Abstract
Background
Multidimensional impairment of older patients may influence the clinical outcome of acute or chronic diseases. Our purpose is to evaluate the usefulness of a multidimensional prognostic index (MPI) based on a comprehensive geriatric assessment (CGA) for predicting mortality risk in older patients with community-acquired pneumonia (CAP).
Methods
This prospective study included 134 hospitalized patients aged 65 and older with a diagnosis of CAP. A standardized CGA that included information on clinical, cognitive, functional, and nutritional status as well as comorbidities, medications, and social support network was used to calculate MPI. The pneumonia severity index (PSI) was also calculated. The predictive value of the MPI for all cause mortality over a 1-year follow-up was evaluated and was compared with that of PSI.
Results
Higher MPI values were significantly associated with higher mortality at 30 days (Grade 1 = 3%, Grade 2 = 12%, Grade 3 = 44%, p < .001), 6 months (Grade 1 = 7%, Grade 2 = 21%, Grade 3 = 50%, p < .001), and 1 year (Grade 1 = 10%, Grade 2 = 33%, Grade 3 = 53%, p < .001). A close agreement was found between the estimated mortality by MPI and the observed mortality. MPI had a significant greater discriminatory power than PSI both at 30 days (area under the receiver operating characteristic [ROC] curve = 0.83 vs 0.71, p = .019) and 6 months (0.79 vs 0.69, p = .035), but not after 1 year of follow-up (0.80 vs 0.75, p = .185).
Conclusions
This MPI, calculated from information collected in a standardized CGA, accurately stratifies hospitalized elderly patients with CAP into groups at varying risk of short- and long-term mortality. The predictive accuracy of the MPI was higher than the predictive value of the PSI.
Keywords: Multidimensional prognostic index, Comprehensive geriatric assessment, Pneumonia, Mortality, Elderly people
PNEUMONIA is one of the most common and significant health problems in elderly people. Its prevalence rate increases from 6/1000 in the 18–39 age group to 34/1000 in persons aged 75 years and older (1). The prevalence of pneumonia is higher in institutionalized older participants than in community dwellers (2). Older patients hospitalized with a diagnosis of community-acquired pneumonia (CAP) have a higher risk of readmission to the hospital than patients hospitalized without CAP (3). In addition, in patients aged 65 years and older, hospital mortality rate for CAP is more than 10% and the 1-year mortality is more than 40% (4). Indeed, hospitalization for CAP implies a prognosis as grim as that for congestive heart failure, stroke, or a major fracture (5).
The potential severity of pneumonia and its economic impact have led to the development of a number of predictive score systems to optimize the care and the treatment of CAP such as the pneumonia severity index (PSI) (6), the modified American Thoracic Society (mATS) rule (7), and the severity score CURB-65 (confusion; urea; respiratory rate; blood pressure) (8). Although all these score systems are useful for predicting the need for intensive care unit treatment and death in adult patients (9), different scores have different strengths and weaknesses as outcome prediction tools, particularly in older patients (10). Indeed, although in adult patients PSI demonstrated being most sensitive than mATS (9,10) and CURB-65 (9) in predicting mortality, recent studies that evaluated the clinical usefulness of pneumonia severity score systems modified for older patients reported conflicting results (11,12).
Because in older participants affected by acute diseases such as pneumonia, clinical outcome and mortality result from a combination of biological, functional, psychological, and environmental factors, tools that effectively identify patients with different life expectancy should be multidimensional in nature (13,14). Recently, a multidimensional prognostic index (MPI) for 1-year mortality derived from a standardized comprehensive geriatric assessment (CGA) was developed and validated in two independent cohorts of elderly patients hospitalized for acute disease or relapse of a chronic disease (15). In both cohorts, a close agreement was found between the estimated and the observed mortality after both 6 months and 1 year of follow-up.
The aim of the present study was to evaluate the prognostic accuracy of an MPI derived from a standardized CGA on evaluating short- (30-day) and long-term (1-year) mortality rates, in elderly patients hospitalized for CAP.
METHODS
Participants
In this prospective study with a 1-year follow-up, patients aged 65 years and older admitted from January 2004 to December 2006 to the Geriatric Unit of the Casa Sollievo della Sofferenza Hospital, Scientific Institute for Research and Health Care (IRCCS), San Giovanni Rotondo, Italy, were screened for eligibility. Inclusion criteria were (a) age ≥ 65 years, (b) diagnosis of CAP, (c) ability to provide an informed consent or availability of a proxy for informed consent and willingness to participate in the study, (d) complete CGA during hospitalization, and (e) availability of mortality/survival information after 1 year from the hospitalization. Patients with acute-care hospitalization lasting 72 hours or more within the previous 15 days were excluded from the study.
At baseline, the following parameters were collected by a structured interview and clinical evaluation: date of birth, gender, clinical history, current pathologies, and medication history. All patients admitted to our unit received a standard CGA for clinical purposes. Vital status up to December 31, 2007, was assessed by directly contacting the participants or consulting the registry offices of the cities where the patients resided at the time of hospital admission. Dates of death were identified from death certificates.
