Abstract
Background
Therapeutic activities to improve mobility often include walking practice and exercises to improve deficits in endurance, strength, and balance. Because walking may also be energy inefficient in people with decreased mobility, another approach is to reduce energy cost by improving timing and coordination (TC) of movement.
Methods
This pilot randomized trial of older adults with slow and variable gait offered two types of therapeutic activity over 12 weeks. One addressed Walking, Endurance, Balance, and Strength (WEBS) and the other focused on TC. Outcomes were energy cost of walking and measures of mobility.
Results
Of 50 participants (mean age, 77.2 ± 5.5 years, 65% women), 47 completed the study. Baseline gait speed was 0.85 ± 0.13 m/s and energy cost of walking was 0.30 ± 0.10 mL/kg/m, nearly twice normal. Both interventions increased gait speed (TC by 0.21 m/s and WEBS by 0.14 m/s, p < .001). TC reduced the energy cost of walking 0.10 ± 0.03 mL/kg/m more than WEBS (p < .001) and reduced the modified Gait Abnormalities Rating Scale 1.5 ± 0.6 more points than WEBS (p < .05). TC had a 9.8 ± 3.5 points greater gain than WEBS in self-reported confidence in walking (p < .01).
Conclusions
In older adults with slow and variable gait, activity focused on TC reduced the energy cost of walking and improved confidence in walking more than WEBS while generating at least equivalent gains in mobility. To optimize mobility, future larger studies should assess various combinations of TC and WEBS over longer periods of time.
Keywords: Exercise, Gait, Energy cost
DIFFICULTY walking results in reduced activity, loss of independence, falls, and injuries (1–4) and has been associated with lower extremity muscle weakness (5–9), poor balance(9,10), and deconditioning (8,11). Therapeutic interventions to improve walking traditionally focus on walking practice and remediation of deficits in strength, balance, and endurance, under the assumption that gains in physiological capacity will contribute to improved walking (10,12–14). Such interventions improve walking speed modestly and may help prevent serious mobility disability (10–13,15–18).
Older adults with difficulty walking also use more energy to walk, which might contribute to the problem (6,19–22). Although normal gait is associated with an energy cost of about 0.15 mL/kg/m (23), gaits with altered timing and posture can double or even triple the cost (21,24,25). Interventions that focus on timing and coordination (TC) of gait might reduce the energy cost of walking. Such interventions are based on concepts of motor learning (26,27) and have been shown to enhance motor skill in animal and human studies (28–32).
This randomized clinical trial was designed to compare effects on mobility and energy cost of walking of two programs of therapeutic activity; (a) gait training with exercise to improve strength, balance, and endurance versus (b) stepping and walking practice that focused on TC of gait.
METHODS
Overview
This 12-week randomized, controlled, single-blind trial compared two forms of therapeutic activity in older adults with objective evidence of walking difficulty. The study was approved by the Pittsburgh Institutional Review Board, and all participants gave informed consent.
Participants
Eligible older adults had mild-to-moderate mobility difficulty based on gait speed and variability. Gait speed was less than or equal to 1.0 and greater than or equal to 0.6 m/s (33). Gait variability was assessed on a pressure-sensitive walkway. Abnormal step length variability was defined as a coefficient of variation (CV) of 4.5% or higher (4). Abnormal step width variability was defined as either too little or too much variability (CV of less than 7% or greater than 30%) because both have been associated with falls risk (34). Participants had to have a Mini-Mental State Examination (35) score greater than or equal to 24.
Measures
All measures were collected at baseline prior to randomization and after 12 weeks of intervention by assessors masked to treatment arm.
Energy cost of walking.—
The energy cost of walking reflects the energy used for all bodily actions during walking (36). Participants walked on a treadmill at a self-selected pace while oxygen consumption data were collected using open circuit spirometry and analysis of expired gases with a VO2000 portable metabolic measurement system, Medgraphics, Minneapolis, MN. All participants had one to two practice sessions to become familiar and comfortable with the treadmill prior to oxygen consumption measurement. The mean rate of oxygen consumption and carbon dioxide production was determined over 3 minutes after steady state was reached (27,36–38). We used two standard methods to estimate cost. The energy cost of walking, reported in mL/kg/m, represents an estimate of energy expenditure per unit of gait speed (23,32,39–43) and relates to metabolic equivalents (METS). It is time independent, repeatable, reflects the physiological cost of gait (36,38,44), is little influenced by fitness (38,44), and can be compared across individuals and over time, regardless of changes in gait speed (38,42,44). The metabolic cost of transport, a dimensionless measure of the energy expenditure of moving a unit weight over a unit distance, was determined from the mean flow rates of oxygen and carbon dioxide collected and the standard equation by Brockway (45–47) to estimate average metabolic power. The average metabolic power was divided by body mass (W/kg), the constant for gravity (9.81 m/s2), and walking speed (m/s) to achieve the dimensionless metabolic cost of transport (W/N/(m/s) (47–49). We estimated total rather than net energy cost. Net estimates require correction for resting energy expenditure, which uses different methods and requires additional testing (50). We were interested in change over time and expected resting energy expenditure to remain constant; thus, we used total energy cost to reduce respondent burden.
Clinical gait assessment.—
The modified Gait Abnormality Rating Scale (GARSM), a seven-item criterion-based, observational rating of gait abnormalities associated with fall risk (51,52) was used to assess gait characteristics. Reliability is excellent (intraclass correlation coefficient 0.95 to 0.99) among experienced assessors. Each item is scored 0–3, for a total score of 0–21. Higher scores reflect poorer performance (51).
Gait speed and variability.—
Participants walked at their usual speed on a 4-m instrumented walkway, the GaitMatII (E.Q. Inc., Chalfont, PA) (53), with 2-m noninstrumented sections at either end to allow for acceleration and deceleration. After two practice walks, two walks were used for data collection. Gait speed was averaged over the two walks. Step length and step width variability were calculated as the CV based on the average standard deviation of all right and left steps over the two walks divided by the mean step length or step width (54,55). The number of steps used to estimate variability is somewhat less than used by some others (3,56–58) but allows for measures of spatial variability during natural walking not on a treadmill and has acceptable reliability (59,60).
