Abstract
The role of tumor estrogen receptors (ERs) and serum estrogen in lung cancer is inconclusive. We investigated the hypothesis that ERs and functional single-nucleotide polymorphisms in the estrogen biosynthesis pathway are associated with poorer lung cancer survival. Lung cancer patients (n = 305) from a National Cancer Institute-Maryland (NCI-MD) case–case cohort in the Baltimore metropolitan area were used as a test cohort. To validate, 227 cases from the NCI-MD case–control cohort and 293 cases from a Norwegian lung cancer cohort were studied. Information on demographics, tobacco and reproductive histories was collected in an interviewer-administered questionnaire. Serum estrogen, progesterone, tumor messenger RNA expression of hormone receptors and germ line DNA polymorphisms were analyzed for associations with lung cancer survival. Patients in the highest tertile of serum estrogen had worse survival in all three cohorts (P combined < 0.001). Furthermore, the variant allele of estrogen receptor alpha (ER-α) polymorphism (rs2228480) was significantly associated with increased tumor ER-α levels and worse survival in all three cohorts [hazard ratio (HR) = 2.59, 95% confidence interval (CI): 1.20– 4.01; HR = 1.76, 95% CI: 1.08–2.87 and HR = 2.85, 95% CI: 1.31–4.36). Other polymorphisms associated with lower serum estrogen correlated with improved survival. Results were independent of gender and hormone replacement therapy. We report a significant association of increased serum estrogen with poorer survival among lung cancer male and female patients. Understanding the genetic control of estrogen biosynthesis and response in lung cancer could lead to improved prognosis and therapy.
Introduction
Lung cancer is the leading cause of cancer death both in the USA and worldwide (1–3). Overall, the 5 years of survival rate for lung cancer is only 16%. Therefore, it is vital to both prevent lung cancer and improve its therapy.
The lung cancer rate has been declining among men in the USA; however, it has reached a plateau among women after steadily increasing over the last 40 years (1). Because of this increase among women and gender differences in prognosis for the same histological type (4,5), the possible role in lung cancer of both exogenous and endogenous estrogens, along with estrogen receptors (ERs) has been investigated. Estrogens stimulate growth in both normal lung epithelial cells (6) and lung tumor cells (6–9). Also, similar to breast cancer, the aromatase enzyme is critical in the synthesis of estrogens in the lung (7). Aromatase is active in normal lung tissue, lung cancer cell lines and lung tumors, with the highest level of activity in tumors (7,9,10). Research on the role of ER expression in lung cancer has been inconclusive with high levels of ER expression detected in lung cancer patients in some studies (11–14) and non-detectable expression (15,16) or very low levels (6,17) in other studies. Both isoforms of ER, ER-alpha (ER-α) and estrogen receptor-beta (ER-β) have been detected in lung cancer tissue and normal lung cells (6,7,18,19).
Estrogen synthesis is a complex pathway (Figure 1) in which the aromatase enzyme, encoded by the CYP19 gene, plays a key role in converting androstenedione and testosterone into estradiol and estrone (21). Thus, the enzyme, 17α-hydroxylase/17,20-lyase, which is encoded by the CYP17 gene, is critical because it catalyzes the production of androstenedione and dehydroepiandrosterone from 17-hydroxyprogesterone and 17-hydroxypregnenolone (21,22). The enzyme, 3β-hydroxysteroid dehydrogenase, which catalyzes the conversion of pregnenolone into progesterone, 17-hydroxypregnenolone into 17-hydroxyprogesterone or dehydroepiandrosterone into androstenedione (23) is also significant in this pathway as progesterone is a necessary precursor to estrogen biosynthesis. Other essential enzymes in this pathway include 17β-hydroxysteroid dehydrogenase type 1, which catalyzes the production of estradiol from estrone (21) and catechol-O-methyltransferase (COMT), which catalyzes the conversion of the carcinogenic metabolite of estradiol (4-hydroxyestradiol) into 4-methoxyestradiol (24).
Fig. 1.
The estrogen biosynthesis pathway. The pathway from cholesterol through estrogen synthesis and metabolism is shown with the enzymes involved. Bolded enzymes are those in which SNPs were investigated in relation to estrogen measures and lung cancer survival (20).
In the present study, we investigated whether serum estrogen levels and tumor ER-α, ER-β and progesterone receptor (PR) gene expression were associated with lung cancer survival in three independent cohorts. In addition, we examined the role of single-nucleotide polymorphisms (SNPs) in genes in the estrogen biosynthesis pathway with lung cancer survival.
Materials and methods
Study design and eligibility criteria
National Cancer Institute-Maryland case–case cohort.
Lung cancer patients were recruited at the time of surgery from hospitals in the greater Baltimore metropolitan area, including the University of Maryland, Baltimore Veterans Administration, Saint Agnes, North West Hospital Center, Sinai, Mercy and Union Memorial Hospitals as described previously (25). The study was approved by the Institutional Review Boards of the National Cancer Institute (NCI) and all the participating hospitals.
The initial case-only cohort consisted of women and men with histologically confirmed primary non-small-cell lung cancer based on the World Health Organization guidelines, who were self-reported African–American or Caucasian as described previously (25). Three-hundred and seventy patients were initially eligible for the case-only study. Nineteen patients refused (16 personal refusals and 3 physician refusals) and 43 patients did not have paraffin-embedded tissue available for the study. Therefore, the final case–case cohort consisted of 308 patients, 103 women and 205 men. In addition, for this report, we eliminated two women that were premenopausal and one woman with unknown menopausal status, so the final cohort consisted of 100 postmenopausal women and 205 men.
Subjects were administered a structured interview after informed consent was obtained. Eighty percent of the interviews were in-person with the patient and 20% were administered to a family member. The interview had questions about demographic history, medical and cancer history, medication use, tobacco use, alcohol use, family medical and cancer history, adult passive smoke exposure, reproductive history and occupational history. Passive smoke exposure was assessed by self-report. Patients were asked if anyone smokes cigarettes in their home, if they are still smoking in the home, how long ago someone smoked in the home, the average number of cigarettes smoked in the home and how many years they smoked in the home. Workplace passive smoke exposure was also assessed by self-report. Patients were asked if they were employed at a job for >5 years where coworkers smoked cigarettes in their immediate area along with how many years they were employed at this job. Blood was also obtained at this time and stored at the Laboratory of Human Carcinogenesis at the NCI at −80°C until use.
National Cancer Institute-Maryland case–control cohort.
Lung cancer patients were recruited from hospitals in the greater Baltimore metropolitan area as described previously (26). Two hundred and twenty-seven cases from the ongoing National Cancer Institute-Maryland (NCI-MD) case–control cohort were selected after evaluation for sufficient DNA for genotyping. Never-smokers smoked <100 cigarettes in their lifetime. Former-smokers reported quitting more than a year before the date of diagnosis. Current-smokers continued to smoke or quit smoking less than a year since the lung cancer diagnosis. The study was approved by the Institutional Review Board of the NCI, the University of Maryland Medical System, the Baltimore VA Medical Center, the Johns Hopkins University School of Medicine, Sinai Hospital, MedStar Research Institute and the Research Ethics Committee of Bon Secours Baltimore Health System.