Diagnosis of Pneumonia
CAP was diagnosed using standard criteria, including chest radiograph demonstrating pneumonia, probable pneumonia, or the presence of a new infiltrate and the presence of at least two of the following symptoms and signs compatible with pneumonia: new or increased cough, new or increased sputum production, fever ≥ 38 °C, pleuritic chest pain, new or increased physical findings on chest examination (rales, rhonchi, wheezes, bronchial breathing), malaise, or difficulty in breathing (16).
The CGA
CGA was carried out using assessment instruments widely employed in geriatric practice. Functional status was evaluated by activities of daily living (ADL) index (17), which defines the level of dependence/independence in six daily care activities including bathing, toileting, feeding, dressing, continence, and transferring, and by instrumental activities of daily living (IADL) scale (18), which assess independence in eight activities that are more cognitively and physically demanding than ADL, including managing finances, taking medications, using telephone, shopping, using transportation, preparing meals, doing housework, and washing.
Cognitive status was assessed by the short portable mental status questionnaire (SPMSQ), a 10-item questionnaire that assesses orientation, memory, attention, calculation, and language (19).
Comorbidity was examined using the cumulative illness rating scale (CIRS) (20). The CIRS uses a 5-point ordinal scale (score 1–5) to estimate the severity of pathology in each of 13 systems, including cardiac, vascular, respiratory, eye–ear–nose–throat, gastrointestinal, hepatic, renal, genitourinary, musculoskeletal, skin, nervous system, endocrine–metabolic, and psychiatric behavioral disorders. Based on the ratings, the comorbidity index (CIRS—CI) score, which reflects the number of concomitant diseases, was derived from the total number of categories in which moderate or severe levels (Grade 3–5) of disease were identified (range 0–13).
Nutritional status was explored with the mini nutritional assessment (MNA) (21), which includes information on (a) anthropometric measures; (b) lifestyle, medication, and mobility; (c) number of meals, food and fluid intake, and autonomy of feeding; and (d) self-perception of health and nutrition.
The Exton-Smith Scale (ESS) was used to evaluate the risk of developing pressure sores. This 5-item questionnaire determines physical and mental condition, activity, mobility, and incontinence. For each item, a score from 1 to 4 is assigned (22).
Medication use was defined according to the Anatomical Therapeutics Chemical Classification code system, and the number of drugs used by patients at admission was recorded. Social aspects included household composition, home service, and institutionalization.
The MPI
We used the MPI developed and validated in two independent cohorts of elderly hospitalized patients as previously reported (15). Briefly, a cluster analysis on CGA data was initially made for evaluating the independence of variables and identifying the most relevant domains of the CGA in predicting mortality outcome. The cluster analysis showed a correlation among ADL, IADL, SPMSQ, ESS, and MNA and evident independency among these variables and comorbidity (CIRS) and medication use, which were correlated with each other and social aspects. Thus, we started to develop an MPI considering only three variables: ADL, medication use, and social aspects. This “three-domain” MPI, although in a Cox regression analysis produced an acceptable separation among the survival curves of the three groups of patients (low, moderate, and severe risk of death), resulted in an unsatisfactory prognostic index for 1-year mortality (OR = 0.634, 95% CI = 0.141–2.871). Following a step-wise method, other domains of the CGA, one at a time, were progressively included in the model and relative Cox and logistic regression analyses performed. Thus, an “eight-domain” MPI, that is, that included a total of 63 items in eight domains of the CGA, resulted the best index for predicting 1-year mortality. For each domain, a tripartite hierarchy was used, that is 0 = no problems, 0.5 = minor problems, and 1 = major problems, based on conventional cut-off points derived from the literature for the SPMSQ (19), MNA (21), ESS (22), and ADL/IADL (23) or by observing the frequency distribution of the patients at various levels to identify points of separation for comorbidities and number of medications (15). The specific thresholds used to define the three categories are shown in Table 1. The sum of the calculated scores from the eight domains was divided by 8 to obtain a final MPI score between 0 and 1. For analytical purposes, absolute values of MPI were not considered, and we expressed MPI as low (MPI value ≤ 0.33), moderate (MPI value between 0.34 and 0.66), and severe risk (MPI > 0.66) according to previous rules-based indices used for exploring multidimensional impairment in elderly participants (24). The approximate time required for collecting data for the CGA was 20 minutes (range from 15 to 25 minutes per person); further 3–5 minutes were needed to include data in the software (www.operapadrepio.it/it/content/view/1091/976/) for calculation of the MPI value. Further details on mathematical methods used to identify the best MPI cut-off points have been previously reported elsewhere (15).
Table 1.