Gait Efficacy Scale.—
The Gait Efficacy Scale is a self-reported 10-item scale of perceived confidence in walking for a range of challenges from level walking to walking on uneven surfaces, curbs, or stairs. Item scores range from 1 for no confidence to 10 for complete confidence, with a possible total score of 10–100 (61,62).
Short Physical Performance Battery.—
The Short Physical Performance Battery (SPPB) consists of three tasks: gait speed, balance, and chair stands, using 4-point scales for each and a summary score that ranges from 0 to 12 (63,64). We used the repeated chair rise task and the balance component to reflect changes in lower extremity strength and balance after intervention.
Comorbidity index.—
This is a self-report of having been told by a doctor you have any of 18 common conditions. Eight domains (cardiovascular, respiratory, musculoskeletal, neurological, general, cancer, diabetes, and visual) are derived (65).
Interventions
General.—
Both protocol driven, physical therapist–led interventions for small groups of two to three participants, lasted 60 minutes twice a week for 12 weeks. The interventions were conducted at different times to avoid cross contamination. Therapists were trained and assessed for study protocol adherence initially and periodically. The protocols defined each activity and gave standards for progression based on accuracy and ease of performance. Time spent on walking alone was monitored to be equal between the two treatment arms (Table 1, summary of interventions). Protocols available on request.
Table 1.
Characteristics of Two Types of Therapeutic Physical Activity*
| Timing and Coordination in Walking | Minutes |
| Flexibility (stretching) | 5 |
| Stepping pattern | 25 |
| Forward and backward steps | |
| Step across—forward and backward | |
| Step across step—forward and backward | |
| Walking patterns | 15 |
| Ovals | |
| Spirals | |
| Serpentines | |
| Complex walking patterns | |
| Cross path of others | |
| Opposite direction of others on ovals | |
| With object manipulation—bounce, toss | |
| Treadmill paced walking | 15 |
| 2–3 min usual pace | |
| 30–60 s at 10% increased speed | |
| 1–2 min at usual speed | |
| Repeat pattern up to 3 times | |
| Total min | 60 |
| Walking, Endurance, Balance, and Strength Practice | |
| Flexibility (stretching) | 5 |
| Strength (cuff weights) | 10 |
| Ankle dorsi and plantar flexors | |
| Knee extensors | |
| Hip extensors, abductors, flexors | |
| Gait training | 25 |
| Heel–toe pattern | |
| Lengthen stride and increase speed | |
| Walk from feet, trunk centered or upright | |
| Balance | 5 |
| Standing balance, tandem, one leg | |
| Stand with reach forward, up, down | |
| Stand with ball catch | |
| Endurance | 15 |
| Nustep, stationary cycle | |
| Total min | 60 |
Note: *All activities progress in difficulty according to a standardized protocol with operational definitions of mastery of each stage.
Walking, Endurance, Balance, and Strength (WEBS). This program was based on current physical therapy standards for gait and balance retraining. Sessions began with leg and trunk stretches. Strengthening used progressive resistance in lower extremity muscles, with increasing repetitions to a maximum of 20 followed by increasing resistance using cuff weights. Balance tasks redistributed the center of mass over the base of support (66), with and without destabilizing activities. Endurance was trained either with a seated, stair climbing–like activity (NuStep; NuStep, Inc., Ann Arbor, MI), or a stationary cycle, at a submaximal workload (self-reported rating of perceived exertion (RPE) of 10–13, somewhat hard) (67). Heart rate and blood pressure were monitored according to guidelines (68,69). After participants progressed to the ability to sustain a 10–13 RPE level for 15 minutes, workload was increased. Gait training involved verbal instructions by the therapist to correct abnormalities of gait or posture during walking on an indoor track.
Timing and coordination.—
This program was based on principles of motor learning that enhance “skill” or smooth and automatic movement control (26,30,31,70–72). TC used goal-oriented progressively difficult stepping and walking patterns to promote the TC of stepping, integrated with the phases of the gait cycle (26,30,71,72). Progression was based on increasing aspects such as speed, amplitude, or accuracy of performance prior to undertaking a more complex task that combined these aspects (73). The progression of stepping patterns was (a) self-paced forward and across, (b) increase speed, (c) alternate side, and (d) alternate forward with backward. Walking patterns incorporated sequences of interlimb timing from stepping into walking. Walking patterns progressed by altering speed, amplitude (eg, narrowing oval width), or accuracy of performance (eg, without straying from the desired path) and then to complex patterns such as walking past other walkers and combined upper extremity tasks such as carrying, bouncing, or tossing a ball (72). Rhythmic stepping was reinforced with treadmill pacing. This treadmill walking was done at preferred walking speed with very brief mildly increased speed intervals to train timing, did not raise the RPE, and was not designed to increase endurance (74).
Data Analysis
All statistical analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC). Participant characteristics and baseline measurements were compared between arms using t tests for continuous variables and chi-square tests for categorical variables. Unadjusted comparison of change in outcomes between treatment arms used t tests. To obtain adjusted comparison of outcomes between treatment arms, we fitted an analysis of covariance model using baseline to follow-up change in each outcome as the response variable; treatment arm as the main factor of interest; and age, gender, and baseline value of the outcome as covariates. To detect possible different treatment effects in those with low and high baseline energy cost of walking, we included treatment arm, baseline energy cost (dichotomized at the median into low and high), and treatment arm × baseline energy cost interaction as primary factors of interest and made appropriately constructed contrasts to compare treatment effect within only those with low (high) baseline energy cost.
RESULTS
Of 286 persons screened, 111 underwent onsite screening. Fifty participants met all criteria and were randomized and 47 completed the study (Figure 1). The three dropouts developed medical conditions unrelated to the study and did not differ in baseline characteristics from completers (Table 1).
Figure 1.