Eligibility criteria have been described previously (26). Briefly, eligibility criteria included being free of known diagnosis of human immunodeficiency virus, hepatitis C virus and hepatitis B virus; a United States citizen, a resident of Baltimore City or adjacent counties of Maryland or the Maryland Eastern Shore; English-speaking; non-institutionalized; not currently taking antibiotics or steroid medications and for cases, not having been interviewed as a control for the study and being within 2 years of diagnosis. Next-of-kin interviews were not conducted when the study participant was deceased. After informed consent was obtained, in-person interviews were administered by a trained interviewer to obtain information on prior medical and cancer history, tobacco use, occupational, spousal and childhood secondhand smoke exposure, family medical history and socioeconomic history. Passive smoke exposure was assessed by self-report. Patients were asked whether anyone smoked cigarettes in their home, along with how many people smoked in the home, their relationship to the patient, whether they smoked lightly, moderately or heavily, the number of cigarettes they smoked in the home, how long they have been smoking in the home and whether they have stopped smoking in the home (and if yes, how long ago). Exposure in the workplace was also assessed by self-report. Patients were asked whether they were exposed to cigarette smoke in the workplace in the last 48 h, if they worked at a job for >5 years where they were exposed to cigarette smoke, how many years they worked at this job and what level of smoke they were exposed to at the job. Blood was obtained by the interviewers at this time and immediately placed on ice. Buffy coat was then separated from red cells and plasma and all were stored at −70°C. Frozen blood components were sent to the Laboratory of Human Carcinogenesis at the NCI and stored at −70°C until use.
Norwegian case-only cohort.
The cohort had 282 lung cancer cases that were recruited at the time of surgery for non-small-cell lung cancer at Haukeland University Hospital in Bergen between 1986 and 2007. The participation rate was 95%. All patients were Caucasians of Norwegian origin. They were interviewed by trained health personnel using standardized questionnaires. Current-smokers were those who were still smoking at the time of recruitment or had stopped smoking less than a year before recruitment. Former-smokers reported quitting more than a year before recruitment and never-smokers smoked <100 cigarettes during their lifetime. The date and cause of death were obtained from Statistics Norway after approval by The Data Inspectorate. Data from Statistics Norway rely on information from the death certificates and from the Cancer Registry of Norway. Lung cancer was registered as the cause of death when stated as the immediate or underlying cause of death on the death certificate. Any mention of lung cancer or another solid cancer death within 2 years of diagnosis on a death certificate was treated as death from lung cancer. Lung cancer was registered as cause of death when stated to be the immediate or underlying cause of death on the death certificate. Blood was collected at the time of surgery. The lung cancer cases were drawn from the lung cancer biobank at the National Institute of Occupational Health (NIOH) Norway. This biobank is authorized for examining genetic variations and biological processes in lung cancer of importance for lung cancer prevention, early diagnosis and prognosis.
Serum estrogen and progesterone analysis
In the NCI-MD case–case cohort, serum samples were available from 191 (63%) of the cases (126 males and 65 females). The initial study only required paraffin-embedded tissue from the patient, therefore, not all study participants had available serum. Cases without serum samples were not significantly different than cases with serum samples in race, age, gender, smoking status or pack-years (data not shown). Serum samples were available for all 227 participants from the NCI-MD case–control cohort and 293 from the Norwegian case-only cohort; however, several estrogen levels were below the detectable limit of the assay (n = 8 and n = 16, respectively. Using 800 μl of the available serum samples, serum estrogen was measured using an immunoassay from the Immulite 2000 of Diagnostic Products Corporation per the manufacturer’s instructions. Serum estrogen was calculated as picogram per milliter. Tertiles were calculated in each study based on the distribution values.
The assay was specific for estradiol, estriol and estrone; therefore, all three principal forms of estrogen were detected by the assay. The cross-reactivity for estradiol was 100%, whereas the cross-reactivity for estriol was 81% and for estrone was 69%. All samples were run in duplicate and all batches contained blinded duplicates. Data regarding hormone therapy were missing from 23 women (23%). Nineteen of the 100 women (19%) from the NCI-MD case–case cohort reported being on hormone therapy at the time of hospital admission. The majority of these women (88%) who took estrogen therapy reported taking Premarin, a conjugated equine estrogen that is converted by the body to active estrogens, mainly estrone. Therefore, the assay detected serum estrogen from estrogen therapy and endogenous production from aromatization. Of the 19 women on estrogen therapy at the time of blood collection, 5 did not have serum for analysis of hormone levels; 1 had serum estrogen levels below the detectable limit and 3 were in the second tertile of estrogen levels, with the remaining women in the third tertile of estrogen levels. All samples were handled in the same manner.
Data on hormone therapy were unknown or missing from 32 (30%) of the women in the NCI-MD case–control cohort. Only two (3%) women from the NCI-MD cohort reported being on hormone therapy at the time of interview. Both reported taking estrogen pills such as Premarin, Estrace, Estratab or Ogen. Of these two women, one woman was in the second tertile of serum estrogen levels while one was in the third tertile. No data were available on hormone replacement therapy in the Norwegian case-only cohort.
All the women from the NCI-MD case–case cohort were postmenopausal as defined by not having a menstrual period within the past 6 weeks due to natural menopause (n = 48) or a previous hysterectomy (n = 52). The ages of women in this cohort ranged from 38 to 84 with a mean age of 65. In the NCI-MD case–control cohort, women were postmenopausal as defined by reporting no menstruation within the past 6 weeks as a result of a doctor telling them they underwent menopause (n = 47) or having a hysterectomy (n = 28). The remaining two women were over the age of 65, therefore postmenopausal status was assumed. The ages of women in this cohort ranged from 43 to 82 with a mean age of 66. There were no data collected from the Norwegian case-only cohort on menopausal status. The ages of women in this cohort ranged from 36 to 81 with a mean age of 66.
Circulating progesterone was also examined in all three cohorts on the Immulite 2000. The lower detection limit for the progesterone assay was 0.20 ng/ml. In the NCI-MD case–case cohort, two (2%) women reported ever taking Prempro, a combination of estrogen plus progesterone. Six (8%) women in the NCI-MD case–control cohort reported ever taking progesterone pills such as Provera and nine (12%) reported ever taking estrogen and progesterone pills such as Prempro; however, none were still on the pills during the study. Similar to the estrogen assay, all samples were run in duplicate. In the NCI-MD case–case cohort, 11 patients (4%) had circulating progesterone levels below the detectable limit. Eleven (5%) and five (2%) cases had circulating progesterone levels below the detectable limit in the NCI-MD case–control cohort and Norway case-only cohort, respectively. Based on the distribution values, circulating progesterone was divided into tertiles for statistical analysis.
ER-α, ER-β and PR expression in lung cancer tissue
In the NCI-MD case–case cohort, tissue samples were available from 178 patients (58%) (119 males and 59 females). These patients were not significantly different from the cases without tissue samples available in terms of race, age, gender, smoking status or pack-years. Frozen tissue was not collected from every participant, as the original study only required having paraffin-embedded tissue. All samples were handled in the same manner. Using these lung cancer samples, ER-α, ER-β and PR expression were examined utilizing quantitative reverse transcription polymerase chain reaction Taqman assays to measure messenger RNA (mRNA). Samples were measured in triplicate using 18S mRNA as the control. All samples amplified for each receptor. Primers and probes were developed that crossed the exon splice site and are as follows:
Primers: ERα-F—5′-ACTTGCTCTTGGACAGGAACCA-3′ and ERα-R—5′-CAAACTCCTCTCCCTGCAGATT-3′, ERβ-F—5′-GCCGACAAGGAGTTGGTACAC-3′ and ERβ-R—5′-AACAGGCTGAGCTCCACAAAG-3′, PR-F—5′-AGTTCTTTGCTGACAAGTCTTAATCAAC-3′ and PR-R—5′-TCGAAAACCTGGCAATGATTTAG-3′; Probes: ERα-probe—5′-TGGCTACATCATCTCGGTTCCGCA-3′, ERβ-probe—5′-TGATCAGCTGGGCCAAGAAGATTCCC-3′ and PR-probe—5′-AGGCGAGAGGCAACTTCTTTCAGTAGTCAAG-3′.
