1 gene expression that are known targets of miR-21. To further validate this notion, we extracted total proteins from all the seven PC cell lines and measured the basal level of expression of E-cadherin, vimentin, FEN-1, PTEN, PDCD4, Maspin, and TPM1 (Figure 5C). The level of expression of PTEN, PDCD4, Maspin, and TPM1 was found to be significantly reduced in drug-resistant cell lines. In contrast, the level of FEN-1 and vimentin was significantly up-regulated in drug-resistant cell lines.
Table 2.
Statistical and clustering analysis of miRNA data between the three groups
| Group 1 | Group 2 | Group 3 | |||
|---|---|---|---|---|---|
| MIAPaCa-2 | MIAPaCa-GR | MIAPaCa-GTR | |||
| No. | Reporter Name | p-value | Mean | Mean | Mean |
| 156 | hsa-miR-1308 | 4.32E-10 | 173 | 745 | 569 |
| 852 | hsa-miR-886-5p | 2.82E-09 | 587 | 132 | 90 |
| 293 | hsa-miR-1975 | 1.69E-08 | 1,715 | 5,918 | 4,848 |
| 247 | hsa-miR-185 | 6.34E-08 | 291 | 673 | 674 |
| 273 | hsa-miR-1915 | 8.97E-08 | 173 | 651 | 527 |
| 868 | hsa-miR-92a | 9.62E-08 | 3,823 | 1,596 | 1,754 |
| 100 | hsa-miR-126 | 1.10E-07 | 534 | 971 | 902 |
| 157 | hsa-miR-130a | 1.12E-07 | 351 | 802 | 634 |
| 432 | hsa-miR-320a | 1.12E-07 | 3,847 | 2,072 | 2,084 |
| 119 | hsa-miR-1275 | 1.16E-07 | 666 | 188 | 291 |
| 817 | hsa-miR-7 | 1.38E-07 | 1,610 | 565 | 423 |
| 824 | hsa-miR-720 | 1.73E-07 | 1,134 | 364 | 370 |
| 433 | hsa-miR-320b | 1.95E-07 | 4,053 | 2,110 | 2,051 |
| 434 | hsa-miR-320c | 2.27E-07 | 4,185 | 2,193 | 2,141 |
| 435 | hsa-miR-320d | 4.31E-07 | 3,040 | 1,599 | 1,624 |
| 126 | hsa-miR-1280 | 5.48E-07 | 1,175 | 478 | 668 |
| 355 | hsa-miR-22 | 5.80E-07 | 236 | 495 | 482 |
| 223 | hsa-miR-15a | 6.85E-07 | 759 | 1,399 | 1,806 |
| 417 | hsa-miR-30a | 7.29E-07 | 912 | 2,288 | 2,541 |
| 780 | hsa-miR-638 | 9.17E-07 | 489 | 853 | 881 |
| 473 | hsa-miR-361-5p | 1.01E-06 | 807 | 1,524 | 1,608 |
| 426 | hsa-miR-30e | 1.42E-06 | 420 | 896 | 1,169 |
| 424 | hsa-miR-30d | 1.76E-06 | 552 | 1,210 | 1,306 |
| 80 | hsa-miR-1246 | 1.86E-06 | 3,605 | 8,071 | 7,683 |
| 871 | hsa-miR-92b | 2.02E-06 | 1,397 | 637 | 720 |
| 327 | hsa-miR-20b | 2.88E-06 | 2,375 | 1,239 | 1,247 |
| 521 | hsa-miR-424 | 3.83E-06 | 250 | 540 | 667 |
| 385 | hsa-miR-27a | 4.38E-06 | 1,538 | 2,819 | 3,093 |
| 292 | hsa-miR-1974 | 6.60E-06 | 15,945 | 9,498 | 8,684 |
| 297 | hsa-miR-1979 | 6.90E-06 | 6,301 | 5,431 | 3,752 |
| 419 | hsa-miR-30b | 8.50E-06 | 453 | 854 | 903 |
| 213 | hsa-miR-151-5p | 1.00E-05 | 883 | 1,432 | 1,313 |
| 230 | hsa-miR-17 | 1.04E-05 | 3,486 | 2,408 | 1,865 |
| 29 | hsa-miR-106a | 1.11E-05 | 3,465 | 2,424 | 1,880 |
| 360 | hsa-miR-221 | 1.59E-05 | 5,421 | 7,191 | 8,200 |
| 325 | hsa-miR-20a | 2.04E-05 | 3,911 | 2,789 | 2,245 |
| 34 | hsa-miR-10a | 2.32E-05 | 480 | 279 | 200 |
| 227 | hsa-miR-16 | 2.43E-05 | 8,590 | 13,443 | 11,781 |
| 380 | hsa-miR-26a | 3.02E-05 | 2,496 | 3,740 | 3,922 |
| 10 | hsa-let-7e | 4.98E-05 | 2,287 | 1,338 | 2,377 |
| 329 | hsa-miR-21 | 6.57E-05 | 16,363 | 18,437 | 24,832 |
| 387 | hsa-miR-27b | 8.97E-05 | 565 | 950 | 1,069 |
| 523 | hsa-miR-425 | 1.06E-04 | 335 | 674 | 613 |
| 890 | hsa-miR-98 | 1.21E-04 | 492 | 436 | 635 |
| 283 | hsa-miR-195 | 1.85E-04 | 221 | 416 | 408 |
| 399 | hsa-miR-29b | 2.39E-04 | 373 | 569 | 662 |
| 478 | hsa-miR-365 | 2.91E-04 | 466 | 164 | 147 |
| 31 | hsa-miR-106b | 7.19E-04 | 1,724 | 2,563 | 2,211 |
| 6 | hsa-let-7c | 8.52E-04 | 4,155 | 3,708 | 4,967 |
| 893 | hsa-miR-99b | 1.14E-03 | 730 | 677 | 550 |
| 33 | hsa-miR-107 | 1.23E-03 | 1,931 | 2,816 | 2,544 |
| 492 | hsa-miR-374b | 1.27E-03 | 586 | 645 | 841 |
| 4 | hsa-let-7b | 1.83E-03 | 2,002 | 2,375 | 2,684 |
| 24 | hsa-miR-103 | 1.90E-03 | 2,130 | 3,091 | 2,599 |
| 397 | hsa-miR-29a | 2.11E-03 | 7,701 | 11,078 | 9,797 |
| 718 | hsa-miR-584 | 2.21E-03 | 895 | 655 | 617 |
| 242 | hsa-miR-1826 | 2.28E-03 | 19,150 | 20,015 | 15,659 |
| 17 | hsa-let-7i | 2.72E-03 | 9,341 | 12,161 | 11,956 |