Multidimensional Prognostic Index Score Assigned to Each Domain Based on the Severity of the Problems
| Problems |
|||
| Assessment | No (Value = 0) | Minor (Value = 0.5) | Severe (Value = 1) |
| Activities of daily living* | 6–5 | 4–3 | 2–0 |
| Instrumental activities of daily living* | 8–6 | 5–4 | 3–0 |
| Short portable mental status questionnaire† | 0–3 | 4–7 | 8–10 |
| Cumulative illness rating scale—comorbidity index‡ | 0 | 1–2 | ≥3 |
| Mini nutritional assessment (MNA)§ | ≥24 | 17–23.5 | <17 |
| Exton-Smith Scale (ESS)‖ | 16–20 | 10–15 | 5–9 |
| Numbers of medications | 0–3 | 4–6 | ≥7 |
| Social support network | Living with family | Institutionalized | Living alone |
Notes: *Number of active functional activities.
Number of errors.
Number of diseases.
MNA score: ≥24, satisfactory nutritional status; 17–23.5, at risk of malnutrition; <17, malnutrition.
ESS score: 16–20, minimum risk; 10–15, moderate risk; 5–9 high risk of developing scores.
The PSI
A validated pneumonia specific risk index, the PSI, was also computed. The PSI categorizes patients into five classes (I–V) based on 20 variables, including age, sex, the presence of comorbid illnesses, vital sign abnormalities, and some laboratory and radiographic abnormalities. Elderly patients were classified into five risk classes; Class I (absent by definition) was not used in this analysis, Class II (<71 points), Class III (71–90 points), Class IV (91–130 points), and Class V (>130 points) (6).
Statistical Analysis
Continuous variables were presented as mean and standard deviation and categorical variables as frequencies and percentages. Comparisons between men and women were made using the Mann–Whitney U test. The Kruskal–Wallis and chi-squared tests were used to compare age, gender, educational level, and mortality across the MPI groups.
The relationship between MPI score group and time to death was analyzed by age- and sex-adjusted Cox proportional hazards regression model. Time to death was calculated as the time between admission and time of death or the end of follow-up, whichever come first. The proportionality of the hazard assumption was graphically checked by plotting log (–log [survival function]).
To test the hypothesis that the prognostic value of the aggregated MPI was superior to the prognostic value of its single components considered individually, a logistic model was carried out on the individual parameters. Age, ADL, IADL, SPMSQ, CIRS, MNA, ESS, and the number of medications were evaluated as continuous variables, whereas social support network and MPI were evaluated as ordinal variables, based on the assumption of equidistance between single-unit values. Sex was analyzed as a dichotomous variable.
We assessed the predictive accuracy of the final model by looking at the two components of accuracy: calibration and discrimination. Calibration of the model was assessed by comparing the predicted mortality with the actual mortality in patients with different grades of MPI. The discrimination of the model was assessed by calculating the area under the receiver operating characteristic (ROC) curves for the MPI and the PSI models. C test was used to compare the areas under the ROC curves of MPI and PSI considered as continuous variables. A p value < .05 was considered for statistical significance.
All analyses were performed using the SPSS version 13 software for Windows (SPSS Inc., Chicago, IL).
RESULTS
Overall Study Population
During the enrollment period, 170 patients were consecutively admitted to our Geriatric Unit with a diagnosis of CAP. Ten patients were excluded because they were younger than 65 years; 4 patients were excluded because information on their vital status after 1-year of follow-up was not available and 22 patients were excluded because the CGA was not completed. Thus, the final study population included 134 patients, 89 men (66%) and 45 women (34 %) with a mean age of 78.7 (8.8) and a range from 65 to 100 years old.
Table 2 reports the characteristics of patients included in the study, divided according to gender; women were significantly older (p < .001), had more ADL (p < .001) and IADL (p = .03) disabilities, lower educational level (p = 0.04), lower cognitive function in SPMSQ (p = 0.04), lower MNA score (p = 0.008), lower Exton-Smith scores (p < .001), higher CIRS—CI (p < .001), and higher CIRS severity Indexes (p < .001) than men. Only eight patients (5.9% of patients) were institutionalized, 3 men and 5 women (3.3% vs 11.1%, p = ns); their mean MPI value was 0.61 ± 0.23.
Table 2.