In the NCI-MD case–control cohort, blood was routinely collected, however, tissue was only collected if the patient went to surgery. The majority of these patients did not go to surgery because they were diagnosed at a late stage; therefore, there was insufficient tissue to examine ER-α, ER-β and PR mRNA levels in this study.
In the Norwegian case-only cohort, RNA was available from only 66 of the cases. Total RNA was extracted from lung tumor tissue using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The same primers and probes were used as described for the NCI-MD case–case cohort in similar Taqman assays.
ER-α expression was divided into tertiles based on the distribution values in that cohort with the lowest tertile classified as ER-α mRNA negative and the highest two tertiles classified as ER-α mRNA positive. ER-β and PR expression were classified in the same manner as ER-α.
DNA isolation and genotyping
DNA was available for ∼80, 100 and 90% of the participants in the NCI-MD case–case cohort, the NCI-MD case–control cohort and the Norwegian case-only cohort, respectively, and was extracted using a phenol–chloroform extraction. There was no difference from cases without available DNA in race, age, gender, smoking status or pack-years (data not shown). In the NCI-MD case–case cohort, some participants did not have available DNA due to depletion of DNA by analysis in other studies. Genotyping was performed at the NCI-Core Genotyping Facility or at Bioserve Biotechnologies (Beltsville, MD) using assays described on the SNP500 website (http://snp500cancer.nci.nih.gov). All genotyping assays contained positive and negative controls as well as at least 10% blinded and randomized duplicates. The following SNPs were analyzed: COMT-01 (rs4680), CYP17a1-01 (rs743572), CYP17a1-05 (rs6162), CYP19a1-01 (rs700518), CYP19a1-06 (rs1065779), CYP19a1-08 (rs4646), CYP19a1-09 (rs10046), CYP19a1-14 (rs767199), ESR1-01 (rs2077647), ESR1-07 (rs2228480), HSD3B1-03 (rs6201) and HSD17B1-03 (rs2830). The genotype concordance for each SNP was at least 99% among duplicates. These SNPs were selected to represent pathways specific to estrogen metabolism (Figure 1).
Statistical analyses
There were no statistically significant differences in genotype frequencies between Caucasians and African–Americans in the NCI-MD case–case cohort or the NCI-MD case–control cohort, therefore, analyses were adjusted for, not stratified by, race. All the participants in the Norwegian case-only cohort were Caucasians.
Cause of death and date of death were obtained by linkage to death certificate data in the National Death Index for the NCI-MD case–case and NCI-MD case–control cohorts and as described earlier for the Norway case-only cohort. Patients were categorized as ‘alive’ or ‘dead’ based on survival status 5 years following diagnosis.
Kaplan–Meier survival curves were computed to illustrate differences in survival based on serum estrogen levels, tumor ER-α and ER-β expression levels, ESR1-07, CYP19a1-08 and HSD17B1-03 genotypes. Survival curves were calculated for 5 years of survival rates.
Cox proportional hazards modeling was used to calculate the hazard ratios (HRs) for lung cancer survival associated with serum hormone levels. The models were adjusted for potential confounding variables including pack-years of smoking, smoking status, age, gender, race (NCI-MD case–case cohort and NCI-MD case–control study) and tumor stage. When the variable changed the β-coefficient by at least 5%, the parameter was classified as a confounding variable. The associations of individual SNPs with tumor ER-α, serum estrogen, tumor ER-β and tumor PR were assessed using one-way analysis of variance after adjustment for confounding variables. Cox proportional hazards models were also used to examine the effect of estrogen-related genotypes on lung cancer survival with adjustment for the same confounders as above, pack-years of smoking, age, gender, race and stage. Staging was calculated using the TNM Classification of Malignant Tumours staging system (27). Participants with missing values for any variables in the Cox proportional hazards models were omitted from the analysis. The Bonferroni-adjusted P values were calculated by multiplying the P-value by the number of SNPs examined (n = 12). All of the above analyses were conducted using Stata Statistical Software (28).
Results
Study population
The overall mean age for lung cancer patients did not differ across the three cohorts (Table I), although there was a slight difference in age among men across cohorts when stratified by gender (P = 0.03). Most of the cases from the NCI-MD case–case cohort and the NCI-MD case–control cohort were Caucasian (77%). Cases from the Norwegian case-only cohort were all Caucasians. Smoking status differed across the cohorts (P = 0.001). Fifty-nine percent of the Norwegian cases were current-smokers, whereas only 46% of the cases from the NCI-MD case–control cohort were current-smokers. There was a significant difference in pack-years smoked among participants in the three cohorts with a higher mean duration in the NCI-MD case–case cohort than the Norwegian case-only cohort.
Table I.
Demographic characteristics of study participants
| NCI-MD case–case study (%), n = 305 | NCI-MD case-control (%), n = 227 | Norwegian case-only study (%), n = 282 | P-value | |
| Age | ||||
| Overall mean (SD) | 64.5 (9) | 65.9 (10) | 66.2 (10) | 0.11 |
| Mean (SD) for women | 64.8 (9) | 65.8 (10) | 64.6 (10) | 0.71 |
| Mean (SD) for men | 64.4 (9) | 66.0 (10) | 67.2 (10) | 0.03 |
| Gender (%) | ||||
| Male | 205 (67) | 151 (67) | 178 (63) | |
| Female | 100 (33) | 76 (33) | 104 (37) | 0.55 |
| Race (%) | ||||
| Caucasian | 235 (77) | 175 (77) | 282 (100) | |
| Males | 157 (67) | 116 (66) | 178 (63) | |
| Females | 78 (33) | 59 (34) | 104 (37) | |
| African–American | 70 (23) | 52 (23) | 0 (0) | |
| Males | 48 (69) | 35 (67) | 0 (0) | |
| Females | 22 (31) | 17 (33) | 0 (0) | <0.001 |
| Smoking history (%)a | ||||
| Never-smoker | 9 (3) | 13 (6) | 19 (7) | |
| Former-smoker | 121 (40) | 109 (48) | 87 (31) | |
| Current-smoker | 175 (57) | 105 (46) | 166 (59) | 0.001 |
| Mean pack-years + SD | 60.2 (31) | 53.9 (27) | 32.5 (18.3) | <0.001 |
| Tumor stage (%)b | ||||
| Stage I | 188 (62) | 66 (29) | 165 (58) | |
| Stage II | 56 (18) | 23 (10) | 59 (21) | |
| Stage III | 51 (17) | 38 (17) | 41 (15) | |
| Stage IV | 5 (2) | 32 (14) | 3 (1) | <0.001 |
Values given in bold are statistically significant.
Ten cases (3%) from the Norwegian case-only study were missing data on smoking status.
Five cases (1%) from the NCI-MD case–case study; 68 (30%) cases from the NCI-MD case–control study and 14 cases (5%) from the Norwegian case-only study were missing data on tumor stage.