| 418 | hsa-miR-30a* | 2.89E-03 | 578 | 739 | 853 |
| 244 | hsa-miR-183 | 4.36E-03 | 573 | 574 | 456 |
| 225 | hsa-miR-15b | 4.40E-03 | 4,731 | 3,494 | 3,966 |
| 125 | hsa-miR-128 | 4.99E-03 | 558 | 671 | 748 |
| 239 | hsa-miR-182 | 5.19E-03 | 1,049 | 1,367 | 1,142 |
| 264 | hsa-miR-191 | 7.20E-03 | 1,812 | 2,120 | 2,091 |
| 421 | hsa-miR-30c | 7.23E-03 | 1,699 | 2,165 | 2,428 |
| 873 | hsa-miR-93 | 7.92E-03 | 2,239 | 2,187 | 1,772 |
| 402 | hsa-miR-29c | 7.98E-03 | 544 | 661 | 776 |
| 362 | hsa-miR-222 | 8.28E-03 | 5,701 | 5,760 | 6,566 |
| 249 | hsa-miR-186 | 8.85E-03 | 429 | 493 | 382 |
| Following transcripts are statistically significant but have low signals (signal < 500) | |||||
| 232 | hsa-miR-181a | 6.03E-09 | 66 | 289 | 177 |
| 204 | hsa-miR-148a | 1.92E-08 | 33 | 215 | 211 |
| 741 | hsa-miR-605 | 5.62E-08 | 8 | 219 | 34 |
| 255 | hsa-miR-18a | 2.63E-07 | 441 | 281 | 189 |
| 161 | hsa-miR-132 | 1.27E-06 | 49 | 90 | 100 |
| 464 | hsa-miR-345 | 1.78E-06 | 51 | 137 | 150 |
| 36 | hsa-miR-10b | 2.03E-06 | 264 | 106 | 81 |
| 127 | hsa-miR-1281 | 2.14E-06 | 20 | 86 | 77 |
| 405 | hsa-miR-301a | 2.50E-06 | 100 | 209 | 95 |
| 475 | hsa-miR-362-5p | 2.92E-06 | 103 | 185 | 172 |
| 770 | hsa-miR-629 | 3.58E-06 | 58 | 109 | 107 |
| 199 | hsa-miR-146b-5p | 4.32E-06 | 28 | 76 | 73 |
| 285 | hsa-miR-196a | 4.57E-06 | 42 | 20 | 9 |
| 275 | hsa-miR-192 | 4.94E-06 | 122 | 319 | 232 |
| 214 | hsa-miR-152 | 5.89E-06 | 177 | 327 | 284 |
| 647 | hsa-miR-532-5p | 6.80E-06 | 186 | 389 | 381 |
| 803 | hsa-miR-660 | 9.13E-06 | 153 | 310 | 330 |
| 22 | hsa-miR-101 | 1.02E-05 | 125 | 272 | 272 |
| 110 | hsa-miR-1268 | 1.12E-05 | 191 | 89 | 86 |
| 159 | hsa-miR-130b | 1.29E-05 | 203 | 368 | 283 |
| 195 | hsa-miR-1469 | 1.80E-05 | 88 | 197 | 183 |
| 707 | hsa-miR-575 | 1.82E-05 | 26 | 64 | 51 |
| 137 | hsa-miR-1290 | 1.85E-05 | 30 | 103 | 95 |
| 737 | hsa-miR-601 | 1.92E-05 | 47 | 29 | 21 |
| 851 | hsa-miR-886-3p | 2.01E-05 | 231 | 99 | 77 |
| 257 | hsa-miR-18b | 2.08E-05 | 171 | 126 | 87 |
| 281 | hsa-miR-194 | 2.32E-05 | 105 | 225 | 216 |
| 176 | hsa-miR-137 | 2.54E-05 | 93 | 43 | 43 |
| 814 | hsa-miR-671-5p | 5.15E-05 | 186 | 104 | 88 |
| 439 | hsa-miR-324-5p | 5.50E-05 | 72 | 139 | 135 |
| 356 | hsa-miR-22* | 5.93E-05 | 90 | 183 | 213 |
| 117 | hsa-miR-1274a | 6.22E-05 | 91 | 62 | 61 |
| 291 | hsa-miR-1973 | 6.79E-05 | 33 | 102 | 78 |
| 196 | hsa-miR-146a | 8.06E-05 | 11 | 35 | 11 |
| 9 | hsa-let-7d* | 8.26E-05 | 312 | 167 | 180 |
| 576 | hsa-miR-500* | 8.41E-05 | 87 | 126 | 130 |
| 518 | hsa-miR-422a | 9.15E-05 | 106 | 48 | 71 |
| 474 | hsa-miR-362-3p | 9.80E-05 | 32 | 62 | 68 |
| 764 | hsa-miR-625 | 1.19E-04 | 14 | 24 | 35 |
| 546 | hsa-miR-455-3p | 1.45E-04 | 90 | 189 | 140 |
| 472 | hsa-miR-361-3p | 1.52E-04 | 26 | 47 | 47 |
| 829 | hsa-miR-760 | 1.53E-04 | 107 | 67 | 41 |
| 26 | hsa-miR-103-as | 1.61E-04 | 44 | 73 | 76 |
| 847 | hsa-miR-877 | 1.86E-04 | 109 | 51 | 50 |
| 554 | hsa-miR-486-5p | 1.89E-04 | 235 | 114 | 177 |
| 771 | hsa-miR-629* | 3.80E-04 | 24 | 52 | 47 |
| 553 | hsa-miR-486-3p | 4.53E-04 | 97 | 57 | 109 |
| 831 | hsa-miR-762 | 4.92E-04 | 180 | 271 | 339 |
| 149 | hsa-miR-1301 | 5.00E-04 | 60 | 114 | 81 |
| 778 | hsa-miR-636 | 5.15E-04 | 20 | 17 | 35 |
| 537 | hsa-miR-450a | 5.94E-04 | 44 | 63 | 99 |
| 236 | hsa-miR-181c | 6.10E-04 | 24 | 55 | 37 |
| 882 | hsa-miR-940 | 6.14E-04 | 105 | 112 | 181 |
| 879 | hsa-miR-937 | 6.24E-04 | 19 | 30 | 34 |
| 289 | hsa-miR-197 | 6.38E-04 | 149 | 92 | 100 |
| 254 | hsa-miR-188-5p | 7.43E-04 | 69 | 34 | 35 |
| 182 | hsa-miR-140-3p | 7.62E-04 | 124 | 197 | 171 |
| 834 | hsa-miR-766 | 7.77E-04 | 20 | 45 | 51 |
| 83 | hsa-miR-1249 | 7.99E-04 | 117 | 44 | 131 |
| 878 | hsa-miR-936 | 8.40E-04 | 36 | 13 | 20 |
| 724 | hsa-miR-589* | 8.64E-04 | 9 | 27 | 23 |
| 511 | hsa-miR-409-3p | 1.03E-03 | 13 | 37 | 25 |
| 470 | hsa-miR-34c-3p | 1.03E-03 | 16 | 42 | 35 |