Baseline Characteristics by Sex
| All | Males | Females | p Value | |
| Patients, no (%) | 134 (100) | 89 (66) | 45 (34) | — |
| Age | ||||
| M (SD), y | 78.7 (8.8) | 76.4 (7.9) | 83.3 (8.9) | <.001 |
| Range, y | 65–100 | 65–93 | 69–100 | — |
| Educational level, M (SD), y | 4.5 (3.6) | 4.9 (4.03) | 3.32 (2.0) | .04 |
| ADL, M (SD), score | 3.7 (2.6) | 4.2 (2.4) | 2.8 (2.5) | .001 |
| IADL, M (SD), score | 3.4 (3.1) | 3.8 (3.1) | 2.6 (3.10) | .03 |
| SPMSQ, M (SD), score | 2.8 (3.1) | 2.5 (2.9) | 3.6 (3.2) | .03 |
| Exton-Smith, M (SD), score | 14.7 (4.3) | 15.7 (3.9) | 12.6 (4.35) | <.001 |
| CIRS—CI, M (SD), score | 3.2 (1.7) | 2.7 (1.3) | 4.0 (2.0) | <.001 |
| CIRS—SI, M (SD), score | 1.7 (0.4) | 1.6 (0.3) | 1.9 (0.4) | <.001 |
| MNA, M (SD), score | 20.6 (6.1) | 21.4 (6.2) | 19.0 (5.4) | .008 |
| Number of medications, M (SD) | 4.1 (2.6) | 4.0 (2.6) | 4.2 (2.8) | .62 |
| Social index, no (%) | ||||
| Living with family | 99 (73.2) | 75 (83.1) | 24 (53.3) | <.001 |
| Institutionalized | 8 (5.9) | 3 (3.4) | 5 (11.1) | .165 |
| Living alone | 27 (20.1) | 11 (12.4) | 16 (35.6) | .003 |
| MPI, M (SD), score | 0.43 (0.25) | 0.38 (0.24) | 0.55 (0.22) | <.001 |
| Mortality, no (%) | ||||
| At 30 d | 22 (16) | 10 (11) | 12 (27) | .02 |
| At 6 mos | 30 (22) | 17 (19) | 13 (29) | .27 |
| At 12 mos | 38 (28) | 21 (24) | 17 (38) | .10 |
Note: ADL = activities of daily living; CIRS—CI = cumulative illness rating scale—comorbidity index; CIRS—SI = cumulative illness rating scale—severity index; IADL = instrumental activities of daily living; MNA = mini nutritional assessment; MPI = multidimensional prognostic index; SPMSQ = short-portable mental status questionnaire.
The overall mortality rate was 16% after 30 days, 22% after 6 months, and 28% after 12 months of follow-up, without significant differences between men and women.
The MPI
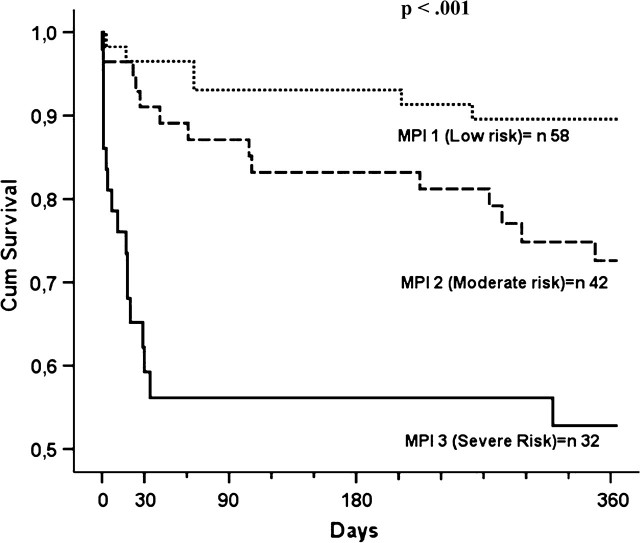
A significantly higher mean MPI value was observed in women than men (0.55 [0.22] vs 0.38 [0.24], p < .001). Higher MPI values were significantly associated with female sex (p < .001), older age (p = .02), and a progressively higher mortality after 30 days (Grade 1 = 3%, Grade 2 = 12%, Grade 3 = 44%; p < .001), 6 months (Grade 1 = 7%, Grade 2 = 21%, Grade 3 = 50%; p < .001), and 1 year (Grade 1 = 10%, Grade 2 = 33%, Grade 3 = 53%; p < .001) of follow-up (Table 3).
Table 3.
Baseline Characteristics by MPI Grade
| MPI 1 (Low Risk) | MPI 2 (Moderate Risk) | MPI 3 (Severe Risk) | p Value | |
| Patients, no (%) | 58 (43) | 42 (31) | 34 (25) | — |
| MPI value , M (SD) | 0.19 (0.08) | 0.49 (0.09) | 0.76 (0.07) | |
| Sex | ||||
| Women, no (%) | 9 (15) | 19 (45) | 17 (50) | .001 |
| Age | ||||
| M (SD), y | 75.9 (8.2) | 80.9 (9.0) | 80.8 (8.6) | .02 |
| Range, y | 65–93 | 66–100 | 68–97 | — |
| Educational levels, M (SD) | 4.9 (3.4) | 4.3 (3.5) | 3.6 (3.8) | .21 |
| Mortality, no (%) | ||||
| At 30 d | 2 (3) | 5 (12) | 15 (44) | <.001 |
| At 6 mos | 4 (7) | 9 (21) | 17 (50) | <.001 |
| At 1 y | 6 (10) | 14 (33) | 18 (53) | <.001 |
| PSI, no (%) | ||||
| I (absent by definition) | — | — | — | — |
| II | 0 (0) | 0 (0) | 0 (0) | — |
| III | 9 (69) | 2 (15) | 2 (15) | .14 |
| IV | 43 (52) | 27 (33) | 12 (15) | .001 |
| V | 6 (15) | 13 (33) | 20 (51) | <.001 |
Note: MPI = multidimensional prognostic index; PSI = pneumonia severity index.
