Abstract
To probe the connection between longevity and stress resistance, we compared the sensitivity of Ames long-lived dwarf mice and control littermates with paraquat, diquat, and dobutamine. In young adult animals, 95% of male and 39% of female controls died after paraquat administration, but no dwarf animals died. When the experiment was repeated at an older age or a higher dosage of paraquat, dwarf mice still showed greater resistance. Dwarf mice also were more resistant to diquat; 80% of male and 60% of female controls died compared with 40% and 20% of dwarf mice, despite greater sensitivity of dwarf liver to diquat. Dwarf mice were also less sensitive to dobutamine-induced cardiac stress and had lower levels of liver and lung F2-isoprostanes. This is the first direct in vivo evidence that long-lived Ames dwarf mice have enhanced resistance to chemical insult, particularly oxidative stressors.
Keywords: Reactive oxygen species, Liver, Ames dwarf mice, Paraquat, Diquat
AMES dwarf mice (1) are the first long-lived mutant mammal reported (2). Male and female Ames dwarf mice live an average of 49% and 64% longer than control littermates, respectively (2). Ames dwarf mice have a 70% lower body weight than control mice, 1.6°C lower core body temperature (2), delayed onset of neoplastic lesions (2,3), and youthful levels of cognitive performance and locomotor activity into advanced age (4–6). The increased life span combined with the postponement of neoplasia and cognitive decline suggests that the increase in longevity arises because of retarded aging. The Ames mutation maps to a single nucleotide substitution in the gene Prophet of Pit-1 (Prop-1) (7). At embryonic days 12.5–13, this mutation prevents the differentiation of pituitary cells that would normally express the homeotic factor Pit-1 that leads to postnatal hypoplasia of the anterior pituitary gland (8,9) and a deficiency in thyroid-stimulating hormone, prolactin, and growth hormone (GH), resulting in low levels of circulating insulin-like growth factor 1 (IGF-1) (10,11).
Since the discovery that Ames dwarf mice have an increased life span (2), investigators have sought mechanisms to explain this phenomenon. The oxidative stress theory of aging, formerly called the free radical theory of aging (12), proposes that resistance to oxidative stress is linked to extended life span. Studies with calorie-restricted (CR) mice (13,14) as well as long-lived mutant Caenorhabditis elegans (15,16) and Drosophila (17,18) support this theory. Oxidative stress is defined as an elevated concentration of reactive oxygen–containing molecules including but not limited to free radicals such as hydroxyl and superoxide (19). Organisms defend against oxidative stress via antioxidant enzymes (such as superoxide dismutases, glutathione peroxidases, and catalase), small molecules that serve as direct scavengers of free radicals or cofactors for antioxidant enzymes, turnover of oxidatively damaged macromolecules, and ultimately cell cycle arrest or apoptosis. To the extent that antioxidant defenses cannot keep pace with oxidative stress, oxidative damage accumulates with the eventual impairment of physiological function.
In Ames dwarf mice, enzyme activity data suggest that the expression and activity of certain antioxidants are altered. However, the direction of change is not always consistent with increased resistance to oxidative stress. The same is true for biomarkers of oxidative damage. The strongest data to support the hypothesis that Ames dwarf mice have increased resistance to oxidative stress come from the Miller laboratory. Using primary cultures of fibroblasts from adult dwarf mice, they showed that fibroblasts from dwarf mice are resistant to paraquat, H2O2, cadmium, and a variety of other stressors (20). However, it is unclear whether the sensitivity of fibroblasts accurately reflects the sensitivity of tissues to oxidative stress in the whole animal.
In this study, we directly tested the sensitivity of Ames dwarf to oxidative stress by treating them with paraquat and diquat, which are inducers of oxidative stress. We also tested the resistance of Ames mice to dobutamine, an inducer of cardiac stress (21). We found that Ames dwarf mice are resistant to paraquat- and diquat-induced toxicity and to dobutamine-induced alteration in cardiac stress. Thus, the increase in longevity reported for Ames dwarf mice is in fact correlated with increased resistance to oxidative stress as well as other types of stress.
MATERIALS AND METHODS
Animals
Ames heterozygous (Prop-1+/−) mice were kindly provided by Dr Holly Brown-Borg (University of North Dakota). To establish our Ames colony, Prop-1+/− male and female mice were mated with each other. The offspring were Prop-1−/− (dwarf) and Prop-1+/− (control, which are phenotypically indistinguishable from wild type). For the calorie restriction experiments, male C57Bl6 (The Jackson Laboratory, Bar Harbor, ME) mice were used. All mice were fed a standard NIH-31 chow and maintained in microisolator cages on a 12-hour dark/light cycle, and age-matched groups were chosen for the experiments reported here. All animal procedures were approved by the subcommittee for animal studies at the Audie L. Murphy Veterans Administration Hospital and the University of Texas Health Science Center Institutional Animal Care and Use Committee. For the isoprostane experiment, calorie restriction was accomplished in Ames mice using a standard pair-feeding regimen adapted from McCarter and colleagues (22). Briefly, from 6 weeks of age onward, the CR mice were fed 70% of the food consumed by age-matched mice fed ad libitum (AL) (i.e., a 30% restriction of food intake). The feeding of the CR mice occurred daily at 3:00 PM. The animals were initially housed four per cage, and the CR group was not given mineral supplements as previous studies in mice and rats have demonstrated that multiple (rather than single) housing does not affect the life-extending effects of calorie restriction and that mineral supplementation is not necessary under these conditions (23,24).
Paraquat and Diquat
Paraquat (Sigma-Aldrich, St Louis, MO) and diquat (Chem Service, Westchester, NY) were stored at room temperature in a desiccator, protected from light. Immediately before use, they were dissolved in 0.9% saline (25 mg/mL), the concentration was verified via absorbance at 308 nm, and the respective compounds were injected interperitoneally at the dosages indicated in the figure legends. A Hamilton syringe demarcated in 2.5-μL increments was used for the injection (the body weight–adjusted doses ranged from 32.5 to 140 μL), making it possible to adjust dosage for body weight differences as small as 0.6 g. For tissue collection, the diquat-treated mice were anesthetized with ketamine/acepromazine/xylazine cocktail. Blood was collected by cardiac puncture and transferred to heparinized storage tubes for plasma isolation. The animals were then euthanized by cervical dislocation; livers and other organs of interest were immediately removed and snap-frozen in liquid nitrogen or preserved in 10% neutral buffered formalin for histology.
Short-Term Survival Monitoring
For survival studies of paraquat- and diquat-treated mice, cages were placed under an array of digital surveillance cameras (Strategic Vista, Ontario, Canada) attached to generic Pentium series computers running Windows XP. These cameras monitored the animals continuously, and the recorded footage was used to determine the time of death with a resolution of less than 1 minute. The time of injection was subtracted from the recorded time of death to obtain the survival time for each animal.
Alanine–Leucine Transaminase Activity
Blood collected from diquat-treated mice was stored on ice in lithium heparin tubes. As soon as possible after collection, plasma was separated from the formed elements by centrifugation for 10 minutes at 1.5 kg in a centrifuge at 4°C. Alanine–leucine transaminase (ALT) activity in the plasma was measured as per manufacturer’s instructions using the ALT Colorimetric Kit from Teco Diagnostics (Anaheim, CA).