Serum estrogen, tumor ER-α and lung cancer survival
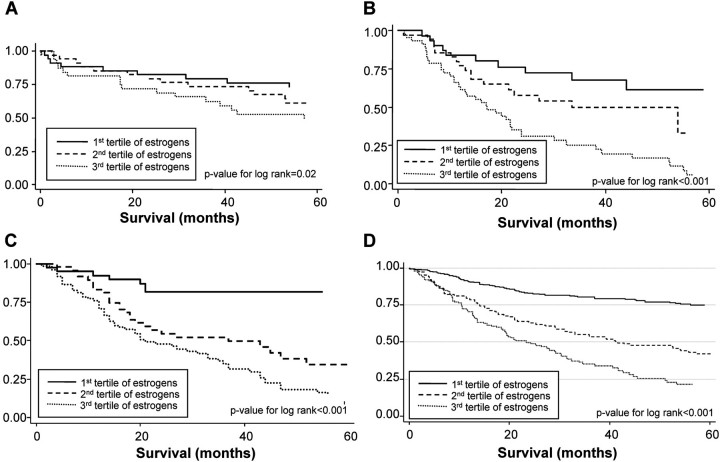
We observed a consistent monotonic trend in higher levels of serum estrogen and poorer survival in the NCI-MD case–case cohort, the NCI-MD case–control cohort and the Norwegian case-only cohort (Figure 2 and Table II, P = 0.001, P < 0.001 and P < 0.001, respectively). The normal physiological levels for estradiol in men range from 14 to 55 pg/ml, whereas for postmenopausal women, it ranges from 10 to 35 pg/ml (29). In all three cohorts, the highest tertile, which was significantly associated with a worse prognosis, was in the upper area of the physiological range (Table II). In all three cohorts, 3% of the men were slightly above the normal physiological range, whereas 10% of the women were in all three cohorts (supplementary Table I is available at Carcinogenesis Online). In addition, in the NCI-MD case–case cohort lung cancer patients that were tumor ER-α mRNA positive had worse survival than those who were tumor ER-α mRNA negative (supplementary Figure 1 is available at Carcinogenesis Online) (HR = 3.53, 95% CI: 1.17–10.62); however, this association was not significant in the Norwegian case-only cohort (HR = 1.22, 95% CI: 0.78–1.90). Patients that were tumor ER-β positive had slightly shorter lung cancer survival than those that were positive in the NCI-MD case–case cohort (P for log-rank = 0.04); however, this was not significant in the Norwegian case-only cohort (P for log-rank = 0.85) (supplementary Figure 2 is available at Carcinogenesis Online). The associations observed between serum estrogen and tumor ER-α mRNA levels with lung cancer survival were independent of gender in all three cohorts (supplementary Tables I and II are available at Carcinogenesis Online). Both increased serum estrogen (supplementary Table I is available at Carcinogenesis Online) and being tumor ER-α positive (supplementary Table II is available at Carcinogenesis Online) was associated with poor prognosis among both men and women. Because the assay for serum estrogen would also detect estrogen present from estrogen therapy, we repeated the analysis in the NCI-MD case–case cohort with removal of the 19 women patients who were currently taking estrogen therapy and in the NCI-MD case–control cohort with the removal of the two patients who were currently taking estrogen therapy (supplementary Table III is available at Carcinogenesis Online). Higher levels of serum estrogen remained associated with poorer lung cancer survival among women not currently taking estrogen therapy.
Fig. 2.
Kaplan–Meier 5 years of cancer survival curves for the association of serum estrogen with lung cancer survival. Serum estrogen is categorized by tertiles and the P-values were calculated for differences in survival curves using the log-rank test. (A) Serum estrogen and lung cancer survival in the NCI-MD case–case cohort (P = 0.02). (B) Serum estrogen and lung cancer survival in the NCI-MD case–control cohort (P < 0.001). (C) Serum estrogen and lung cancer survival in the Norwegian case-only cohort (P < 0.001). (D) Serum estrogen and lung cancer survival in all three cohorts combined (P < 0.001).
Table II.
Multivariate analysis of serum estrogen levels and lung cancer survival
| NCI-MD case–case study |
NCI-MD case–control study |
Norwegian case-only study |
|||||||
| n (%) | HR (95% CI)a | P-value | n (%) | HR (95% CI)a | P-value | n (%) | HR (95% CI)b | P-value | |
| Serum estrogen (tertiles)c | |||||||||
| First tertile (pg/ml) | 59 (31) | 1.00 (reference) (6–21) | 73 (33) | 1.00 (reference) (5–20) | 95 (36) | 1.00 (reference) (5–19) | |||
| Second tertile (pg/ml) | 66 (35) | 1.39 (1.21–1.73) (22–31) | 0.003 | 80 (37) | 1.65 (0.71–3.87) (21–32) | 0.248 | 86 (32) | 2.28 (1.14–4.19) (20–28) | 0.005 |
| Third tertile (pg/ml) | 66 (35) | 1.66 (1.38–2.16) (32–91) | 0.030 | 66 (30) | 3.87 (1.92–7.81) (33–73) | <0.001 | 85 (32) | 5.17 (2.88–9.02) (29–96) | <0.001 |
| P-trend | 0.001 | <0.001 | <0.001 | ||||||
Adjusted for smoking status, pack-years, age, gender, race and tumor stage.
Adjusted for smoking status, pack-years, age, gender and tumor stage.
n = 114 in the NCI-MD case–case study were missing data on serum estrogen levels because no serum was available; n = 8 in the NCI-MD case–control study and n = 16 in the Norwegian case-only study had serum estrogen levels below the detectable limits of the assay and were not included in the analysis.
Estrogen-related genetic polymorphisms, tumor ER-α mRNA levels and serum estrogen
SNPs in genes involved in the estrogen biosynthesis pathway were investigated to assess whether they were associated with tumor ER-α mRNA levels, PR mRNA levels or serum estrogen to provide evidence of functionality. Several SNPs in the aromatase gene were associated with serum estrogen levels (supplementary Table IV is available at Carcinogenesis Online). Specifically, the variant genotypes of CYP19a1-01, CYP19a1-08 and CYP19a1-14, which are in linkage disequilibrium (supplementary Figure 3 is available at Carcinogenesis Online), were all associated with lower levels of serum estrogen compared with the referent genotype. The variants of CYP19a1-01, CYP19a1-08 and CYP19a1-14 were significantly associated with lower serum estrogen levels in all three cohorts. The same trend was observed in CYP19a1-06 and CYP19a1-09; however, the P for trend was not statistically significant in all three cohorts.
ESR1-07, a SNP in exon 8 of the ER-α gene, was associated with tumor ER-α mRNA expression levels in the NCI-MD case–case cohort such that the variant genotype was associated with higher levels of tumor ER-α mRNA compared with the wild-type genotype (P < 0.0001) (supplementary Table V is available at Carcinogenesis Online). The same trend was observed with this SNP in the Norwegian case-only cohort although it did not reach statistical significance (P = 0.06). This SNP was not associated with serum estrogen levels. The variant genotype of ESR1-07 was also associated with increased expression levels of tumor PR mRNA, an ER-α-regulated gene, in the NCI-MD case–case cohort (P < 0.0001) and the Norwegian case-only study (P = 0.003).
Estrogen-related polymorphisms and lung cancer survival
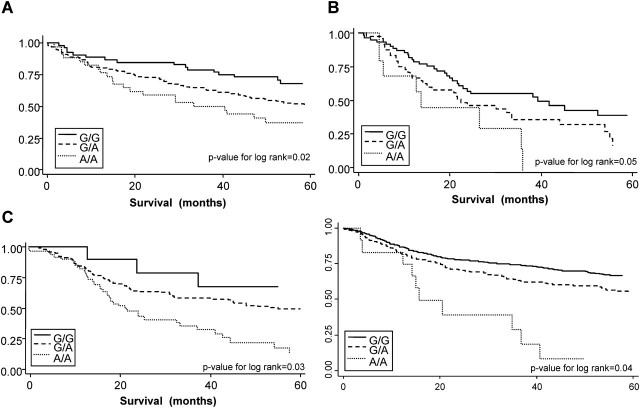
The estrogen-related polymorphisms that were significantly associated with either serum estrogen (supplementary Table IV is available at Carcinogenesis Online) or tumor ER-α mRNA expression (supplementary Table V is available at Carcinogenesis Online) were evaluated for associations with lung cancer survival (Table III). Of those 12 SNPs, 3 were significantly associated with lung cancer survival (Table III). The variant genotype of CYP19a1-08, which was significantly associated with lower serum estrogen levels in all three cohorts was also associated with better lung cancer survival in all three cohorts (HR = 0.41, 95% CI: 0.19–0.88, HR = 0.62, 95% CI: 0.30–1.26 and HR = 0.46, 95% CI: 0.23–0.88) including when all three cohorts were combined (HR = 0.65, 95% CI: 0.48–0.88) (supplementary Figure 4 is available at Carcinogenesis Online). The variant genotype of ESR1-07, which was significantly associated with increased expression levels of tumor ER-α mRNA, was also significantly associated with poorer survival in all three cohorts (HR=2.59, 95% CI: 1.20–4.01, HR = 1.76, 95% CI: 1.08–2.87 and HR = 2.86, 95% CI: 1.32–4.36) including all three cohorts combined (HR = 2.47, 95% CI: 1.15–3.88) (Figure 3). Finally, the variant genotype of HSD17B1-03 was associated with better lung cancer survival compared with the wild-type genotype in all three cohorts (HR = 0.64, 95% CI: 0.38–0.96, HR = 0.46 95% CI: 0.24–0.87 and HR = 0.51, 95% CI: 0.30–0.98) including all three cohorts combined (HR = 0.69, 95% CI: 0.12–0.97) (supplementary Figure 5 is available at Carcinogenesis Online).