Figure 1 shows the age- and sex-adjusted survival curves for different grades of MPI: patients with higher MPI demonstrated higher mortality rates (p < .001). Table 4 reports the corresponding mortality estimated by means of hazard ratio and 95% CIs and the observed mortality in patients with different grades of MPI. A very close agreement was found between the estimated mortality by the three MPI grades and the observed mortality after 30 days, 6 months, and 1 year of follow-up (Table 4).
Figure 1.
Survival curves, adjusted for age and gender, for different grades of multidimensional prognostic index as obtained in patients with pneumonia.
Table 4.
Mortality by MPI
| EM |
||||
| MPI Grade | Time | HR (95% CI) | OM | Δ* |
| 1 (low risk) | 30 d | 0.039 (–0.03 to 0.10) | 0.034 | 0.005 |
| 6 mos | 0.074 (0.00 to 0.15) | 0.069 | 0.005 | |
| 12 mos | 0.110 (0.02 to 0.19) | 0.103 | 0.007 | |
| 2 (moderate risk) | 30 d | 0.096 (0.01 to 0.18) | 0.119 | –0.023 |
| 6 mos | 0.204 (0.07 to 0.33) | 0.214 | –0.010 | |
| 12 mos | 0.318 (0.18 to 0.45) | 0.333 | –0.015 | |
| 3 (high risk) | 30 d | 0.485 (0.33 to 0.64) | 0.441 | 0.044 |
| 6 mos | 0.623 (0.47 to 0.78) | 0.500 | 0.123 | |
| 12 mos | 0.639 (0.48 to 0.80) | 0.529 | 0.110 |
Notes: EM = estimated mortality; HR = hazard ratio; OM = observed mortality; MPI = multidimensional prognostic index.
Delta indicates the difference between HR and OM values.
Logistic analyses demonstrated that MPI was significantly associated with the mortality rates after 30 days, 6 months, and 1 year of follow-up. Standardized beta coefficients showed that both the short- (30 days) and the long-term (6 months and 1 year) prognostic values of MPI were higher compared with those of the individual parameters (Table 5).
Table 5.
Individual Risk Factors for Mortality at Follow-Up
| 30 d |
6 mos |
1 y |
|||||||
| Risk Factors | β Coefficient* | OR (95% CI) | p | β Coefficient | OR (95% CI) | p | β Coefficient | OR (95% CI) | p Value |
| MPI | 3.80 | 4.58 (2.09–10.04) | <.001 | 3.92 | 3.48 (1.87–6.50) | <.001 | 3.54 | 2.82 (1.59–5.00 | <.001 |
| Age | 2.24 | 1.07 (1.01–1.14) | .02 | 2.68 | 1.08 (1.02–1.13) | .007 | 3.42 | 1.11 (1.04–1.17) | <.001 |
| Sex (male) | –1.21 | 0.53 (0.19–1.48) | .23 | –0.15 | 0.93 (0.37–2.35) | .88 | –0.06 | 0.97 (0.40–2.37) | .95 |
| ADL | 2.91 | 1.36 (1.11–1.68) | .004 | 2.95 | 1.31 (1.09–1.56) | .003 | 1.81 | 1.16 (0.99–1.38) | .07 |
| IADL | 2.08 | 1.24 (1.01–1.51) | .038 | 2.54 | 1.25 (1.05–1.49) | .01 | 1.91 | 1.15 (1.00–1.34) | .06 |
| SPMSQ | 0.34 | 1.03 (0.85–1.26) | .74 | 1.22 | 1.10 (0.94–1.28) | .22 | 1.04 | 1.08 (0.93–1.26) | .30 |
| CIRS—CI | 0.69 | 1.10 (0.84–1.44) | .49 | 0.31 | 1.04 (0.81–1.34) | .75 | 0.54 | 1.07 (0.84–1.37) | .59 |
| MNA | 0.64 | 1.03 (0.94–1.13) | .52 | 1.39 | 1.05 (0.98–1.13) | .16 | 1.05 | 1.04 (0.97–1.12) | .29 |
| ESS | 3.15 | 1.25 (1.09–1.43) | .002 | 3.23 | 1.22 (1.08–1.37) | .001 | 3.00 | 1.19 (1.06–1.33) | .003 |
| Number of medications | 1.84 | 1.18 (0.99–1.41) | .06 | 1.65 | 1.14 (0.97–1.34) | .01 | 1.99 | 1.17 (1.00–1.37) | .04 |
| Social network | 0.29 | 1.19 (0.38–3.75) | .77 | 0.74 | 1.47 (0.52–4.14) | .46 | 0.88 | 1.57 (0.57–4.32) | .38 |
Notes: ADL = activities of daily living; CIRS—CI = cumulative illness rating scale—comorbidity index; ESS = Exton-Smith Scale; IADL = instrumental activities of daily living; MNA = mini nutritional assessment; MPI = multidimensional prognostic index; SPMSQ = short-portable mental status questionnaire.
