Abstract
Emerging reports reveal that activating Toll-like receptor-2 (TLR2)–MyD88 signals in CD8 T lymphocytes enhances cytokine production and cytotoxicity; however, the signaling pathway remains undefined. In the present study, we examined the physiologic significance and molecular mechanisms involved in this process. We found that TLR2 engagement on T-cell receptor transgenic CD8 OT-1 T cells increased T-bet transcription factor levels consequently, augmenting effector transcript and protein levels both in vivo and in vitro. In contrast, TLR2 agonist did not costimulate TLR2−/−OT-1 or MyD88−/−OT-1 T cells. Elevated T-bet levels in TLR2-MyD88–activated T cells was a consequence of increased biosynthesis resulting from the enhanced acti- vation of the mammalian target of the rapamycin (mTOR) pathway. Inhibiting mTOR, Akt, or protein kinase C in T cells abolished the costimulatory effects of the TLR2 agonist. In vivo, activating TLR2–MyD88 signals in T cells increased effector-molecule levels and enhanced the clearance of Listeria monocytogenes-Ova. These results help define a signaling pathway linking the TLR-MyD88 and mTOR pathway in an Akt- and protein kinase C–dependent manner. These results highlight a critical role for MyD88 signaling in T-cell activation and cytotoxicity. Furthermore, these findings offer the opportunity for improving the efficacy of vaccines and T cell–based immunotherapies by targeting TLR-MyD88 signaling within T cells.
Introduction
The molecular and cellular bases for the costimulatory effects of Toll-like receptor (TLR) agonists are being gradually unraveled, adding to our understanding of how TLR signals enhance T cell–mediated immune responses. Expressed primarily on cells of the innate immune system, such as dendritic cells (DCs), TLRs recognize microbial-derived molecules. Certain TLRs, such as TLR1 and TLR2, can form heterodimers to facilitate the detection of a broader array of microbes.1 TLR engagement on professional antigen-presenting cells (APCs) induces their maturation, resulting in the increased expression of costimulatory molecules and cytokines necessary for optimal T-cell activation.1 Thus, given their potent effects on APCs, TLR agonists are believed to influence T-cell responses principally by stimulating TLRs on cells of the innate immune system.1,2
However, recent advances by several groups, including ours, indicate that the adjuvant effects of certain TLR agonists may also be attributed to the activation of TLRs and the TLR adapter molecule myeloid differentiation factor (MyD88) directly in T cells. Both CD43–11 and CD810,12,13 T cells express functional TLRs. In CD8 T cells, concomitant engagement of the T-cell receptor (TCR) and TLR3 enhanced interferon-γ (IFN-γ) production,10 whereas TLR2 engagement on CD8 T cells increased the expression levels of granzyme B in vitro.13 We recently reported that TLR2 engagement on CD8 T cells also increased IFN-γ, granzyme B, and perforin production (referred to as effector molecules), and, consequently, enhanced T-cell cytotoxicity both in vivo and in vitro.12 However, the molecular pathway by which TLR-MyD88 signals in T cells augment effector function is poorly defined. Only recently have studies implicated the PI3K signaling pathway in TLR2-mediated T-cell activation14; however, the molecular mechanisms through which these signals influence activation and cytotoxicity remain largely undefined.
Among the various signals involved in T-cell activation, 2 transcription factors, T-bet and eomesodermin (EOMES), profoundly influence CD8 T-cell differentiation into effector cytotoxic T lymphocytes (CTLs).15 Both these transcription factors can regulate IFN-γ, perforin, and granzyme B transcription, and in their absence, CD8 T cells fail to effectively transition from a naive T cell into an effector or memory cell.15–17 T-bet expression is known to be induced by signals mediated via the TCR and IFN-γR; however, additional signals capable of influencing the expression of these transcription factors remains limited.