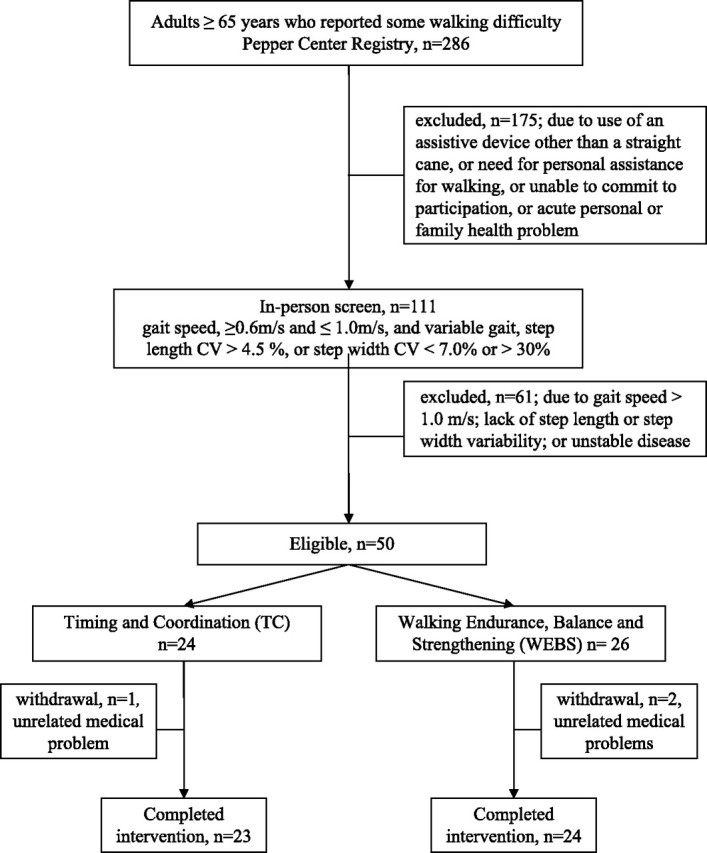
Study flow chart.
Participants had a mean age of 77.2 years and slow and variable gait (Table 2). The baseline mean energy cost of walking was 0.30 mL/kg/m, almost twice the energy cost of normal walking (0.15 mL/kg/m) (23), and the metabolic cost of transport was 0.63, also twice usual (0.34) (47). The mean GARSM rating of gait abnormalities was 6.6, twice that found in community-dwelling older adults without walking problems (51).
Table 2.
Baseline Participant Characteristics by Intervention Group
| Total (n = 50) | Completed Intervention Treatment Group |
Dropout (n = 3) | ||
| TC (n = 23) | WEBS (n = 24) | |||
| Age, y, M (SD) | 77.2 (5.4) | 76.2 (5.5) | 78.4 (5.5) | 76.0 (3.5) |
| MMSE, 0–30, M (SD) | 28.7 (1.4) | 28.5 (1.5) | 28.8 (1.3) | 29.0 (1.7) |
| Comorbidities, number, M (SD)* | 2.6 (1.1) | 2.8 (1.1) | 2.3 (1.2) | 3.3 (0.6) |
| Gender, female, n (%) | 32 (65.3) | 17 (73.9) | 13 (54.2) | 3 (100) |
| Race, black, n (%) | 6 (12.2) | 3 (13.0) | 2 (8.3) | 1 (33.3) |
| Education, ≥high school, n (%) | 33 (67.4) | 16 (69.6) | 15 (62.5) | 2 (66.7) |
| Difficulty walking 2–3 blocks, n (%)† | 34 (72.3) | 17 (73.9) | 17 (70.8) | 2 (66.7) |
| Disease history n (%)* | ||||
| Cardiac | 7 (14.9) | 4 (17.4) | 3 (12.5) | 1 (33.3) |
| Arthritis | 34 (72.3) | 17 (73.9) | 17 (73.9) | 2 (66.7) |
| Osteoporosis | 18 (38.3) | 10 (43.5) | 8 (33.3) | 1 (33.3) |
| Diabetes | 4 (8.5) | 2 (8.7) | 2 (8.7) | 0 (0.0) |
| Depression | 8 (17.0) | 5 (21.7) | 3 (12.5) | 2 (66.7) |
| Vision | 31 (66.0) | 14 (60.9) | 17 (70.8) | 2 (66.7) |
| Hearing | 15 (31.9) | 6 (26.1) | 9 (37.5) | 1 (33.3) |
| Gait speed, m/s, M (SD)‡ | 0.85 (0.13) | 0.88 (0.13) | 0.82 (0.13) | 0.75 (0.20) |
| Step length variability, CV, n (%)§ | 39 (78.0) | 18 (50.0) | 18 (50.0) | 3 (100.0) |
| Step width variability, CV, n (%)‖ | 39 (78.0) | 19 (82.6) | 17 (70.8) | 3 (100.0) |
Notes: *Based on the Comorbidity Index of 8 domains.
In response to query, “How much difficulty do you have walking several blocks”? Dichotomized as none versus any difficulty (a little, some, quite a lot, cannot do).
Between-group mean difference at baseline, marginally significant, p ≤ .12.
n (%) of participants with step length CV greater than 4.5%.
n (%) of participants with step width CV less than 7% or greater than 30%. CV = coefficient of variation; MMSE = Mini-Mental State Examination.
Despite randomization, there were baseline differences between the two treatment arms, with lower energy cost of walking, metabolic cost of transport, and fewer gait abnormalities in the TC compared with the WEBS group. There were nonsignificant but potentially meaningful differences between groups in gender and gait speed. All 47 completers participated in at least 22 of 24 sessions.