Apoptosis
Liver samples preserved in 10% neutral buffered formalin were paraffin embedded, sectioned, and fixed on slides. Apoptotic cells in these sections were identified on the basis of double-strand DNA breaks using the ApopTag Kit from Chemicon (Temecula, CA). Cell nuclei that were both dark and compacted were identified under a light microscope as apoptotic, and the number of such nuclei was exhaustively counted for entire liver cross sections. To ensure that no fields were repeated or skipped, a semitransparent, self-adhesive grid was applied to the slides and used to navigate them. The number of apoptotic nuclei in the entire cross section was divided by the cross-sectional area (arbitrary grid units) to give the number of apoptotic cells per cross-sectional area of liver.
F2-Isoprostanes
The levels of 8-Iso-PGF2α (F2-isoprostanes) in the liver were determined using gas chromatography/mass spectrometry (GC/MS) as described by Roberts and Morrow (25). Tissue samples were homogenized in ice-cold Folch solution (2:1 chloroform:methanol) containing 5 mg/100 mL butylated hydroxytoluene. Lipids were extracted and hydrolyzed with 15% KOH. After acidification, the F2-isoprostanes were extracted with a C18 Sep-Pak column and a silica Sep-Pak column (Waters, Milford, MA). The F2-isoprostanes were converted to pentafluororobenzyl esters and purified by thin layer chromatography, derivatized to trimethysilyl ether derivatives, and quantified by GC/MS using [2H4] 8-Iso-PGF2α as an internal standard. The levels of F2-isoprostane in liver are expressed as nanogram per gram tissue.
Echocardiography and Dobutamine-Induced Cardiac Stress
For the echocardiographic studies, the mice were anesthetized with isofluorane (1%) in a 100% oxygen mix, which allowed the mice to breathe spontaneously, and the body temperature was maintained at 37°C using an isothermal pad. Electrocardiograms and heart rates were continuously monitored. From a transthoracic approach, two-dimensional targeted M-mode echocardiographic recordings were obtained using the Vevo 770 High-Resolution In Vivo Imaging System (Visual Sonics, Toronto, Ontario, Canada), which provides spatial resolution to 30 m. A perpendicular view with respect to the left ventricular long axis was obtained in order to observe the two-dimensional parasternal short axis. From these views, the dimensions, wall thickness, and volumes were measured for three cycles and averaged. Temporary cardiac stress was induced by administering dobutamine, a β receptor agonist that increases heart rate by stimulating myocyte contractility (26). Dobutamine was given at a dose of 1.5 μg/g body weight by intraperitoneal injection. Echocardiograms were recorded at baseline (preinjection) and 10 minutes after injection.
Statistics
All data in bar charts are expressed as mean ± SEM. The Cox–Mantel log-rank test was used to evaluate all survival curves. The likelihood ratio test and the Wald test were also performed, and in all cases, the p values returned agreed to the first significant digit with the log-rank test. The Student’s t test was used to compare ALT levels, the number of apoptotic cells per unit of cross-sectional area, and tissue isoprostane levels. When multiple comparisons were done on the same set of samples (in the isoprostane experiments), Holm’s method (27) was used to adjust the p values returned by the t test. All p values are expressed to the first significant digit in the graphs. Left ventricular dimensions and wall thicknesses were expressed as percent change between baseline (pre) and dobutamine injection (post) values and evaluated by Student’s t test. All statistical tests were performed using the R statistical language (28).
RESULTS
Sensitivity of Ames Dwarf Mice to Paraquat
The sensitivity of Ames dwarf and control mice to paraquat was studied because paraquat is a standard model for inducing oxidative stress in cells and whole animals. Paraquat (N,N′-dimethyl-4,4′-bipyridinium dichloride) is a bipyridyl compound that is a relatively pure catalytic generator of superoxide anions (29). Paraquat is reduced by nicotinamide adenine dinucleotide phosphate to its free radical form, immediately transferring one electron to oxygen, producing superoxide anion, and reforming the original paraquat cation. This reaction occurs in the cytoplasm (30), microsomes (31,32), and the mitochondria (33–35).
Using a novel video system that we developed, we were able to measure the postparaquat survival time of mice with 1-minute resolution. Figure 1 shows the survival of young adult (4–6 months of age) male and female dwarf and control mice after exposure to 50-mg paraquat/kg body weight. None of the dwarf mice died from this dose of paraquat compared with 95% of the male (Figure 1A) and 39% of the female (Figure 1B) control mice. Because no dwarf mice died at this dose and less than 40% of the female control mice died at this dose, we studied the effect of a higher dose of paraquat (75 mg/kg) on the survival of young adult female dwarf and control mice. All the female control mice (Figure 1C) died from this dose within 4 days after the administration of paraquat; however, only 20% of the dwarf mice died after 5 days of administration. To determine whether the difference in paraquat toxicity is also observed in old mice, we measured the survival of old (14–20 months of age) male dwarf and control mice after injection of 50-mg paraquat/kg body weight. Again, we observed that the old dwarf mice exhibited a significant increase in resistance to paraquat-induced toxicity compared with control mice: 50% of the dwarf mice were dead after 6 days compared with 98% of the control mice (Figure 1D).
Figure 1.
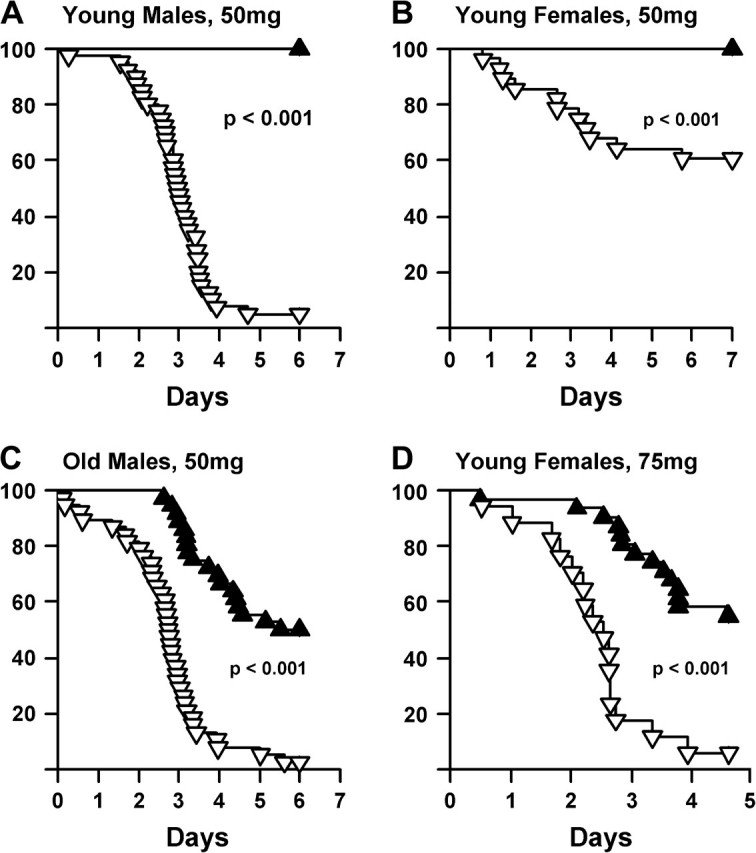
Survival of Ames mice after paraquat exposure. Paraquat (50 mg/kg) was administered to 40 male control mice and 30 male dwarf mice (A) as well as to 28 female control mice and 31 female dwarf mice (B). All mice were 4.1–6.3 mo old. In (C), paraquat (50 mg/kg) was administered to 38 male control mice 11.9–17.9 mo of age and 36 male dwarf mice 14.0–19.9 mo of age. In (D), a higher dose of paraquat (75 mg/kg) was administered to 9 control and 15 dwarf female mice 4.1–6.3 mo of age. The dwarf mice are shown in solid triangles, and the control littermates in open triangles. The survival of the mice was followed over 6 days and statistically analyzed using the log-rank test as described in the Experimental Procedures.