In the present study, we investigated the cellular and molecular mechanisms through which TLR2-MyD88 signals within CD8 T cells enhance the production of effector molecules and augment cytotoxicity. We used TCR transgenic OT-1, MyD88−/−OT-1 or TLR2−/−OT-1 CD8 T cells, which recognize an epitope from the chicken ovalbumin protein, to examine the costimulatory effects of TLR-MyD88 signals. We found that TLR2 ligation on OT-1 T cells, but not TLR2−/−OT-1, MyD88−/−OT-1, or T-bet−/−OT-1, increased T-bet protein levels, resulting in the increased binding to the promoters of IFN-γ, granzyme B, and perforin and, consequently, elevated transcript and protein levels. TLR2-MyD88 signals increased T-bet biosynthesis, in part, by enhancing the activation of the mammalian target of the mTOR pathway in a manner dependent on Akt and PKC signaling. The biologic significance of activating TLR2-MyD88 signals within CD8 T cells is underscored by, in fact, that OT-1 T cells, transferred into MyD88−/− or wild-type (WT) mice, cleared Listeria monocytogenes more effectively than did MyD88−/−OT-1 or TLR2−/−OT-1 T cells. Furthermore, in experiments where we cotransferred OT-1 and MyD88−/−OT-1, we detected higher levels of effector transcripts and T-bet protein in OT-1 cells after treatment with TLR2 ligand. These findings add to our understanding of how TLR-MyD88 signals enhance T-cell activation by revealing a mechanistic regulation of effector molecules and T-bet expression and by uncovering a physiologically relevant role for TLR-MyD88 signals within T cells in the resolution of an intracellular bacterial infection.
Methods
Mice
Studies were reviewed and approved by the Institutional Animal Care and Use Committee. BL6 mice were obtained from Charles River Laboratories, MyD88−/− mice were a gift from Dr Douglas Golenbock (Boston University, Boston, MA), B6.129-TLR2tm1kir/J (TLR2−/−), T-bx21 (T-bet−/−),IFN-γR−/−, and OT-1 mice were purchased from The Jackson Laboratory. CD8 T cells were used from the 7th or later generation of MyD88−/−OT-1, TLR2−/−OT-1, and T-bet–/–OT-1 mice. All genotypes and phenotypes were determined by polymerase chain reaction (PCR) and by flow cytometry using antibodies specific to CD8, and the TCR chains, Vα2 and Vβ5.1.
T-cell sorting, activation, and use of inhibitors
CD8 T cells were purified by negative selection (StemCell Technologies), followed by positive selection (Miltenyi Biotec). T-cell purity routinely yielded 99%. For in vitro studies, OT-1 T cells were stimulated with antigen-pulsed MyD88−/− splenocytes (1nM SIINFEKL for 2 hours at 37°C). In some experiments, CD8 T cells were activated using plate-bound anti-CD3 antibody (Ab) at 2 μg/mL. For the detection of cytokines, T cells were activated in the presence of the TLR1/2 ligand, (tripalmitoyl-S-(bis(palmitoyloxy)propyl)-Cys-Ser-(Lys)3-Lys), (InvivoGen) for 3-5 days, followed by intracellular staining (BD Bioscience) or enzyme-linked immunosorbence assay (ELISA; eBioscience). In some experiments, mTOR was inhibited with rapamycin (2μM; Sigma-Aldrich) during the last 4-6 hours of culture. Alternatively, protein kinase C (PKC)–inhibitor rottlerin (0.7μM; Calbiochem) or Akt inhibitor (0.7μM; Calbiochem) were added with the TLR2 ligand for 12 hours and then washed off, and placed into culture for an additional 60 hours, followed by cytokine detection or cultured for 36 hours for detection of T-bet and EOMES protein levels. In some experiments, inhibitors were serially diluted 2-fold.
qRT-PCR
RNA transcript numbers were determined by quantitative real-time PCR (qRT-PCR). Briefly, RNA was extracted from purified CD8 T cells or for in vivo experiments, then RNA was extracted immediately after sorting by fluorescence-activated cell sorting (FACS). All PCR primer sets were purchased from SuperArray Bioscience Corporation using RT2 SYBR Green with ROX (SuperArray). Serial dilutions of all transcripts were used to generate the standard curve and then normalized to β-actin transcript levels. Thermal cycling conditions were 95°C (10 minutes), followed by 40 cycles of 95°C (15 seconds) for denaturation, and 60°C (1 minute) for annealing and extension.