β Value standardized.
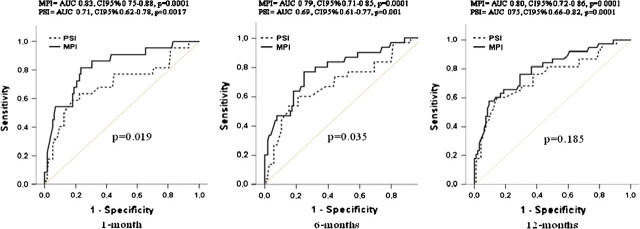
The discrimination of the MPI was also good, with an ROC area for mortality of 0.83, 95% CI = 0.75–0.87 (sensitivity = 81.8%, specificity = 76.8%, cut-off value > 0.178) at 30 days, of 0.79, 95% CI = 0.71–0.85 (sensitivity = 76.7%, specificity = 75.0%, cut-off value > 0.248) at 6 months, and of 0.80, 95% CI = 0.72–0.86 (sensitivity = 57.9%, specificity = 90.6%, cut-off value > 0.439) at 1-year of follow-up (Figure 2).
Figure 2.
Receiver operating characteristic (ROC) curves for multidimensional prognostic index (MPI) and pneumonia severity index (PSI) at 1 month, 6 months, and 12 months of follow-up.
Comparison Between the MPI and the PSI
As shown in Table 3, with increasing MPI grades, a decrease in the prevalence of patients of PSI Class III and IV was observed. Conversely, the prevalence of patients of PSI Class V significantly increased with increasing MPI grades.
Mortality rates significantly increased from PSI Class III to PSI Class V either after 30 days (PSI Class III = 8% vs Class IV = 11% vs Class V = 31%, p = .01), 6 months (PSI Class III = 8% vs Class IV = 17% vs Class V = 38%, p = .01), and 1 year of follow-up (PSI Class III = 8% vs Class IV = 22% vs Class V = 49%, p = 0.002). The discrimination of PSI, as evaluated by the ROC area, was 0.71, 95% CI 0.62–0.78 (sensitivity = 63.6%, specificity = 77.7%, cut-off value > 121) at 30 days, 0.69, 95% CI 0.61–0.77 (sensitivity = 60%, specificity = 78.8%, cut-off value > 144) at 6 months, and 0.75, 95% CI 0.65–0.82 (sensitivity = 60.5%, specificity = 85.4%, cut-off value > 141) at 1 year of follow-up. Figure 2 compares ROC areas for models with MPI and PSI: the model with MPI demonstrated a significantly higher prognostic value for mortality than did the model with PSI after 30 days (p = .019) and after 6 months (p = .035). After 1 year of follow-up, although the prognostic value of the model with MPI was higher than that for the model with PSI, this difference was not statistically significant (p = .185).
DISCUSSION
In the present study, we demonstrated that MPI derived from a CGA can be used to stratify hospitalized older patients with CAP into categories that experienced a highly differential short- and long-term mortality. The MPI is derived from parameters obtained from a standardized CGA and was excellent in predicting 1-month to 1-year mortality risk as demonstrated by the very close agreement between the estimated mortality and the observed mortality as well as by the three distinct and uncrossed survival curves corresponding with the three MPI groups.
The overall 30-day mortality rate observed in this study was 16%, which is in agreement with the 30-day mortality rates of 16 and 19% recently reported in two studies carried out in elderly patients hospitalized with CAP in Japan (12) and the United States (25), respectively. In this study, the 1-year mortality rate was 28%, confirming previous observations that there is a substantial long-term mortality after hospitalization for CAP in the elderly patients (4,26).
Predicting the outcome in elderly patients diagnosed with CAP is very important in clinical decision-making process. Previous epidemiological studies (4,27) suggested that old age, male sex, and comorbidities were important predictors of mortality in the elderly population diagnosed with CAP. More recently, two studies have explored the role of the functional assessment in elderly patients with CAP. The first study (26) reported that functional status, evaluated by means of the ADL Barthel Index in 99 elderly patients hospitalized with CAP, was an independent predictor of short- and long-term mortality. The second study, carried out in 112 elderly patients with CAP (25), demonstrated that the patients who were functionally dependent according to a 29-item scale that included ADL, IADL, mobility, communication, and cognitive assessment had significantly higher 1-year mortality than the patients who were functionally independent (50% vs 23%), and the difference remained significant after adjusting for comorbidity and severity of CAP. Indeed, a retrospective study of 193 patients with CAP aged 80 and older (12) reported that using a performance status assessment according to the European Cooperative Oncology Group performance status scale (28) significantly improved the predictive value of the PSI for 30-day mortality. Thus, all the forementioned studies suggest that severity indices for CAP should possibly be adjusted in the elderly population, to include an assessment of functional status.
