Abstract
Mucosal immunization with a killed whole-cell pneumococcal vaccine, given with enterotoxin-related adjuvants, has been shown to confer multi-serotype protection against colonization of the nasopharynx and middle ear in mice. However, because novel mucosal immunization strategies may be difficult to implement, here we evaluated subcutaneous injection. Strain RM200 was engineered to be capsule-negative, autolysin-negative, and to express a non-toxic mutant pneumolysoid. Liter-scale and 60-L Good Manufacturing Practice (GMP) cultures were grown in bovine-free soy-based medium, killed with chloroform or beta-propiolactone, and injected into C57Bl/6 mice without or with aluminum adjuvant. The adjuvant Al(OH)3 strongly increased responses, particularly if pre-treated with phosphate. Protection was found in several tested model infections: nasal colonization with a serotype 6B strain and fatal aspiration-sepsis with strains of serotype 3 and 5. Protection against colonization was mechanistically dependent on the presence of CD4+ T cells at the time of challenge; in contrast, in the type 3 aspiration-sepsis model, CD4+ T cells were not required for protection at the time of challenge, suggesting that antibody alone was sufficient to protect against death in this model. Rabbits receiving sequential intramuscular injections in a pilot toxicity study displayed local reactogenicity at injection sites but no clinical signs. The rabbit antiserum thus produced was active in an in vitro phagocytic killing assay and passively protected mice in the type 3 aspiration-sepsis model. Approval is being sought for human trials of this vaccine.
Keywords: Streptococcus pneumoniae, vaccine, colonization, sepsis
INTRODUCTION
Streptococcus pneumoniae (pneumococcus) persists as a major pathogen, particularly among children in low-income countries [1]. Capsular polysaccharide conjugate vaccines provide type-specific immunity, but have the disadvantages of limited serotype coverage, increasing disease from non-vaccine serotypes, and relatively high cost [2, 3]. Therefore, potentially more economical serotype-independent vaccines based upon species-common protein antigens are being investigated [4]. We have been investigating the use of killed cells of non-capsulated pneumococci, maximizing the exposure of a variety of species-common sub-capsular antigens, thus potentially providing synergistic immunity to multiple pneumococcal targets. This preparation, designated “whole cell antigen” (WCA) or, when given with suitable adjuvant, whole cell vaccine (WCV), was initially intended for mucosal administration to reduce colonization. Intranasal (i.n.) vaccination using cholera toxin as adjuvant prevents fatal serotype 3 pneumonia in rats and reduces nasopharygeal (NP) and middle ear colonization in mice by strains of serotype 6B or 23F [5, 6]. Serum antibodies are raised, but the accelerated pneumococcal clearance in mice can be induced in the absence of antibodies by a CD4+ T cell-dependent, IL-17A-mediated mechanism [7, 8].
Considering the potency and low cost, PATH supported the further development of WCV and generation under “Good Manufacturing Practice” (GMP) at Instituto Butantan (Sao Paulo, Brazil). Anticipating human testing, WCA was made from cells expressing a non-toxic variant of pneumolysin (carrying three mutations -- W433F, D385N, C428G -- which greatly reduce hemolytic capacity) and cultured in medium lacking bovine components [9, 10]. Potency was increased by killing with agents such as chloroform or beta-propiolactone so that released soluble components are retained. In preclinical studies, we have investigated several mucosal adjuvants other than cholera toxin, and different routes (such as buccal, sublingual or transcutaneous) [9]. However, at present, mucosal administration may be difficult to implement in developing countries and current international recommendations favor injection, so here we have tested WCA in mice by the subcutaneous (s.c.) route, evaluating aluminum salts as an adjuvant. In addition to nasopharyngeal colonization with serotype 6B, protection was tested in models of fatal aspiration-sepsis with isolates of serotype 3 and 5. A pilot toxicology study including an immunogenicity endpoint was conducted with multiple intramuscular injections in rabbits, and the antiserum produced was shown to induce phagocytic killing in vitro and protection following passive transfer to mice in the pneumonia model.
MATERIALS AND METHODS
Materials
Aluminum hydroxide (alum) was from Brenntag North America (2% Alhydrogel). Beta-propiolactone (BPL) was from Fisher, and saline was from B. Braun Medical Inc. (Bethlehem, PA). All other reagents were obtained from Sigma.
Antigen preparations
For non-GMP grade material, pneumococcal strain RM200 (a capsule-negative, autolysin-negative, pneumolysoid-expressing strain derived from Rx1 as described in [9]) was grown at 37°C with 5% CO2 to A600 1.0 in animal protein-free medium [10] at which viable count was approximately 6×108 CFU/ml. Further steps were at 4 °C. The cells were collected by centrifugation, and washed twice with Lactated Ringer’s solution (LR) (102 mM NaCl, 28 mM NaC3H5O3, 1.5 mM CaCl2 and 4 mM KCl). For killing with chloroform, washed cells in LR with 0.2% glucose at A600=32 were mixed with chloroform 1/40 v/v for 2 hours (this preparation is named WCC). Killed cells were then lyophilized, which eliminates residual organic solvent. For killing with BPL, washed cells in LR with 10% sucrose were mixed with BPL, 1/4000 v/v for 24 hours at 4°C followed by a 2-hour incubation at 37°C to inactivate BPL; cells were subsequently frozen or lyophilized (this preparation is called WCB). Protein concentration was determined using the Total Protein Kit with bovine serum albumin as standard (Sigma).
