Abstract
We took a polygenic approach to evaluate the effects of 41 potentially functional single-nucleotide polymorphisms (SNPs) in microRNAs (miRNAs)-related genes on survival and recurrence among renal cell carcinoma (RCC) patients. During a median follow-up of 21.8 months, among 316 RCC patients, 64 died and 56 developed recurrence. In single-SNP analysis, we identified seven SNPs significantly associated with RCC survival and five SNPs with recurrence. The most significant associations were SNPs in GEMIN4 with the variant alleles of both rs7813 and rs910925 associated with 1.74-fold [95% confidence interval (CI) = 1.15–2.62] increased risk of death, whereas the variant allele of rs3744741 conferred a decreased risk of death [hazard ratio (HR) = 0.39; 95% CI = 0.19–0.77]. Several SNPs belonging to the pre-miRNA and were identified to be significantly associated with RCC recurrence. Haplotypes of DICER and DROSHA were also associated with altered patient survival and recurrence. More importantly, we observed cumulative effects of multiple SNPs on RCC survival. Compared with subjects carrying zero to two unfavorable genotypes, those carrying three to five and six and more unfavorable genotypes had an increased risk of death with a HR of 2.49 (95% CI = 1.24–5.00) and 6.66 (95% CI = 2.49–17.86), respectively, with significant dose–response trend (P for trend<0.001). As the first study of miRNA-related genetic polymorphisms on RCC clinical outcome, our results strongly suggested that miRNA-related SNPs may impact the recurrence and survival in RCC patients. Future investigation in larger populations and functional characterizations are necessary to validate these results.
Introduction
Renal cell carcinoma (RCC) represents the third leading cause of death among genitourinary malignancies. In 2009, ∼57 760 new cases of kidney and renal pelvis cancers are expected and ∼12 980 deaths will occur in the USA (1). RCC is the most lethal urological malignancy with >40% of patients eventually die of the disease (2,3). The 5 year cancer-specific survival rates for stages I to IV were 81, 74, 53 and 8%, respectively (4). The majority (80–90%) of RCC has the clear cell histology. Surgical resection remains the best curative therapy for RCC (5). However, after the curative nephrectomy, 20–40% patients will develop recurrence (6).
MicroRNAs (miRNA) are a group of endogenous, small non-coding RNA molecules of 22 nucleotides (7). To date, ∼700 human miRNAs have been cataloged in the miRBase registry, with the total number predicted to be 1000 (8). It has been estimated that miRNAs regulate the expression of approximately one-third of human genes (9,10). MiRNAs are generated in a precisely coordinated two-step pathway. Most miRNAs reside in intergenic or intronic regions and are transcribed as a part of a long transcript through RNA polymerases II (11). These primary miRNA transcripts (pri-miRNA) are processed in the nucleus by the microprocessor machinery, which contains the Drosha RNase and the double-strand RNA-binding protein DGCR8 (12). A hairpin precursor miRNA molecule of 70–100 nucleotides (pre-miRNA) is then produced, which translocates to the cytoplasm through the assistance of RAN GTPase and Exportin 5 (XPO5), where it is further processed by a protein complex that includes DICER, TRBP, AGO1 and AGO2, leading to the production of mature miRNAs (13,14). The global or specific deregulation of key genes in the miRNA biogenesis pathway has been associated with malignant transformation (11,13). The mature miRNAs negatively affect the expression level of their target genes through binding to the 3′ untranslated region of the target genes at the posttranscriptional level.
With the increasing choices of treatment modalities that potentially improve survival, it is of great clinical benefit to predict clinical outcome of RCC patients. Large-scale profiling of miRNA expressions using microarray or real-time polymerase chain reaction techniques has revealed significant associations between miRNA expression signatures and the etiology and prognosis of various cancers (9,15–17). Moreover, identification of germ line genetic variants that predict RCC clinical outcome has become an increasingly promising approach that complements to somatic biomarkers. Although genetic polymorphisms have been widely implicated in cancer treatment response (18,19), such evidence is lacking for the miRNA-related genes. In this study, we tested the hypothesis that common sequence variants in genes of miRNA and of the miRNA biogenesis pathway affect RCC clinical outcomes. We evaluated the effects of 41 potentially functional miRNA-related single-nucleotide polymorphisms (SNPs) as well as took a polygenic approach to evaluate the cumulative effects of these SNPs on survival and recurrence among RCC patients.
Materials and methods
Study population
The study population and enrollment have been described previously (20,21). Briefly, incident RCC cases were recruited from The University of Texas M. D. Anderson Cancer Center in Houston, Texas. M. D. Anderson Cancer Center staff interviewers identified RCC cases through a daily review of computerized appointment schedules for the Departments of Urology and Genitourinary Medical Oncology. All cases were individuals with newly diagnosed histologically confirmed RCC. There was no age, gender, ethnicity or cancer stage restrictions on recruitment. Demographic and epidemiological information were collected by in-person interview. Data collected including age, gender, ethnicity, as well as occupation history, tobacco use history, medical history and family history of cancer. After the interview, a 40 ml blood sample was drawn into coded heparinized tubes and sent to the laboratory for molecular analyses. All study participants were followed on treatments, survival and tumor recurrence. Clinical and follow-up data were abstracted from medical records. The study end point was overall survival and recurrence. The study was approved by the Institutional Review Board of M. D. Anderson Cancer Center. All participants signed an informed consent prior to participation in the study. An individual who had never smoked or had smoked <100 cigarettes in his or her lifetime was defined as a never-smoker. An individual who had smoked at least 100 cigarettes in his or her lifetime but had quit >12 months before diagnosis was coded as a former-smoker. Current smokers were those who were currently smoking or had quit <12 months before diagnosis.
SNP selection and genotyping
The selection of genes and SNPs have been described previously in detail (22). We identified 41 potential functional polymorphisms: 24 SNPs in 11 genes in the miRNA biogenesis pathway, 7 SNPs in 7 pre-miRNAs and 10 SNPs in 8 pri-miRNAs. All SNPs have a reported minor allele frequency of >0.01 in Caucasians and were located in exons, promoters (within 2 kb of the gene) or untranslated regions. When we selected SNPs in the miRNA biogenesis pathway, except for two AGO1 SNPs (rs636832 and rs595961) located in introns, all other polymorphisms reside in functional regions, including exons, UTRs and promoters (within 2 kb of the genes). In the case of multiple potentially functional SNPs within the same haplotype block (defined by the linkage coefficient r2 > 0.8), only one SNP was included. One exception is GEMIN4 rs7813 because it is the only SNP in the miRNA biogenesis pathway that has been reported in previous studies to be significantly associated with cancer risk (21). For SNPs in pri-miRNAs but not in pre-miRNAs, because we identified >200 such SNPs with a minor allele frequency of >0.01 in Caucasians, we included 10 SNPs from 8 pri-miRNAs whose mature counterparts have been extensively implicated in cancer etiology or clinical outcome. The genotyping procedures were also described previously in detail (22). All polymorphisms were genotyped using the SNPlex assay according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA). Genotypes were called by GeneMapper software (Applied Biosystems) using a template file provided with each custom SNPlex assay. Internal quality controls and negative controls were used to ensure genotyping accuracy, and 5% of all samples were randomly selected and genotyped in duplicate with 100% concordance.
Statistical analysis
The χ2 test or Fisher’s exact test was applied separately to compare the distribution of selected demographic and clinical variables by vital status as well as recurrence status. The Cox proportional hazard model was used to assess the effect of individual SNPs on overall survival, defined as the time from the date of surgical resection to the date of death or last follow-up. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated by fitting the Cox model while adjusting for age, sex, smoking status, tumor stage, grade and treatments. Similarly, we used Cox proportional hazards to analyze recurrence-free survival time, defined as the time from surgery to recurrence or last follow-up. Kaplan–Meier curves and log-rank tests were used to assess the differences in survival and recurrence-free survival times by individual polymorphisms.