| 646 | hsa-miR-532-3p | 1.12E-03 | 139 | 183 | 256 |
| 583 | hsa-miR-505 | 1.17E-03 | 53 | 71 | 88 |
| 706 | hsa-miR-574-5p | 1.28E-03 | 354 | 236 | 211 |
| 575 | hsa-miR-500 | 1.30E-03 | 75 | 94 | 108 |
| 806 | hsa-miR-663 | 1.40E-03 | 98 | 139 | 209 |
| 183 | hsa-miR-140-5p | 1.57E-03 | 38 | 52 | 60 |
| 160 | hsa-miR-130b* | 1.76E-03 | 85 | 58 | 69 |
| 677 | hsa-miR-548q | 2.00E-03 | 27 | 43 | 34 |
| 73 | hsa-miR-1237 | 2.53E-03 | 61 | 37 | 90 |
| 888 | hsa-miR-96 | 2.82E-03 | 66 | 90 | 97 |
| 209 | hsa-miR-149* | 2.91E-03 | 99 | 139 | 166 |
| 794 | hsa-miR-652 | 3.29E-03 | 46 | 64 | 52 |
| 490 | hsa-miR-374a | 3.40E-03 | 449 | 291 | 349 |
| 88 | hsa-miR-1254 | 3.52E-03 | 66 | 14 | 15 |
| 877 | hsa-miR-935 | 3.76E-03 | 31 | 17 | 27 |
| 172 | hsa-miR-135b | 4.12E-03 | 87 | 47 | 54 |
| 881 | hsa-miR-939 | 4.15E-03 | 43 | 24 | 26 |
| 567 | hsa-miR-494 | 4.67E-03 | 20 | 38 | 51 |
| 579 | hsa-miR-502-3p | 4.91E-03 | 79 | 98 | 119 |
| 48 | hsa-miR-1201 | 4.92E-03 | 32 | 30 | 17 |
| 74 | hsa-miR-1238 | 5.15E-03 | 40 | 28 | 68 |
| 446 | hsa-miR-331-3p | 5.30E-03 | 81 | 136 | 135 |
| 822 | hsa-miR-718 | 5.52E-03 | 29 | 36 | 59 |
| 449 | hsa-miR-335* | 5.55E-03 | 9 | 32 | 14 |
| 238 | hsa-miR-181d | 5.97E-03 | 78 | 90 | 60 |
| 651 | hsa-miR-542-3p | 6.70E-03 | 31 | 50 | 71 |
| 208 | hsa-miR-149 | 6.86E-03 | 44 | 55 | 89 |
| 206 | hsa-miR-148b | 7.01E-03 | 294 | 377 | 380 |
| 270 | hsa-miR-1913 | 7.25E-03 | 65 | 44 | 130 |
| 524 | hsa-miR-425* | 7.49E-03 | 19 | 31 | 35 |
| 496 | hsa-miR-376a* | 7.57E-03 | 4 | 22 | 0 |
| 887 | hsa-miR-95 | 7.61E-03 | 12 | 17 | 30 |
| 245 | hsa-miR-183* | 7.69E-03 | 55 | 47 | 34 |
| 699 | hsa-miR-568 | 7.90E-03 | 19 | 21 | 10 |
| 16 | hsa-let-7g* | 7.92E-03 | 26 | 13 | 20 |
| 372 | hsa-miR-23a* | 9.40E-03 | 38 | 22 | 23 |
| 377 | hsa-miR-24-2* | 9.41E-03 | 51 | 71 | 75 |
Table 3.
Statistical and clustering analysis of miRNAdata between the three groups
| Group 1 | Group 2 | Group 3 | |||
|---|---|---|---|---|---|
| AsPC-1 | AsPC-1-OR | AsPC-1-GTR | |||
| No. | Reporter Name | p-value | Mean | Mean | Mean |
| 196 | hsa-miR-146a | 5.42E-14 | 50 | 803 | 1,163 |
| 156 | hsa-miR-1308 | 3.23E-09 | 541 | 166 | 996 |
| 232 | hsa-miR-181a | 7.36E-09 | 581 | 260 | 853 |
| 293 | hsa-miR-1975 | 1.30E-08 | 1,209 | 1,148 | 2,965 |
| 311 | hsa-miR-200c | 6.91E-08 | 488 | 778 | 1,486 |
| 17 | hsa-let-7i | 1.69E-07 | 6,313 | 2,572 | 4,862 |
| 295 | hsa-miR-1977 | 2.43E-07 | 494 | 513 | 1,125 |
| 273 | hsa-miR-1915 | 2.75E-07 | 396 | 360 | 880 |
| 385 | hsa-miR-27a | 4.55E-07 | 2,159 | 2,484 | 3,886 |
| 399 | hsa-miR-29b | 5.84E-07 | 395 | 319 | 695 |
| 285 | hsa-miR-196a | 8.73E-07 | 465 | 533 | 323 |
| 29 | hsa-miR-106a | 9.65E-07 | 5,619 | 3,240 | 2,793 |
| 891 | hsa-miR-99a | 1.05E-06 | 472 | 800 | 417 |
| 435 | hsa-miR-320d | 1.21E-06 | 1,891 | 1,185 | 1,105 |
| 817 | hsa-miR-7 | 1.27E-06 | 1,792 | 1,619 | 703 |
| 297 | hsa-miR-1979 | 1.63E-06 | 2,708 | 1,505 | 2,623 |
| 868 | hsa-miR-92a | 1.71E-06 | 4,903 | 3,497 | 2,902 |
| 80 | hsa-miR-1246 | 1.77E-06 | 493 | 1,264 | 1,504 |
| 824 | hsa-miR-720 | 1.79E-06 | 625 | 1,023 | 424 |
| 230 | hsa-miR-17 | 1.86E-06 | 5,860 | 3,254 | 2,916 |
| 718 | hsa-miR-584 | 2.02E-06 | 550 | 446 | 227 |
| 871 | hsa-miR-92b | 2.32E-06 | 2,070 | 1,450 | 1,197 |
| 433 | hsa-miR-320b | 3.09E-06 | 2,516 | 1,632 | 1,408 |
| 10 | hsa-let-7e | 3.94E-06 | 3,747 | 4,542 | 2,438 |
| 434 | hsa-miR-320c | 4.03E-06 | 2,578 | 1,697 | 1,445 |
| 387 | hsa-miR-27b | 4.85E-06 | 1,553 | 1,521 | 2,065 |
| 20 | hsa-miR-100 | 5.33E-06 | 1,269 | 2,118 | 1,270 |
| 893 | hsa-miR-99b | 5.83E-06 | 715 | 732 | 410 |
| 235 | hsa-miR-181b | 6.36E-06 | 906 | 516 | 663 |
| 292 | hsa-miR-1974 | 7.10E-06 | 3,357 | 5,102 | 5,715 |
| 426 | hsa-miR-30e | 7.56E-06 | 389 | 318 | 778 |
| 325 | hsa-miR-20a | 8.05E-06 | 6,502 | 4,076 | 3,463 |
| 242 | hsa-miR-1826 | 8.17E-06 | 11,001 | 9,759 | 15,410 |