One day prior to immunization, vaccines were prepared as follows. Frozen aliquots were thawed or lyophilized vials were reconstituted with sterile water, diluted to the appropriate concentration, and mixed with Al(OH)3 at the indicated concentration in a 15 ml conical tube, which was then tumbled overnight at 4°C to allow for adsorption.
Immunization and challenge of mice
C57BL/6J mice (Jackson Laboratories, Bar Harbor, Maine) were used in all the experiments. The age at time of first immunization was between 4–6 weeks. Gently restrained, nonanesthetized mice received 2 or 3 subcutaneous injections of 200 μl of adjuvant with or without antigen in the lower part of the back at 2-week intervals. Blood was drawn 1 or 2 weeks after the last immunization, and assayed for antibody and for IL-17A production in vitro after stimulation with WCA.
NP colonization model
To determine susceptibility to NP colonization, i.n. challenge with live encapsulated pneumococci was performed as described [5]: 3 weeks after the last immunization, mice were challenged with 107 colony-forming units (CFU) of serotype 6B strain 0603 in 10 μl of PBS applied as described. To determine NP colonization, an upper respiratory culture was done 10 days later by instilling sterile saline retrograde through the transected trachea, collecting the first 6 drops (about 0.1 ml) from the nostrils, and plating neat or diluted samples on blood agar plates containing 2.5 μg gentamicin/ml. The figures show the CFU per nasal wash sample of individual mice; the geometric means (GM) are displayed as a horizontal bar. For ease of statistical analysis, a sterile sample was assigned half the lower limit of detection (1.6 CFU/nasal wash), or 0.8 CFU/nasal wash.
Aspiration-sepsis challenge model
Two weeks after the last immunization, mice were gently anesthetized with isoflorane, held supine, and given a 100 μl intranasal inoculation containing an inoculum of type 3 strain WU-2 or type 5 strain DBL5 (a kind gift of Dr. David Briles) using a model we have described before [11] but with the modification that mice were not intranasally exposed to pneumococcus 2 days prior to aspiration challenge. This model induces sepsis and death within 3–4 days in nonimmunized mice. Mice are monitored twice daily and sacrificed by C02 inhalation and terminal exsanguination when demonstrating signs of illness following which a blood culture is obtained; in all cases, these blood cultures reliably demonstrate the presence of pneumococcal bacteremia. For passive protection studies, one day prior to induction of aspiration, mice were injected intraperitoneally with either 500 μl saline or 300 μl plus 200 μl of heat inactivated (56°C for 30 minutes) serum obtained from rabbits immunized with aluminum hydroxide with or without WCA as described below. All animal studies were approved by our local animal ethics committees.
Rabbit immunization and toxicology studies
All rabbit immunizations were performed at MPI (Mattawan, MI). Female New Zealand White rabbits in groups of three were given 0.5 ml injections of saline, Al(OH)3 alone (containing 0.6 mg of Al), Al(OH)3-adsorbed WCB at doses of 50, 500 or 5000 μg or a whole cell Diphtheria-Tetanus-Pertussis whole cell (DTwP) vaccine (clinical product from Instituto Butantan) intramuscularly on day 1, 15, 29 and 43. Sera were obtained before each immunization and at a terminal bleed on day 45 and shipped frozen to Children’s Hospital Boston for measurement of antibodies. Observations for morbidity, mortality, clinical signs, body temperature and food and water consumption were conducted on a regular basis for all animals. Dermal irritation scores were evaluated prior to each dose and daily for 3 consecutive days following each dose (with the exception of the last dose). Clinical pathology was performed at baseline, on day 2 and at termination. At study termination (two days post last dose), macroscopic examinations were performed, organ weights were recorded, and the injection sites were microscopically examined.
Enzyme-linked immunosorbent assay (ELISA)
Assays for murine antibodies to WCA were done in Immulon 2 HB 96-microwell plates (Thermo Scientific, Waltham, MA) coated with WCA 100 μg of protein per ml in PBS. Plates were blocked with 1% BSA in PBS. Antibody diluted in PBS-T was added and incubated at room temperature for 2 hours. Plates were washed with PBS-T, and secondary HRP-conjugated antibody to mouse immunoglobulin G, G1 or G2 (all from Sigma) was added and incubated at room temperature for one hour. The plates were washed and developed with SureBlue TMB Microwell Peroxidase Substrate (KPL, Gaithersburg, MD).
Assay of IL-17A production in whole blood samples
Fifty μl of heparinized blood was added to 450 μl DMEM (BioWhittaker, Walkersville, MD) containing 10% low-endotoxin defined FBS (Hyclone, Logan, UT), 50 μM 2-mercaptoethanol (Sigma) and ciprofloxacin (10 μg/ml, Cellgro, Manassas, VA). Except for the nonstimulated control, the cultures were incubated at 37°C for 6 days with 107 cells of pneumococcal WCA. Supernatants were collected following centrifugation and stored at −80°C until analyzed by ELISA for IL-17A concentration (R&D Systems, Minneapolis, MN).