We performed a joint analysis by counting the number of putative unfavorable genotypes showing significant association with clinical outcomes (P < 0.05) in the Cox model. HRs and 95% CIs were calculated using multivariate Cox proportional hazard models after adjusting for appropriate variables. STATA software (version 10.0; STATA Corp., College Station, TX) was used for the above analyses. To take into account of the issue of multiple comparisons, we used the false discovery rate calculation based on the Benjamini–Hochberg method (23). We calculated the false discovery rate-adjusted Q-values at the 5% level to see whether the observed P-values remained statistically significant after adjusting for multiple comparisons.
Haplotype and diplotype frequencies were analyzed using the HelixTree Genetics Analysis Software (Golden Helix, Bozeman, MT). Haplotypes were inferred using the expectation–maximization algorithm implemented in the HelixTree software. The adjusted HRs and 95% CI for each haplotype and/or diplotype were calculated using multivariate Cox regression using the most abundant haplotype and/or diplotype as the reference group.
Survival tree analyses using recursive partitioning were performed to investigate higher order gene–gene interactions and to identify subgroups of individuals at higher risk of death or recurrence using a modified STREE program (http://peace.med.yale.edu/pub/stree/) (24). The tree starts with the root node that includes all the study participants and uses a log-rank statistic to select the optimal split that distinguishes patients into better and worse survival. The recursive procedure continues to produce subsequent nodes that are more homogeneous (with respect to survival) than the original node. The final model is a tree structure with many binary splits, and each terminal node represents a group of patients with different survival patterns based on distinct genotype combinations. HRs and 95% CIs were calculated for terminal nodes using multivariate Cox proportional hazard models after adjusting for appropriate variables.
Results
The study included 316 patients with RCC. During a median follow-up of 21.8 months, 64 patients died (Table I). There was no significant difference in the distribution of sex by survival status (P = 0.94). The mean ages of patients that survived and did not survive were 58.88 and 59.36 years, respectively (P = 0.75). There was no significant difference in the distribution of smoking status (P = 0.41). Among ever-smokers, pack-years of smoking did not differ significantly by survival status (P = 0.64). Among clinical variables, there were no significant differences in terms of histology (P = 0.21), radiotherapy (P = 0.27) or molecular targeted therapy (P = 0.26). However, there was a significant difference in survival by tumor grade (P < 0.001), stage (P < 0.001) and tumor size (P < 0.001). Furthermore, alive patients were less probably to have received cytokine treatment (P < 0.001) or chemotherapy (P = 0.006) than patients who had died.
Table I.
Characteristics of study populationa
| Dead (N = 64) | Alive (N = 252) | P-value | Recurrence (N = 56) | No recurrence (N = 181) | P-value | |
| Sex, N (%) | ||||||
| Male | 42 (65.63) | 164 (65.08) | 0.94 | 40 (71.43) | 115 (63.54) | 0.28 |
| Female | 22 (34.38) | 88 (34.92) | 16 (28.57) | 66 (36.46) | ||
| Ethnicity, N (%) | ||||||
| White | 54 (84.38) | 206 (81.75) | 0.82 | 46 (82.14) | 146 (80.66) | 0.48 |
| Hispanic | 2 (3.13) | 12 (4.76) | 1 (1.79) | 10 (5.52) | ||
| Black | 8 (12.50) | 34 (13.49) | 9 (16.07) | 25 (13.81) | ||
| Age, mean (SD) | 59.36 (10.21) | 58.88 (10.57) | 0.75 | 59.36 (9.74) | 59.37(10.83) | 0.99 |
| Smoking status, N (%) | ||||||
| Never | 28 (43.75) | 133 (52.78) | 0.41 | 31 (55.36) | 93 (51.38) | 0.63 |
| Former | 27 (42.19) | 86 (34.13) | 17 (30.36) | 67 (37.02) | ||
| Current | 9 (14.06) | 33 (13.10) | 8 (14.29) | 21 (11.60) | ||
| Pack-years, mean (SD) | 26.47 (31.39) | 28.78 (23.82) | 0.64 | 39.42 (33.20) | 24.91 (21.32) | 0.01 |
| Stage, N (%) | ||||||
| I | 6 (9.38) | 128 (53.33) | <0.001 | 4 (7.14) | 111 (65.29) | <0.001 |
| II | 5 (7.81) | 20 (8.33) | 7 (12.50) | 15 (8.82) | ||
| III | 19 (29.69) | 60 (25.00) | 36 (64.29) | 40 (23.53) | ||
| IV | 34 (53.13) | 32 (13.33) | 9 (16.07) | 4 (2.35) | ||
| Grade, N (%) | ||||||
| I | 0 (0.00) | 3 (1.19) | <0.001 | 0 (0.00) | 2 (1.10) | <0.001 |
| II | 7 (10.94) | 79 (31.35) | 8 (14.29) | 69 (38.12) | ||
| III | 22 (34.38) | 126 (50.00) | 23 (41.07) | 90 (49.72) | ||
| IV | 35 (54.69) | 38 (15.08) | 25 (44.64) | 14 (7.73) | ||
| Unknown | 0 (0.00) | 6 (2.38) | 0 (0.00) | 6 (3.31) | ||
| Tumor size, mean (SD) | 9.30 (4.14) | 6.03 (3.78) | <0.001 | 9.68 (3.58) | 5.24 (3.39) | <0.001 |
| Histology, N (%) | ||||||
| Conventional | 55 (85.44) | 199 (78.97) | 0.21 | 46 (82.14) | 145 (80.11) | 0.74 |
| Other | 9 (14.06) | 53 (21.03) | 10 (17.86) | 36 (19.89) | ||
| Cytokine treatment, N (%) | ||||||
| Yes | 15 (23.81) | 6 (2.44) | <0.001 | 0 | 1 (0.56) | 0.58 |
| No | 48 (76.19) | 240 (97.56) | 55 (100.00) | 179 (99.44) | ||
| Chemotherapy, N (%) | ||||||
| Yes | 11 (17.46) | 16 (6.45) | 0.006 | 6 (10.91) | 5 (2.78) | 0.012 |
| No | 52 (82.54) | 232 (93.55) | 49 (89.09) | 175 (97.22) | ||
| Molecular targeted therapy, N (%) | ||||||
| Yes | 7 (11.11) | 17 (6.85) | 0.26 | 1 (1.82) | 3 (1.67) | 0.94 |
| No | 56 (88.89) | 231 (93.15) | 54 (98.18) | 177 (98.33) | ||
| Radiotherapy, N (%) | ||||||
| Yes | 2 (3.17) | 3 (1.22) | 0.27 | 0 (0.00) | 1 (0.56) | 0.58 |
| No | 61 (96.83) | 243 (98.78) | 55 (100.00) | 179 (99.44) | ||
Numbers in some categories do not add up to total due to missing values
During the same follow-up period, 56 patients developed recurrence, whereas 181 patients had no recurrence. Again, there was no significant difference in sex, age, ethnicity or smoking status by recurrence status. Significant differences were found between pack-years of smoking, tumor stage, grade, tumor size and whether patients ever received chemotherapy (Table I).