Immunoblots and ChIP
CD8 T cells were activated with plate-bound anti-CD3 antibodies for 48 hours before extracting nuclear protein. Nuclear proteins (10 μg/lane) were resolved in 12.5% Tris-glycine sodium dodecyl sulfate (SDS) gels and transferred to polyvinylidene difluoride membranes. The membrane was blocked for 4 hours with 5% milk in phosphate-buffered saline and 0.05% Tween-20, followed by incubation with a monoclonal anti-T bet (Cell Signaling), EOMES, or lamin antibodies (Santa Cruz Biotechnology) overnight at 4°C and, subsequently, incubated in horseradish peroxidase–conjugated secondary Ab, and detected using enhanced chemilumine-scence (ECL; Amersham Pharmacia Biotech). Alternatively, the levels of phosphorylated and nonphosphorylated mTOR, factor 4E binding protein 1 (4E-BP1), P70S6 kinase (P70S6K), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Cell Signaling) were determined using total lysate by immunoblot at the time points indicated in the figure legends. The chromatin immunoprecipitation (ChIP) procedure was conducted as described by the manufacturer (USB Corporation), using polyclonal antibodies (Santa Cruz Biotechnology). Primer sequences were as follows18: IFN-γ; GAGAAATTCACATTACAAGGGC, TTAAGATGGTGACAGATAGGTGG; granzyme B; ATGCTCCTGATTACCCTCAC, CAGAGAACCACCACTTACAG, perforin; GTACTAGCCTGCTCAAACCT, CTAATCACAGTGTCCCATGAG, β-actin; CGATATCCACGTGACATCCA, AGAGAAAGCGAGATTGAGGA.
Pulse-chase experiment
CD8 T cells from BL6 mice were activated with plate-bound anti-CD3 Ab for 44 hours, washed 4× in L-methionine–free RPMI 1640, seeded at a density of 2 × 106 cells/mL in L-methionine–free RPMI 1640 for 1.5 hours, and then pulsed with 0.5 mCi (9.25 MBq) [35S] methionine for 4 hours. Cells were washed twice with phosphate-buffered saline, followed by nuclear protein extraction. Nuclear proteins were precleared with protein-G Sepharose for 30 minutes and immunoprecipitated with the indicated antibodies and agarose-conjugated protein-G. The immunoprecipitates were separated on 12.5% SDS–polyacrylamide gel electrophoresis (PAGE) and subsequently transferred to a membrane.
Polysome analysis
Purified CD8 T cells were activated with plate-bound anti-CD3 Ab for 48 hours and then lysed in buffer containing 100 μg/mL cycloheximide. After the removal of nuclei and mitochondria, supernatants were layered onto 10%-50% sucrose gradients and spun at 38 000 rpm (2 hours at 4°C) in an SW 40 rotor (Beckman Instruments). Centrifuged gradients were fractionated into 12 1-mL fractions and the polysome profile was determined via ultraviolet (UV) absorbance at 260 nm followed by RNA extraction, reverse transcription, and quantification by RT-PCR.
Bacterial infection of mice
Purified OT-1, TLR2–/–OT-1, or MyD88–/–OT-1 T cells (2.5 × 105) were intravenously injected into mice, followed by injection (intravenous) 24 hours later with L monocytogenes–expressing Ova. For determination of bacterial burdens in mice, spleens were collected at various time points after infection and homogenized in distilled water. Serial dilutions of homogenates were plated onto brain-heart infusion (BHI) broth (BD Bioscience) agar. Colonies were counted after 24 hours of incubation at 37°C. Colony-forming unit (CFU) data were analyzed by analysis of variance (ANOVA) models after logarithm transformation and allowing for unequal variances.
Gene transcript and T-bet protein analysis after in vivo T-cell activation
CD90.1+ CD45.2+ OT-1 and CD90.2+CD45.2+ MyD88−/−OT-1 T cells were purified, as described in “T-cell sorting, activation, and use of inhibitors,” mixed to a 1:1 ratio, and injected (intravenously; 0.25 × 106) into CD45.1+CD90.2+ BL6 mice. One day later, mice were injected with CD45.1 splenocytes pulsed with SIINFEKL antigen and coinjected with or without TLR2 ligand (5 μg). Five days later, spleens and lymph nodes were pooled and OT-1 and MyD88−/−OT-1 cells were sorted by FACS, RNAwas extracted, and gene expression was analyzed using RT-PCR. Alternatively, the intracellular levels of T-bet in T cells were determined by flow cytometry.