Surface killing assay
Neutrophil surface killing assays were performed as described previously [8, 12]. Briefly, type 6B strain 0603 [5] was grown to mid-log phase and frozen in THY/10% glycerol at −80°. On the day of the experiment, bacteria were thawed and diluted to 100 CFU/μL in RPMI supplemented with 10% FBS and opsonized with normal rabbit serum or serum from rabbits that had been immunized three times with WCV as described above for 30 minutes at 37°C. In all cases, rabbit sera were heated for 30 min at 52°C to inactivate complement. Polymorphonuclear leukocytes were purified from the peripheral blood of human volunteers using a Histopaque 1077, 1119 gradient (Sigma-Aldrich, St. Louis, MO) according to the manufacturers instructions and used immediately. Five μl of the opsonized bacterial suspension, diluted to contain 30 cfu, was spotted at room temperature on trypticase soy agar with 5% defibrinated sheep blood (TSA II) (BD) with 5 replicates per plate, and the fluid was allowed to absorb, requiring about 15 minutes. Ten microliters of neutrophils (3×106 cells/mL; resulting in a bacterial:neutrophil ratio of approximately 1:60) were then overlaid, allowed to absorb, and incubated overnight at 37° with 5% CO2. Controls included bacteria spotted in the absence of neutrophils or with neutrophils but no serum.
Statistical analysis
Antibody and IL-17A concentrations and NP colonization densities were compared by the Mann-Whitney U test using PRISM (version 4.0a, GraphPad Software, Inc). Differences in survival were analyzed with the Kaplan-Meier test, using PRISM as well. For the toxicology study, all comparisons were made to the group receiving the alum adjuvant alone. Comparisons of body weight, food consumption, body temperature, hematology (except leukocyte counts) coagulation parameters, clinical chemistry values, and organ weights were performed by group pair-wise comparisons using either ANOVA or Welch’s test, with appropriate adjustment for multiple comparisons. For leukocyte counts and urinalyses, due to lack of normality, data were log and rank transformed, respectively, and transformed data were analyzed as above. Erythema, eschar and edema formation were analyzed by Cochran Mantel Haenszel Test.
RESULTS
General approach
Dosage of WCA is quoted in μg of protein, of which about 85% is cellular and ca 15% is soluble [9]; 1 μg corresponds approximately to a total dry weight of 1.7 μg and to 106 CFU before killing. For active immunization of mice, two or three sequential injections were given two weeks apart, blood was taken 1 or 2 weeks after the last injection for assays of priming for IL-17A expression in vitro and of serum IgG antibody to WCA, the animals were challenged with pneumococcus in either the colonization model or the aspiration-sepsis model, and the outcome in individual mice compared to the in vitro assay values.
Evaluation of aluminum adsorption by immunological assays and protection in the colonization model
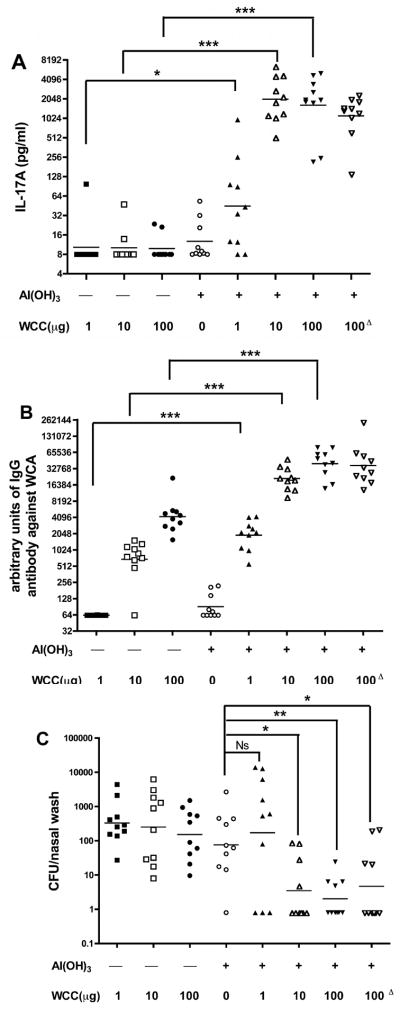
Ascending doses of WCC - 1, 10, and 100 μg - were tested in a 3-injection sequence without and with adsorption onto Al(OH)3, 0.21 mg Al per dose. Without adjuvant there was no measurable IL-17A response (Figure 1A), while with Al(OH)3 there was a significant response even to the 1 μg dose. Without and with adsorption there were dose-dependent antibody responses, but with about 100-fold potentiation by the Al(OH)3 (Figure 1B). Without adsorption there was no protection against experimental colonization with serotype 6B strain 0603, but with Al(OH)3 the two higher WCC doses gave significant reduction of CFU (Figure 1C). A similar requirement for Al(OH)3 for IL-17A responses and enhancement of antibody response was noted when WCB was used (data not shown).
Figure 1.
Effect of Al(OH)3 adsorption on the immunogenicity of chloroform-killed whole-cell antigen (WCC). The antigen was incubated 18 hours at 4 °C with gentle mixing so as to provide 1, 10, or 100 μg of protein and 0.21 mg of Al per 0.2 ml dose. Adsorbed or nonabsorbed antigen or Al(OH)3 alone was injected under the skin in the lower back area three times at 2-week intervals. In data column 8 the WCC-Al(OH)3 preparation was incubated 1 month at 37°C then stored at 4°C before injection (this group is indicated by the Δ superscript). A. Priming for IL-17A responses to WCA in vitro by blood samples taken 2 weeks post-2nd injection. B. IgG antibody to WCA assayed by ELISA in those blood samples. C. Clearance of serotype 6B from the nasopharynx. The mice were challenged 2 weeks post-3rd injection with pneumococcal type 6B strain 0603. Ten days post-challenge the CFU recovered from nasal wash samples was determined. In this and subsequent Figures, unless indicated, the significance of differences was compared to adjuvant alone, calculated by Mann-Whitney U, is shown by asterisks: * p<0.05, ** p<0.01 and *** p<0.001.