Table III.
Multivariate analysis of estrogen-related SNPs associated with lung cancer survival
| NCI-MD case–case study |
NCI-MD case–control study |
Norwegian case-only study |
|||||||
| SNP | n (%) | HR (95% CI)ab | P-value | n (%) | HR (95% CI)ac | P-value | n (%) | HR (95% CI)de | P-value |
| Comt-01 | |||||||||
| G/G | 80 (35) | 1.00 (reference) | 59 (28) | 1.00 (reference) | 86 (35) | 1.00 (reference) | |||
| G/A | 100 (44) | 0.97 (0.60–1.58) | 0.911 | 103 (48) | 0.65 (0.32–1.36) | 0.256 | 114 (46) | 0.90 (0.56–1.46) | 0.682 |
| A/A | 47 (21) | 1.33 (0.77–2.29) | 0.306 | 51 (24) | 0.99 (0.55–1.80) | 0.979 | 47 (19) | 1.50 (0.87–2.60) | 0.146 |
| Cyp17a1-01 | |||||||||
| T/T | 84 (35) | 1.00 (reference) | 80 (37) | 1.00 (reference) | 93 (37) | 1.00 (reference) | |||
| T/C | 114 (48) | 1.57 (0.99–2.49) | 0.053 | 108 (50) | 0.87 (0.51–1.48) | 0.610 | 123 (49) | 0.63 (0.32–1.24) | 0.183 |
| C/C | 39 (16) | 1.30 (0.71–2.38) | 0.397 | 27 (13) | 1.25 (0.61–2.52) | 0.543 | 33 (13) | 0.78 (0.50–1.21) | 0.265 |
| Cyp17a1-05 | |||||||||
| C/C | 29 (34) | 1.00 (reference) | 81 (37) | 1.00 (reference) | 92 (37) | 1.00 (reference) | |||
| C/T | 42 (49) | 1.11 (0.48–2.56) | 0.800 | 106 (48) | 0.93 (0.46–1.88) | 0.848 | 119 (48) | 1.27 (0.69–2.35) | 0.449 |
| T/T | 15 (17) | 0.69 (0.26–1.81) | 0.449 | 33 (15) | 0.90 (0.46–1.78) | 0.763 | 38 (15) | 1.62 (0.85–3.09) | 0.145 |
| Cyp19a1-01 | |||||||||
| A/A | 69 (29) | 1.00 (reference) | 77 (36) | 1.00 (reference) | 67 (27) | 1.00 (reference) | |||
| A/G | 98 (41) | 0.80 (0.36–1.77) | 0.582 | 91 (43) | 0.93 (0.43–2.03) | 0.864 | 121 (50) | 1.43 (0.79–2.60) | |
| G/G | 70 (30) | 1.08 (0.44–2.62) | 0.866 | 43 (20) | 1.51 (0.86–2.67) | 0.152 | 56 (23) | 1.25 (0.72–2.20) | |
| Cyp19a1-06 | |||||||||
| T/T | 64 (27) | 1.00 (reference) | 74 (35) | 1.00 (reference) | 70 (28) | 1.00 (reference) | |||
| T/G | 118 (50) | 0.97 (0.60–1.55) | 0.888 | 96 (45) | 0.98 (0.46–2.08) | 0.964 | 123 (50) | 0.86 (0.55–1.35) | 0.502 |
| G/G | 56 (24) | 0.84 (0.48–1.50) | 0.564 | 43 (20) | 1.57 (0.79–3.12) | 0.194 | 55 (22) | 0.53 (0.28–1.00) | 0.053 |
| Cyp19a1-08 | |||||||||
| T/T | 132 (55) | 1.00 (reference) | 123 (56) | 1.00 (reference) | 131 (53) | 1.00 (reference) | |||
| T/G | 96 (40) | 0.32 (0.15–0.72) | 0.004 | 78 (36) | 0.72 (0.34–1.52) | 0.388 | 100 (40) | 0.69 (0.40–1.10) | 0.130 |
| T/T | 12 (5) | 0.41 (0.19–0.88) | 0.034 | 18 (8) | 0.62 (0.30–1.26) | 0.185 | 18 (7) | 0.46 (0.23–0.88) | 0.020 |
| Cyp19a1-09 | |||||||||
| C/C | 70 (30) | 1.00 (reference) | 79 (36) | 1.00 (reference) | 70 (28) | 1.00 (reference) | |||
| C/T | 98 (42) | 0.75 (0.35–1.61) | 0.462 | 96 (44) | 0.87 (0.40–1.89) | 0.724 | 123 (50) | 0.84 (0.54–1.33) | 0.460 |
| T/T | 66 (28) | 0.71 (0.26–1.94) | 0.506 | 45 (20) | 1.50 (0.88–2.58) | 0.140 | 55 (22) | 0.53 (0.28–1.00) | 0.052 |
| Cyp19a1-14 | |||||||||
| C/C | 75 (31) | 1.00 (reference) | 90 (41) | 1.00 (reference) | 66 (27) | 1.00 (reference) | |||
| C/T | 114 (48) | 0.98 (0.58–1.68) | 0.951 | 89 (41) | 1.19 (0.56–2.53) | 0.656 | 122 (49) | 0.81 (0.51–1.29) | 0.367 |
| T/T | 50 (21) | 1.14 (0.65–1.98) | 0.652 | 40 (18) | 1.58 (0.78–3.20) | 0.206 | 59 (24) | 0.62 (0.34–1.13) | 0.115 |
| Esr1-01 | |||||||||
| T/T | 60 (30) | 1.00 (reference) | 56 (26) | 1.00 (reference) | 73 (29) | 1.00 (reference) | |||
| T/C | 90 (44) | 0.95 (0.56–1.61) | 0.856 | 118 (54) | 1.16 (0.50–2.65) | 0.732 | 128 (51) | 0.97 (0.60–1.57) | 0.910 |
| C/C | 53 (26) | 1.01 (0.57–1.81) | 0.965 | 45 (21) | 2.22 (1.11–4.45) | 0.024 | 49 (20) | 1.41 (0.80–2.49) | 0.232 |
| Esr1-07 | |||||||||
| G/G | 53 (25) | 1.00 (reference) | 142 (65) | 1.00 (reference) | 159 (64) | 1.00 (reference) | |||
| G/A | 126 (59) | 1.69 (1.07–2.67) | 0.050 | 73 (33) | 1.08 (0.26–4.48) | 0.917 | 82 (33) | 1.54 (1.01–2.36) | 0.040 |
| A/A | 34 (16) | 2.59 (1.20–4.01) | 0.005 | 5 (2) | 1.76 (1.08–2.87) | 0.025 | 9 (4) | 2.86 (1.32–4.36) | 0.010 |
| Hsd3b1-03 | |||||||||
| A/A | 204 (86) | 1.00 (reference) | 23 (11) | 1.00 (reference) | 2 (1) | 1.00 (reference) | |||
| A/G | 26 (11) | 0.46 (0.14–1.52) | 0.203 | 186 (89) | 0.30 (0.17–0.56) | <0.001 | 248 (99) | 1.70 (0.42–6.91) | 0.460 |
| G/G | 6 (3) | 0.57 (0.20–1.62) | 0.295 | 0 (0) | - | ||||
| Hsd17b1-03 | |||||||||
| G/G | 84 (35) | 1.00 (reference) | 72 (33) | 1.00 (reference) | 82 (33) | 1.00 (reference) | |||
| G/A | 106 (44) | 0.61 (0.37–0.86) | 0.035 | 101 (46) | 0.81 (0.48–1.38) | 0.445 | 115 (46) | 0.67 (0.42–1.13) | 0.260 |
| A/A | 50 (21) | 0.64 (0.38–0.96) | 0.042 | 47 (21) | 0.46 (0.24–0.87) | 0.017 | 52 (21) | 0.51 (0.30–0.98) | 0.030 |
Values given in bold are statistically significant.