Sensitivity of Ames Dwarf Mice to Diquat
We measured the sensitivity of mice to diquat in two ways. First, we measured the survival of dwarf mice after diquat injection; second, we measured diquat-induced hepatotoxicity. Diquat (1,1′-ethylene-2,2′-bipyridyldiylium dibromide) is a compound chemically similar to paraquat and catalyzes superoxide production in the same manner (36). Whereas paraquat is actively taken up by and concentrated in pneumocytes (37,38), diquat is distributed to multiple organs, including but not limited to the liver (39–41). Thus, diquat can generate oxidative stress in different tissues than paraquat. Six days after injection of young adult (4–6 months of age) male and female dwarf and control mice with diquat (100 mg/kg), 80% of the male and 60% of the female control mice had died, whereas only 40% of the male and 20% of the female dwarf mice had died (Figure 2).
Figure 2.
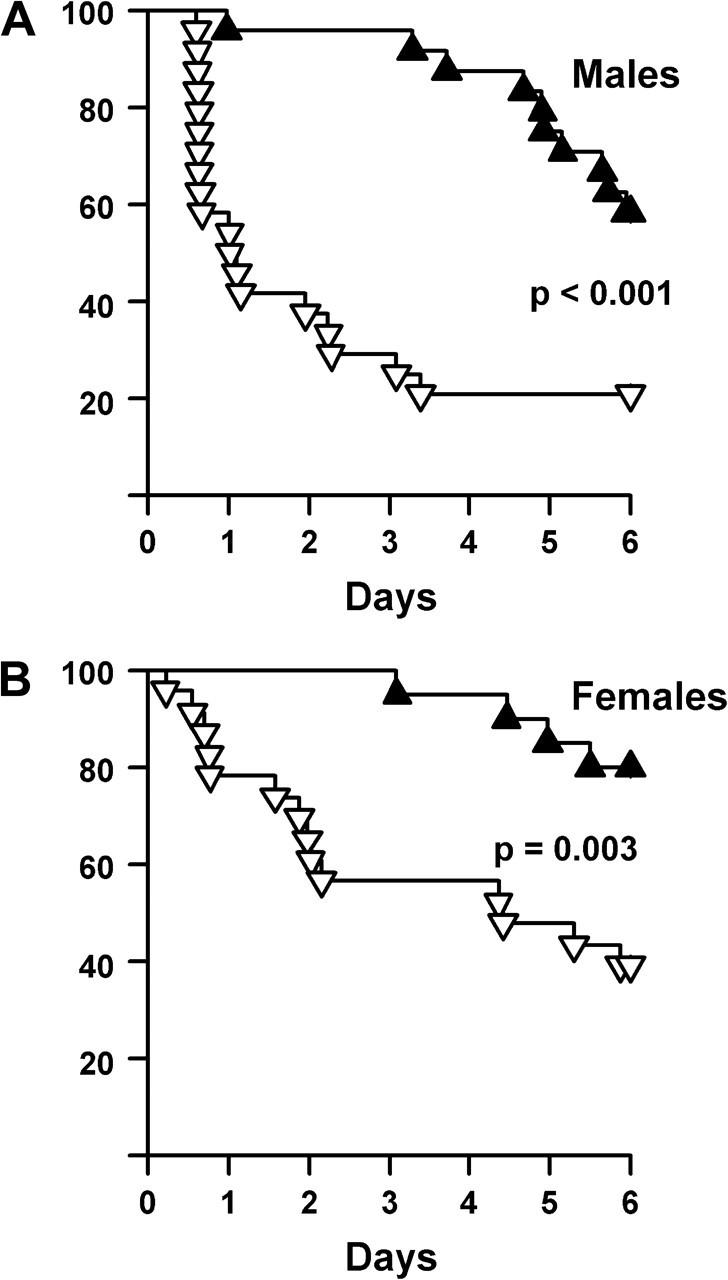
Survival of Ames mice after diquat exposure. Diquat (50 mg/kg) was administered to 24 control and 24 dwarf male mice (A) as well as to 23 control and 20 dwarf female mice (B). All the animals were between 4.2 and 5.8 mo of age. The dwarf mice are shown in solid triangles, and the control littermates in open triangles. The survival of the mice was followed over 6 days and statistically analyzed using the log-rank test as described in the Experimental Procedures.
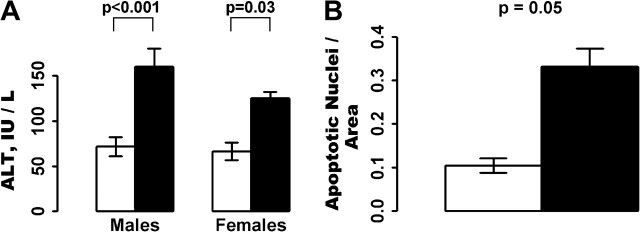
We also measured the sensitivity of dwarf and control mouse livers to diquat by assaying the activity of ALT in the plasma after diquat treatment (Figure 3A). ALT is a cytoplasmic liver enzyme, and its release from the liver and appearance in blood are used to measure loss of hepatocyte cell membrane integrity in humans and animals, that is, hepatoxicity (42). Surprisingly, we observed a significant increase in ALT levels after diquat treatment in both male and female dwarf mice compared with their control littermates, indicating that the dwarf mice experienced increased hepatotoxicity after diquat treatment. To confirm the ALT data, we measured the induction of apoptosis by diquat treatment in the livers of female dwarf and control mice. Livers from the dwarf mice had a 2.8-fold increase in apoptosis as measured by double-strand DNA breaks (Figure 3B). Thus, the data in Figure 3 show that dwarf mice exhibited increased hepatotoxicity in response to diquat treatment.
Figure 3.
Sensitivity of Ames mice to diquat-induced hepatotoxicity. Ames control and dwarf mice were treated with diquat (50 mg/kg), and 6 h after treatment the mice were killed. Hepatotoxicity was measured by the increase in alanine–leucine transaminase (ALT) activity in the plasma and apoptosis in the liver as described in the Experimental Procedures. (A) The mean and SEM ALT activities measured in the plasma of 10 male control mice, 11 male dwarf mice, 8 female control mice, and 8 female dwarf mice (all animals were 12.3–13.9 mo of age). (B) The mean and SEM levels of apoptosis measured in the liver of nine male control mice and nine male dwarf mice (12.3–13.7 mo of age). In (A) and (B), the open bars represent control mice and the solid bars represent dwarf mice. The mean values are displayed on the bars.