Routinely, adsorption was done for 18–22 hours at 4°C, and the preparations were tested immediately. To test the stability of the adsorbed antigen, Al(OH)3 with WCC at 100 μg per dose was incubated at 37°C for one month before testing in the above-described experiment. The IL-17A responses, antibody titers and protection results did not differ from the same dosage of freshly prepared WCC-Al(OH)3 (Figures A, B, C, last column, indicated by the Δ superscript).
Phosphate pretreatment of alum has been shown to have important effects on the immunogenicity of aluminum-adjuvanted vaccines [13]. To evaluate this, Al(OH)3 was preequilibrated 7 days with sodium phosphate buffer, pH 7.5 in a range of concentrations from 0–100 mM, then washed and used at a limiting Al dosage (0.085 mg) to adsorb WCC at the limiting dose of 10 μg. Phosphate pre-treatment at 10–100 mM gave potentiations of 8–10 fold in IL-17A response (Figure 2A) and 2–3 fold increase in antibody titer (Figure 2B). However, in neither assay did the phosphate treatment raise the response beyond that of the higher dosage of Al(OH)3 (0.21 mg of Al), which was routinely used in further studies.
Figure 2.
Effect of phosphate pre-treatment of Al(OH)3 on adjuvancy. Portions of Al(OH)3 were pre-incubated 7 days at 25°C with sodium phosphate buffer, pH 7.5 at the concentrations in mM indicated, then incubated for adsorption in amounts corresponding to doses of 10 μg of WCC and 0.085 mg of Al per 0.2-ml dose. Controls were non-phosphate-exposed Al(OH)3 at 0.21 mg/dose, given alone or with 10 μg of WCC (columns 1 and 2). The schedule and assays were as in Figure 1. A. Priming for IL-17A, B. Serum IgG antibody to WCA.
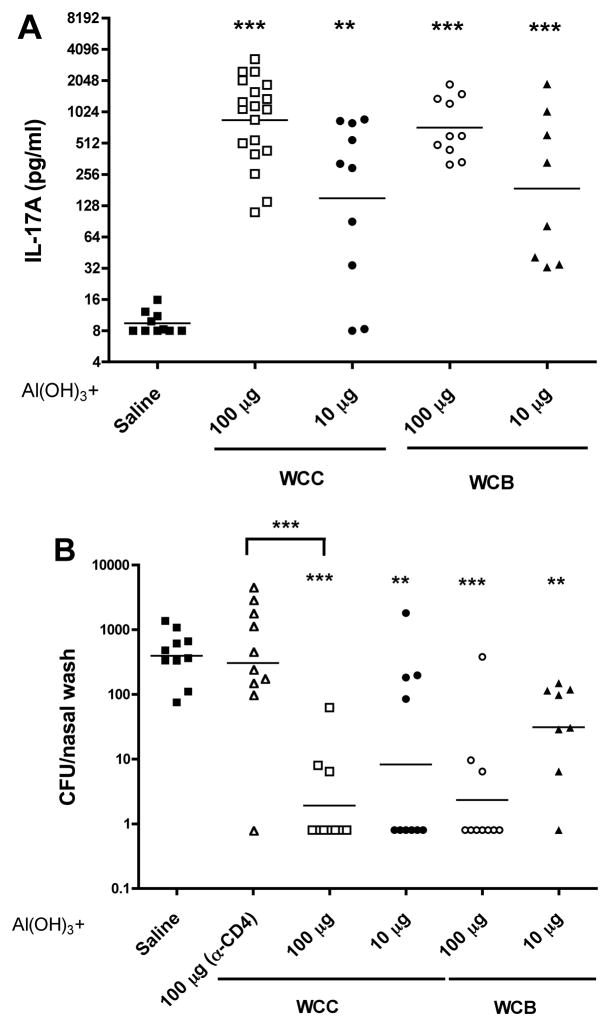
Comparison of WCC and WCB and role of CD4+ T cells in the colonization model
WCC and WCB at doses of 10 or 100 μg, adsorbed onto Al(OH)3 were compared. The dose-dependent IL-17A responses did not differ (Figure 3A). Clearance of serotype 6B from the nasopharynx also was indistinguishable (Figure 3B). Dependence upon the CD4+ pathway was evaluated in a group of WCC-immunized animals given anti-CD4+ antiserum just prior to challenge to eliminate these cells as effectors of protection; in these mice the protection was eliminated (2nd column, Figure 3B). Since beta-propiolactone is used in several other human vaccines, including rabies vaccines [14], it was judged the preferred agent of killing for industrial production at Instituto Butantan, and the studies below used WCB made there, some under GMP.
Figure 3.
Comparison of WCC with antigen made with beta-propiolactone-killed cells (WCB); and role of the CD4+ cells in the colonization model. Schedule, assays, and challenge were as in Figure 1. A. Priming for IL-17A. B. Clearance of a serotype 6B strain from the nasopharynx. Dependence upon CD4+ cells was evaluated in a group of WCC-immunized animals given CD4 antiserum 1 day prior to and two, five, eight days after challenge (in the column indicated by α-CD4).