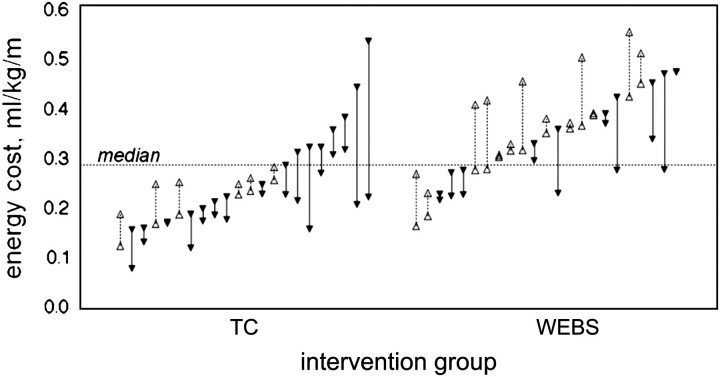
Forty-five of the 47 completers performed both preintervention and postintervention tests of the energy cost of walking. The energy cost of walking decreased in the TC group but did not change in the WEBS group. The adjusted difference in change of energy cost between groups was −0.10 mL/kg/m (p = .0001) Table 3. Similar results and between-group differences were found for the metabolic cost of transport (Table 3). There was a marginally significant interaction between baseline energy cost of walking and intervention group (p = .0587). Among the 21 completers with energy cost below the median (TC n = 14; WEBS, n = 7), there was no evidence of a treatment effect with either intervention (Table 3). Among the 24 participants with energy cost above the median (TC n = 8; WEBS, n = 16), TC reduced energy cost 0.15 mL/kg/m more than WEBS (Table 3). The relation between baseline energy cost and treatment effect was plotted for each individual (Figure 2). Among the participants with higher baseline energy cost, WEBS intervention resulted in four with decreased, four with increased, and eight with no change in energy cost, whereas all eight in the TC intervention reduced energy cost. TC compared with WEBS yielded greater gains in clinical gait assessment and marginally greater gains in gait speed (Table 3). Gait variability did not change with either intervention. Confidence in walking improved 10.8 points in TC but did not change in WEBS (Table 3).
Table 3.
Baseline Status and Postintervention Outcomes by Treatment Group
| Treatment Group* |
Adjusted Group Differences |
|||||||||
| TC |
WEBS |
Adjusted Difference (SE) | ||||||||
| Pre-Mean (SD) | Post-Mean (SD) | Change Mean (SD) | p | Pre-Mean (SD) | Post-Mean (SD) | Change Mean (SD) | p | p | ||
| Energy expenditure | ||||||||||
| Energy cost of walking, mL/kg/m† | 0.26 (0.10) | 0.21 (0.06) | −0.04 (0.10) | .0266 | 0.34 (0.09) | 0.35 (0.10) | 0.01 (0.10) | .6647 | −0.10 (0.03) | .0002 |
| Below Md <0.284 mL/kg/m | 0.20 (0.04) | 0.19 (0.06) | −0.00 (0.05) | .9452 | 0.24 (0.05) | 0.28 (0.09) | 0.04 (0.08) | .1993 | −0.05 (0.04) | .1509 |
| Above Md >0.284 mL/kg/m | 0.37 (0.08) | 0.24 (0.05) | −0.13(0.10) | .0076 | 0.38 (0.06) | 0.38 (0.10) | −0.01 (0.10) | .7861 | −0.15(0.03) | <.0001 |
| Metabolic cost of transport, W/N/(m/s)† | 0.54 (0.21) | 0.44 (0.13) | −0.10 (0.20) | .0232 | 0.71 (0.18) | 0.72 (0.22) | 0.02 (0.21) | .6755 | −0.21(0.05) | .0003 |
| Observed gait and mobility | ||||||||||
| GARSM, 0–21† | 5.7 (2.5) | 3.0 (2.3) | −2.6 (1.5) | <.0001 | 7.4 (2.5) | 6.0 (2.9) | −1.46 (2.1) | .0025 | −1.5 (0.6) | .0221 |
| Gait speed, m/s‡ | 0.88 (0.13) | 1.09 (0.13) | 0.21 (0.14) | <.0001 | 0.82 (0.13) | 0.96 (0.19) | 0.14 (0.15) | .0002 | 0.07 (0.04) | .1038 |
| Self-reported mobility | ||||||||||
| Gait Efficacy Scale, 0–100 | 69.5 (20.2) | 80.3 (13.9) | 10.8 (17.2) | .0065 | 72.6 (13.5) | 71.3 (11.2) | −1.3 (13.8) | .6504 | 9.8 (3.5) | .0081 |
Notes: *n = 23 for TC and 24 for WEBS except for energy expenditure data where n = 22 for TC and 23 for WEBS.
Between-group mean difference at baseline, p ≤ .05.
Between-group mean difference at baseline, p ≤ .12. GARSM = modified Gait Abnormality Rating Scale; TC = Timing and Coordination; WEBS = Walking, Endurance, Balance, and Strength.
Figure 2.
Individual participant change in energy cost from before to after intervention by intervention group, Timing and Coordination (TC) n = 22; Walking, Endurance, Balance, and Strength (WEBS), n = 23.
We assessed the effect of the two interventions on strength and balance using components of the SPPB. Repeated chair rise time is a reflection of lower extremity strength (63,75–77). Chair rise time in seconds changed somewhat, although not significantly more with WEBS than TC (mean change ± SD for TC, −2.08 ± 2.50, p = .0025; −2.44 ± 2.12, p = .0001 for WEBS), supporting a strength training effect of WEBS. The balance component of the SPPB did not change with either intervention (0.04 ± 1.11, p = .852 in TC and 0.13 ± 0.90, p = .503 in WEBS).
DISCUSSION
Among older adults with walking difficulty, a therapeutic activity program based on TC of gait resulted in greater improvement in the energy cost of walking, clinical gait assessment, speed, and perceived confidence in walking compared with a program of gait training based on walking practice and remediation of deficits in endurance, balance, and strength. The treatment effects were greatest in persons with higher energy cost of walking. A focus on TC might help make walking “easier” energetically (31) and appears to offer an additional strategy to reduce walking difficulty. Perhaps, the optimal intervention should combine aspects of both interventions and reduce deficits and improve timing.