Age-Related Levels of Lipid Peroxidation in Ames Dwarf Mice
Tissue isoprostanes have been found to increase with age (43) and under conditions of oxidative stress in lung (44), plasma, liver, and kidney (45). Because these compounds are chemically stable, are not affected by dietary lipid content, and can be detected with precision in plasma, urine, and most tissues (25,44), they are a reliable marker for oxidative damage (46). Although data on age-related accumulation of F2-isoprostanes in the liver are available for CR animals (47) and as of recently, for Ames dwarf mice (48), there has not yet been a side-by-side comparison of both treatments using the same strain background. In our experiment, both dwarf and control mice showed a significant increase (83% and 110%, respectively) in liver isoprostanes with age (Figure 4A). Both the young adult and old dwarf mice had significantly lower liver isoprostane levels (26% and 15% decrease, respectively) compared with age-matched control mice. Old CR mice had significantly lower levels of isoprostanes (18% less) than old control mice and comparable levels to old dwarf mice (Figure 4A). Lung isoprostane levels showed a similar direction of age-related increase (23% and 29%, respectively, for dwarf and normal mice; Figure 4B), the old dwarf mice had 15% lower levels of lung isoprostanes than old normal mice, and old CR mice also had significantly lower levels of lung isoprostanes than old normal mice (22%) but showed no significant difference from old dwarf mice. Young dwarf mice had 11% lower levels of lung isoprostanes, but unlike liver isoprostanes, this difference was not statistically significant. These data indicate that lifelong accumulation of isoprostanes in multiple tissue types is diminished in the long-lived dwarf mice and that the acute diquat-induced hepatotoxicity data are not predictive of age-related accumulation of lipid oxidation. Furthermore, these data show that dietary restriction in phenotypically normal mice on the Ames background afforded a degree of protection against lipid oxidation that is comparable to dwarfism.
Figure 4.
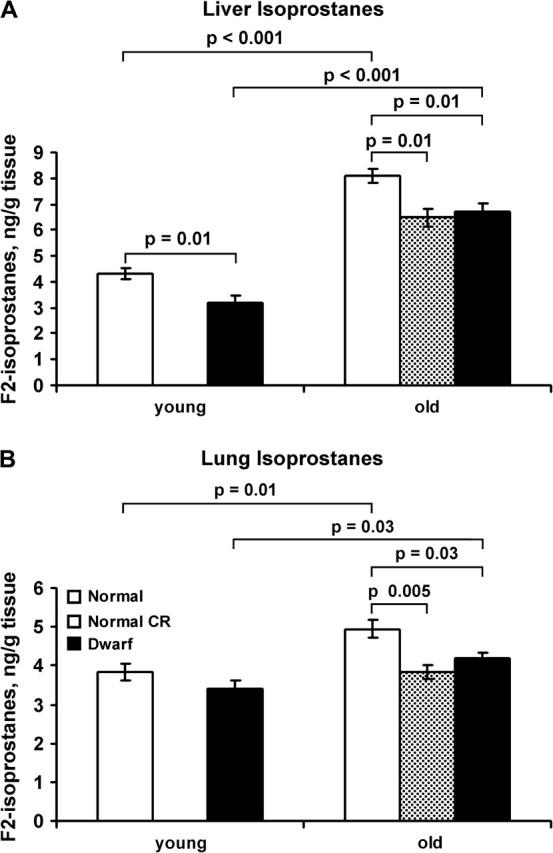
Changes in hepatic isoprostane levels with age, genotype, and calorie restriction. Isoprostane levels were measured in lungs (A) and livers (B) collected from control mice (open bars, n = 5 young and 11 old), control old mice fed a CR diet (stippled bars, n = 4 mice), and dwarf mice (solid bars, n = 5 young and 9 old) as described in the Methods. Each bar represents the mean and SEM for mice of the young (6–8 mo of age) and old (19–33 mo of age) groups. The data were statistically analyzed using the Student’s t test (with Holm’s correction) as described in the Methods, and p values are shown for significant differences.
Sensitivity of Ames Dwarf Mice to Cardiac Stress
Dobutamine, a positive inotropic drug that increases heart rate and myocardial contractility, is frequently used clinically to determine maximum stress response in older patients who cannot exercise on a treadmill. We used dobutamine stress echocardiography to determine stress sensitivity of wild-type and dwarf mice to increased cardiac work. Because of the large disparity in gross body size and heart size between dwarf and control mice, the baseline dimension and wall thickness data were adjusted to tibia length (Table 1). As shown in Table 2, dobutamine increased contractile parameters in wild-type mice, consistent with maximal inotropic stimulation (49). However, dwarf mice had a significantly smaller increase in fractional shortening and systolic and diastolic wall thicknesses than wild-type mice, despite a similar increase in heart rate. Furthermore, the dwarf mice had a smaller decline in end-systolic and end-diastolic dimensions. These data suggest that dwarf mice increased myocyte contractility in response to increased inotropic stimulus to a lesser degree than wild-type mice, that is, they appear to be more resistant to dobutamine-induced cardiac stress.
Table 1.
Baseline Echocardiographic Parameters in Wild-Type and Ames Dwarf Mice
| Wild Type (N = 7) | Dwarf (N = 8) | |
| Age (mo) | 11.9 ± 0.2 | 11.8 ± 0.1 |
| Heart rate (beats/min) | 420 ± 5 | 385 ± 16 |
| Tibia (mm) | 16.6 ± 0.3 | 12.2 ± 0.7* |
| End-diastolic dimensions/tibia (mm/mm) | 0.25 ± 0.01 | 0.26 ± 0.02 |
| End-systolic dimensions/tibia (mm/mm) | 0.16 ± 0.01 | 0.17 ± 0.01 |
| Fractional shortening (%) | 36 ± 2 | 35 ± 2 |
| Diastolic wall thickness/tibia (mm/mm) | 0.054 ± 0.002 | 0.045 ± 0.005 |
| Systolic wall thickness/tibia (mm/mm) | 0.078 ± 0.004 | 0.068 ± 0.006 |
Note: Physiological parameters of cardiac function were measured in eight wild-type and seven Ames dwarf mice. Data are shown as M ± SEM. *p < .05 vs wild type.
Table 2.
Dobutamine Response in Wild-Type and Ames Dwarf Mice
| Wild Type (% change; N = 7) | Dwarf (% change; N = 8) | |
| Heart rate | 24 ± 4 | 23 ± 5 |
| End-diastolic dimensions | −14 ± 3 | −4 ± 1* |
| End-systolic dimensions | −40 ± 4 | −15 ± 4* |
| Fractional shortening | 57 ± 9 | 23 ± 9* |
| Diastolic wall thickness | 26 ± 6 | 4 ± 4* |
| Systolic wall thickness | 33 ± 5 | 10 ± 6* |
Note: Physiological parameters of cardiac function were measured in eight wild-type and seven Ames dwarf mice at 10–11 mo of age. The percent change from the resting baseline state was calculated as (postinjection − preinjection/preinjection) × 100 and is shown as M ± SEM. *p < .05 vs wild type.