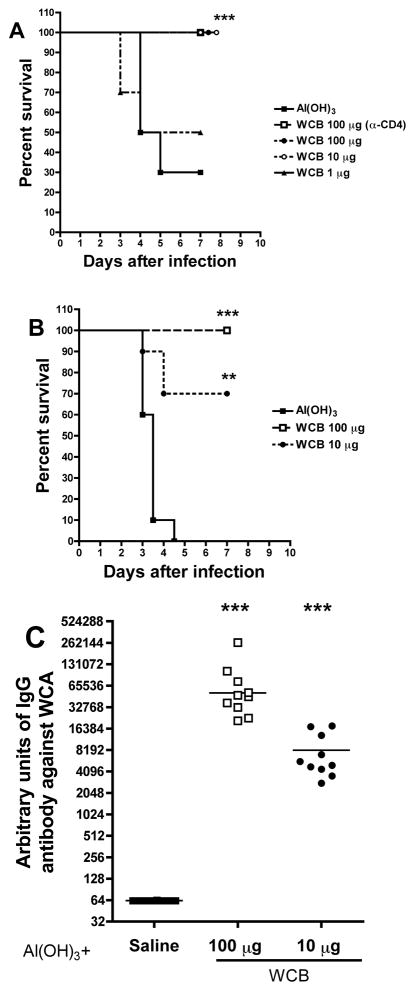
Protection against fatal aspiration-sepsis
WCB-Al(OH)3 at doses of 1, 10, and 100 μg were tested (in the 3-injection schedule) in a model of fatal aspiration-sepsis with serotype 3 strain WU-2 [11, 15]. Of the controls receiving Al(OH)3 alone, 7/10 died (Figure 4A) and 2 of the 3 survivors were bacteremic by the close of the 7-day observation period (not shown). There was dose-dependent protection by WCB: half the mice receiving 1 μg and all receiving 10 or 100 μg survived the 7-day observation period; none of the surviving vaccinated mice was bacteremic. As expected, there were dose-dependent increases in IL-17A and antibody responses (not shown). ELISA was performed using HRP-conjugated secondary antimouse IgG1 or IgG2 antibodies: IgG1 was readily detectable, but mice sera had very little antipneumococcal IgG2 (data not shown). Treatment of the mice with CD4+ antibodies did not prevent protection (Figure 4A), indicating that CD4+ T cell responses were not needed at time of challenge and that the antibodies were sufficient to protect. Overall, protection was uniformly observed when the serum antipneumococcal IgG antibody response in mice exceeded 10,000 arbitrary units.
Figure 4.
Effect of 3-injection or 2-injection schedules of WCB upon fatal serotype 3 aspiration-sepsis. WCB at doses of 1, 10, or 100 μg was adsorbed to Al(OH)3 (0.21 mg Al/dose) and given at two-week intervals. A. In the 3-injection schedule, 8 days post-3rd dose, the mice were anaesthetized with isofluorane and given 106 CFU of strain WU2 in 100 μl PBS i.n. Survival curves are shown. Mice alive after 7 days were sacrificed and cultured for pneumococcal bacteremia--the only bacteremic animals were 2 of the 3 Al(OH)3-only controls. Dependence of the protection upon CD4+ cells was evaluated in the indicated group that was given CD4 antiserum 1 day prior to and 3 days after challenge. B. In the 2-injection schedule, the mice were challenged 9 days post-2nd, and survival is graphed as in A. C. Effect of the 2-immunization schedule on serum IgG antibody titers to WCA, determined 2 days pre-challenge.
Protection in this model was tested also with just two injections. This was done with WCB prepared in a 60-liter fermentation under GMP. Doses of 10 or 100 μg were given 2 weeks apart, followed by blood sampling one week later and challenge 2 days thereafter. The 10-μg dosage was partially and the 100-μg dose completely protective against death or bacteremia (Figure 4B); the serum IgG antibody response in the 2-injection schedule was substantial; titers were about 7-folder greater after the higher dose (Figure 4C). Similar results were obtained when the vaccine was administered intramuscularly (data not shown).
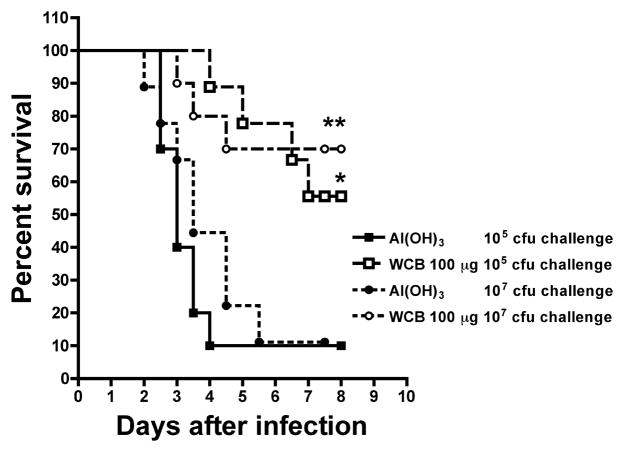
To demonstrate expanded serotype coverage, the aspiration-sepsis was induced with a serotype not previously used: challenge with type 5 strain DBL5 was fatal with as little as 105 CFU/aspiration. The 2-injection sequence with 100-μg doses of WCB-Al(OH)3 was tested and found highly protective against challenges with 105 or 107 CFU/aspiration (Figure 5).
Figure 5.
Effect of alum-adsorbed WCB on survival in a model of fatal aspiration-sepsis induced with a serotype 5 strain. The mice were immunized twice at a 2-week interval with 100 μg of WCB on Al(OH)3 (0.21 mg of Al) and challenged 8 days later by isofluorane-induced aspiration of either 105 or 107 CFU of serotype 5 strain DBL5. Survival curves are shown.
Preliminary toxicology assessment and immunogenicity in rabbits
New Zealand White females in groups of 3 were injected intramuscularly on day 1, 15, 29 and 43 with saline, Al(OH)3 alone (0.21 mg of Al), Al(OH)3-adsorbed WCB at doses of 50, 500 or 5000 μg, or - as a known toxicity control - a commercial DTwP vaccine. No test article-related clinical signs, dermal irritation, loss of appetite or temperature changes were observed throughout. Upon necropsy, there were no definitive test article-related macroscopic findings in any of the groups in this study.