In older adults with slow gait speed (eg, <1.0 m/s), prior exercise trials have shown mean improvements in gait speed of about 6% (range, 0%–13%) with strength, flexibility, balance and aerobic exercise training (10,11,13,16,17,78,79), functional training (80), and Tai Chi (81). Few studies have examined the effect of exercise on energy cost of walking. Mian and colleagues (14) found that a 12-month conditioning exercise for healthy older adults improved balance and walking ability but did not change the metabolic cost of walking. Survivors of a stroke who participated in aerobic training improved in mean peak workload capacity more than expected based on the mean increase in maximal oxygen consumption (32,82). The authors attributed the extra gain to increases in gait efficiency (32).
How might reducing the energy cost of walking benefit the older adult with walking difficulty? Consider a typical older adult with chronic stable cardiopulmonary disease and an estimated maximum aerobic capacity of 5 METS or 17.5 mL/kg/min oxygen rate (83). At the mean energy cost of walking and gait speed found in our study (0.30 mL/kg/m, 0.85 m/s), usual walking will consume 15.3 mL/kg/min of oxygen or 4.4 METS, approximately 87.4% of maximum aerobic capacity. If the energy cost of walking improved by 0.10 to 0.20 mL/kg/m, as in this study, then the energy cost of walking at 0.85 m/s is reduced to 10.2 mL/kg/min or 2.91 METS, only 58% of maximum aerobic capacity. In older adults with high energy cost of walking, reducing the energy cost of walking could change the amount of work required to perform daily activities, perhaps reducing fatigue and increasing activity.
This study has substantial strengths. We targeted a highly clinically relevant population with demonstrated deficits in walking and typical comorbid conditions. We adhered to numerous quality standards for clinical trials, including randomization, predefined primary outcome measures, masked outcome assessment, and protocolized exercise interventions. Our dropout rate was low. The interventions should be feasible in clinical practice; they employed low- to moderate-intensity exercise and a relatively low frequency of treatment of twice a week.
The study has limitations as well. The study was powered to detect treatment differences in physiological and performance measures but not in more distal outcomes. We did not directly measure endurance using a 400-m walk or submaximal treadmill test. Despite randomization, treatment groups had differences at baseline. However, because the TC group appeared to have lower energy cost of walking at baseline, several possible sources of bias are unlikely. If regression to the mean influenced the findings, the WEBS group would have tended to greater decreases in energy cost. Because the TC benefit to energy cost appears to be attenuated in persons with lower baseline energy cost and the TC group had more participants with low baseline energy cost, then the overall opportunity to detect change in TC was reduced but was still highly significant. Our TC intervention did not appear to change gait variability. The best way to measure gait variability is not known (57–59,84). Our measures might have been insensitive to change or the TC intervention worked through a mechanism other than gait variability. The TC intervention paced gait using a treadmill. Because the energy cost of walking is measured on a treadmill, familiarity with the treadmill may have biased the measurement of energy cost. We think this is unlikely because all participants had 1–2 treadmill sessions at baseline in order to become familiar with the treadmill and comfortable with testing. We also note that independent measures of gait such as speed and GARSM changed in a way that is consistent with a true chance in energy cost. Finally, there was overlap between the two interventions: both included walking practice for 20–30 minutes per session. The approaches were designed to differ mainly in the approach to gait training. The treadmill was expected to “pace walking” to train sequencing and timing to become more “automatic”, whereas the therapist-led gait training was based on cueing, instructions, and feedback typical in rehabilitation practice.
Interventions that reduce the energy cost of walking have potential to improve gait speed and mobility performance, especially among older adults with an initially high energy cost of walking. Future studies should assess gait coordination interventions, perhaps in combination with other training approaches, on longer term clinical and physiological effects and more distal mobility outcomes.
FUNDING
University of Pittsburgh Older American's Independence Center grants (1 P30 AG024827 and AG023641). J.S.B. is supported by a National Institutes on Aging and American Federation of Aging Research Paul Beeson Career Development Award (1 K23 AG026766-01).
Acknowledgments
An abstract of the preliminary report of the findings was presented at the American Geriatrics Society annual meeting, May 2008, Washington, DC.
References
- 1.Brach JS, Studenski S, Perera S, VanSwearingen JM, Newman AB. Gait variability and the risk of incident mobility disability. J Gerontol A Biol Sci Med Sci. 2007;62A:983–988. doi: 10.1093/gerona/62.9.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hausdorff JM, Edelberg HK, Mitchell SL, Goldberger AL, Wei JY. Increased gait unsteadiness in community-dwelling elderly fallers. Arch Phys Med Rehabil. 1997;78(3):278–283. doi: 10.1016/s0003-9993(97)90034-4. [DOI] [PubMed] [Google Scholar]