DISCUSSION
Hypomorphic alleles of the age-1 (50,51) and daf-2 (52) genes can extend the life span of nematodes by 50%–100%. These mutants are also resistant to a number of stressors including ultraviolet (UV) (53), H2O2 (15), heavy metals (54), heat (55,56), paraquat (16), and 1-methyl-4phenyl-1,2,3,6-tetrahydropyridine (57). Age-1 and daf-2 are nematode orthologs of the mammalian insulin and IGF-1 receptors, and in recent years a number of long-lived mouse mutants have been developed with various impairments in IGF-1 signaling (58–60), among them, Ames dwarf mice. (10,11). Mice that are long lived due to calorie restriction also have suppressed somatotropic signaling, for example, reduced plasma levels of GH (61), IGF-1 (62,63), and insulin (64).
Currently, researchers are studying whether these various long-lived mouse models are also resistant to oxidative stress like the long-lived nematodes. It has been accepted that both rats and mice on a CR diet accumulate less oxidative damage and have increased resistance to oxidative stress (65). In contrast to calorie restriction, it is not obvious that Ames dwarf mice show increased resistance to oxidative stress. Although it appears that some components of the antioxidant defense system are enhanced in Ames dwarf mice in some tissues (66–68), other components are reduced (66,69,70). Markers of oxidative damage to proteins, nucleic acids, and lipids have also been measured and were found to be decreased, unchanged, or even elevated depending on the tissue being examined (71,72). The most direct and strongest line of evidence that Ames dwarf mice show increased resistance to oxidative stress is that cultured tail skin fibroblasts from Ames dwarf mice (and from Snell dwarf mice) are resistant to a wide variety of stressors (20,73). In these studies, cells from Ames dwarf mice were significantly more resistant than those from normal controls to UV irradiation, H2O2, cadmium, and, in the case of Snell dwarf mice, paraquat. However, it is uncertain whether the increased resistance of fibroblasts to stress translates to whole animals because the type and degree of oxidative stress applied in cell cultures may not be physiologically relevant. Currently there are no published data on the in vivo sensitivity of Ames dwarf to oxidative stress resistance.
The sensitivity of Ames dwarf and control mice to paraquat was studied because paraquat is a standard model for inducing oxidative stress in cells and whole animals due to its ability to catalyze production of superoxide anions within the cell (29). Our data demonstrate that Ames dwarf mice, both males and females, were much more resistant to paraquat than were normal littermates, and this resistance to paraquat toxicity was maintained with age in the Ames dwarf mice. Paraquat is actively taken up by the lung pneumocytes (37,38), causing death due to pulmonary edema or, after a longer period, pulmonary fibrosis. Therefore, the differences in sensitivity to oxidative stress generated by paraquat could be specific to lung, especially because it has been found that the age-related onset of lung adenocarcinoma is delayed in Ames dwarf mice (3). To establish that the Ames dwarf mice show in vivo resistance to oxidative stress, we went on to test the sensitivity of Ames dwarf mice to diquat. Diquat, which also generates superoxide anions, affects a variety of organs (other than lung) including liver (74), kidney (75), gall bladder (39), and the gastrointestinal tract (39). We observed that the Ames dwarf mice were also more resistant to diquat toxicity than control mice, as measured by overall survival after a lethal dose. Thus, our whole animal data show that Ames dwarf mice showed increased resistance to toxicity generated by superoxide anions, in agreement with the cultured fibroblast data of Salmon and colleagues (20). In all experiments, we adjusted paraquat and diquat dosage to body weight. Therefore, it could be argued that if the size of liver and kidneys in dwarf mice is larger in proportion to body weight, we could have administered an insufficient dose to the dwarf mice. We found that most internal organs other than brain of dwarf mice in fact trend toward being smaller relative to body weight. This trend attained significance in young female mice (6–8 months old) for liver (18% smaller than control), kidneys (19% smaller than control), spleen (63% smaller than control), and brain (68% larger than control; data not shown). Therefore, if organ size had any bearing on the ability to excrete or metabolize paraquat and diquat, the enhanced survival of female dwarf mice may actually be underreported. However, we do not completely discount the possibility that altered metabolism of toxins also contributes to the enhanced survival of dwarf mice because the expression of some genes involved in xenobiotic metabolism is altered in Ames dwarf mice (76,77). Indeed, clearance of toxins may itself be a physiologically important mechanism for withstanding oxidative stress that merits further study. We have performed time course experiments comparing plasma paraquat levels in dwarf and control mice and have not found any significant difference at any time point between 0.5 and 12 hours (data not shown).
One surprising result from our study was that even though the Ames dwarf mice showed increased resistance to diquat at the whole animal level, their livers appeared to sustain greater diquat-induced damage than those of normal littermates. These data illustrate that the same animal model can show tissue differences in sensitivity to oxidative stress, underscoring the importance of studying a variety of stressors in multiple tissues when assessing the in vivo sensitivity of animal models to oxidative stress. Previous studies also indicate that the liver of Ames dwarf mice might be more vulnerable to stress. For example, cultured hepatocytes from Ames mice have lower levels of catalase activity (69), and Harper and colleagues (78) found that the livers of Ames dwarf mice are more sensitive to acetaminaphen in vivo. Acetaminophen in large doses causes liver damage via oxidative stress (79). Kennedy and colleagues (80) observed elevated basal levels of Procaspase-3 and Bcl-2 in cultured hepatocytes from Ames dwarf mice compared with control mice and increased levels of H2O2-induced caspase-3 induction. Thus, the liver of Ames dwarf mice may be particularly vulnerable to a bolus of oxidative stress. However, we observed that the livers of young and old Ames dwarf mice showed a significant reduction in F2-isoprostane levels, suggesting that the liver of Ames dwarf mice is more resistant to endogenous levels of oxidative stress.
We also compared F2-isoprostane levels in old, normal CR mice on the Ames background with those of age-matched Ames dwarf and normal mice fed AL. The levels F2-isoprostane in livers and lungs of old, Ames normal CR mice were similar to Ames dwarf mice fed AL and were significantly lower than those of old, normal AL mice. In other words, it appears that old Ames normal CR and Ames dwarf AL mice showed similar resistance to endogenous levels of oxidative stress.
Because we observed that the liver of the Ames dwarf mice was more sensitive to diquat, we were interested in studying the sensitivity of other tissues from Ames dwarf mice to stress. Dobutamine is a stressor that simulates physical exertion by increasing heart rate, which decreases ventricular dimensions and increases in wall thickness. As a result, dobutamine alters both preload and after load (81). The dwarf mice resisted the stress challenge, despite an increase in heart rates, indicating that less of a departure from baseline wall thickness and ventricular volumes occurred for an equal stimulus. These data suggest an increased functional capacity in the Ames dwarf heart. In contrast, old mice (82) and mice that have had myocardial infarction (26) have a diminished response to dobutamine as measured by increased baseline dimensions and a decrease in the maximal rate of change in left ventricular pressure. The pattern of resistance to dobutamine stress that we observed in Ames is consistent with previous published results in the Ghrhr−/− (Little) mice, which also have a lower response to dobutamine compared with wild-type mice at young and old ages (82). Interestingly, these parameters remain constant with age in the Ghrhr−/− mice and decline in wild-type mice, suggesting a preservation of cardiac function with age in the Ghrhr−/− mice. Furthermore, caloric restriction reverses age-dependent declines in the ratio of early diastolic filling to atrial filling and reduces the fraction of ventricular filling due to atrial systole (83). Combined, these studies demonstrate that long-lived mouse models display increased cardiac resistance to inotropic stress and show improved cardiac aging.