The associations between single SNPs and RCC survival were shown in Table II. Overall, there were six SNPs significantly associated with RCC survival and one SNP with borderline significance (P = 0.055). Dominant model was the best-fitting model for rs910924, rs3744741 and rs4968104 in GEMIN4; additive model was the best-fitting model for rs7813 and rs910925 in GEMIN4, as well as rs4919510 in mir608. One SNP, rs3742330 in DICER, was borderline associated with RCC survival. Note that the most significant associations were observed in SNPs in GEMIN4. In particular, the variant alleles of both rs7813 and rs910925 were each associated with 1.74-fold (95% CI = 1.15–2.62; P = 0.009) increased risk of death, whereas the variant allele of rs3744741 conferred a significantly decreased risk of death with a HR of 0.39 (95% CI = 0.19–0.77; P = 0.007). The associations remained significant after adjusting for multiple comparisons. Similar results were obtained when we restricted the analysis in clear cell histology (Table II).
Table II.
HRs of single SNPs and RCC survival
| Gene | SNP | Allelesa | Minor allele frequency (dead/alive) | Best-fitting genetic model (All histology types) |
Best-fitting genetic model (Clear cell carcinoma) |
||||
| Model | HR (95% CI)b | P-valuec | Model | HR (95% CI)b | P-value | ||||
| Biogenesis pathway | |||||||||
| DROSHA | rs6877842 | G/C | 0.15/0.16 | DOM | 0.79 (0.40–1.55) | 0.494 | DOM | 0.91 (0.43–1.92) | 0.811 |
| rs10719 | C/T | 0.26/0.26 | DOM | 0.68 (0.36–1.30) | 0.246 | DOM | 0.59 (0.29–1.21) | 0.151 | |
| DGCR8 | rs417309 | G/A | 0.05/0.07 | DOM | 1.04 (0.42–2.54) | 0.935 | DOM | 1.20 (0.48–2.99) | 0.694 |
| rs3757 | G/A | 0.20/0.24 | REC | 0.44 (0.10–1.91) | 0.273 | DOM | 0.91 (0.46–1.77) | 0.771 | |
| rs1640299 | G/T | 0.50/0.48 | DOM | 1.55 (0.82–2.95) | 0.179 | ADD | 1.21 (0.77–1.89) | 0.406 | |
| XPO5 | rs11077 | A/C | 0.52/0.42 | ADD | 1.37 (0.91–2.05) | 0.130 | DOM | 1.85 (0.89–3.83) | 0.099 |
| RAN | rs14035 | C/T | 0.29/0.28 | ADD | 1.25 (0.80–1.94) | 0.324 | REC | 1.72 (0.62–4.79) | 0.302 |
| DICER | rs3742330 | A/G | 0.08/0.07 | DOM | 2.17 (0.98–4.79) | 0.055 | DOM | 1.85 (0.78–4.39) | 0.164 |
| rs13078 | T/A | 0.25/0.17 | ADD | 1.47 (0.96–2.27) | 0.080 | ADD | 1.63 (0.98–2.72) | 0.061 | |
| TRBP | rs784567 | C/T | 0.48/0.39 | DOM | 1.54 (0.79–2.99) | 0.204 | ADD | 1.19 (0.79–1.80) | 0.415 |
| AGO1 | rs636832 | G/A | 0.13/0.15 | DOM | 1.16 ( 0.58–2.29) | 0.678 | DOM | 0.96 (0.43–2.11) | 0.912 |
| rs595961 | A/G | 0.18/0.22 | REC | 1.63 (0.40–6.65) | 0.494 | ADD | 0.88 (0.47–1.66) | 0.699 | |
| AGO2 | rs4961280 | C/A | 0.20/0.19 | DOM | 1.15 ( 0.63–2.10) | 0.644 | ADD | 1.14 (0.66–1.96) | 0.637 |
| GEMIN4 | rs910924 | C/T | 0.22/0.23 | DOM | 2.04 (1.11–3.76) | 0.022 | DOM | 2.27 (1.13–4.57) | 0.021 |
| rs2740348 | G/C | 0.16/0.15 | DOM | 1.59 (0.87–2.90) | 0.132 | DOM | 1.39 (0.72–2.70) | 0.325 | |
| rs7813 | T/C | 0.37/0.36 | ADD | 1.74 (1.15–2.62) | 0.009 | ADD | 1.71 (1.08–2.70) | 0.021 | |
| rs910925 | G/C | 0.37/0.37 | ADD | 1.74 (1.15–2.62) | 0.009 | ADD | 1.71 (1.08–2.70) | 0.021 | |
| rs3744741 | C/T | 0.16/0.18 | DOM | 0.39 (0.19–0.77) | 0.007 | DOM | 0.40 (0.18–0.85) | 0.018 | |
| rs1062923 | T/C | 0.19/0.16 | DOM | 0.75 (0.40–1.42) | 0.379 | DOM | 0.83 (0.41–1.68) | 0.604 | |
| rs4968104 | T/A | 0.21/0.21 | DOM | 1.88 (1.03–3.42) | 0.040 | DOM | 2.01 (1.02–3.98) | 0.044 | |
| GEMIN3 | rs197414 | C/A | 0.12/0.14 | DOM | 0.59 (0.30–1.17) | 0.134 | DOM | 0.62 (0.30–1.26) | 0.184 |
| rs197388 | T/A | 0.21/0.21 | ADD | 0.88 (0.54–1.43) | 0.611 | REC | 0.28 (0.03–2.59) | 0.259 | |
| rs197412 | T/C | 0.40/0.45 | ADD | 0.70 (0.47–1.05) | 0.087 | ADD | 0.62 (0.40–0.98) | 0.041 | |
| HIWI | rs1106042 | G/A | 0.02/0.06 | DOM | 0.49 (0.11–2.12) | 0.341 | DOM | 0.48 (0.11–2.10) | 0.333 |
| Pre-miRNA | |||||||||
| mir146a | rs2910164 | G/C | 0.25/0.27 | DOM | 0.77 (0.43–1.40) | 0.396 | DOM | 0.56 (0.29–1.08) | 0.08 |
| mir196a-2 | rs11614913 | C/T | 0.34/0.37 | REC | 1.44 (0.66–3.16) | 0.359 | DOM | 0.80 (0.43–1.50) | 0.481 |
| mir423 | rs6505162 | C/A | 0.49/0.46 | REC | 0.95 (0.47–1.91) | 0.886 | REC | 0.84 (0.39–1.81) | 0.647 |
| mir492 | rs2289030 | C/G | 0.06/0.06 | DOM | 1.49 (0.58–3.82) | 0.407 | DOM | 1.40 (0.51–3.87) | 0.516 |
| mir604 | rs2368392 | C/T | 0.21/0.27 | ADD | 0.73 (0.43–1.24) | 0.247 | ADD | 0.70 (0.40–1.23) | 0.217 |
| mir608 | rs4919510 | C/G | 0.25/0.21 | ADD | 1.61 (1.00–2.57) | 0.048 | ADD | 1.64 (0.96–2.79) | 0.071 |
| mir631 | rs5745925 | CT/— | 0.10/0.09 | DOM | 0.77 (0.37–1.59) | 0.481 | DOM | 0.65 (0.27–1.57) | 0.335 |
| Pri-miRNA | |||||||||
| let7f-2 | rs17276588 | G/A | 0.04/0.03 | DOM | 0.33 (0.09–1.15) | 0.081 | DOM | 0.41 (0.12–1.47) | 0.172 |
| mir26a-1 | rs7372209 | C/T | 0.37/0.28 | DOM | 1.51 (0.86–2.66) | 0.154 | DOM | 1.33 (0.70–2.50) | 0.384 |
| mir30a | rs1358379 | A/G | 0.06/0.03 | DOM | 1.34 (0.59–3.07) | 0.482 | DOM | 1.19 (0.45–3.17) | 0.721 |
| mir30c-1 | rs16827546 | C/T | 0.02/0.04 | DOM | 0.26 (0.03–2.11) | 0.208 | N/A | N/A | N/A |
| mir100 | rs1834306 | C/T | 0.47/0.46 | REC | 0.78 (0.38–1.59) | 0.491 | REC | 0.81 (0.37–1.77) | 0.597 |
| mir124-1 | rs531564 | C/G | 0.16/0.12 | DOM | 1.57 (0.85–2.88) | 0.150 | DOM | 1.42 (0.72–2.81) | 0.307 |
| mir219-1 | rs107822 | G/A | 0.20/0.26 | DOM | 0.75 (0.40–1.42) | 0.380 | ADD | 0.70 (0.36–1.37) | 0.296 |
| rs213210 | T/C | 0.06/0.07 | DOM | 0.88 (0.34–2.28) | 0.790 | DOM | 0.70 (0.24–2.03) | 0.511 | |
| mir373 | rs12983273 | C/T | 0.15/0.14 | DOM | 1.75 (0.96–3.18) | 0.066 | DOM | 2.04 (1.04–3.98) | 0.038 |
| rs10425222 | C/A | 0.08/0.05 | DOM | 2.04 (0.89–4.68) | 0.09 | DOM | 1.94 (0.67–5.62) | 0.220 | |
Major/minor alleles.