- 3.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82(8):1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 4.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear? J Am Geriatr Soc. 1997;45(3):313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 5.Brown M, Sinacore DR, Host H. The relationship of strength to function in the older adult. J Gerontol A Biol Sci Med Sci. 1995;50A:55–59. doi: 10.1093/gerona/50a.special_issue.55. [DOI] [PubMed] [Google Scholar]
- 6.Chandler JM, Duncan PW, Kochersberger G, Studenski S. Is lower extremity strength gain associated with improvement in physical performance and disability in frail, community-dwelling elders? Arch Phys Med Rehabil. 1998;79(1):24–30. doi: 10.1016/s0003-9993(98)90202-7. [DOI] [PubMed] [Google Scholar]
- 7.Buchner DM, Wagner EH. Preventing frail health. Clin Geriatr Med. 1992;8:1–17. [PubMed] [Google Scholar]
- 8.Buchner DM, Beresford SAA, Larson EB, LaCroix AZ, Wagner EH. Effects of physical activity on health status in older adults II: intervention studies. Annu Rev Public Health. 1992;13:469–488. doi: 10.1146/annurev.pu.13.050192.002345. [DOI] [PubMed] [Google Scholar]
- 9.Wolfson L, Judge J, Whipple R, King M. Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol A Biol Sci Med Sci. 1995;50:64–67. doi: 10.1093/gerona/50a.special_issue.64. [DOI] [PubMed] [Google Scholar]
- 10.Judge JO. Balance training to maintain mobility and prevent disability. Am J Prev Med. 2003;25(3 suppl 2):150–156. doi: 10.1016/s0749-3797(03)00178-8. [DOI] [PubMed] [Google Scholar]
- 11.Brown M, Holloszy JO. Effects of a low intensity exercise program on selected physical performance characteristics of 60- to 71-year olds. Aging (Milano) 1991;3:129–139. doi: 10.1007/BF03323989. [DOI] [PubMed] [Google Scholar]
- 12.Fiatarone MA, Marks MA, Ryan EC, Meredith ND, Lipsitz CN, Evans WJ. High intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990;263:3029–3034. [PubMed] [Google Scholar]
- 13.Judge JO, Underwood M, Gennosa T. Exercise to improve gait velocity in older adults. Arch Phys Med Rehabil. 1993;74:400–406. [PubMed] [Google Scholar]
- 14.Mian OS, Thom JM, Ardigo LP, Morse CI, Narici MV, Minetti AE. Effect of a 12-month physical conditioning programme on the metabolic cost of walking in healthy older adults. Eur J Appl Physiol. 2007;100:499–505. doi: 10.1007/s00421-006-0141-9. [DOI] [PubMed] [Google Scholar]
- 15.Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 16.Sauvage LR, Myklebust BM, Crow-Pan J, et al. A clinical trial of strengthening and aerobic exercise to improve gait and balance in elderly male nursing home residents. Am J Phys Med Rehabil. 1992;71:333–342. doi: 10.1097/00002060-199212000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Topp R, Mikesky A, Wigglesworth J, Holt W, Edwards JE. The effect of a 12-week dynamic resistance strength training program on gait velocity and balance of older adults. Gerontologist. 1993;33(4):501–506. doi: 10.1093/geront/33.4.501. [DOI] [PubMed] [Google Scholar]
- 18.LIFE Study Investigators. Effects of a physical activity intervention on measures of physical performance: results of the Lifestyle Interventions and independence for elders pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61A:1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 19.Ferrucci L, Baninelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48(12):1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 20.Fried LP. Conference on the physiologic basis of frailty. Aging Clin Exp Res. 1992;4:251–252. [PubMed] [Google Scholar]
- 21.Malatesta D, Simar D, Dauvilliers Y, Candau R, Borrani F, Prefaut C. Energy cost of walking and gait instability in healthy 65- and 80-yr-olds. J Appl Physiol. 2003;95:2248–2256. doi: 10.1152/japplphysiol.01106.2002. [DOI] [PubMed] [Google Scholar]
- 22.Rantanen T, Guralnik JM, Ferrucci L, et al. Coimpairments as predictors of severe walking disability in older women. J Am Geriatr Soc. 2001;49:21–27. doi: 10.1046/j.1532-5415.2001.49005.x. [DOI] [PubMed] [Google Scholar]
- 23.Waters R. Energy expenditure. In: Perry J, editor. Gait Analysis: Normal and Pathologic Function. Thorofare, NJ: Slack; 2004. pp. 443–489. [Google Scholar]
- 24.Donelan JM, Kram R, Kuo AD. Mechanical work for step-to-step transitions is a major determinant of the metabolic cost of human walking. J Exp Biol. 2002;205(23):3717–3727. doi: 10.1242/jeb.205.23.3717. [DOI] [PubMed] [Google Scholar]
- 25.McGibbon CA, Krebs DE, Puniello MS. Mechanical energy analysis identifies compensatory strategies in disabled elders’ gait. J Biomech. 2001;34:481–490. doi: 10.1016/s0021-9290(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 26.Brooks VB. The Neural Basis of Motor Control. New York: Oxford University Press; 1986. [Google Scholar]
- 27.McArdle WD, Katch FI, Katch VL. Exercise Physiology: Energy, Nutrition, and Human Performance. 5th ed. Baltimore, MD: Lippincott Williams & Williams; 2001. [Google Scholar]
- 28.Anderson BJ, Alcantara AA, Greenough WT. Motor-skill learning: changes in synaptic organization of the rat cerebellar cortex. Neurobiol Learn Mem. 1996;66:221–229. doi: 10.1006/nlme.1996.0062. [DOI] [PubMed] [Google Scholar]
- 29.Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem. 2000;74:27–55. doi: 10.1006/nlme.1999.3934. [DOI] [PubMed] [Google Scholar]
- 30.Daly JJ, Ruff RL. Construction of efficacious gait and upper limb functional interventions based on brain plasticity evidence and model-based measures for stroke patients. Scientific WorldJournal. 2007;7:2031–2045. doi: 10.1100/tsw.2007.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman MA, Dawes H, van den Berg M, Wade DT, Burridge J, Izadi H. Can aerobic treadmill training reduce the effort of walking and fatigue in people with multiple sclerosis: a pilot study. Mult Scler. 2007;13:113–119. doi: 10.1177/1352458506071169. [DOI] [PubMed] [Google Scholar]