Although not clearly dose-dependent, neutrophils and monocytes showed mild to moderate increases (1.58 to 1.87-fold relative to alum adjuvant) in all WCB groups. Fibrinogen increased dose-dependently and progressively from day 2 (1.16 to 1.94-fold relative to alum adjuvant) to day 45 (1.31 to 2.62-fold relative to alum adjuvant) in animals receiving the mid- and high WCB doses. Globulins were mildly elevated in the groups that received WCB 500 μg and WCB 5,000 μg. Changes in these clinical pathology parameters are consistent with an inflammatory response to the vaccine.
Numerous microscopic findings were seen in the intramuscular injection sites, with findings varying between the different control groups and the WCB test article injections. These findings were generally greatest for injection site 4 (day 45) and showed ongoing recovery over time (injection site 1, day 1). The only slight evidence of dose dependence between the WCB immunized groups was seen in the finding of subcutaneous hemorrhage/inflammation/necrosis.
Overall, findings of muscle and subcutaneous inflammation were increased to some degree in all of the WCB groups compared to the Group 2 controls, with no consistent evidence of dose dependence for either severity or incidence of any of these findings across all injection sites. WCB groups, as a whole, did not consistently have increased incidence or severity of any microscopic findings compared to the DTwP controls.
In conclusion, the test article, WCB was examined at three dose levels, 50 μg, 500 μg, or 5,000 μg. Based upon findings limited to inflammation (fibrinogen and microscopic pathology), the no observed adverse effect level (NOAEL) of this study was 50 μg/animal of WCB adsorbed to aluminum hydroxide.
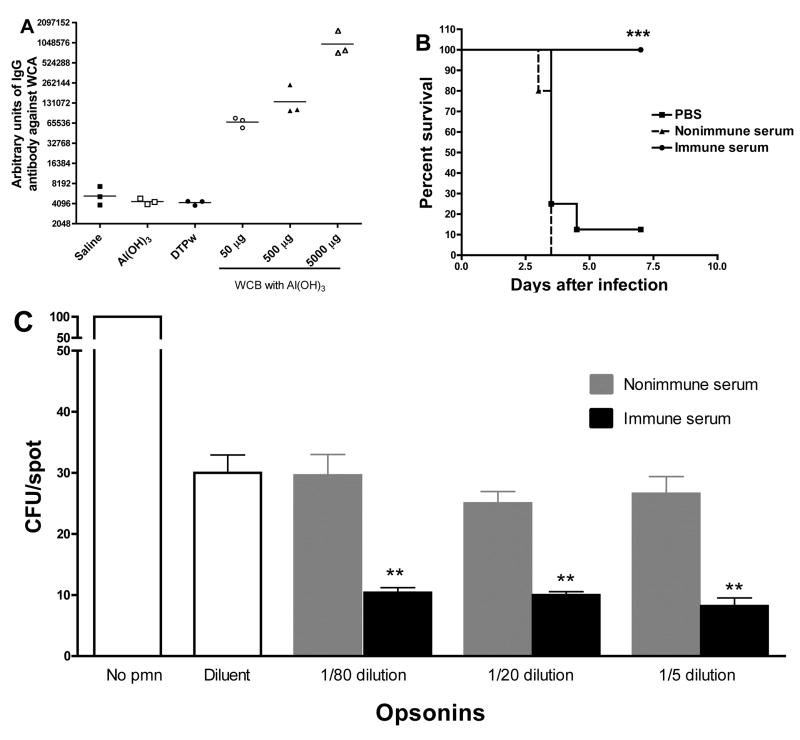
Serum IgG antibody titers rose progressively and with dose-dependency in samples taken pre-injection on days 1,15, 29, and 43; the day −43 titers are shown in Figure 6A. Pools of the day −45 sera from alum and WCB immunized rabbits were tested for passive protection in the mouse aspiration-sepsis model with serotype 3: the day −45 pool was highly protective (Figure 6B). Heat-treated terminal bleed sera from rabbits that had been immunized with 500 μg of Al(OH)3-adsorbed WCB or alum alone was evaluated in a surface killing assay as described previously [8, 12]. As shown in Figure 6C, at the three dilutions tested, immune sera from rabbits significantly enhanced killing of a strain of type 6B pneumococcus by human neutrophils compared to pre-immune sera, indicating that immunization with WCA in alum induces opsonophagocytic antibodies.
Figure 6.
Rabbit immunization studies. Female New Zealand White animals in groups of 3 were given saline, Al(OH)3 alone (0.6 mg of Al), Al(OH)3-adsorbed WCB at doses of 50, 500 or 5000 μg, or DTwP vaccine, intramuscularly on day 1, 15, and 29. Sera were taken before immunization and at day 43. A. Serum IgG antibody titers against WCA at day 43. B. Passive protections. Pools of the day −45 sera from Al(OH)3 and Al(OH)3-adsorbed WCB at dose 500 μg immunized rabbits were tested for passive protection in the mouse pneumonia model with serotype 3. Serum was heated at 56°C for 30 min to inactivate complement. Mice were given 200 μl of either pool with 300 μl PBS intraperitoneally one day before being challenged with strain WU2 as described in Fig 4A. Survival curves are shown. C. Opsonophagocytic killing assay. Heat-treated sera from rabbits immunized three times with alum alone or alum-adsorbed WCB (500 μg per dose) were used in a surface killing assay using a serotype 6B pneumococcal strain (0603). At the three dilutions tested (1/5, 1/20 and 1/80), immune sera significantly increased opsonophagocytic killing of pneumococci compared to preimmune sera at the same dilution. **P<0.008 by Mann-Whitney U.