To the best of our knowledge, this is the first description of a prognostic index based on data available from a standard CGA for older patients hospitalized with CAP in an acute care setting. Adjusting for age and sex, the prognostic effect of MPI on mortality was highly significant. Moreover, logistic regression analysis confirmed that the aggregate MPI was significantly associated with mortality, and its prognostic value was higher than those provided by the individual parameters utilized for constructing the MPI, that is ADL, IADL, SPMSQ, MNA, ESS, CIRS, medications, and social network. In this population, other than MPI, only the ADL and IADL scores, the ESS, and the number of medications were factors independently associated with mortality. These data are in agreement with studies carried out in older patients hospitalized for an acute disorder or relapse of a chronic disease independently from the diagnoses (15) as well as in elderly patients hospitalized for an acute upper gastrointestinal bleeding (29). Overall these findings support the concept that considering multidimensional aggregate information is very important for predicting short-term and long-term mortality in older patients with acute diseases, including pneumonia.
As shown by the areas under the ROC curves, in this population the predictive value of MPI was significantly higher than the predictive value of PSI. This difference was statistically significant at both 30 days and 6 months but not after 1 year of follow-up. This finding was unexpected considering that PSI is an index especially designed to predict short-term mortality, and it has not been related to long-term outcomes in previous studies. Indeed, the original PSI was developed to identify patients with CAP with a low risk of death who can be potentially be treated as outpatients (6). It should be noted that the present study included older patients (mean age = 78.7 years) who were hospitalized and possibly at high risk for mortality. This finding is in agreement with a previous study, which reported that PSI did not perform well in predicting mortality risk in elderly patients aged 80 and older who were admitted to the hospital with CAP (12).
In this population, the prognostic value of MPI was good either at 30 days or after longer periods of follow-up. As shown by the ROC curves in Figure 2, although at 30 days MPI demonstrated satisfactory sensitivity (82%) and specificity (77%) with an area under the ROC curve of 0.83 (cut-off value >0.178), after 1 year of follow up, the sensitivity decreased to 57.9%, whereas its specificity increased to 91%, resulting an area under the ROC curve of 0.80 (cut-off value > 0.439). Conversely, in agreement with data from other studies (10,12), sensitivity of PSI was relatively low at both 30 days (64%) and 1 year (60.5%), and its overall prognostic value for mortality was similar when evaluated at 30 days and 1 year, with areas under the ROC curves (0.71 at 30 days, cut-off > 121 and 0.75 at 1 year, cut-off > 141), lower than the areas yielded from MPI. These findings suggest that in our population, the multidimensional impairment may influence 1- and 6-month mortality related to an acute disease more than the severity of pneumonia as evaluated by PSI.
The present study has some limitations. Because the study population comprised selected patients, that is admitted to a geriatric unit, twice as many men than women, it is possible that the sample is unrepresentative of older population hospitalized with CAP. Secondly, because the MPI focused on hospitalized older patients, it is possible that it is not applicable to institutionalized or ambulatory participants. Indeed, in different groups of elderly participants, the MPI would probably include other predictor variables that take into account of the different setting populations. Moreover, because MPI needs a complete CGA assessment in an acute setting, it is possible that it is a complex bedside index to use in all elderly patients. Finally, the study population was relatively small, and the patients were recruited within a single hospital. Larger prospective multicenter studies are needed to confirm the findings.
In conclusion, we have described an MPI in hospitalized elderly patients with CAP. This index was a sensitive measure of the multidimensional risk assessment that might be useful in identifying elderly patients with CAP at different risk of mortality who probably need a different intensity of clinical interventions.
Acknowledgments
This work was fully supported by grants from Ministero della Salute, IRCCS Research Program 2006–2008, Line 2: “Malattie di rilevanza sociale.” This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
References
- 1.Viegi G, Pistelli R, Cazzola M, et al. Epidemiological survey on incidence and treatment of community acquired pneumonia in Italy. Respir Med. 2006;100(1):46–55. doi: 10.1016/j.rmed.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Janssens JP. Pneumonia in the elderly (geriatric) population. Curr Opin Pulm Med. 2005;11(3):226–230. doi: 10.1097/01.mcp.0000158254.90483.1f. [DOI] [PubMed] [Google Scholar]
- 3.Bohannon RW, Maljanian RD. Hospital readmissions of elderly patients hospitalized with pneumonia. Conn Med. 2003;67(10):599–603. [PubMed] [Google Scholar]