| 780 | hsa-miR-638 | 1.17E-05 | 1,219 | 1,192 | 2,307 |
| 304 | hsa-miR-19b | 1.60E-05 | 2,040 | 1,031 | 1,023 |
| 397 | hsa-miR-29a | 1.91E-05 | 8,567 | 9,222 | 14,033 |
| 383 | hsa-miR-26b | 1.96E-05 | 2,074 | 2,817 | 1,573 |
| 97 | hsa-miR-125b | 2.13E-05 | 1,382 | 2,274 | 1,816 |
| 360 | hsa-miR-221 | 2.25E-05 | 2,869 | 2,202 | 3,833 |
| 706 | hsa-miR-574-5p | 2.33E-05 | 190 | 454 | 159 |
| 432 | hsa-miR-320a | 2.73E-05 | 2,292 | 1,598 | 1,350 |
| 366 | hsa-miR-224 | 2.93E-05 | 1,379 | 1,578 | 997 |
| 327 | hsa-miR-20b | 3.15E-05 | 4,047 | 2,600 | 1,967 |
| 225 | hsa-miR-15b | 3.27E-05 | 1,666 | 2,408 | 1,390 |
| 873 | hsa-miR-93 | 3.71E-05 | 1,096 | 784 | 889 |
| 307 | hsa-miR-200a | 3.75E-05 | 502 | 334 | 694 |
| 302 | hsa-miR-19a | 6.86E-05 | 573 | 321 | 328 |
| 424 | hsa-miR-30d | 7.13E-05 | 763 | 692 | 1,263 |
| 1 | hsa-let-7a | 1.23E-04 | 12,377 | 16,225 | 11,303 |
| 4 | hsa-let-7b | 2.08E-04 | 5,034 | 6,342 | 3,822 |
| 281 | hsa-miR-194 | 2.58E-04 | 4,993 | 6,724 | 8,032 |
| 264 | hsa-miR-191 | 3.31E-04 | 1,079 | 1,537 | 1,425 |
| 473 | hsa-miR-361-5p | 5.53E-04 | 420 | 482 | 363 |
| 419 | hsa-miR-30b | 5.61E-04 | 1,304 | 2,092 | 2,015 |
| 8 | hsa-let-7d | 5.88E-04 | 7,252 | 9,036 | 6,765 |
| 421 | hsa-miR-30c | 8.23E-04 | 1,100 | 1,416 | 1,613 |
| 96 | hsa-miR-125a-5p | 1.83E-03 | 2,796 | 2,369 | 2,049 |
| 6 | hsa-let-7c | 2.14E-03 | 9,427 | 11,381 | 8,282 |
| 126 | hsa-miR-1280 | 2.61E-03 | 1,413 | 1,554 | 1,039 |
| 239 | hsa-miR-182 | 2.86E-03 | 987 | 1,303 | 1,076 |
| 223 | hsa-miR-15a | 4.42E-03 | 847 | 691 | 857 |
| 31 | hsa-miR-106b | 4.74E-03 | 867 | 726 | 936 |
| 212 | hsa-miR-151-3p | 6.59E-03 | 776 | 653 | 595 |
| Following transcripts are statistically significant but have low signals (signal < 500) | |||||
| 199 | hsa-miR-146b-5p | 2.61E-10 | 34 | 118 | 146 |
| 221 | hsa-miR-155 | 4.46E-09 | 15 | 63 | 54 |
| 184 | hsa-miR-141 | 1.16E-08 | 39 | 45 | 276 |
| 119 | hsa-miR-1275 | 2.63E-08 | 206 | 385 | 115 |
| 195 | hsa-miR-1469 | 3.37E-08 | 189 | 404 | 228 |
| 478 | hsa-miR-365 | 6.99E-08 | 346 | 91 | 117 |
| 761 | hsa-miR-623 | 7.19E-08 | 19 | 59 | 16 |
| 888 | hsa-miR-96 | 1.10E-07 | 70 | 74 | 143 |
| 22 | hsa-miR-101 | 1.63E-07 | 89 | 57 | 139 |
| 296 | hsa-miR-1978 | 1.69E-07 | 235 | 178 | 387 |
| 255 | hsa-miR-18a | 2.22E-07 | 265 | 172 | 94 |
| 546 | hsa-miR-455-3p | 2.22E-07 | 146 | 263 | 91 |
| 490 | hsa-miR-374a | 2.66E-07 | 338 | 427 | 167 |
| 544 | hsa-miR-454 | 4.63E-07 | 397 | 447 | 223 |
| 472 | hsa-miR-361-3p | 6.88E-07 | 35 | 20 | 53 |
| 278 | hsa-miR-193a-5p | 7.21E-07 | 250 | 129 | 83 |
| 460 | hsa-miR-340 | 1.06E-06 | 68 | 33 | 43 |
| 677 | hsa-miR-548q | 1.11E-06 | 52 | 91 | 94 |
| 214 | hsa-miR-152 | 1.45E-06 | 93 | 35 | 53 |
| 741 | hsa-miR-605 | 1.65E-06 | 104 | 10 | 22 |
| 209 | hsa-miR-149* | 1.68E-06 | 155 | 162 | 351 |
| 567 | hsa-miR-494 | 1.75E-06 | 33 | 18 | 106 |
| 102 | hsa-miR-1260 | 1.89E-06 | 107 | 105 | 46 |
| 254 | hsa-miR-188-5p | 1.96E-06 | 43 | 87 | 27 |
| 521 | hsa-miR-424 | 2.08E-06 | 189 | 189 | 357 |
| 832 | hsa-miR-764 | 2.35E-06 | 47 | 12 | 17 |
| 707 | hsa-miR-575 | 2.39E-06 | 57 | 55 | 172 |
| 814 | hsa-miR-671-5p | 4.28E-06 | 113 | 237 | 88 |
| 890 | hsa-miR-98 | 4.67E-06 | 270 | 334 | 161 |
| 492 | hsa-miR-374b | 5.03E-06 | 302 | 404 | 235 |
| 349 | hsa-miR-218 | 5.09E-06 | 5 | 43 | 69 |
| 260 | hsa-miR-1908 | 6.42E-06 | 47 | 32 | 111 |
| 160 | hsa-miR-130b* | 6.67E-06 | 59 | 38 | 23 |
| 110 | hsa-miR-1268 | 6.72E-06 | 108 | 180 | 79 |
| 257 | hsa-miR-18b | 7.32E-06 | 105 | 72 | 54 |
| 852 | hsa-miR-886-5p | 7.70E-06 | 91 | 98 | 185 |
| 696 | hsa-miR-564 | 9.49E-06 | 16 | 49 | 22 |
| 572 | hsa-miR-498 | 1.01E-05 | 31 | 76 | 27 |
| 236 | hsa-miR-181c | 1.18E-05 | 172 | 47 | 282 |
| 211 | hsa-miR-150* | 1.25E-05 | 42 | 81 | 43 |
| 249 | hsa-miR-186 | 1.30E-05 | 195 | 103 | 127 |
| 392 | hsa-miR-296-5p | 1.44E-05 | 41 | 81 | 37 |