DISCUSSION
There are many potential advantages in considering a pneumococcal vaccine such as WCV for mucosal administration [16]. However, the World Health Organization’s “Target Product Profile” for Advance Market Commitment for Pneumococcal Vaccine prefers intramuscular or subcutaneous injection [17] thus it was cogent to test WCA as an injectable vaccine, evaluating currently approved aluminum adjuvants. Adsorption on Al(OH)3 greatly increased potency in C57BL/6J mice. Subcutaneous injections protected against intranasal colonization, which necessarily precedes pneumococcal diseases [18]. In addition to our serotype 6B colonization model, which is non-lethal, a model was used that starts with aspiration of pneumococci, in which intranasal challenge of anaesthetized mice with a highly encapsulated serotype 3 strain produces bacteremia and death. Here WCV by the s.c. route was potent—three sequential injections of as little as 1 μg protein, or two of 10 μg, were significantly protective. In this model, the serum antibody response, which is unnecessary for clearance of pneumococci from the nasopharynx [7], appears to participate in protection--as shown both with non-abrogation by CD4 antibodies and transfer by serum. Pilot toxicology studies performed in rabbits were reassuring, with a NOAEL of 50 μg/animal of WCB adsorbed to aluminum hydroxide. Antibodies elicited by immunization of rabbits have opsonophagocytic properties in the absence of complement. Thus, both the IL-17A and antibody mechanisms appear to participate when WCV is given parenterally. To extend the observation to additional serotypes, the pneumonia model was reproduced and protection demonstrated with a type 5 strain, a frequent serotype in developing countries childhood infections [19].
For such a complex antigen, straightforward standardization and quality control by biochemical criteria is not feasible. A potency assay based upon animal immunization is required; but animal challenge tests as an endpoint are not ideal, and correlates determined by in vitro assay are preferable. Priming of mice for generation of IL17A in vitro is a correlate of protection by WCV given by many routes [9], but this assay requires tissue culture facilities that may not be readily available in many developing countries. As shown here, when WCV was given s.c. serum IgG antibody to WCA determined by ELISA is both a correlate and an agent of protection against invasive disease. Therefore, the planned initial clinical evaluation of WCV is as an aluminum-adsorbed antigen for intramuscular injection, and the primary potency criterion will be by mouse immunization and ELISA for IgG antibody after two sequential injections. The IL-17A assay could potentially serve as a supplementary criterion. Like the IgG ELISA, the IL-17A assay can be conducted with small samples of human blood, so both would be useful biomarkers in clinical trials.
The remarkable success of the 7-valent pneumococcal conjugate vaccine (PCV) against invasive disease in the US and the exciting results from clinical trials in South Africa and The Gambia of a 9-valent PCV [20, 21], understandably led to the inference that, given enough serotype coverage with future generation conjugate vaccines and lowering of cost, pneumococcal disease could be significantly controlled, if not eradicated, in both developed and developing countries. The recent licensure in Europe and the US, respectively, of so-called “second generation” 10- and 13-valent pneumococcal vaccines represents further important advances in this area. However, the emergence of serotypes not included in the first generation 7-valent conjugate vaccine [3], and the demonstration that these strains are important causes of disease, morbidity and mortality [22, 23] is a cause for concern. While current efforts to promote the use of appropriately broad pneumococcal conjugate vaccines in developed and developing countries are continuing, the need for alternative approaches to vaccination against pneumococcus remains a priority.
Over the past decade, several groups, including our own, have focused on the development of species-specific pneumococcal vaccines that would lead to clinical trials [24–28]. It is nevertheless sobering that, to date, no such vaccine has progressed to Phase III clinical trials. Furthermore, it is clear that the development of a species-specific pneumococcal vaccine faces several important hurdles for development and licensure, including, but not limited to, the choice of study population, endpoints and ascertainment of efficacy, comparisons to the currently-approved conjugate pneumococcal vaccines, route of administration and potential need for adjuvants for optimal stimulation of mucosal immunity.
We believe that our preclinical studies strongly support studying the WCA adsorbed on alum and administered parenterally in humans. We suggest that immunization with the WCV in this fashion may generate immunity to pneumococcus by a two-pronged mechanism: noncapsular antibody-mediated protection against invasive disease and CD4+ IL-17A-mediated protection against colonization and possibly also mucosal disease. Preparations are underway to seek regulatory approval for a Phase I clinical trial in healthy adults.