Adjusted for age, sex, ethnicity, smoking status, stage, grade and treatments. P-value < 0.05 was in bold and used in the unfavorable genotype analysis.
Underlined P-values remained significant after false discovery rate adjustment at 0.05 level.
The association between RCC recurrence and single SNPs was shown in Table III. A total of five SNPs showed significant association with RCC recurrence. Dominant model was the best-fitting model for rs2910164 in mir146a, rs11614913 in mir196a-2 and rs5745925 in mir631. Additive model was the best-fitting model for rs4919510 in mir608; recessive model was the best-fitting model for rs6505162 in mir423. Similar results were obtained if the analysis was restricted in clear cell histology (Table III). One SNP, rs4919510 in mir608, exhibited significant association with both RCC recurrence and survival (Tables II and III) with HR of 1.88 (95% CI = 1.12–3.16; P = 0.017) and 1.61 (95% CI = 1.00–2.57; P = 0.048), respectively.
Table III.
HRs of single SNPs and RCC recurrence
| Gene | SNP | Allelesa | Minor allelle frequency (recurrence/no recurrence) | Best-fitting genetic model (All histology types) |
Best-fitting genetic model (Clear cell carcinoma) |
||||
| Model | HR (95% CI)b | P-valuec | Model | HR (95% CI)b | P-value | ||||
| Biogenesis pathway | |||||||||
| DROSHA | rs6877842 | G/C | 0.13/0.14 | DOM | 0.52 (0.23–1.18) | 0.118 | DOM | 0.36 (0.13–0.98) | 0.046 |
| rs10719 | C/T | 0.21/0.26 | ADD | 0.61 (0.34–1.10) | 0.102 | ADD | 0.63 (0.33–1.20) | 0.161 | |
| DGCR8 | rs417309 | G/A | 0.05/0.05 | DOM | 0.52 (0.17–1.65) | 0.269 | DOM | 0.79 (0.24–2.55) | 0.693 |
| rs3757 | G/A | 0.17/0.24 | DOM | 0.61 (0.30–1.22) | 0.163 | DOM | 0.57 (0.25–1.30) | 0.182 | |
| rs1640299 | G/T | 0.45//0.51 | DOM | 0.95 (0.49–1.86) | 0.889 | DOM | 0.86 (0.40–1.87) | 0.708 | |
| XPO5 | rs11077 | A/C | 0.45/0.44 | DOM | 0.57 (0.29–1.13) | 0.109 | DOM | 0.36 (0.16–0.85) | 0.020 |
| RAN | rs14035 | C/T | 0.28/0.29 | DOM | 0.55 (0.27–1.11) | 0.097 | DOM | 0.50 (0.23–1.09) | 0.080 |
| DICER | rs3742330 | A/G | 0.04/0.06 | DOM | 0.75 (0.23–2.45) | 0.640 | DOM | 0.52 (0.13–2.04) | 0.348 |
| rs13078 | T/A | 0.14/0.19 | DOM | 0.77 (0.39–1.55) | 0.468 | DOM | 0.61 (0.26–1.41) | 0.244 | |
| TRBP | rs784567 | C/T | 0.47/0.40 | DOM | 0.79 (0.37–1.68) | 0.534 | REC | 1.31 (0.60–2.86) | 0.493 |
| AGO1 | rs636832 | G/A | 0.10/0.15 | DOM | 0.82 (0.32–2.11) | 0.680 | DOM | 0.69 (0.24–1.99) | 0.497 |
| rs595961 | A/G | 0.16/0.22 | REC | 3.52 (0.88–14.02) | 0.075 | REC | 3.65 (0.71–18.68) | 0.120 | |
| AGO2 | rs4961280 | C/A | 0.20/0.20 | REC | 2.74 (0.30–24.66) | 0.369 | DOM | 0.62 (0.27–1.47) | 0.280 |
| GEMIN4 | rs910924 | C/T | 0.26/0.23 | DOM | 1.53 (0.76–3.09) | 0.234 | DOM | 1.27 (0.54–3.02) | 0.585 |
| rs2740348 | G/C | 0.16/0.15 | DOM | 0.88 (0.42–1.84) | 0.740 | DOM | 1.15 (0.50–2.64) | 0.744 | |
| rs7813 | T/C | 0.41/0.36 | REC | 1.69 (0.67–4.28) | 0.268 | ADD | 1.38 (0.80–2.37) | 0.246 | |
| rs910925 | G/C | 0.41/0.36 | REC | 1.69 (0.67–4.28) | 0.268 | ADD | 1.38 (0.80–2.37) | 0.246 | |
| rs3744741 | C/T | 0.16/0.17 | DOM | 0.62 (0.30–1.27) | 0.190 | DOM | 0.67 (0.28–1.57) | 0.354 | |
| rs1062923 | T/C | 0.16/0.15 | DOM | 1.09 (0.50–2.38) | 0.837 | DOM | 0.88 (0.33–2.35) | 0.801 | |
| rs4968104 | T/A | 0.23/0.21 | DOM | 1.63 (0.85–3.12) | 0.144 | DOM | 1.30 (0.55–3.05) | 0.546 | |
| GEMIN3 | rs197414 | C/A | 0.12/0.13 | DOM | 0.72 (0.30–1.71) | 0.46 | REC | 0.70 (0.28–1.74) | 0.44 |
| rs197388 | T/A | 0.22/0.21 | DOM | 0.57 (0.28–1.15) | 0.12 | REC | 0.43 (0.19–0.98) | 0.05 | |
| rs197412 | T/C | 0.42/0.44 | REC | 0.51 (0.21–1.24) | 0.137 | REC | 0.39 (0.14–1.08) | 0.069 | |
| HIWI | rs1106042 | G/A | 0.05/0.07 | DOM | 0.52 (0.15–1.74) | 0.288 | DOM | 0.54 (0.16–1.84) | 0.325 |
| Pre-miRNA | |||||||||
| mir146a | rs2910164 | G/C | 0.23/0.27 | DOM | 0.47 (0.24–0.92) | 0.029 | DOM | 0.42 (0.19–0.90) | 0.026 |
| mir196a-2 | rs11614913 | C/T | 0.34/0.38 | DOM | 0.37 (0.19–0.73) | 0.004 | DOM | 0.29 (0.14–0.64) | 0.002 |
| mir423 | rs6505162 | C/A | 0.43/0.48 | REC | 0.39 (0.17–0.89) | 0.026 | REC | 0.46 (0.19–1.13) | 0.091 |
| mir492 | rs2289030 | C/G | 0.06/0.08 | DOM | 0.79 (0.25–2.48) | 0.692 | DOM | 0.58 (0.13–2.62) | 0.476 |
| mir604 | rs2368392 | C/T | 0.22/0.28 | REC | 0.27 (0.04–1.99) | 0.197 | REC | 0.28 (0.04–2.16) | 0.223 |
| mir608 | rs4919510 | C/G | 0.29/0.22 | ADD | 1.88 (1.12–3.16) | 0.017 | ADD | 2.21 (1.24–3.92) | 0.007 |
| mir631 | rs5745925 | CT/— | 0.11/0.08 | DOM | 3.93 (1.69–9.17) | 0.002 | DOM | 4.62 (1.72–12.40) | 0.002 |
| Pri-miRNA | |||||||||
| let7f-2 | rs17276588 | G/A | 0.05/0.03 | DOM | 1.05 (0.32–3.43) | 0.929 | DOM | 1.43 (0.41–4.98) | 0.573 |
| mir26a-1 | rs7372209 | C/T | 0.26/0.29 | DOM | 0.84 (0.43–1.65) | 0.622 | ADD | 0.67 (0.33–1.37) | 0.274 |
| mir30a | rs1358379 | A/G | 0.06/0.03 | DOM | 1.36 (0.44–4.18) | 0.595 | DOM | 1.41 (0.36–5.56) | 0.620 |