| 100 | hsa-miR-126 | 2.04E-05 | 325 | 203 | 187 |
| 764 | hsa-miR-625 | 2.35E-05 | 49 | 54 | 93 |
| 784 | hsa-miR-642 | 2.90E-05 | 98 | 34 | 53 |
| 523 | hsa-miR-425 | 3.77E-05 | 162 | 191 | 295 |
| 287 | hsa-miR-196b | 4.20E-05 | 93 | 46 | 68 |
| 486 | hsa-miR-371-5p | 4.64E-05 | 28 | 53 | 28 |
| 244 | hsa-miR-183 | 4.94E-05 | 211 | 313 | 195 |
| 56 | hsa-miR-1208 | 5.11E-05 | 27 | 62 | 39 |
| 716 | hsa-miR-582-5p | 6.62E-05 | 147 | 106 | 81 |
| 118 | hsa-miR-1274b | 7.89E-05 | 57 | 86 | 82 |
| 517 | hsa-miR-421 | 8.12E-05 | 37 | 58 | 23 |
| 501 | hsa-miR-378 | 9.22E-05 | 203 | 114 | 208 |
| 418 | hsa-miR-30a* | 1.34E-04 | 210 | 348 | 189 |
| 238 | hsa-miR-181d | 1.64E-04 | 368 | 228 | 254 |
| 276 | hsa-miR-192* | 1.77E-04 | 53 | 57 | 93 |
| 881 | hsa-miR-939 | 1.90E-04 | 50 | 78 | 41 |
| 60 | hsa-miR-1224-5p | 1.97E-04 | 40 | 77 | 45 |
| 446 | hsa-miR-331-3p | 1.99E-04 | 97 | 64 | 120 |
| 806 | hsa-miR-663 | 3.08E-04 | 186 | 135 | 260 |
| 518 | hsa-miR-422a | 3.20E-04 | 134 | 72 | 147 |
| 554 | hsa-miR-486-5p | 3.49E-04 | 184 | 138 | 105 |
| 289 | hsa-miR-197 | 4.43E-04 | 72 | 43 | 42 |
| 125 | hsa-miR-128 | 4.75E-04 | 206 | 174 | 129 |
| 820 | hsa-miR-7-1* | 6.23E-04 | 117 | 118 | 74 |
| 388 | hsa-miR-27b* | 6.32E-04 | 48 | 41 | 29 |
| 169 | hsa-miR-134 | 6.43E-04 | 19 | 42 | 14 |
| 14 | hsa-let-7f-2* | 6.53E-04 | 35 | 19 | 14 |
| 331 | hsa-miR-210 | 6.65E-04 | 33 | 43 | 56 |
| 151 | hsa-miR-1303 | 6.83E-04 | 35 | 37 | 19 |
| 502 | hsa-miR-378* | 7.01E-04 | 22 | 16 | 28 |
| 869 | hsa-miR-92a-1* | 8.47E-04 | 48 | 32 | 23 |
| 475 | hsa-miR-362-5p | 8.99E-04 | 36 | 24 | 39 |
| 520 | hsa-miR-423-5p | 9.19E-04 | 249 | 319 | 185 |
| 182 | hsa-miR-140-3p | 9.31E-04 | 151 | 200 | 238 |
| 400 | hsa-miR-29b-1* | 9.53E-04 | 114 | 141 | 89 |
| 149 | hsa-miR-1301 | 9.99E-04 | 39 | 25 | 23 |
| 688 | hsa-miR-556-3p | 1.19E-03 | 40 | 45 | 28 |
| 35 | hsa-miR-10a* | 1.32E-03 | 138 | 97 | 144 |
| 579 | hsa-miR-502-3p | 1.38E-03 | 28 | 14 | 35 |
| 280 | hsa-miR-193b* | 1.54E-03 | 71 | 48 | 25 |
| 703 | hsa-miR-572 | 1.54E-03 | 31 | 44 | 54 |
| 66 | hsa-miR-1228 | 1.62E-03 | 63 | 32 | 36 |
| 2 | hsa-let-7a* | 1.64E-03 | 42 | 28 | 23 |
| 9 | hsa-let-7d* | 1.72E-03 | 82 | 94 | 59 |
| 429 | hsa-miR-31* | 1.79E-03 | 37 | 52 | 47 |
| 83 | hsa-miR-1249 | 1.84E-03 | 70 | 122 | 70 |
| 771 | hsa-miR-629* | 1.92E-03 | 51 | 29 | 31 |
| 803 | hsa-miR-660 | 1.93E-03 | 54 | 34 | 54 |
| 831 | hsa-miR-762 | 2.01E-03 | 207 | 273 | 297 |
| 851 | hsa-miR-886-3p | 2.22E-03 | 37 | 39 | 59 |
| 427 | hsa-miR-30e* | 2.27E-03 | 233 | 288 | 179 |
| 206 | hsa-miR-148b | 2.55E-03 | 213 | 134 | 175 |
| 157 | hsa-miR-130a | 2.76E-03 | 38 | 27 | 22 |
| 69 | hsa-miR-1231 | 2.92E-03 | 37 | 37 | 70 |
| 511 | hsa-miR-409-3p | 3.35E-03 | 32 | 15 | 18 |
| 277 | hsa-miR-193a-3p | 3.40E-03 | 27 | 11 | 22 |
| 797 | hsa-miR-654-5p | 3.72E-03 | 43 | 18 | 53 |
| 322 | hsa-miR-206 | 4.31E-03 | 10 | 34 | 9 |
| 291 | hsa-miR-1973 | 4.57E-03 | 12 | 8 | 28 |
| 270 | hsa-miR-1913 | 4.68E-03 | 61 | 95 | 53 |
| 5 | hsa-let-7b* | 4.96E-03 | 67 | 53 | 46 |
| 576 | hsa-miR-500* | 5.37E-03 | 29 | 21 | 36 |
| 172 | hsa-miR-135b | 5.43E-03 | 208 | 194 | 275 |
| 553 | hsa-miR-486-3p | 5.58E-03 | 77 | 84 | 56 |
| 117 | hsa-miR-1274a | 6.19E-03 | 29 | 29 | 48 |
| 882 | hsa-miR-940 | 6.27E-03 | 95 | 151 | 93 |
| 448 | hsa-miR-335 | 6.28E-03 | 97 | 99 | 133 |
| 229 | hsa-miR-16-2* | 6.46E-03 | 118 | 105 | 137 |
| 449 | hsa-miR-335* | 6.54E-03 | 72 | 51 | 45 |
| 183 | hsa-miR-140-5p | 6.61E-03 | 53 | 39 | 68 |
| 161 | hsa-miR-132 | 7.00E-03 | 32 | 44 | 49 |
| 847 | hsa-miR-877 | 7.23E-03 | 35 | 47 | 34 |
| 541 | hsa-miR-452 | 7.56E-03 | 182 | 173 | 134 |
| 389 | hsa-miR-28-3p | 7.84E-03 | 101 | 85 | 85 |
| 241 | hsa-miR-1825 | 8.22E-03 | 44 | 22 | 27 |
| 550 | hsa-miR-484 | 8.70E-03 | 91 | 58 | 64 |
| 368 | hsa-miR-2276 | 9.29E-03 | 14 | 29 | 15 |
| 40 | hsa-miR-1180 | 9.34E-03 | 41 | 37 | 24 |
Figure 5.