- 4.Kaplan V, Clermont G, Griffin MF, et al. Pneumonia: still the old man's friend? Arch Intern Med. 2003;163(3):317–323. doi: 10.1001/archinte.163.3.317. [DOI] [PubMed] [Google Scholar]
- 5.Yende S, Angus DC, Ali IS, et al. Influence of comorbid conditions on long-term mortality after pneumonia in older people. J Am Geriatr Soc. 2007;55(4):518–525. doi: 10.1111/j.1532-5415.2007.01100.x. [DOI] [PubMed] [Google Scholar]
- 6.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. New Engl J Med. 1997;336(4):243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 7.Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA, Campbell GD. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Resp Crit Care Med. 2001;163(7):1730–1754. doi: 10.1164/ajrccm.163.7.at1010. [DOI] [PubMed] [Google Scholar]
- 8.Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spindler C, Ortqvist A. Prognostic score systems and community-acquired bacteraemic pneumococcal pneumonia. Eur Respir J. 2006;28(4):816–823. doi: 10.1183/09031936.06.00144605. [DOI] [PubMed] [Google Scholar]
- 10.Buising KL, Thursky KA, Black JF, et al. A prospective comparison of severity scores for identifying patients with severe community-acquired pneumonia: reconsidering what is meant by severe pneumonia. Thorax. 2006;61(5):419–424. doi: 10.1136/thx.2005.051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myint PK, Kamath AV, Vowler SL, Maisey DN, Harrison BD British Thoracic Society. Severity assessment criteria recommended by the British Thoracic Society (BTS) for community-acquired pneumonia (CAP) and older patients. Should SOAR (systolic blood pressure, oxygenation, age and respiratory rate) criteria be used in older people? A compilation study of two prospective cohorts. Age Ageing. 2006;35(3):286–291. doi: 10.1093/ageing/afj081. [DOI] [PubMed] [Google Scholar]
- 12.Naito T, Suda T, Yasuda K, et al. A validation and potential modification of the pneumonia severity index in elderly patients with community-acquired pneumonia. J Am Geriatr Soc. 2006;54(8):1212–1219. doi: 10.1111/j.1532-5415.2006.00825.x. [DOI] [PubMed] [Google Scholar]
- 13.Solomon D, Sue Brown A, Brummel-Smith K, et al. Best paper of the 1980s: National Institutes of Health Consensus Development Conference Statement: geriatric assessment methods for clinical decision-making 1988. J Am Geriatr Soc. 2003;51(10):1490–1494. doi: 10.1046/j.1532-5415.2003.51471.x. [DOI] [PubMed] [Google Scholar]
- 14.Rubenstein LZ, Joseph T. Freeman award lecture: comprehensive geriatric assessment: from miracle to reality. J Gerontol Med Sci. 2004;59(5):473–477. doi: 10.1093/gerona/59.5.m473. [DOI] [PubMed] [Google Scholar]
- 15.Pilotto A, Ferrucci L, Franceschi M, et al. Development and validation of a multidimensional prognostic index for 1-year mortality from the comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res. 2008;11(1):151–161. doi: 10.1089/rej.2007.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartlett JG, Mundy LM. Community-acquired pneumonia. N Engl J Med. 1995;333(24):1618–1624. doi: 10.1056/NEJM199512143332408. [DOI] [PubMed] [Google Scholar]
- 17.Katz S, Downs TD, Cash HR, Grotz RC. Progress in the development of an index of ADL. Gerontologist. 1970;10(1):20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 18.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 19.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 20.Linn B, Linn M, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 21.Guigoz Y, Vellas B. The mini nutritional assessment (MNA) for grading the malnutrition states of elderly patients: presentation of the MNA, history and validation. Nestle Nutr Workshop Ser Clin Perform Programme. 1999;1:3–11. doi: 10.1159/000062967. [DOI] [PubMed] [Google Scholar]
- 22.Bliss MR, McLaren R, Exton-Smith AN. Mattresses for preventing pressure sores in geriatric patients. Mon Bull Minis Health Public Health Lab Serv. 1966;25:238–268. [PubMed] [Google Scholar]
- 23.Thomas VS, Rockwood K, McDowell I. Multidimensionality in instrumental and basic activities of daily living. J Clin Epidemiol. 1998;51(4):315–321. doi: 10.1016/s0895-4356(97)00292-8. [DOI] [PubMed] [Google Scholar]
- 24.Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hebert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353(9148):205–206. doi: 10.1016/S0140-6736(98)04402-X. [DOI] [PubMed] [Google Scholar]
- 25.Mody L, Sun R, Bradley SF. Assessment of pneumonia in older adults: effect of functional status. J Am Geriatr Soc. 2006;54(7):1062–1067. doi: 10.1111/j.1532-5415.2006.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres OH, Munoz J, Ruiz D, et al. Outcome predictors of pneumonia in elderly patients: importance of functional assessment. J Am Geriatr Soc. 2004;52(10):1603–1609. doi: 10.1111/j.1532-5415.2004.52492.x. [DOI] [PubMed] [Google Scholar]
- 27.Koivula I, Sten M, Makela PH. Prognosis after community-acquired pneumonia in the elderly: a population-based 12-years follow-up study. Arch Intern Med. 1999;159(14):1550–1555. doi: 10.1001/archinte.159.14.1550. [DOI] [PubMed] [Google Scholar]
- 28.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 29.Pilotto A, Ferrucci L, Scarcelli C, et al. Usefulness of the comprehensive geriatric assessment in older patients with upper gastrointestinal bleeding: a two-year follow-up study. Dig Dis. 2007;25(2):124–128. doi: 10.1159/000099476. [DOI] [PMC free article] [PubMed] [Google Scholar]