CR mice have been reported to have an enhanced resistance to oxidative stress, for example, reduced levels of oxidative damage [reviewed in Bokov and colleagues (65)] as well as sensitivity to oxidative stress, such as paraquat (13,14). We have confirmed these observations in C57BL/6 mice (Supplement 1). However, unlike Ames dwarf mice, CR C57BL/6 mice have reduced levels of ALT and fewer apoptotic cells after diquat exposure compared with AL controls. It should be noted, however, that due to differing strain backgrounds, caution should be used in comparing the oxidative stress sensitivity data from C57BL/6 mice and the Ames mice presented here.
Gamma radiation is a nonchemical source of oxidative stress (84), and some mutant mice deficient in antioxidant enzymes are more sensitive to gamma radiation than wild-type littermates (85,86). However, we found no difference in survival between dwarf mice and normal littermates of either sex after exposure to 11 Gy of gamma radiation (Supplement 2). This may be due to dwarf mice not being protected against direct macromolecular effects of gamma radiation (e.g., chromosome breakage). It is also possible that a difference was masked by the relatively high dose of radiation used; however, the dose used was chosen because the mutant group in this case was expected to have longer rather than shorter survival times as was the case for the earlier studies at our institution (85,86).
In conclusion, we find that Ames dwarf mice have increased resistance to the oxidative stressors, paraquat, and diquat. In fact, Ames dwarf mice were more resistant to oxidative stress induced by paraquat than CR mice, and these data are in agreement with the oxidative stress theory of aging. However, the organ and tissue sensitivity of Ames dwarf mice to oxidative stress may vary as shown by the increased sensitivity of liver from Ames dwarf mice to diquat-induced hepatotoxicity. Further experiments elucidating tissue-by-tissue differences in resistance to oxidative damage are likely to yield important insights into the interrelation of the somatotropic axis, aging, and oxidative stress.
FUNDING
The authors gratefully acknowledge support from the National Institutes of Health: AGO-21890 (A.F.B); AG-19316, AG-13319, AG-23843, and AG-26557 (A.R.); HL-75360 (M.L.L.); the San Antonio Nathan Shock Aging Center (1P30-AG-13319) (A.R.); Department of Veterans Affairs Merit Grant (A.R.); and a Research Enhancement Award Program from the Department of Veterans Affairs (A.R.).
SUPPLEMENTARY MATERIAL
Supplementary material can be found at: http://biomed.gerontologyjournals.org/
References
- 1.Schaible R, Gowen JW. A new dwarf mouse. Genetics. 1961;46:896. [Google Scholar]
- 2.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384(6604):33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 3.Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in Ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003;58(4):291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- 4.Kinney BA, Meliska CJ, Steger RW, Bartke A. Evidence that ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Horm Behav. 2001;39(4):277–284. doi: 10.1006/hbeh.2001.1654. [DOI] [PubMed] [Google Scholar]
- 5.Kinney BA, Coschigano KT, Kopchick JJ, Steger RW, Bartke A. Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. Physiol Behav. 2001;72(5):653–660. doi: 10.1016/s0031-9384(01)00423-1. [DOI] [PubMed] [Google Scholar]
- 6.Kinney-Forshee BA, Kinney NE, Steger RW, Bartke A. Could a deficiency in growth hormone signaling be beneficial to the aging brain? Physiol Behav. 2004;80(5):589–594. doi: 10.1016/j.physbeh.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Sornson MW, Wu W, Dasen JS, et al. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- 8.Ward RD, Stone BM, Raetzman LT, Camper SA. Cell proliferation and vascularization in mouse models of pituitary hormone deficiency. Mol Endocrinol. 2006;20(6):1378–1390. doi: 10.1210/me.2005-0409. [DOI] [PubMed] [Google Scholar]
- 9.Watkins-Chow DE, Camper SA. How many homeobox genes does it take to make a pituitary gland? vol. 14, pg 284, 1998. Trends Genet. 1998;14(10):434. doi: 10.1016/s0168-9525(98)01476-0. [DOI] [PubMed] [Google Scholar]
- 10.Chandrashekar V, Bartke A. Induction of endogenous insulin-like growth factor-I secretion alters the hypothalamic-pituitary-testicular function in growth hormone-deficient adult dwarf mice. Biol Reprod. 1993;48(3):544–551. doi: 10.1095/biolreprod48.3.544. [DOI] [PubMed] [Google Scholar]
- 11.Pell JM, Bates PC. Differential action of growth hormone and insulin-like growth factor-I on tissue protein metabolism in dwarf mice. Endocrinology. 1992;130(4):1942–1950. doi: 10.1210/endo.130.4.1547721. [DOI] [PubMed] [Google Scholar]
- 12.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 13.Richardson A, Liu F, Adamo ML, Van Remmen H, Nelson JF. The role of insulin and insulin-like growth factor-1 in mammalian ageing. Best Pract Res Clin Endocrinol Metab. 2004;18(3):393–406. doi: 10.1016/j.beem.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Sun DX, Muthukumar AR, Lawrence RA, Fernandes G. Effects of calorie restriction on polymicrobial peritonitis induced by cecum ligation and puncture in young C57BL/6 mice. Clin Diagn Lab Immunol. 2001;8(5):1003–1011. doi: 10.1128/CDLI.8.5.1003-1011.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsen PL. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1993;90(19):8905–8909. doi: 10.1073/pnas.90.19.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanfleteren JR. Oxidative stress and aging in Caenorhabditis elegans. Biochem J. 1993;292:605–608. doi: 10.1042/bj2920605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleming JE, Reveillaud I, Niedzwiecki A. Role of oxidative stress in Drosophila aging. Mutat Res. 1992;275(3–6):267–279. doi: 10.1016/0921-8734(92)90031-j. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Pot D, Kachman SD, Nuzhdin SV, Harshman LG. A quantitative trait locus analysis of natural genetic variation for Drosophila melanogaster oxidative stress survival. J Hered. 2006;97(4):355–366. doi: 10.1093/jhered/esl009. [DOI] [PubMed] [Google Scholar]
- 19.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 20.Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005;289(1):E23–E29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- 21.Jewitt D, Birkhead J, Mitchell A, Dollery C. Clinical cardiovascular pharmacology of dobutamine—selective inotropic catecholamine. Lancet. 1974;2(7877):363–367. doi: 10.1016/s0140-6736(74)91754-1. [DOI] [PubMed] [Google Scholar]
- 22.McCarter R, Mejia W, Ikeno Y, et al. Plasma glucose and the action of calorie restriction on aging. J Gerontol A Biol Sci Med Sci. 2007;62(10):1059–1070. doi: 10.1093/gerona/62.10.1059. [DOI] [PubMed] [Google Scholar]