Comparative expression analysis of (A) The expression of miR-21 family and miR-221 in MIAPaCa-2, MIAPaCa-GR, MIAPaCa-GTR, AsPc-1, AsPc-1OR, AsPc-1GTR as determined by miRNA microarray profiling; (B) Comparative expression analysis of miR-21 and miR-221 in BxPC-3, MIAPaCa-2, MIAPaCa-GR, MIAPaCa-GTR, Aspc-1, AsPc-1OR, AsPc-1GTR cells by qRT-PCR. There was a significant up-regulation of both miR-21 and miR-221 by both the methods of microarray profiling and qRT-PCR methods. P values were calculated by the paired t-test (C) Western blots analysis showing the basal level expression of several proteins some of which are targets of miR-21 (PTEN, PDCD4, Maspin and TPM1).
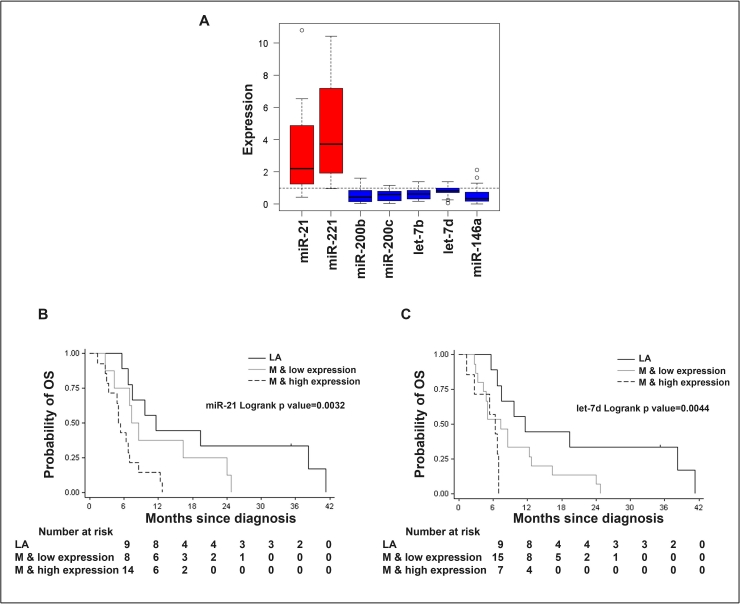
Correlation between miR-21 and let-7d expression and the survival of PC patients
The expression levels of all seven miRNAs are presented as box plot in Figure 6A. The miR-21 and let-7d plasma expression levels in patients with PC were associated with overall survival (Figure 6B and 6C). These results suggest that higher expression of miR-21 could serve as a biomarker for worse survival of PC patients, and thus could serves as an important prognostic marker. In contrast, the overall low level of let-7d expression could serve as an independent prognostic marker for PC patients. While the survival rate of PC patients with low expression levels of miR-221 trended to be longer than patients with higher levels, the difference was not statistically significant. Similarly, low expression levels of let-7b, miR-146a, miR-200b, and miR-200c were not statistically significant when compared between PC to healthy controls. This would strengthen the argument that miR-21 would be a useful plasma marker for predicting tumor aggressiveness and overall survival in patients with PC.
Figure 6.
(A) Box plot representing the expression of seven miRNAs as assessed by qRT-PCR. (B). The Kaplan-Meier curves and log-rank tests for miR-21 expression and survival; (C) The Kaplan-Meier curves and log-rank tests for let-7d expression and survival.
Discussion
Although, many miRNAs are expressed in tissues and tumor cells, their development as biomarkers requires tissue collection by invasive methods as opposed to the more convenient approach of studying peripheral blood. A high correlation of miRNA expression between tumor tissue and matching plasma was demonstrated in patients with breast cancer [23]; however, such studies are lacking in PC. Since early diagnosis may hold the key for our success in improving the outcome of patients with PC the identification and validation of miRNA as potential biomarkers for early detection and predicting tumor aggressiveness may have a major impact on design of future strategies in the diagnosis and treatment of PC. Emerging evidence clearly support the importance of circulating miRNAs in many tumor types including breast, prostate, colorectal, lung, ovarian, and pancreatic cancer [24,9,25,26,10]. Studies have shown that miR-21 is up-regulated in most of these cancers [13,27,28,29,30,31,32,33,34,35,15,36] support the concept that discovery of other miRNAs from the plasma of PC patients would be useful for earlier diagnosis and, predicting tumor behavior. In addition the discovery of agents that can favorably alter the expression of miRNAs would provide opportunities for novel strategies to improve the survival of patients with PC.
Identification of differentially expressed plasma miRNAs from pooled plasma samples by miRNA profiling followed by qRT-PCR validation of individual patient samples may serve as a novel approach for specific tumor types. In this report, we found 91 miRNAs that were differentially expressed in the plasma of PC patients compared to normal controls. This finding supports the utility of miRNA profiling helping differentiating PC patients from healthy individuals and a potential novel approach for non-invasive bio-marker test for diagnosis and risk stratification. Similar to other studies, our results demonstrated that miR-21 was up-regulated in PC patients supporting its suggested oncogenic role. Our data are also consistent with four resistant cell lines data as presented in this report. We and other investigators have reported earlier that miR-21 targets PTEN and inversely regulates its expression, which in turn, inversely regulates the Akt pathway [11,32]. Here we show that there are several other genes that are regulated by miR-21, which not only includes the PTEN but also PDCD4, Maspin and TPM1 that were significantly decreased in all four drug-resistant PC cell lines (Figure 5C), resulted from the increased expression of miR-21 in all drug-resistant aggressive cell lines.
Our results on the high expression of miR-21 in the plasma and its correlation with worse survival are consistent with several other reports. A recent study showed that the loss of PTEN function may cause accelerated cancer progression, and decreased survival in the K-ras mouse model of pancreatic cancer [37]. Others have shown that miR-21 serves as a driver and negative regulator of the Ras/MEK/ERK pathway in transgenic mice of NSCLC model [38]. Zhu et al demonstrated that ionizing radiation up-regulates miR-21, which activates AP-1 and ErbB/Stat3 pathway and promotes liver carcino-genesis [39]. Up-regulation of miR-21 is not limited to pancreatic, lung, or liver cancers but also seen in colon carcinoma cells as downstream effectors of TGF-β by directly targeting Rac GTPase and facilitating invasion and metastasis [40]. Moreover, studies have shown shorter disease-free interval in patients with a higher expression of miR-21 in colorectal carcinoma tissue and colorectal cancer liver metastasis relative to adjacent normal colonic tissues [41]. Furthermore, growth of laryngeal squamous cell carcinoma xenograft tumors and Ras protein expression was significantly reduced by anti-sense miRNA (ASO-miR-21 lenti-virus) [42]. Surprisingly, the functional role of miR-21 is not only limited to cancer because it has been found to be up-regulated in cardiovascular diseases by affecting fibrosis and heart failure [43]. Taken together, miR-21 appears to be an interesting molecular target for the development of therapeutic strategies against many forms of cancer as well as for cardiovascular diseases.