- 32.Macko RF, Smith GV, Dobrovolny NA, Sorkin JD, Goldeberg AP, Silver KH. Treadmill training improves fitness reserve in chronic stroke patients. Arch Phys Med Rehabil. 2001;82:879–884. doi: 10.1053/apmr.2001.23853. [DOI] [PubMed] [Google Scholar]
- 33.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. JAGS. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 34.Brach JS, Berlin JE, VanSwearingen JM, Newman AB, Studenski SA. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J Neuroeng Rehabil. 2005;2(21) doi: 10.1186/1743-0003-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 36.Boyd R, Rodda J, Olesch C, et al. High- or low-technology measurements of energy expenditure in clinical gait analysis? Dev Med Child Neurol. 1999;41:676–682. doi: 10.1017/s0012162299001395. [DOI] [PubMed] [Google Scholar]
- 37.Ijzerman KJ, Baardman G. van’t Hof MA, Boom HB, Hermens HJ, Veltnik PH. Validity and reproducibility of crutch force and heart rate measurements to assess energy expenditure of paraplegic gait. Arch Phys Med Rehabil. 1999;80:1017–1023. doi: 10.1016/s0003-9993(99)90054-0. [DOI] [PubMed] [Google Scholar]
- 38.Stott FD, Raftery EB, Goulding L, editors. The Objective Measurement of Physical Performance with Long term Ambulatory Physiological Surveillance Equipment (LAPSE) London: Academic Press; 1979. [Google Scholar]
- 39.Bernardi M, Macaluso A, Sproviero E, et al. Cost of walking and locomotor impairment. J Electromyogr Kinesiol. 1999;9(2):149–157. doi: 10.1016/s1050-6411(98)00046-7. [DOI] [PubMed] [Google Scholar]
- 40.Gersten J, Orr W. External work of walking in hemiparetic patients. Scand J Rehabil Med. 1971;3:85–88. [PubMed] [Google Scholar]
- 41.Macko RF, DeSouza CA, Tretter LD, et al. Treadmill aerobic exercise training reduces the energy expenditure and cardiovascular demands of hemiparetic gait in chronic stroke patients: a preliminary report. Stroke. 1997;28:326–330. doi: 10.1161/01.str.28.2.326. [DOI] [PubMed] [Google Scholar]
- 42.Waters RL, Lunsford BR. Energy cost of paraplegic ambulation. J Bone Joint Surg. 1985;67:1245–1250. [PubMed] [Google Scholar]
- 43.Waters RL, Banres G, Husserl T, Silver L, Liss R. Comparable energy expenditure following arthrodesis of the hip and ankle. J Bone Joint Surg. 1988;70:1032–1037. [PubMed] [Google Scholar]
- 44.Hood VL, Granat MH, Maxwell DJ, Hasler JP. A new method of using heart rate to represent energy expenditure: the total heart beat index. Arch Phys Med Rehabil. 2002;83:1266–1273. doi: 10.1053/apmr.2002.34598. [DOI] [PubMed] [Google Scholar]
- 45.Brockway JM. Derivation of formulae used to calculate energy expenditure in man. Hum Nutr Clin Nutr. 1987;41C:463–467. [PubMed] [Google Scholar]
- 46.Donelan JM, Shipman DW, Kram R, Kuo AD. Mechanical and metabolic requirements for active lateral stabilization in human walking. J Biomech. 2004;37:827–835. doi: 10.1016/j.jbiomech.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Norris JA, Granata KP, Mitros MR, Byrne EM, Marsh AP. Effect of augmented plantarflexion power on preferred walking speed and economy in young and older adults. Gait Posture. 2007;25:620–627. doi: 10.1016/j.gaitpost.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Saibene F, Minetti AE. Biomechanical and physiological aspects of legged locomotion in humans. Eur J Appl Physiol. 2003;88:297–316. doi: 10.1007/s00421-002-0654-9. [DOI] [PubMed] [Google Scholar]
- 49.Donelan JM, Kram R, Kuo AD. Mechanical and metabolic determinants of the preferred step width in human walking. Proc R Soc Lond. 2001;268:1985–1992. doi: 10.1098/rspb.2001.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gleim GW, Stachenfeld NS, Nicholas JA. The influence of flexibility on the economy of walking and jogging. J Orthop Res. 1990;8:814–823. doi: 10.1002/jor.1100080606. [DOI] [PubMed] [Google Scholar]
- 51.VanSwearingen JM, Paschal KA, Bonino P, Yang JF. The Modified Gait Abnormality Rating Scale and recognizing recurrent fall risk of community-dwelling, frail older veterans. Phys Ther. 1996;76:994–1002. doi: 10.1093/ptj/76.9.994. [DOI] [PubMed] [Google Scholar]
- 52.VanSwearingen JM, Paschal KA, Bonino P, Chen T. Assessing recurrent fall risk of community-dwelling, frail older veterans using specific tests of mobility and the Physical Performance Test of function. J Gerontol A Biol Sci Med Sci. 1998;53A(6):M457–M464. doi: 10.1093/gerona/53a.6.m457. [DOI] [PubMed] [Google Scholar]
- 53.Walsh JP. Foot Fall Measurement Technology. In: Craik RL, Oatis CA, editors. Gait Analysis: Theory and Application. St. Louis, MO: Mosby-Year Book; 1995. pp. 125–142. [Google Scholar]
- 54.Brach JS, Berthold R, Craik R, VanSwearingen JM, Newman AB. Gait variability in community-dwelling older adults. J Am Geriatr Soc. 2001;49:1646–1650. doi: 10.1046/j.1532-5415.2001.t01-1-49274.x. [DOI] [PubMed] [Google Scholar]
- 55.Brach JS, Studenski S, Perera S, VanSwearingen JM, Newman AB. Stance time and step width variability have unique contributing impairments in older persons. Gait Posture. 2008;27:431–439. doi: 10.1016/j.gaitpost.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grabiner PC, Biswas T, Grabiner MD. Age-related changes in spatial and temporal gait variables. Arch Phys Med Rehabil. 2001;82(1):31–35. doi: 10.1053/apmr.2001.18219. [DOI] [PubMed] [Google Scholar]
- 57.Owings TM, Grabiner MD. Measuring step kinematic variability on an instrumented treadmill: how many steps are enough? J Biomech. 2003;36(8):1215–1218. doi: 10.1016/s0021-9290(03)00108-8. [DOI] [PubMed] [Google Scholar]