- 23.Ikeno Y, Hubbard GB, Lee S, et al. Housing density does not influence the longevity effect of calorie restriction. J Gerontol A Biol Sci Med Sci. 2005;60(12):1510–1517. doi: 10.1093/gerona/60.12.1510. [DOI] [PubMed] [Google Scholar]
- 24.Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo EJ, Yu BP. Influence of the restriction of individual dietary components on longevity and age-related disease of Fischer rats: the fat component and the mineral component. J Gerontol. 1988;53:B13–B21. doi: 10.1093/geronj/43.1.b13. [DOI] [PubMed] [Google Scholar]
- 25.Roberts LJ, Morrow JD. Measurement of F-2-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28(4):505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 26.Wiesmann F, Ruff J, Engelhardt S, et al. Dobutamine-stress magnetic resonance microimaging in mice—acute changes of cardiac geometry and function in normal and failing murine hearts. Circ Res. 2001;88(6):563–569. doi: 10.1161/01.res.88.6.563. [DOI] [PubMed] [Google Scholar]
- 27.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 28.R Development Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 29.Farrington JA, Ebert M, Land EJ, Fletcher K. Bipyridylium quaternary salts and related compounds. V. Pulse radiolysis studies of the reaction of paraquat radical with oxygen. Implications for the mode of action of bipyridyl herbicides. Biochim Biophys Acta. 1973;314(3):372–381. doi: 10.1016/0005-2728(73)90121-7. [DOI] [PubMed] [Google Scholar]
- 30.Hirai KI, Ikeda K, Wang GY. Paraquat damage of rat liver mitochondria by superoxide production depends on extramitochondrial NADH. Toxicology. 1992;72:1–16. doi: 10.1016/0300-483x(92)90081-o. [DOI] [PubMed] [Google Scholar]
- 31.Bus JS, Gibson JE. Paraquat—model for oxidant-initiated toxicity. Environ Health Perspect. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gage JC. Action of paraquat and diquat on respiration of liver cell fractions. Biochem J. 1968;109(5):757–761. doi: 10.1042/bj1090757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cocheme HM, Murphy MP. Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem. 2008;283:1786–1798. doi: 10.1074/jbc.M708597200. [DOI] [PubMed] [Google Scholar]
- 34.Fukushima T, Yamada K, Isobe A, Shiwaku K, Yamane Y. Mechanism of cytotoxicity of paraquat .1. Nadh oxidation and paraquat radical formation via complex-I. Exp Toxicol Pathol. 1993;45(5–6):345–349. doi: 10.1016/S0940-2993(11)80424-0. [DOI] [PubMed] [Google Scholar]
- 35.Shimada H, Hirai KI, Simamura E, Pan JH. Mitochondrial NADH-quinone oxide reductase of the outer membrane is responsible for paraquat cytotoxicity in rat livers. Arch Biochem Biophys. 1998;351(1):75–81. doi: 10.1006/abbi.1997.0557. [DOI] [PubMed] [Google Scholar]
- 36.Stancliffe TC, Pirie A. The production of superoxide radicals in reactions of the herbicide diquat. FEBS Lett. 1971;17(2):297–299. doi: 10.1016/0014-5793(71)80168-0. [DOI] [PubMed] [Google Scholar]
- 37.Hoet PHM, Lewis CPL, Dinsdale D, Demedts M, Nemery B. Putrescine uptake in hamster lung slices and primary cultures of type-Ii pneumocytes. Am J Physiol Lung Cell Mol Physiol. 1995;13(5):L681–L689. doi: 10.1152/ajplung.1995.269.5.L681. [DOI] [PubMed] [Google Scholar]
- 38.Smith LL, Wyatt I. The accumulation of putrescine into slices of rat lung and brain and its relationship to the accumulation of paraquat. Biochem Pharmacol. 1981;30(10):1053–1058. doi: 10.1016/0006-2952(81)90441-x. [DOI] [PubMed] [Google Scholar]
- 39.Litchfield MH, Daniel JW, Longshaw S. The tissue distribution of the bipyridylium herbicides diquat and paraquat in rats and mice. Toxicology. 1973;1:155–165. doi: 10.1016/0300-483x(73)90029-2. [DOI] [PubMed] [Google Scholar]
- 40.Rose MS, Smith LL, Wyatt I. Evidence for energy-dependent accumulation of paraquat into rat lung. Nature. 1974;252(5481):314–315. doi: 10.1038/252314b0. [DOI] [PubMed] [Google Scholar]
- 41.Awad JA, Burk RF, Roberts LJ. Effect of selenium deficiency and glutathione-modulating agents on diquat toxicity and lipid-peroxidation in rats. J Pharmacol Exp Ther. 1994;270(3):858–864. [PubMed] [Google Scholar]
- 42.Winawer SJ, Sullivan LW, Herbert V, Zamcheck N. The jejunal mucosa in patients with nutritional folate deficiency and megaloblastic anemia. N Engl J Med. 1965;272:892–895. doi: 10.1056/NEJM196504292721705. [DOI] [PubMed] [Google Scholar]
- 43.Roberts LJ, Reckelhoff JF. Measurement of F-2-isoprostanes unveils profound oxidative stress in aged rats. Biochem Biophys Res Commun. 2001;287(1):254–256. doi: 10.1006/bbrc.2001.5583. [DOI] [PubMed] [Google Scholar]
- 44.Janssen LJ. Isoprostanes: an overview and putative roles in pulmonary pathophysiology. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1067–L1082. doi: 10.1152/ajplung.2001.280.6.L1067. [DOI] [PubMed] [Google Scholar]
- 45.Morrow JD, Awad JA, Kato T, et al. Formation of novel non-cyclooxygenase-derived prostanoids (F2-Isoprostanes) in carbon-tetrachloride hepatotoxicity—an animal-model of lipid-peroxidation. J Clin Invest. 1992;90(6):2502–2507. doi: 10.1172/JCI116143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rikans LE, Hornbrook KR. Lipid peroxidation, antioxidant protection and aging. Biochim Biophys Acta-Molecular Basis of Disease. 1997;1362(2–3):116–127. doi: 10.1016/s0925-4439(97)00067-7. [DOI] [PubMed] [Google Scholar]
- 47.Ward WF, Qi WB, Van Remmen H, Zackert WE, Roberts LJ, Richardson A. Effects of age and caloric restriction on lipid peroxidation: measurement of oxidative stress by F-2-isoprostane levels. J Gerontol A Biol Sci Med Sci. 2005;60(7):847–851. doi: 10.1093/gerona/60.7.847. [DOI] [PubMed] [Google Scholar]
- 48.Choksi KB, Roberts L, Deford JH, Rabek JP, Papaconstantino J. Lower levels of F-2-isoprostanes in serum and livers of long-lived Ames dwarf mice. Biochem Biophys Res Commun. 2007;364:761–764. doi: 10.1016/j.bbrc.2007.10.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295(5564):2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 50.Klass M, Nguyen PN, Dechavigny A. Age-correlated changes in the DNA template in the nematode Caenorhabditis elegans. Mech Ageing Dev. 1983;22:253–263. doi: 10.1016/0047-6374(83)90080-5. [DOI] [PubMed] [Google Scholar]
- 51.Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118(1):75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 53.Murakami S, Johnson TE. A genetic pathway conferring life extension and resistance to UV stress in Caenorhabditis elegans. Genetics. 1996;143(3):1207–1218. doi: 10.1093/genetics/143.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barsyte D, Lovejoy DA, Lithgow GJ. Longevity and heavy metal resistance in daf-2 and age-1 long-lived mutants of Caenorhabditis elegans. FASEB J. 2001;15(3):627–634. doi: 10.1096/fj.99-0966com. [DOI] [PubMed] [Google Scholar]