| mir30c-1 | rs16827546 | C/T | 0.00/0.04 | N/A | N/A | N/A | N/A | N/A | N/A |
| mir100 | rs1834306 | C/T | 0.40/0.49 | REC | 0.51 (0.21–1.24) | 0.139 | REC | 0.53 (0.20–1.42) | 0.206 |
| mir124-1 | rs531564 | C/G | 0.13/0.11 | DOM | 1.83 (0.83–4.07) | 0.136 | DOM | 1.95 (0.83–4.59) | 0.127 |
| mir219-1 | rs107822 | G/A | 0.27/0.27 | DOM | 1.85 (0.89–3.83) | 0.097 | DOM | 2.18 (0.94–5.09) | 0.071 |
| rs213210 | T/C | 0.08/0.07 | DOM | 1.47 (0.60–3.59) | 0.401 | DOM | 1.47 (0.56–3.85) | 0.433 | |
| mir373 | rs12983273 | C/T | 0.14/0.14 | DOM | 1.68 (0.80–3.53) | 0.174 | DOM | 1.23 (0.52–2.87) | 0.640 |
| rs10425222 | C/A | 0.06/0.05 | DOM | 1.84 (0.64–5.27) | 0.258 | DOM | 2.39 (0.69–8.26) | 0.168 | |
Major/minor alleles.
Adjusted for age, sex, ethnicity, smoking status, stage, grade and treatments. P-value < 0.05 was in bold and used in the unfavorable genotype analysis.
Underlined P-values remained significant after false discovery rate adjustment at 0.05 level.
To further assess the cumulative effects of miRNA-related genetic variants on RCC survival, we did a joint analysis by including the seven SNPs showing a significant or borderline significant association in single-SNP analysis above. The unfavorable genotypes were defined as following: rs3742330 (WM and MM), rs910924 (WM and MM), rs7813 (WM and MM), rs910925 (WM and MM), rs3744741 (WW), rs4968104 (WM and MM), rs4919510 (WM and MM), where WW is the wild-type genotype, WM is the heterozygous genotype and MM is the homozygous variant genotype. In case of closely linked SNPs, only one SNP was used to be summed up with other variants. Compared with the reference group, subjects carrying zero to two unfavorable genotypes, those carrying three to five and six or more unfavorable genotypes had an HR of 2.49 (95% CI = 1.24–5.00; P = 0.01), 6.66 (95% CI = 2.49–17.86; P < 0.001), respectively (P for trend<0.001) (Table IV).
Table IV.
Number of unfavorable genotype and RCC clinical outcome
| Death | Alive | HR (95% CI)a | P-value | |
| 0–2 | 29 (50.00) | 125 (52.52) | Ref. | |
| 3–5 | 21 (36.21) | 97 (40.76) | 2.49 (1.24–5.00) | 0.01 |
| ≥6 | 8 (13.79) | 16 (6.72) | 6.66 (2.49–17.86) | <0.001 |
| P-trend | <0.001 | |||
| Recurrence | No recurrence | |||
| 0 or 1 | 4 (9.30) | 43 (25.44) | Ref. | |
| 2 | 16 (37.21) | 56 (33.14) | 4.10 (1.29–13.06) | 0.017 |
| 3 | 13 (30.23) | 57 (33.73) | 6.84 (2.08–22.57) | 0.002 |
| ≥4 | 10 (30.77) | 13 (7.69) | 25.06 (7.00–89.76) | <0.001 |
| P-trend | <0.001 |
Adjusted for age, sex, ethnicity, smoking status, stage, grade and treatments.
We performed similar joint analysis to assess the cumulative effects of SNPs on recurrence by including the five SNPs showing a significant association in single-SNP analysis: rs2910164 (WW), rs11614913 (WW), rs6505162 (WW + WM), rs4919510 (WM + MM) and rs5745925 (WM + MM). Again, only one SNP was used to be summed up in case of closely linked SNPs. We found that compared with the reference group, subjects carrying one or zero unfavorable genotype, subjects carrying two unfavorable genotypes were at 4.10-fold (95% CI = 1.29–13.06; P = 0.017) increased risk of recurrence and the risk further increased to 6.84-fold (95% CI = 2.08–22.57; P = 0.002) among subjects carrying three unfavorable genotypes. The risk was 25.06-fold (95% CI = 7.00–89.76; P < 0.001) among subjects carrying four or more unfavorable genotypes (P for trend<0.001) (Table IV).
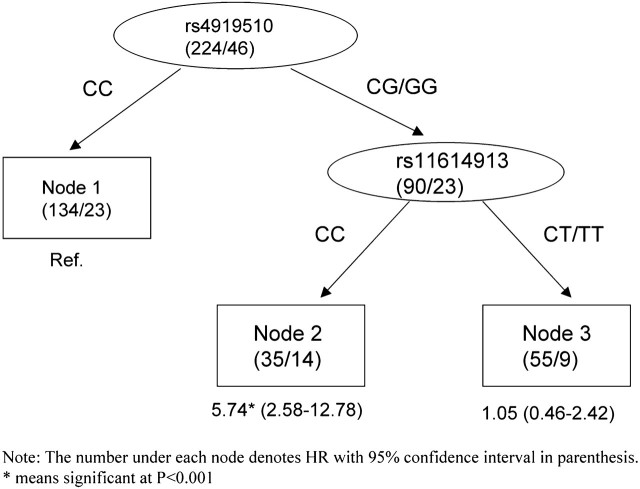
To further explore potential higher order interactions among SNPs, we performed a survival tree analysis. For overall survival, the tree structure resulted in three terminal nodes with a range of low to high-risk subgroups (Figure 1). The initial split was rs3742330 of DICER, followed by rs4919510 of mir608. We selected terminal node 1 (subjects carrying the wild genotype of both SNPs) as the reference group for our analyses. The HRs for terminal nodes 2 (subjects carrying wild-type genotype of rs3742330 and at least one variant allele of rs4919510) and 3 (subjects carrying at least one variant allele of rs3742330) were 2.45 (95% CI = 1.31–4.59; P = 0.005), 3.54 (95% CI = 1.47–8.53; P = 0.005), respectively (Figure 1). We also performed the survival tree analysis to assess gene–gene interactions that affect RCC recurrence (Figure 2). The analysis resulted in three terminal nodes as defined primarily by two SNPs, rs4919510 in mir608 and rs11614913 in mir196a-2. When using terminal node 1 (subjects carrying wild-type genotype of rs4919510) as the reference group, we found that the HRs for terminal nodes 2 (subjects carrying at least one variant allele of the rs4919510 and subjects carrying the wild-type genotype of rs11614913) and 3 (subjects carrying at least one variant allele of the rs4919510 and subjects carrying at least one variant allele of rs11614913) were 5.74 (95% CI = 2.58–12.78; P < 0.001) and 1.05 (95% CI = 0.46–2.42; P = 0.91), respectively (Figure 2).