Adjusted for smoking status, pack-years, age, gender, race and tumor stage.
Approximately 20% of cases were missing DNA and were not included in the analysis.
Three of the 227 cases were missing DNA and were not included in the analysis.
Adjusted for smoking status, pack-years, age, gender and tumor stage.
Approximately 10% of the cases were missing DNA and were not included in the analysis.
Fig. 3.
Kaplan–Meier 5 years of cancer survival curves for the association of ESR1_07 with lung cancer survival. The survival rates of patients with G/G genotypes were compared with patients with G/A genotypes and A/A genotypes. Differences between survival curves were calculated using the log-rank test. (A) ESR1_07 genotypes associated with lung cancer survival in the NCI-MD case–case cohort (P = 0.02). (B) ESR1_07 genotypes associated with lung cancer survival in the NCI-MD case–control cohort (P = 0.05). (C) ESR1_07 genotypes associated with lung cancer survival in the Norwegian case-only cohort (P = 0.03). (D) ESR1_07 genotypes associated with lung cancer survival in all three cohorts combined (P = 0.04).
Serum progesterone and lung cancer survival
Higher levels of progesterone were associated with worse survival among men only in the NCI-MD case–case cohort (supplementary Table VI is available at Carcinogenesis Online). Men in the highest tertile of serum progesterone had significantly worse survival compared with those in the lowest tertiles (HR = 3.25, 95% CI: 1.58–6.68). There was no association between serum progesterone levels and survival among men in the NCI-MD case–control study or the Norwegian case-only study. Similarly, no associations were observed among women in any of the three studies between serum progesterone levels and lung cancer survival.
Discussion
We report an association between serum estrogen, tumor ER-α mRNA expression levels, estrogen-related SNPs and lung cancer survival in independent cohorts. Serum estrogen and estrogen-related SNPS were analyzed in three cohorts, whereas tumor ER-α and ER-β gene expression was analyzed in the two cohorts with available RNA. Higher serum estrogen levels, as well as tumor ER-α mRNA positive status, were associated with worse lung cancer prognosis. We also demonstrated that specific estrogen-related SNPs, which were associated with either higher serum estrogen levels or tumor ER-α mRNA levels, were also associated with poorer lung cancer prognosis. To our knowledge, this is the first study to demonstrate a significant association between serum estrogen and poorer lung cancer survival and to support functionality of estrogen-related SNPs in lung cancer prognosis.
The associations between serum estrogen and tumor ER-α mRNA expression and lung cancer survival were independent of gender and race. One previous study demonstrated that tumor ER expression in the lung was dependent on gender (14); however, our studies were in accordance with another study reporting no difference in tumor ER expression between men and women (13). Our data support the notion that the association we observe between serum estrogen levels and lung cancer survival is not due to estrogen therapy. First, we observe this association not only among females but also among males. Second, the removal of women who were taking estrogen therapy in each study did not alter our findings. Our studies also agree with previous research demonstrating an association between tumor ER-α expression and poor survival (13). We also observed a stronger association of tumor ER-α expression with lung cancer survival among adenocarcinomas in the NCI-MD case–case cohort (supplementary Table VII is available at Carcinogenesis Online). Finally, much of the previous research examining the association between tumor ER-α expression and lung cancer focused on tumor ER-α protein expression measured by immunohistochemistry. Our studies are complementary with these past studies, because we examined ER-α mRNA levels via quantitative reverse transcription polymerase chain reaction, which is more quantitative and less subjective. Our studies also agree with a recent study in which ER-α and ER-β mRNA examined via quantitative reverse transcription polymerase chain reaction were higher in lung carcinoma cells compared with normal epithelium (30). Although ER gene amplification is common in breast cancer (31–33), there are no studies examining whether the same phenomenon exists in lung cancer.
Our study demonstrated a significant positive association between serum estrogen levels and lung cancer survival, independent of histological subtype (data not shown). Although some researchers have suggested that nicotine may have an anti-estrogenic effect (34), neither serum estrogen nor ER-α levels differed by smoking status or pack-years (data not shown). In animal models, estrogen increases lung tumor growth (6,10). Taioli and Wynder (35) suggested that estrogen plays a role in the development of lung cancer among women, similar to its effect on breast cancer development, which is supported by our results. Their study demonstrated that an early age at menopause was associated with a significant decrease in risk of lung adenocarcinoma, whereas use of estrogen replacement therapy was associated with an increased risk (35). Another study using Surveillance, Epidemiology and End Results data supported the detrimental effect of estrogen on lung cancer through its observation that premenopausal women were more probably to have poorly differentiated tumors and to present at a more advanced stage than postmenopausal women (36). Other studies also observed associations between lung cancer risk and earlier age of menarche (37), greater number of menstrual cycles (38), higher parity (39,40) and earlier age at menopause (35,37). Adami et al. (41) observed that women who took estrogen replacement therapy had a higher risk of developing lung cancer, however, they did not report on survival. In another study, median lung cancer survival was shorter in patients taking hormone replacement therapy (42). Other studies also observed a higher risk of lung cancer among women taking hormone replacement therapy, although many findings were not significant (43–45). Recently, data have demonstrated that women on hormone replacement therapy of combined estrogen and progesterone for >5 years have a higher risk of lung cancer risk and mortality (46,47). We observed an association of serum progesterone with lung cancer survival among men in the NCI-MD case–case cohort; however, this was not validated by the other two cohorts. Furthermore, there was no association with serum progesterone and lung cancer survival among the women in any of the cohorts.
To our knowledge, this is the first study to report associations between estrogen-related SNPs and lung cancer prognosis. Specifically, the variant genotype (A/A) of the ESR1-07 SNP was associated with higher tumor ER-α mRNA levels and poorer lung cancer prognosis. This SNP is present in exon 8 of the ER-α gene, which translates to the F domain of the ER-α protein (48,49). The significance of this domain has been widely researched although its exact function is still unknown. The F domain is often combined with the E domain when investigating function, therefore, this E/F domain contains the ligand-binding domain, the dimerization domain and a second activation function (AF2) (20). Transcriptional activity of ER-α is mediated, in part, by the AF2 domain and appears to be hormone dependent (20,48,50,51). Our study is consistent with the above observations because we observe an association between the A/A variant genotype of ESR1-07 and increased tumor PR mRNA, an estrogen-regulated gene (52), suggesting that the variant genotypes (G/A or A/A) of ESR1-07 has higher transcriptional activity than the referent genotype (G/G). It is possible that the variant genotype of ESR1-07 results in a longer half-life of the ER protein and in turn, higher levels of tumor PR mRNA. This increase in ER levels, along with an increase in transcriptional activity, may help explain why patients who are tumor ER-α positive have a poorer prognosis in our study.