Acknowledgments
We thank John Robbins for helpful suggestions. This work was supported by PATH. RM also acknowledges support from NIH (R01 AI066013).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009 Sep 12;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 2.Ray GT. Pneumococcal conjugate vaccine: economic issues of the introduction of a new childhood vaccine. Expert Rev Vaccines. 2002 Jun;1(1):65–74. doi: 10.1586/14760584.1.1.65. [DOI] [PubMed] [Google Scholar]
- 3.Hanage WP. Serotype-specific problems associated with pneumococcal conjugate vaccination. Future microbiology. 2008 Feb;3(1):23–30. doi: 10.2217/17460913.3.1.23. [DOI] [PubMed] [Google Scholar]
- 4.Tai SS. Streptococcus pneumoniae protein vaccine candidates: properties, activities and animal studies. Crit Rev Microbiol. 2006;32(3):139–53. doi: 10.1080/10408410600822942. [DOI] [PubMed] [Google Scholar]
- 5.Malley R, Lipsitch M, Stack A, Saladino R, Fleisher G, Pelton S, et al. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect Immun. 2001 Aug;69(8):4870–3. doi: 10.1128/IAI.69.8.4870-4873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malley R, Morse SC, Leite LCC, Mattos Areas AP, Ho PL, Kubrusly FS, et al. Multi-serotype Protection of Mice Against Pneumococcal Colonization of the Nasopharynx and Middle Ear by Killed Nonencapsulated Cells Given Intranasally with a Non-toxic Adjuvant. Infect Immun. 2004 Jul;72(7):4290–2. doi: 10.1128/IAI.72.7.4290-4292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci U S A. 2005 Mar 29;102(13):4848–53. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008 Sep;4(9):e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu YJ, Yadav P, Clements JD, Forte S, Srivastava A, Thompson CM, et al. Options for inactivation, adjuvant, and route of administration of a killed unencapsulated pneumococcal whole-cell vaccine. Clinical and Vaccine Immunology. 2010 doi: 10.1128/CVI.00036-10. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liberman C, Takagi M, Cabrera-Crespo J, Sbrogio-Almeida ME, Dias WO, Leite LC, et al. Pneumococcal whole-cell vaccine: optimization of cell growth of unencapsulated Streptococcus pneumoniae in bioreactor using animal-free medium. Journal of industrial microbiology & biotechnology. 2008 Nov;35(11):1441–5. doi: 10.1007/s10295-008-0445-3. [DOI] [PubMed] [Google Scholar]
- 11.Lu YJ, Forte S, Thompson CM, Anderson PW, Malley R. Protection against Pneumococcal colonization and fatal pneumonia by a trivalent conjugate of a fusion protein with the cell wall polysaccharide. Infect Immun. 2009 May;77(5):2076–83. doi: 10.1128/IAI.01554-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberger DM, Trzcinski K, Lu YJ, Bogaert D, Brandes A, Galagan J, et al. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathog. 2009 Jun;5(6):e1000476. doi: 10.1371/journal.ppat.1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen B, Belfast M, Soung G, Song L, Egan PM, Capen R, et al. Effect of the strength of adsorption of hepatitis B surface antigen to aluminum hydroxide adjuvant on the immune response. Vaccine. 2009 Feb 5;27(6):888–92. doi: 10.1016/j.vaccine.2008.11.078. [DOI] [PubMed] [Google Scholar]
- 14.Plotkin SA. Rabies. Clin Infect Dis. 2000 Jan;30(1):4–12. doi: 10.1086/313632. [DOI] [PubMed] [Google Scholar]
- 15.Malley R, Srivastava A, Lipsitch M, Thompson CM, Watkins C, Tzianabos A, et al. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect Immun. 2006 Apr;74(4):2187–95. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005 Apr;11(4 Suppl):S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 17.WHO. Target Product Profile (TPP) for the Advance Market Commitment (AMC) for Pneumococcal Conjugate Vaccines. 2008 [Google Scholar]
- 18.Austrian R. Some aspects of the pneumococcal carrier state. J Antimicrob Chemother. 1986;18( Suppl A):35–45. doi: 10.1093/jac/18.supplement_a.35. [DOI] [PubMed] [Google Scholar]
- 19.Hausdorff WP, Bryant J, Paradiso PR, Siber GR. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I [In Process Citation] Clin Infect Dis. 2000;30(1):100–21. doi: 10.1086/313608. [DOI] [PubMed] [Google Scholar]
- 20.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003 Oct 2;349(14):1341–8. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 21.Cutts FT, Zaman SM, Enwere G, Jaffar S, Levine OS, Okoko JB, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005 Mar 26–Apr 1;365(9465):1139–46. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 22.Singleton RJ, Hennessy TW, Bulkow LR, Hammitt LL, Zulz T, Hurlburt DA, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. Jama. 2007 Apr 25;297(16):1784–92. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 23.Hsu HE, Shutt KA, Moore MR, Beall BW, Bennett NM, Craig AS, et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med. 2009 Jan 15;360(3):244–56. doi: 10.1056/NEJMoa0800836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nabors GS, Braun PA, Herrmann DJ, Heise ML, Pyle DJ, Gravenstein S, et al. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine. 2000 Mar 6;18(17):1743–54. doi: 10.1016/s0264-410x(99)00530-7. [DOI] [PubMed] [Google Scholar]
- 25.Briles DE, Hollingshead SK, King J, Swift A, Braun PA, Park MK, et al. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J Infect Dis. 2000 Dec;182(6):1694–701. doi: 10.1086/317602. [DOI] [PubMed] [Google Scholar]
- 26.Giefing C, Meinke AL, Hanner M, Henics T, Bui MD, Gelbmann D, et al. Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J Exp Med. 2008 Jan 21;205(1):117–31. doi: 10.1084/jem.20071168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliveira ML, Areas AP, Campos IB, Monedero V, Perez-Martinez G, Miyaji EN, et al. Induction of systemic and mucosal immune response and decrease in Streptococcus pneumoniae colonization by nasal inoculation of mice with recombinant lactic acid bacteria expressing pneumococcal surface antigen A. Microbes Infect. 2006 Apr;8(4):1016–24. doi: 10.1016/j.micinf.2005.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogunniyi AD, Folland RL, Briles DE, Hollingshead SK, Paton JC. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect Immun. 2000;68(5):3028–33. doi: 10.1128/iai.68.5.3028-3033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]