Fig. 1.
Survival tree analysis of RCC survival.
Fig. 2.
Survival tree analysis of RCC recurrence.
In haplotype analysis, only haplotypes of DICER showed significant association with RCC survival. Specifically, compared with the AT haplotype (in order of rs3742330 and rs13078), the haplotype AA had a HR of 1.51 (95% CI = 0.99–2.31) and the haplotype GA was associated with increased HR of 2.04 (95% CI = 1.00–4.15) (Table V). Similarly, in diplotype analysis, using the AT-AT as the reference group, the diplotype AT-GT was associated with a 2.86-fold increased risk (95% CI = 1.11–7.34; P = 0.03) and the diplotype AA-AA was at 3.48-fold increased risk (95% CI = 1.21–9.97; P = 0.02). No significant associations were observed in other diplotypes. Regarding the association between haplotype/diplotype and recurrence, only haplotypes/diplotypes of DROSHA showed significant associations. Compared with the CG haplotype (in order of rs10719 and rs6877842), a 57% reduction in recurrence risk was observed for the CC haplotype (HR = 0.43; 95% CI = 0.22–0.87; P = 0.02) and the HR was 0.50 (95% CI = 0.27–0.90; P = 0.02) for the TG haplotype. Compared with the CG-CG diplotype, the diplotype CG-CC had a HR of 0.34 (95% CI = 0.12–0.96; P = 0.04), the diplotype TG-TG had HR of 0.18 (95% CI = 0.04–0.94; P = 0.04) and the HR of the diplotype TG-CC was 0.075 (95% CI = 0.008–0.67; P = 0.02) (Table V).
Table V.
DICER and DROSHA haplotype and diplotype and RCC outcome
| Death | Alive | HR (95% CI)a | P-value | |
| DICER | ||||
| Haplotype | ||||
| A-T | 87 (67.97) | 385 (76.39) | Ref. | |
| A-A | 31 (24.22) | 85 (16.87) | 1.51 (0.99–2.31) | 0.06 |
| G-A | 10 (7.81) | 34 (6.75) | 2.04 (1.00–4.15) | 0.049 |
| Diplotype | ||||
| A_T-A_T | 30 (46.88) | 146 (57.94) | Ref. | |
| A_T-A_A | 19 (29.69) | 68 (26.98) | 1.37 (0.72–2.61) | 0.34 |
| A_T-G_T | 8 (12.50) | 25 (9.92) | 2.86 (1.11–7.34) | 0.03 |
| A_A-A_A | 5 (7.81) | 5 (1.98) | 3.48 (1.21–9.97) | 0.02 |
| G_T-A_A | 2 (3.13) | 7 (2.78) | 2.02 (0.40–10.14) | 0.39 |
| G_T-G_T | 0 (0.00) | 1 (0.40) | N/A | N/A |
| Recurrence | No recurrence | HR (95% CI)a | P-value | |
| DROSHA | ||||
| Haplotype | ||||
| C-G | 77 (68.75) | 223 (61.94) | Ref. | |
| C-C | 15 (13.39) | 50 (13.89) | 0.43 (0.22–0.87) | 0.02 |
| T-G | 19 (16.96) | 85 (23.61) | 0.50 (0.27–0.90) | 0.02 |
| T-C | 1 (0.89) | 2 (0.56) | 0.87 (0.12–6.61) | 0.9 |
| Diplotype | ||||
| C_G-C_G | 29 (51.79) | 74 (41.11) | Ref. | |
| C_G-C_C | 7 (12.50) | 30 (16.67) | 0.34 (0.12–0.96) | 0.04 |
| C_G-T_G | 12 (21.43) | 45 (25.00) | 0.69 (0.28–1.67) | 0.41 |
| C_C-C_C | 3 (5.36) | 4 (2.22) | 0.42 (0.07–2.40) | 0.33 |
| C_C-T_C | 0 (0.00) | 2 (1.11) | N/A | N/A |
| T_G-C_C | 2 (3.57) | 10 (5.56) | 0.075 (0.008–0.67) | 0.02 |
| T_G-T_G | 2 (3.57) | 15 (8.33) | 0.18 (0.04–0.94) | 0.04 |
| T_G-T_C | 1 (1.79) | 0 (0.00) | 7.37 (0.64–84.14) | 0.11 |
Bold numbers denote P-value < 0.05.
Adjusted for age, sex, ethnicity, smoking status, stage, grade and treatments.
Discussion
In this systematic evaluation of the influence of genetic variations in the miRNA biosynthesis and miRNA genes on the clinical outcome of RCC patients, we identified seven SNPs that were significantly (including one SNPs with borderline significance) associated with RCC survival and five SNPs with RCC recurrence. We have previously reported that potentially functional SNPs in miRNA-related genes were associated with the etiology of multiple malignancies including RCC (21). This is the first epidemiological study evaluating the effects of these SNPs on RCC clinical outcome.
For individual associations with RCC survival, the most significant SNPs were in GEMIN4 (rs7813 and rs910925, rs3744741). The minor alleles of both rs7813 and rs910925 were associated with 1.74-fold increased death risk for RCC, whereas the minor allele of rs3744741 was associated with 61% reduction in risk. GEMIN4 belong to a family of genes whose products are components of a motor neuron complex and are involved in pre-mRNA splicing and ribonucleoprotein assembly (25). Previously, we have reported that genetic variants of GEMIN4 and GEMIN3 genes were associated with renal, bladder and esophageal cancer risks (21,22,26). In particular, in our previous case–control study, we found that the SNP rs7813 was associated with decreased risk of developing RCC (21). However, in the current study, the same SNP conferred an increased death risk of RCC. In addition, in the current study, the SNP rs3744741 was associated with better RCC survival, but in our previous study (26), the same SNP conferred reduced risk of esophageal cancer. It is not unprecedented that the same genetic trait may have differential effect on cancer susceptibility and cancer prognosis/treatment response. For example, weaker DNA repair capacity was associated with increased cancer risk but was also linked to better cisplatin response due to less removal of DNA–drug adducts (27). Both rs7813 and rs910925 are non-synonymous SNPs in exon 1 of GEMIN4, whose minor alleles resulted in the amino acid substitution of cysteine to arginine and glycine to alanine respectively (dbSNP; http://www.ncbi.nlm.nih.gov/projects/SNP/index). Linkage analysis showed that the two SNPs were in linkage disequilibrium (r2 > 0.8). Expression of these variant forms of GEMIN4 protein in hepatocellular cancer cells led to increased cellular proliferation and reduced apoptosis and DNA repair (28), suggesting a causal physiological role for these genetic variants. Besides GEMIN SNPs, borderline significant association was found in one SNP in DICER (rs3742330). Haplotype and diplotype analyses also showed that genetic variants of DICER were associated with increased risk of RCC mortality. Proteins encoded by DICER are members of the ribonuclease III family of double-stranded RNA endonucleases, which participate in RNA maturation and decay pathways (29). Lowered DICER1 protein expression was associated with incidence of ovarian cancer and advanced tumor stage (30). Using comparative genomic hybridization, Zhang et al. (31) found copy number changes in DICER1, Argonaute2 and other miRNA-related genes in breast and ovarian cancer as well as melanoma. There has been no report on DICER expression profiling in RCC. The correlation between DICER expression and DICER genotypes has yet to be elucidated. However, DICER expression has been examined in other cancers, such as ovarian (30), lung (32,33), esophageal (34) and prostate cancers (35). Decreased DICER expression was associated with advanced ovarian tumor stage and poor patient survival (30), whereas high DICER expression was a poor prognostic factor in patients with prostate and esophageal carcinoma (34,35). The differences in these reports have not been fully elucidated; however, these data suggest that the regulation and the effect of DICER expression may be tumor specific. Besides GEMIN4 and DICER, one SNP in mir608 (rs4919510) was significantly associated with both RCC survival and recurrence with the variant allele conferred significantly increased risk of survival and recurrence. The polymorphism of mir608 (rs4919510) has been predicted by in silico algorithms to show differential binding to its target genes, which include INSR (insulin receptor) and TP53 (36). It is possible that genetic variants of miRNA gene could alter its target gene specificities.