Drug-resistance is considered as one of the major reasons for treatment failure in patients with PC [4]. At this time no therapeutics are able to overcome drug-resistance. However, recent studies have shown that low expression of miR-21 was associated with benefit from adjuvant treatment of PC and consistent with increased anticancer drug activity in vitro, suggesting that miR-21 may allow stratification for adjuvant therapy [44]. We and others have reported earlier that PC cells are more aggressive and resistant to gemcitabine if they have higher expression of miR-21 [11,45], and thus strategies for down-regulation of miR-21 could improve the outcome of conventional therapeutics. In fact, Mei et al reported that combining miR-21 inhibitor gene therapy with paclitaxel may represent a promising novel therapeutic approach for the treatment of breast cancer [46].
Based on our data on the expression profiles of miRNAs in plasma from patients with PC as well as the relationship between miRNA expression and drug sensitivity in PC cell lines, we suggest that miR-21 is predictive marker of clinical drug-resistance and tumor aggressiveness through regulation of several genes, such as those regulating cell survival. Consequently, strategies to down-regulate miR-21 by natural or synthetic agents, gene-based therapies, or synthetic miRNA antagonists, could be useful for overcoming drug-resistance, in patients with PC, which will lead to improve the overall survival of patients diagnosed with this deadly disease.
Acknowledgments
We sincerely thank Guido and Puschelberg Foundation for providing some financial support to complete our study. We also acknowledge the financial support provided through R01 grant funding from the National Cancer Institutes, National Institute of Health awarded to FHS (5R01CA131151, 3R01CA131151-02S1, and 5R01CA132794).
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, lacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321(5897):1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Kong D, Wang Z, Sarkar FH. Regulation of microRNAs by natural agents: an emerging field in chemoprevention and chemotherapy research. Pharm Res. 2010;27(6):1027–1041. doi: 10.1007/s11095-010-0105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkar FH, Li Y, Wang Z, Kong D, Ali S. Implication of microRNAs in drug resistance for designing novel cancer therapy. Drug Resist Updat. 2010;13(3):57–66. doi: 10.1016/j.drup.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Li Y, Ahmad A, Azmi AS, Kong D, Banerjee S, Sarkar FH. Targeting miRNAs involved in cancer stem cell and EMT regulation: An emerging concept in overcoming drug resistance. Drug Resist Updat. 2010 doi: 10.1016/j.drup.2010.07.001. Epub; ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffiths-Jones S, Saini HK, van DS, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36(Database issue):D154–D158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR) Methods. 2010;50(4):298–301. doi: 10.1016/j.ymeth.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Chen J, Chang P, LeBlanc A, Li D, Abbruzzesse JL, Frazier ML, Killary AM, Sen S. MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev Res (Phila Pa) 2009;2(9):807–813. doi: 10.1158/1940-6207.CAPR-09-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Zhang Y, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Wang J, Zen K, Zhang J, Zhang CY. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 10.Resnick KE, Alder H, Hagan JP, Richardson DL, Croce CM, Cohn DE. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol. 2009;112(1):55–59. doi: 10.1016/j.ygyno.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 11.Ali S, Ahmad A, Banerjee S, Padhye S, Dominiak K, Schaffert JM, Wang Z, Philip PA, Sarkar FH. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res . 2010;70(9):3606–3617. doi: 10.1158/0008-5472.CAN-09-4598. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally down-regulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27(15):2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 13.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65(14):6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 14.Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner MR, Frankel WL, Morgan DL, Postier RG, Brackett DJ, Schmittgen TD. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer. 2007;120(5):1046–1054. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slaby O, Svoboda M, Fabian P, Smerdova T, Knoflickova D, Bednarikova M, Nenutil R, Vyzula R. Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology. 2007;72(5-6):397–402. doi: 10.1159/000113489. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, VandenBoom TG, Kong D, Wang Z, Ali S, Philip PA, Sarkar FH. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69(16):6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, Mitsudomi T, Takahashi T. Reduced expression of the let-7 microR-NAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64(11):3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 18.Shi M, Guo N. MicroRNA expression and its implications for the diagnosis and therapeutic strategies of breast cancer. Cancer Treat Rev. 2009;35(4):328–334. doi: 10.1016/j.ctrv.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Ribas J, Lupold SE. The transcriptional regulation of miR-21, its multiple transcripts, and their implication in prostate cancer. Cell Cycle. 2010;9(5):923–929. doi: 10.4161/cc.9.5.10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moriyama T, Ohuchida K, Mizumoto K, Yu J, Sato N, Nabae T, Takahata S, Toma H, Nagai E, Tanaka M. MicroRNA-21 modulates biological functions of pancreatic cancer cells including their proliferation, invasion, and chemoresis-tance. Mol Cancer Ther. 2009 doi: 10.1158/1535-7163.MCT-08-0592. Epub; ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Park JK, Lee EJ, Esau C, Schmittgen TD. Antisense inhibition of microRNA-21 or -221 arrests cell cycle, induces apoptosis, and sensitizes the effects of gemcitabine in pancreatic adenocarcinoma. Pancreas. 2009;38(7):e190–e199. doi: 10.1097/MPA.0b013e3181ba82e1. [DOI] [PubMed] [Google Scholar]
- 22.Ali S, El-Rayes BF, Sarkar FH, Philip PA. Simultaneous targeting of the epidermal growth factor receptor and cyclooxygenase-2 pathways for pancreatic cancer therapy. Mol Cancer Ther. 2005;4(12):1943–1951. doi: 10.1158/1535-7163.MCT-05-0065. [DOI] [PubMed] [Google Scholar]