- 55.Lithgow GJ, White TM, Hinerfeld DA, Johnson TE. Thermotolerance of a long-lived mutant of Caenorhabditis elegans. J Gerontol. 1994;49(6):B270–B276. doi: 10.1093/geronj/49.6.b270. [DOI] [PubMed] [Google Scholar]
- 56.Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal-stress. Proc Natl Acad Sci U S A. 1995;92(16):7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson TE, Henderson S, Murakami S, et al. Longevity genes in the nematode Caenorhabditis elegans also mediate increased resistance to stress and prevent disease. J Inherit Metab Dis. 2002;25(3):197–206. doi: 10.1023/a:1015677828407. [DOI] [PubMed] [Google Scholar]
- 58.Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309(5742):1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Migliaccio E, Giorgio M, Mele S, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 60.Holzenberger M, Dupont J, Ducos B, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421(6919):182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 61.Campbell GA, Kurcz M, Marshall S, Meites J. Effects of starvation in rats on serum levels of follicle-stimulating hormone, luteinizing-hormone, thyrotropin, growth-hormone and prolactin—response to Lh-releasing hormone and thyrotropin-releasing-hormone. Endocrinology. 1977;100(2):580–587. doi: 10.1210/endo-100-2-580. [DOI] [PubMed] [Google Scholar]
- 62.Breese CR, Ingram RL, Sonntag WE. Influence of age and long-term dietary restriction on plasma insulin-like growth factor-1 (IGF-1), IGF-1 gene expression, and IGF-1 binding proteins. J Gerontol. 1991;46(5):B180–B187. doi: 10.1093/geronj/46.5.b180. [DOI] [PubMed] [Google Scholar]
- 63.Gautsch TA, Kandl SM, Donovan SM, Layman DK. Response of the IGF-I system to prolonged undernutrition and its involvement in somatic and skeletal muscle growth retardation in rats. Growth Dev Aging. 1998;62(1–2):13–25. [PubMed] [Google Scholar]
- 64.Argentino DP, Dominici FP, Munoz MC, Al Regaiey K, Bartke A, Turyn D. Effects of long-term caloric restriction on glucose homeostasis and on the first steps of the insulin signaling system in skeletal muscle of normal and Ames dwarf (Prop1(df)/Prop1(df)) mice. Exp Gerontol. 2005;40(1–2):27–35. doi: 10.1016/j.exger.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 65.Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mech Ageing Dev. 2004;125(10–11):811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 66.Brown-Borg HM, Bode AM, Bartke A. Antioxidative mechanisms and plasma growth hormone levels—potential relationship in the aging process. Endocrine. 1999;11(1):41–48. doi: 10.1385/ENDO:11:1:41. [DOI] [PubMed] [Google Scholar]
- 67.Brown-Borg HM, Rakoczy SG. Catalase expression in delayed and premature aging mouse models. Exp Gerontol. 2000;35(2):199–212. doi: 10.1016/s0531-5565(00)00079-6. [DOI] [PubMed] [Google Scholar]
- 68.Hauck SJ, Bartke A. Effects of growth hormone on hypothalamic catalase and Cu/Zn superoxide dismutase. Free Radic Biol Med. 2000;28(6):970–978. doi: 10.1016/s0891-5849(00)00186-6. [DOI] [PubMed] [Google Scholar]
- 69.Brown-Borg HM, Rakoczy SG, Romanick MA, Kennedy MA. Effects of growth hormone and insulin-like growth factor-1 on hepatocyte antioxidative enzymes. Exp Biol Med. 2002;227(2):94–104. doi: 10.1177/153537020222700203. [DOI] [PubMed] [Google Scholar]
- 70.Romanick MA, Rakoczy SG, Brown-Borg HM. Long-lived Ames dwarf mouse exhibits increased antioxidant defense in skeletal muscle. Mech Ageing Dev. 2004;125(4):269–281. doi: 10.1016/j.mad.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 71.Brown-Borg HM, Johnson WT, Rakoczy S, Romanick M. Mitochondrial oxidant generation and oxidative damage in ames dwarf and GH transgenic mice. AGE. 2001;24(3):85–96. doi: 10.1007/s11357-001-0012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanz A, Bartke A, Barja G. Long-lived Ames dwarf mice: oxidative damage to mitochondrial DNA in heart and brain. AGE. 2002;25(3):119–122. doi: 10.1007/s11357-002-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murakami S, Salmon A, Miller RA. Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J. 2003;17(9):1565–1566. doi: 10.1096/fj.02-1092fje. [DOI] [PubMed] [Google Scholar]
- 74.Smith CV, Hughes H, Lauterburg BH, Mitchell JR. Oxidant stress and hepatic-necrosis in rats treated with diquat. J Pharmacol Exp Ther. 1985;235(1):172–177. [PubMed] [Google Scholar]
- 75.Rose MS, Smith LL. Tissue uptake of paraquat and diquat. [Review] [23 refs] Gen Pharmacol. 1977;8(3):173–176. doi: 10.1016/0306-3623(77)90045-3. [DOI] [PubMed] [Google Scholar]
- 76.Amador-Noguez D, Yagi K, Venable S, Darlington G. Gene expression profile of long-lived Ames dwarf mice and Little mice. Aging Cell. 2004;3(6):423–441. doi: 10.1111/j.1474-9728.2004.00125.x. [DOI] [PubMed] [Google Scholar]
- 77.Boylston WH, Deford JH, Papaconstantinou J. Identification of longevity-associated genes in long-lived Snell and Ames dwarf mice. Age. 2006;28(2):125–144. doi: 10.1007/s11357-006-9008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harper JM, Salmon AB, Chang YY, Bonkowski M, Bartke A, Miller RA. Stress resistance and aging: Influence of genes and nutrition. Mech Ageing Dev. 2006;127(8):687–694. doi: 10.1016/j.mad.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Masubuchi Y, Suda C, Horie T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol. 2005;42(1):110–116. doi: 10.1016/j.jhep.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 80.Kennedy MA, Rakoczy SG, Brown-Borg HM. Long-living Ames dwarf mouse hepatocytes readily undergo apoptosis. Exp Gerontol. 2003;38(9):997–1008. doi: 10.1016/s0531-5565(03)00164-5. [DOI] [PubMed] [Google Scholar]
- 81.Hayat SA, Senior R. Contrast echocardiography for the assessment of myocardial viability. Curr Opin Cardiol. 2006;21(5):473–478. doi: 10.1097/01.hco.0000240585.68720.e7. [DOI] [PubMed] [Google Scholar]
- 82.Reddy AK, Amador-Noguez D, Darlington GJ, et al. Cardiac function in young and old Little mice. J Gerontol A Biol Sci Med Sci. 2007;62(12):1319–1325. doi: 10.1093/gerona/62.12.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taffet GE, Pham TT, Hartley CJ. The age-associated alterations in late diastolic function in mice are improved by caloric restriction. J Gerontol A Biol Sci Med Sci. 1997;52(6):B285–B290. doi: 10.1093/gerona/52a.6.b285. [DOI] [PubMed] [Google Scholar]
- 84.Riley PA. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 85.Van Remmen H, Qi WB, Sabia M, et al. Multiple deficiencies in antioxidant enzymes in mice result in a compound increase in sensitivity to oxidative stress. Free Radic Biol Med. 2004;36(12):1625–1634. doi: 10.1016/j.freeradbiomed.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 86.Yant LJ, Ran QT, Rao L, et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med. 2003;34(4):496–502. doi: 10.1016/s0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]