Individual SNPs associated with RCC recurrence were all pre-miRNA SNPs (Table 3), including mir146a (rs2910164), mir196a-2 (rs11614913), mir423 (rs6505162), mir608 (rs4919510) and mir631 (rs5745925). The mir146a gene has been implicated in the development of multiple cancers and the regulation of inflammation induced via the innate immune response (37). Decreased mir146a expression has been associated with hormone refractory prostate cancer (38) and upregulation of this gene was found to suppress breast cancer metastasis (39). Variant allele of rs2910164 has been associated with increased risk for breast, ovarian and hepatocellular carcinomas (40,41), whereas in the current study, the variant allele was associated with decreased risk of RCC recurrence. More importantly, rs2910164 has been shown to be a functional polymorphism, which affects the production of the mature transcript as well as its binding to target mRNA, such as that of BRCA1 (40,41).
Our data showed that the variant T allele of the mir196a-2 rs11614913 was associated with a decreased risk for RCC recurrence. High mir196a level has been shown to promote the oncogenic phenotype in colorectal cancer (42) and its expression is a potential biomarker of progression during transformation of Barrett’s esophagus to adenocarcinoma (43). One of the known targets of mir196a is the annexin A1 gene (ANXA1) and suppression of ANXA1 expression by mir196a led to increased cell proliferation and anchorage-independent growth and decreased apoptosis (44). Moreover, homozygous wild-type (CC) allele of rs11614913 in mir196a has been associated with increased expression of the mature transcript and decreased survival for non-small cell lung cancer in a Chinese population (45).
We have previously reported that rs6505162 of mir423 was associated with decreased risk for esophageal cancer (26). In the current study, we found that variant A allele of rs6505162 was associated with 61% reduced risk for RCC recurrence. Increased expression of mir423 has been associated with elevated risk for endometrial cancer (46). One of the putative target of mir423 is a Kruppel-like factor 2 (KLF2) gene, which is involved in the regulation of endothelial proinflammatory function and angiogenesis by inhibiting hypoxia-inducible factor-1 alpha (HIF-1α) expression (46,47). Perhaps the variant allele of mir423 has decreased capacity to target KLF2 mRNA, which leads to increased inhibition of the inflammatory and angiogenic pathways and protects against risk of cancer recurrence. Further research is warranted to test the potential functional effects of these and other miRNA polymorphisms in RCC cells.
We also found that one SNP (rs5745925) in mir631 was associated with increased risk of RCC recurrence. We previously reported that the same SNP was associated with increased risk of esophageal cancer (26). There has been no report on functional characterization of this SNP.
Several haplotypes and diplotypes of the DROSHA SNPs (rs6877842 and rs10719) showed significant association with RCC recurrence. As DICER, proteins encoded by DROSHA are members of the ribonuclease III family of double-stranded RNA endonucleases, which participate in RNA maturation and decay pathways (29). It is interesting that the significant associations were not observed in single-SNP analysis but became evident in haplotype and diplotype analysis, suggesting that single SNPs in DROSHA may affect RCC recurrence jointly.
To explore the higher order interactions among the SNPs, we applied the survival tree analysis to further define high- versus low-risk subgroups. With this analytic approach, subgroups of individuals with different risk profile were identified based on SNP combinations. Because of the moderate sample size of this study, the number of subjects became small in terminal nodes. This analysis was therefore exploratory in nature and these results should be interpreted with caution.
Due to the relatively limited number of RCC cases, some of the SNPs we identified might be chance findings. However, the significant associations identified remained significant after adjusting for multiple comparisons. Furthermore, to increase detection power of the test, we took a pathway-based polygenic strategy to further elucidate the accumulative influences of multiple miRNA polymorphisms on RCC outcome. We identified an accumulative effect with an increasing number of unfavorable genotypes that occurred in a dose-dependent manner. Specifically, when multiple SNPs were analyzed together, a strong dose–response trend emerged, suggesting increased RCC death–recurrence risk with increasing number of adverse genotypes. Moreover, survival tree, haplotype and diplotype analyses indicated the joint effects of these miRNA genetic variants on RCC survival and recurrence, consistent with the polygenic nature of RCC clinical outcome and supporting the idea that polymorphisms of the miRNA-related genes may influence clinical outcome of renal cancer.
In conclusion, this is the first study that systematically evaluated the association between genetic variants in miRNA-related genes and RCC clinical outcome. Our results identified several putative variants that impact RCC survival and recurrence. The results from the cumulative analysis, haplotype analysis as well as higher order gene–gene interactions strongly suggested that these genetic variants may influence RCC clinical outcome jointly. However, the molecular mechanisms associated with these SNPs would depend on the functioanl impact of these SNPs on miRNAs and the interaction of specific miRNAs with target mRNAs in kidney tissues. Thus, future functional experiments are needed to elucidate the underlying molecular mechanisms associated with the SNPs. Nevertheless, validating current findings in independent patient populations is warranted.
Funding
National Cancer Institute (R01CA 98897, K07 CA134831).