- 23.Wang F, Zheng Z, Guo J, Ding X. Correlation and quantitation of microRNA aberrant expression in tissues and sera from patients with breast tumor. Gynecol Oncol. 2010 doi: 10.1016/j.ygyno.2010.07.021. Epub; ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Brase JC, Johannes M, Schlomm T, Faith M, Haese A, Steuber T, Beissbarth T, Kuner R, Sultmann H. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int J Cancer. 2010 doi: 10.1002/ijc.25376. Epub; ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Heneghan HM, Miller N, Lowery AJ, Sweeney KJ, Newell J, Kerin MJ. Circulating microRNAs as novel minimally invasive biomarkers for breast cancer. Ann Surg. 2010;251(3):499–505. doi: 10.1097/SLA.0b013e3181cc939f. [DOI] [PubMed] [Google Scholar]
- 26.Kong X, Du Y, Wang G, Gao J, Gong Y, Li L, Zhang Z, Zhu J, Jing Q, Qin Y, Li Z. Detection of Differentially Expressed microRNAs in Serum of Pancreatic Ductal Adenocarcinoma Patients: miR-196a Could Be a Potential Marker for Poor Prognosis. Dig Dis Sci. 2010 doi: 10.1007/s10620-010-1285-3. Epub; ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Chan SH, Wu CW, Li AF, Chi CW, Lin WC. miR-21 microRNA expression in human gastric carcinomas and its clinical association. Anticancer Res. 2008;28(2A):907–911. [PubMed] [Google Scholar]
- 28.Conti A, Aguennouz M, La TD, Tomasello C, Cardali S, Angileri FF, Maio F, Cama A, Germano A, Vita G, Tomasello F. miR-21 and 221 upregulation and miR-181b downregulation in human grade II-IV astrocytic tumors. J Neurooncol. 2009;93(3):325–332. doi: 10.1007/s11060-009-9797-4. [DOI] [PubMed] [Google Scholar]
- 29.du Rieu MC, Torrisani J, Selves J, Al ST, Souque A, Dufresne M, Tsongalis GJ, Suriawinata AA, Carrere N, Buscail L, Cordelier P. MicroRNA-21 is induced early in pancreatic ductal adenocarcinoma precursor lesions. Clin Chem. 2010;56(4):603–612. doi: 10.1373/clinchem.2009.137364. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Huang H, Sun L, Yang M, Pan C, Chen W, Wu D, Lin Z, Zeng C, Yao Y, Zhang P, Song E. MiR-21 indicates poor prognosis in tongue squamous cell carcinomas as an apoptosis inhibitor. Clin Cancer Res. 2009;15(12):3998–4008. doi: 10.1158/1078-0432.CCR-08-3053. [DOI] [PubMed] [Google Scholar]
- 31.Marquez RT, Wendlandt E, Galle CS, Keck K, McCaffrey AP. MicroRNA-21 is upregulated during the proliferative phase of liver regeneration, targets Pellino-1, and inhibits NF-kappaB signaling. Am J Physiol Gastrointest Liver Physiol. 2010;298(4):G535–G541. doi: 10.1152/ajpgi.00338.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133(2):647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian B, Katsaros D, Lu L, Preti M, Durando A, Arisio R, Mu L, Yu H. High miR-21 expression in breast cancer associated with poor disease-free survival in early stage disease and high TGF-betal. Breast Cancer Res Treat. 2009;117(1):131–140. doi: 10.1007/s10549-008-0219-7. [DOI] [PubMed] [Google Scholar]
- 34.Seike M, Goto A, Okano T, Bowman ED, Schetter AJ, Horikawa I, Mathe EA, Jen J, Yang P, Sugimura H, Gemma A, Kudoh S, Croce CM, Harris CC. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Natl Acad Sci U S A. 2009;106(29):12085–12090. doi: 10.1073/pnas.0905234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selaru FM, Olaru AV, Kan T, David S, Cheng Y, Mori Y, Yang J, Paun B, Jin Z, Agarwal R, Hamilton JP, Abraham J, Georgiades C, Alvarez H, Vivekanandan P, Yu W, Maitra A, Torbenson M, Thuluvath PJ, Gores GJ, LaRusso NF, Hruban R, Meltzer SJ. MicroRNA-21 is overexpressed in human cholangiocarcinoma and regulates programmed cell death 4 and tissue inhibitor of metalloproteinase 3. Hepatology. 2009;49(5):1595–1601. doi: 10.1002/hep.22838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao Q, Xu H, Zhang QQ, Zhou H, Qu LH. MicroRNA-21 promotes cell proliferation and down-regulates the expression of programmed cell death 4 (PDCD4) in HeLa cervical carcinoma cells. Biochem Biophys Res Commun. 2009;388(3):539–542. doi: 10.1016/j.bbrc.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 37.Hill R, Calvopina JH, Kim C, Wang Y, Dawson DW, Donahue TR, Dry S, Wu H. PTEN Loss Accelerates KrasG12D-Induced Pancreatic Cancer Development. Cancer Res. 2010 doi: 10.1158/0008-5472.CAN-10-1649. Epub; ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hatley ME, Patrick DM, Garcia MR, Richardson JA, Bassel-Duby R, van RE, Olson EN. Modulation of K-Ras-Dependent Lung Tumorigenesis by MicroRNA-21. Cancer Cell. 2010;18(3):282–293. doi: 10.1016/j.ccr.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Y, Yu X, Fu H, Wang H, Wang P, Zheng X, Wang Y. MicroRNA-21 is involved in ionizing radiation-promoted liver carcinogenesis. Int J Clin Exp Med. 2010;3(3):211–222. [PMC free article] [PubMed] [Google Scholar]
- 40.Cottonham CL, Kaneko S, Xu L. miR-21 and miR-31 converge on TIAM1 to regulate migration and invasion of colon carcinoma cells. J Biol Chem. 2010 doi: 10.1074/jbc.M110.160069. Epub; ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kulda V, Pesta M, Topolcan O, Liska V, Treska V, Sutnar A, Rupert K, Ludvikova M, Babuska V, Holubec L, Jr, Cerny R. Relevance of miR-21 and miR-143 expression in tissue samples of colorectal carcinoma and its liver metastases. Cancer Genet Cytogenet. 2010;200(2):154–160. doi: 10.1016/j.cancergencyto.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Ren J, Zhu D, Liu M, Sun Y, Tian L. Down-regulation of miR-21 modulates Ras expression to promote apoptosis and suppress invasion of Laryngeal squamous cell carcinoma. Eur J Cancer. 2010 doi: 10.1016/j.ejca.2010.07.047. Epub; ahead of print. [DOI] [PubMed] [Google Scholar]
- 43.Bonci D. MicroRNA-21 as Therapeutic Target in Cancer and Cardiovascular Disease. Recent Pat Cardiovasc Drug Discov. 2010 doi: 10.2174/157489010793351962. Epub; ahead of print. [DOI] [PubMed] [Google Scholar]
- 44.Hwang JH, Voortman J, Giovannetti E, Steinberg SM, Leon LG, Kim YT, Funel N, Park JK, Kim MA, Kang GH, Kim SW, Del CM, Peters GJ, Giaccone G. Identification of microRNA-21 as a bio-marker for chemoresistance and clinical outcome following adjuvant therapy in resectable pancreatic cancer. PLoS One. 2010;5(5):e10630. doi: 10.1371/journal.pone.0010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giovannetti E, Funel N, Peters GJ, Del CM, Erozenci LA, Vasile E, Leon LG, Pollina LE, Groen A, Falcone A, Danesi R, Campani D, Verheul HM, Boggi U. MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res. 2010;70(11):4528–4538. doi: 10.1158/0008-5472.CAN-09-4467. [DOI] [PubMed] [Google Scholar]
- 46.Mei M, Ren Y, Zhou X, Yuan XB, Han L, Wang GX, Jia Z, Pu PY, Kang CS, Yao Z. Downregulation of miR-21 enhances chemotherapeutic effect of taxol in breast carcinoma cells. Technol Cancer Res Treat. 2010;9(1):77–86. doi: 10.1177/153303461000900109. [DOI] [PubMed] [Google Scholar]