- 58.Owings TM, Grabiner MD. Step width variability, but not step length variability or step time variability, discriminates gait of healthy young and older adults during treadmill locomotion. J Biomech. 2004;37(6):935–938. doi: 10.1016/j.jbiomech.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 59.Brach JS, Perera S, Studenski S. Reliability and validity of measures of gait variability in community-dwelling older adults. Arch Phys Med Rehabil. 2008;89:2293–2296. doi: 10.1016/j.apmr.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perera S, Brach JS, Talkowski JB, Wert D, Studenski SA. Measuring stride time variability: estimating test-retest reliability and required walk length using bootstrapping [abstract] Program & Abstracts of the ISPGR 18th International Conference. 2007:55–56. [Google Scholar]
- 61.McAuley E, Mihalko SL, Rosengren KS. Self-efficacy and balance correlates of fear of falling in the elderly. J Aging Phys Act. 1997;5:329–340. [Google Scholar]
- 62.Rosengren KS, McAuley E, Mihalko SL. Gait adjustments in older adults: activity and efficacy influences. Psychol Aging. 1998;13:375–380. doi: 10.1037//0882-7974.13.3.375. [DOI] [PubMed] [Google Scholar]
- 63.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 64.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rigler SK, Studenski S, Wallace D, Reker D, Duncan PW. Comorbidity adjustments for functional outcomes in community-dwelling older adults. Clin Rehabil. 2002;16(420):428. doi: 10.1191/0269215502cr515oa. [DOI] [PubMed] [Google Scholar]
- 66.Shumway-Cook A, Woollacott MH. Motor Control; Theory and Practical Applications. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 67.Borg GAV. Psychological bases of perceived exertion. Med Sci Sports Exerc. 2007;4:377–381. [PubMed] [Google Scholar]
- 68.Kenney WL, Humphrey RH, Bryant CX, Mahler DA, Froelicher VF, Miller NH, York TD. ACSM’s Guidelines for Exercise Testing and Prescription. 5th ed. Baltimore, MD: Williams & Wilkins; [Google Scholar]
- 69.Gill TM, DiPietro L, Krumholz HM. Role of exercise stress testing and safety monitoring for older persons starting and exercise program. JAMA. 2000;284:342–349. doi: 10.1001/jama.284.3.342. [DOI] [PubMed] [Google Scholar]
- 70.Nelson WL. Physical principles for economies of skilled movements. Biol Cybern. 1983;46:135–147. doi: 10.1007/BF00339982. [DOI] [PubMed] [Google Scholar]
- 71.Lay BS, Sparrow WA, Hughes KM, O’Dwyer NJ. Practice effects on coordination and control, metabolic energy expenditure, and muscle activation. Hum Mov Sci. 2002;21:807–830. doi: 10.1016/s0167-9457(02)00166-5. [DOI] [PubMed] [Google Scholar]
- 72.Gentile A. Skill acquisition: action, movement, and neuromotor processes. In: Carr JH, Shepherd RB, Gordon J, Gentile AM, Held JM, editors. Movement Sciences. Rockville, NI: Aspen Publishers; 1987. pp. 93–154. [Google Scholar]
- 73.Schmidt RA. Organizing and scheduling practice. In: Schmidt RA, editor. Motor Learning and Practice: From Principles to Practice. Champaign, IL: Human Kinetics Books; 1991. pp. 199–225. [Google Scholar]
- 74.Pohl M, Mehrholz J, Ritschel C, Ruckriem S. Speed-dependent treadmill training in ambulatory hemiparetic stroke patients: a randomized controlled trial. Stroke. 2002;33:553–558. doi: 10.1161/hs0202.102365. [DOI] [PubMed] [Google Scholar]
- 75.Jette AM, Jette DU, Ng J, Plotkin DJ, Bach MA. The Musculokeletal Impairment (MSI) Study Group. Are performance-based measures sufficiently reliable for use in multicenter trials? J Gerontol A Biol Sci Med Sci. 1999;54A(1):M3–M6. doi: 10.1093/gerona/54.1.m3. [DOI] [PubMed] [Google Scholar]
- 76.Jones CJ, Rikli RE, Beam W. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70(2):113–119. doi: 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- 77.Rossiter-Fornoff JE, Wolfe SL, Wolfson L, Buchner DM FICSIT Group. A cross-sectional validation study of the FICSIT common data base static balance measures. J Gerontol A Biol Sci Med Sci. 1995;50A(6):M291–M297. doi: 10.1093/gerona/50a.6.m291. [DOI] [PubMed] [Google Scholar]
- 78.Buchner DM, Cress ME, de Lateur BJ, et al. The effects of strength and endurance training on gait, balance, fall risk, and health services use in community-living older adults. J Gerontol A Biol Sci Med Sci. 1997;52A(4):M218–M224. doi: 10.1093/gerona/52a.4.m218. [DOI] [PubMed] [Google Scholar]
- 79.Helbostad JL, Sletvold O, Moe-Nilssen R. Home training with and without additional group training in physically frail older people living at home: effect on health-related quality of life and ambulation. Clin Rehabil. 2004;18:498–508. doi: 10.1191/0269215504cr761oa. [DOI] [PubMed] [Google Scholar]
- 80.Manini T, Marko M, VanArnam T, et al. Efficacy of resistance and task-specific exercise in older adults who modify tasks of everyday life. J Gerontol A Biol Sci Med Sci. 2007;62A:616–623. doi: 10.1093/gerona/62.6.616. [DOI] [PubMed] [Google Scholar]
- 81.Wolf SL, O’Grady M, Easley KA, Guo Y, Kressig RW, Kutner M. The influence of intense Tai Chi training on physical performance and hemodynamic outcomes in transitionally frail, older adults. J Gerontol A Biol Sci Med Sci. 2006;61A:184–189. doi: 10.1093/gerona/61.2.184. [DOI] [PubMed] [Google Scholar]
- 82.Potempa K, Lopez M, Braun L, Szidon P, Fogg L, Tincknell T. Physiological outcomes of aerobic exercise training in hemiparetic stroke patients. Stroke. 1995;26(1):101–105. doi: 10.1161/01.str.26.1.101. [DOI] [PubMed] [Google Scholar]
- 83.Fletcher GF, Balady GJ, Amsterdam EA, et al. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104:1694–1740. doi: 10.1161/hc3901.095960. [DOI] [PubMed] [Google Scholar]
- 84.Hausdorff JM, Ashkenazy Y, Peng CK, Ivanov PC, Stanley HE, Goldberger AL. When walking becomes random walking: fractal analysis and modeling of gait rhythm fluctuations. Physica A. 2001;302(1–4):138–147. doi: 10.1016/s0378-4371(01)00460-5. [DOI] [PubMed] [Google Scholar]