Glossary
Abbreviations
- CI
confidence interval
- HR
hazard ratio
- miRNA
microRNA
- RCC
renal cell carcinoma
- SNP
single-nucleotide polymorphism
References
- 1.Jemal A, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Janzen NK, et al. Laparoscopic radical nephrectomy and minimally invasive surgery for kidney cancer. Cancer Treat. Res. 2003;116:99–117. doi: 10.1007/978-1-4615-0451-1_6. [DOI] [PubMed] [Google Scholar]
- 3.Lam JS, et al. Prognostic factors and selection for clinical studies of patients with kidney cancer. Crit. Rev. Oncol. Hematol. 2008;65:235–262. doi: 10.1016/j.critrevonc.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. http://www.cancer.org/docroot/CRI/content/CRI_2_4_3X_How_is_kidney_cancer_staged_22.asp?rnav=cri (11 August 2009, date last accessed) [Google Scholar]
- 5.Escudier B. Advanced renal cell carcinoma: current and emerging management strategies. Drugs. 2007;67:1257–1264. doi: 10.2165/00003495-200767090-00002. [DOI] [PubMed] [Google Scholar]
- 6.Janzen NK, et al. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol. Clin. North Am. 2003;30:843–852. doi: 10.1016/s0094-0143(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 7.Lee RC, et al. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 8.Bentwich I, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat. Genet. 2005;37:766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- 9.Lu J, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 10.Lim LP, et al. Microarray analysis shows that some microRNAs down-regulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 11.Esquela-Kerscher A, et al. Oncomirs—microRNAs with a role in cancer. Nat. Rev. Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 12.Han J, et al. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell. 2006;125:887–901. doi: 10.1016/j.cell.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 13.Yue J, et al. MicroRNA trafficking and human cancer. Cancer Biol. Ther. 2006;5:573–578. doi: 10.4161/cbt.5.6.2872. [DOI] [PubMed] [Google Scholar]
- 14.Chendrimada TP, et al. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng F, et al. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology. 2006;130:2113–2129. doi: 10.1053/j.gastro.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 16.Yanaihara N, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Calin GA, et al. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc. Natl Acad. Sci. USA. 2004;101:11755–11760. doi: 10.1073/pnas.0404432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu J, et al. The pharmacogenetic impact of inflammatory genes on bladder cancer recurrence. Pharmacogenomics. 2005;6:575–584. doi: 10.2217/14622416.6.6.575. [DOI] [PubMed] [Google Scholar]
- 19.Wu X, et al. Germline genetic variations in drug action pathways predict clinical outcomes in advanced lung cancer treated with platinum-based chemotherapy. Pharmacogenet. Genomics. 2008;8:955–965. doi: 10.1097/FPC.0b013e32830efdd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J, et al. Case-control analysis of nucleotide excision repair pathway and the risk of renal cell carcinoma. Carcinogenesis. 2008;29:2112–2119. doi: 10.1093/carcin/bgn189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horikawa Y, et al. Single nucleotide polymorphisms of microRNA machinery genes modify the risk of renal cell carcinoma. Clin. Cancer Res. 2008;14:7956–7962. doi: 10.1158/1078-0432.CCR-08-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang H, et al. Evaluation of genetic variants in microRNA-related genes and risk of bladder cancer. Cancer Res. 2008;68:2530–2536. doi: 10.1158/0008-5472.CAN-07-5991. [DOI] [PubMed] [Google Scholar]
- 23.Benjamini Y, et al. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. A. 1995;B57:289–300. [Google Scholar]
- 24.Zhang HP, et al. Recursive Partitioning in the Health Sciences. New York, NY: Springer; 1999. [Google Scholar]
- 25.Shpargel KB, et al. Gemin proteins are required for efficient assembly of Sm-class ribonucleoproteins. Proc. Natl Acad. Sci. USA. 2005;102:17372–17377. doi: 10.1073/pnas.0508947102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye Y, et al. Genetic variations in microRNA-related genes are novel susceptibility loci for esophageal cancer risk. Cancer Prev. Res. 2008;14:460–469. doi: 10.1158/1940-6207.CAPR-08-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spitz MR, et al. Integrative epidemiology: from risk assessment to outcome prediction. J. Clin. Oncol. 2005;23:267–275. doi: 10.1200/JCO.2005.05.122. [DOI] [PubMed] [Google Scholar]
- 28.Wan D, et al. Two variants of the human hepatocellular carcinoma-associated HCAP1 gene and their effect on the growth of the human liver cancer cell line Hep3B. Genes Chromosomes Cancer. 2004;9:48–58. doi: 10.1002/gcc.10293. [DOI] [PubMed] [Google Scholar]
- 29.MacRae IJ, et al. Structural determinants of RNA recognition and cleavage by Dicer. Nat. Struct. Mol. Biol. 2007;14:934–940. doi: 10.1038/nsmb1293. [DOI] [PubMed] [Google Scholar]
- 30.Merritt WM, et al. Dicer, Drosha, and outcomes in patients with ovarian cancer. N. Engl. J. Med. 2008;359:2641–2650. doi: 10.1056/NEJMoa0803785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, et al. microRNAs exhibit high frequency genomic alterations in human cancer. Proc. Natl Acad. Sci. USA. 2006;103:9136–9141. doi: 10.1073/pnas.0508889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiosea S, et al. Overexpression of Dicer in precursor lesions of lung adenocarcinoma. Cancer Res. 2007;67:2345–2350. doi: 10.1158/0008-5472.CAN-06-3533. [DOI] [PubMed] [Google Scholar]
- 33.Karube Y, et al. Reduced expression of Dicer associated with poor prognosis in lung cancer patients. Cancer Sci. 2005;96:111–115. doi: 10.1111/j.1349-7006.2005.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugito N, et al. RNASEN regulates cell proliferation and affects survival in esophageal cancer patients. Clin. Cancer Res. 2006;12:7322–7328. doi: 10.1158/1078-0432.CCR-06-0515. [DOI] [PubMed] [Google Scholar]
- 35.Chiosea S, et al. Up-regulation of dicer, a component of the MicroRNA machinery, in prostate adenocarcinoma. Am. J. Pathol. 2006;169:1812–1820. doi: 10.2353/ajpath.2006.060480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landi D, et al. A catalog of polymorphisms falling in microRNA-binding regions of cancer genes. DNA Cell Biol. 2008;27:35–43. doi: 10.1089/dna.2007.0650. [DOI] [PubMed] [Google Scholar]
- 37.Williams AE, et al. Role of miRNA-146a in the regulation of the innate immune response and cancer. Biochem. Soc. Trans. 2008;36:1211–1215. doi: 10.1042/BST0361211. [DOI] [PubMed] [Google Scholar]
- 38.Lin SL, et al. Loss of mir-146a function in hormone-refractory prostate cancer. RNA. 2008;14:417–424. doi: 10.1261/rna.874808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hurst DR, et al. Breast cancer metastasis suppressor 1 up-regulates miR-146, which suppresses breast cancer metastasis. Cancer Res. 2009;69:1279–1283. doi: 10.1158/0008-5472.CAN-08-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen J, et al. A functional polymorphism in the miR-146a gene and age of familial breast/ovarian cancer diagnosis. Carcinogenesis. 2008;29:1963–1966. doi: 10.1093/carcin/bgn172. [DOI] [PubMed] [Google Scholar]
- 41.Xu T, et al. A functional polymorphism in the miR-146a gene is associated with the risk for hepatocellular carcinoma. Carcinogenesis. 2008;29:2126–2131. doi: 10.1093/carcin/bgn195. [DOI] [PubMed] [Google Scholar]
- 42.Schimanski CC, et al. High miR-196a levels promote the oncogenic phenotype of colorectal cancer cells. World J. Gastroenterol. 2009;15:2089–2096. doi: 10.3748/wjg.15.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maru DM, et al. MicroRNA-196a is a potential marker of progression during Barrett's metaplasia-dysplasia-invasive adenocarcinoma sequence in esophagus. Am. J. Pathol. 2009;174:940–948. doi: 10.2353/ajpath.2009.080718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luthra R, et al. MicroRNA-196a targets annexin A1: a microRNA-mediated mechanism of annexin A1 downregulation in cancers. Oncogene. 2008;27:6667–6678. doi: 10.1038/onc.2008.256. [DOI] [PubMed] [Google Scholar]
- 45.Hu Z, et al. Genetic variants of miRNA sequences and non-small cell lung cancer survival. J. Clin. Invest. 2008;118:2600–2608. doi: 10.1172/JCI34934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boren T, et al. MicroRNAs and their target messenger RNAs associated with endometrial carcinogenesis. Gynecol. Oncol. 2008;110:206–215. doi: 10.1016/j.ygyno.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 47.Kawanami D, et al. Kruppel-like factor 2 inhibits hypoxia-inducible-factor-1 alpha expression and function in the endothelium. J. Biol. Chem. 2009;284:20522–20530. doi: 10.1074/jbc.M109.025346. [DOI] [PMC free article] [PubMed] [Google Scholar]