Aromatase inhibitors have been shown to inhibit lung cancer epithelial cell proliferation in vitro, lung tumor growth in vitro and lung tumor xenograft growth in nude mice, supporting their role in lung carcinogenesis (7–9). Our study illustrates that the variant genotype of an SNP in the aromatase gene, CYP19a1-08, is associated with decreased levels of serum estrogen and a better prognosis among lung cancer patients. Previous research demonstrated that another SNP in the aromatase gene, CYP19a1-09, which is similar to CYP19a1-08, is also located in the 3′ untranslated region and is associated with lower serum estrogen levels in postmenopausal women (53). We confirmed this result in our study. The variant genotype of CYP19a1-08 may impair aromatase function and thus reduce serum estrogens, which result in better lung cancer survival.
There are some limitations to this study. First, we do not have serum hormone, hormone receptor or SNP data on all the patients in the NCI-MD case–case cohort. This study began recruitment in 1984; therefore, many of the samples were depleted before our analyses. However, comparison of race, age, gender, smoking status and pack-years illustrated no significant difference between the study participants that underwent serum hormone, hormone receptor or SNP analysis and those that did not. Significantly, we were able to confirm the results of the test cohort with two independent validation cohorts. Second, although lung cancer diagnosis typically occurs at a later age and our study results are independent of gender, it is important to confirm whether these associations are present in premenopausal women. It is possible that premenopausal women may have a poorer survival than postmenopausal women due to the effect of serum estrogen. The one study that has examined this comparison did not report a difference in survival (36), however, in this previous study, menopausal status was determined solely by age with women <50 categorized as premenopausal and therefore, the potential for misclassification was high. The associations between an increased lung cancer risk and earlier age at menarche, later age at menopause, increased number of menstrual cycles, and lower parity suggest that estrogen plays a role in lung carcinogenesis (35–39).
In conclusion, we observed that serum estrogen and tumor ER-α levels were inversely associated with lung cancer prognosis in three independent cohorts. In addition, specific genotypes affecting serum estrogen and tumor ER-α expression were associated with prognosis. Our findings suggest that further research investigating the use of therapeutic agents to inhibit estrogen synthesis or ER activation in lung cancer patients may be warranted.
Supplementary material
Supplementary Figures 1–5 and Tables I–VII can be found at http://carcin.oxfordjournals.org/
Funding
Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research and the Norwegian Cancer Society (0971); National Cancer Institute Cancer Prevention Fellowship to S.O.-M.
Supplementary Material
Acknowledgments
We thank Donna Perlmutter, Raymond Jones, Dean Mann, Leoni Leondaridis, Glenwood Trivers, John Cottrell, Audrey Salabes, Rex Yung and Mark Krasna for their contributions to patient accrual and tissue collection. We thank Tracey Bosworth for conducting the hormone analysis. We thank Lodve Stangeland for providing human tissue and patient data.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- CI
confidence interval
- ER
estrogen receptor
- ER-α
estrogen receptor-alpha
- ER-β
estrogen receptor-beta
- HR
hazard ratio
- mRNA
messenger RNA
- NCI
National Cancer Institute
- NCI-MD
National Cancer Institute-Maryland
- PR
progesterone receptor
- SNP
single-nucleotide polymorphism
References
- 1.American Cancer Society. Cancer Facts and Figures 2009. 2009. [Google Scholar]
- 2.Mathers CD, et al. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathers CD, et al. Global and regional estimates of cancer mortality and incidence by site: I. Application of regional cancer survival model to estimate cancer mortality distribution by site. BMC Cancer. 2002;2:36. doi: 10.1186/1471-2407-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee YT. Better prognosis of many cancers in female: a phenomenon not explained by study of steroid receptors. J. Surg. Oncol. 1984;25:255–262. doi: 10.1002/jso.2930250408. [DOI] [PubMed] [Google Scholar]
- 5.Ramalingam S, et al. Lung cancer in young patients: analysis of a surveillance, epidemiology, and end results database. J. Clin. Oncol. 1998;16:651–657. doi: 10.1200/JCO.1998.16.2.651. [DOI] [PubMed] [Google Scholar]
- 6.Stabile LP, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62:2141–2150. [PubMed] [Google Scholar]
- 7.Weinberg OK, et al. Aromatase inhibitors in human lung cancer therapy. Cancer Res. 2005;65:11287–11291. doi: 10.1158/0008-5472.CAN-05-2737. [DOI] [PubMed] [Google Scholar]
- 8.Pietras RJ, et al. Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids. 2005;70:372–381. doi: 10.1016/j.steroids.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Marquez-Garban DC, et al. Targeting aromatase and estrogen signaling in human non-small cell lung cancer. Ann. N. Y. Acad. Sci. 2009;1155:194–205. doi: 10.1111/j.1749-6632.2009.04116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mah V, et al. Aromatase expression predicts survival in women with early-stage non small cell lung cancer. Cancer Res. 2007;67:10484–10490. doi: 10.1158/0008-5472.CAN-07-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mollerup S, et al. Expression of estrogen receptors alpha and beta in human lung tissue and cell lines. Lung Cancer. 2002;37:153–159. doi: 10.1016/s0169-5002(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 12.Canver CC, et al. Sex hormone receptors in non-small-cell lung cancer in human beings. J. Thorac. Cardiovasc. Surg. 1994;108:153–157. [PubMed] [Google Scholar]
- 13.Kawai H, et al. Estrogen receptor alpha and beta are prognostic factors in non-small cell lung cancer. Clin. Cancer Res. 2005;11:5084–5089. doi: 10.1158/1078-0432.CCR-05-0200. [DOI] [PubMed] [Google Scholar]
- 14.Fasco MJ, et al. Gender-dependent expression of alpha and beta estrogen receptors in human nontumor and tumor lung tissue. Mol. Cell. Endocrinol. 2002;188:125–140. doi: 10.1016/s0303-7207(01)00750-x. [DOI] [PubMed] [Google Scholar]
- 15.Di NL, et al. Estrogen and progesterone receptors in non-small cell lung cancer in 248 consecutive patients who underwent surgical resection. Arch. Pathol. Lab. Med. 2000;124:1467–1470. doi: 10.5858/2000-124-1467-EAPRIN. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz AG, et al. Nuclear estrogen receptor beta in lung cancer: expression and survival differences by sex. Clin. Cancer Res. 2005;11:7280–7287. doi: 10.1158/1078-0432.CCR-05-0498. [DOI] [PubMed] [Google Scholar]
- 17.Radzikowska E, et al. Estrogen and progesterone receptors in non small cell lung cancer patients. Ann. Thorac. Cardiovasc. Surg. 2002;8:69–73. [PubMed] [Google Scholar]
- 18.Ivanova MM, et al. Activity and intracellular location of estrogen receptors alpha and beta in human bronchial epithelial cells. Mol. Cell. Endocrinol. 2009;305:12–21. doi: 10.1016/j.mce.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali G, et al. Different estrogen receptor beta expression in distinct histologic subtypes of lung adenocarcinoma. Hum. Pathol. 2008;39:1465–1473. doi: 10.1016/j.humpath.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Pearce ST, et al. The biological role of estrogen receptors alpha and beta in cancer. Crit. Rev. Oncol. Hematol. 2004;50:3–22. doi: 10.1016/j.critrevonc.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki T, et al. Intracrine mechanism of estrogen synthesis in breast cancer. Biomed. Pharmacother. 2003;57:460–462. doi: 10.1016/j.biopha.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Olson SH, et al. Variants in estrogen biosynthesis genes, sex steroid hormone levels, and endometrial cancer: a HuGE review. Am. J. Epidemiol. 2007;165:235–245. doi: 10.1093/aje/kwk015. [DOI] [PubMed] [Google Scholar]
- 23.Mason JI, et al. The regulation of 3 beta-hydroxysteroid dehydrogenase expression. Steroids. 1997;62:164–168. doi: 10.1016/s0039-128x(96)00176-6. [DOI] [PubMed] [Google Scholar]
- 24.Jefcoate CR, et al. Tissue-specific synthesis and oxidative metabolism of estrogens. J. Natl Cancer Inst. Monogr. 2000;27:95–112. doi: 10.1093/oxfordjournals.jncimonographs.a024248. [DOI] [PubMed] [Google Scholar]
- 25.Mechanic LE, et al. Polymorphisms in XPD and TP53 and mutation in human lung cancer. Carcinogenesis. 2005;26:597–604. doi: 10.1093/carcin/bgh344. [DOI] [PubMed] [Google Scholar]
- 26.Zheng YL, et al. Bleomycin-induced chromosome breaks as a risk marker for lung cancer: a case-control study with population and hospital controls. Carcinogenesis. 2003;24:269–274. doi: 10.1093/carcin/24.2.269. [DOI] [PubMed] [Google Scholar]
- 27.Lababede O, et al. TNM staging of lung cancer: a quick reference chart. Chest. 1999;115:233–235. doi: 10.1378/chest.115.1.233. [DOI] [PubMed] [Google Scholar]
- 28.StataCorp. Stata Statistical Software: Release 9. College Station, TX: StataCorp LP; 2005. [Google Scholar]
- 29.Gardner D, et al. Greenspan's Basic & Clinical Endocrinology: Eighth Edition, 8th edn. McGraw-Hill Medical, New York, NY; 2007. [Google Scholar]
- 30.Kerr A, et al. Steroid receptor and growth factor receptor expression in human nonsmall cell lung cancers using cells procured by laser-capture microdissection. Adv. Exp. Med. Biol. 2008;617:377–384. doi: 10.1007/978-0-387-69080-3_36. [DOI] [PubMed] [Google Scholar]
- 31.Brown LA, et al. ESR1 gene amplification in breast cancer: a common phenomenon? Nat. Genet. 2008;40:806–807. doi: 10.1038/ng0708-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horlings HM, et al. ESR1 gene amplification in breast cancer: a common phenomenon? Nat. Genet. 2008;40:807–808. doi: 10.1038/ng0708-807. [DOI] [PubMed] [Google Scholar]
- 33.Holst F, et al. Estrogen receptor alpha (ESR1) gene amplification is frequent in breast cancer. Nat. Genet. 2007;39:655–660. doi: 10.1038/ng2006. [DOI] [PubMed] [Google Scholar]
- 34.Baron JA. Beneficial effects of nicotine and cigarette smoking: the real, the possible and the spurious. Br. Med. Bull. 1996;52:58–73. doi: 10.1093/oxfordjournals.bmb.a011533. [DOI] [PubMed] [Google Scholar]
- 35.Taioli E, et al. Re: endocrine factors and adenocarcinoma of the lung in women. J. Natl Cancer Inst. 1994;86:869–870. doi: 10.1093/jnci/86.11.869. [DOI] [PubMed] [Google Scholar]
- 36.Moore KA, et al. Menopausal effects on presentation, treatment, and survival of women with non-small cell lung cancer. Ann. Thorac. Surg. 2003;76:1789–1795. doi: 10.1016/s0003-4975(03)01024-5. [DOI] [PubMed] [Google Scholar]
- 37.Brenner AV, et al. Menstrual and reproductive factors and risk of lung cancer among Chinese women, Eastern Gansu Province, 1994–1998. J. Epidemiol. 2003;13:22–28. doi: 10.2188/jea.13.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao ML, et al. A study of the association between squamous cell carcinoma and adenocarcinoma in the lung, and history of menstruation in Shanghai women, China. Lung Cancer. 1996;14(suppl. 1):S215–S221. doi: 10.1016/s0169-5002(96)90224-x. [DOI] [PubMed] [Google Scholar]
- 39.Seow A, et al. Diet, reproductive factors and lung cancer risk among Chinese women in Singapore: evidence for a protective effect of soy in nonsmokers. Int. J. Cancer. 2002;97:365–371. doi: 10.1002/ijc.1615. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz AG, et al. Reproductive factors, hormone use, estrogen receptor expression and risk of non small-cell lung cancer in women. J. Clin. Oncol. 2007;25:5785–5792. doi: 10.1200/JCO.2007.13.3975. [DOI] [PubMed] [Google Scholar]
- 41.Adami HO, et al. Risk of cancer in women receiving hormone replacement therapy. Int. J. Cancer. 1989;44:833–839. doi: 10.1002/ijc.2910440515. [DOI] [PubMed] [Google Scholar]
- 42.Ganti AK, et al. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J. Clin. Oncol. 2006;24:59–63. doi: 10.1200/JCO.2005.02.9827. [DOI] [PubMed] [Google Scholar]
- 43.Wu AH, et al. Personal and family history of lung disease as risk factors for adenocarcinoma of the lung. Cancer Res. 1988;48:7279–7284. [PubMed] [Google Scholar]
- 44.Elliott AM, et al. Use of exogenous hormones by women and lung cancer: evidence from the Royal College of General Practitioners' Oral Contraception Study. Contraception. 2006;73:331–335. doi: 10.1016/j.contraception.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Kabat GC, et al. Reproductive and hormonal factors and risk of lung cancer in women: a prospective cohort study. Int. J. Cancer. 2007;120:2214–2220. doi: 10.1002/ijc.22543. [DOI] [PubMed] [Google Scholar]
- 46.Chlebowski RT, et al. Oestrogen plus progestin and lung cancer in postmenopausal women (Women's Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet. 2009;374:1243–1251. doi: 10.1016/S0140-6736(09)61526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slatore CG, et al. Lung cancer and hormone replacement therapy: association in the vitamins and lifestyle study. J. Clin. Oncol. 2010;28:1540–1546. doi: 10.1200/JCO.2009.25.9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peters GA, et al. Estrogen receptor domains E and F: role in dimerization and interaction with coactivator RIP-140. Mol. Endocrinol. 1999;13:286–296. doi: 10.1210/mend.13.2.0244. [DOI] [PubMed] [Google Scholar]
- 49.Nilsson S, et al. Estrogen receptor transcription and transactivation: basic aspects of estrogen action. Breast Cancer Res. 2000;2:360–366. doi: 10.1186/bcr81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montano MM, et al. The carboxy-terminal F domain of the human estrogen receptor: role in the transcriptional activity of the receptor and the effectiveness of antiestrogens as estrogen antagonists. Mol. Endocrinol. 1995;9:814–825. doi: 10.1210/mend.9.7.7476965. [DOI] [PubMed] [Google Scholar]
- 51.Hart LL, et al. The estrogen receptor: more than the average transcription factor. Biochem. Cell Biol. 2002;80:335–341. doi: 10.1139/o02-038. [DOI] [PubMed] [Google Scholar]
- 52.Graham JD, et al. Physiological action of progesterone in target tissues. Endocr. Rev. 1997;18:502–519. doi: 10.1210/edrv.18.4.0308. [DOI] [PubMed] [Google Scholar]
- 53.Dunning AM, et al. Polymorphisms associated with circulating sex hormone levels in postmenopausal women. J. Natl Cancer Inst. 2004;96:936–945. doi: 10.1093/jnci/djh167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.