Results
TLR2 engagement on CD8 T cells augments T-bet protein levels and IFN-γ, perforin, and granzyme B expression
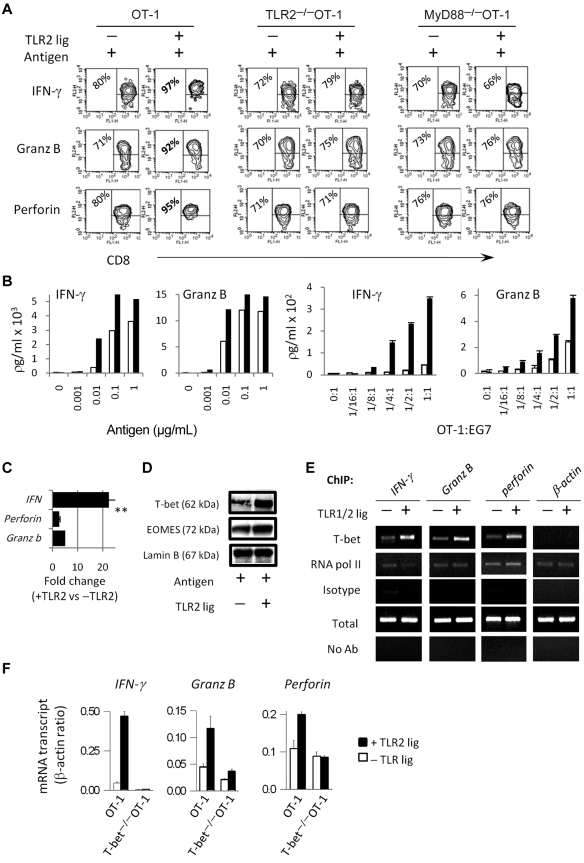
We examined the protein and mRNA levels of IFN-γ, granzyme B, and perforin in OT-1, TLR2−/−OT-1, and MyD88−/−OT-1 T cells after TCR and TLR2 engagement. Purified OT-1 CD8 T cells were activated with antigen-pulsed MyD88−/− splenocytes in the presence or absence of the TLR1/2 agonist, Pam3Cys4. Because TLR1/2 signal transduction requires the adapter molecule MyD88, the use of MyD88−/−APCs ensures that the costimulatory effects of TLR1/2 ligand occur through TLR stimulation on CD8 T cells.19,20 As shown in Figure 1A, TLR2 stimulation on OT-1 T cells increased the percentage of cells that produced IFN-γ, perforin, and granzyme B. In contrast, TLR2 ligand did not increase the production of effector molecules by TLR2−/−OT-1 or MyD88−/−OT-1 T cells (Figure 1A). Purified CD8 T cells stimulated with plate-bound anti-CD3 Ab, in the absence of APCs, also produced higher levels of effector molecules, confirming that the costimulatory effects of TLR2 agonists occurred by acting directly on T cells (Figure 1B). Furthermore, TLR2 stimulation lowered the activation threshold (Figure 1B). However, in agreement with previous reports,12,13 in the absence of antigen, TLR2 ligand did not costimulate T cells, highlighting the requirement for concomitant TCR stimulation.
Figure 1.
TLR2 stimulation on CD8 T cells increases the expression levels of effectors molecules and T bet. (A,F) Purified CD8 OT-1 T cells were activated with antigen-pulsed MyD88−/− splenocytes in each of the absence (white bar) or presence of the TLR2 agonist, Pam3Cysk4 (10 μg/mL; black bar). Four days later, the intracellular level of granzyme-B, perforin, and IFN-γ were determined by flow cytometry. (B) The production of IFN-γ and granzyme B by OT-1 T cells, in response to varying concentrations of antigen, was determined by ELISA. (C) The IFN-γ, perforin, and granzyme-B transcript levels in OT-1 T cells were determined by RT-PCR. The fold changes in gene expression are shown. (D) The protein levels of T-bet and EOMES in nuclear extracts of OT-1 T cells were determined by Western blot analysis. (E) T-bet binding to the promoter of effectors molecules was examined by ChIP assays 3 days after T-cell activation. (F) Gene expression in OT-1 or T-bet−/−OT-1 T cells was determined by RT-PCR. The data shown for panels A through D are representative of at least 4 independent experiments, each yielding similar results, whereas data shown in panels E and F are representative of 2 experiments. The fold changes in gene expression between TLR-stimulated and non–TLR-stimulated cells were analyzed by ANOVA; **P < .001, *P < .05
Because the expression of effectors molecules in CD8 T cells is regulated primarily at the level of transcription, we com-pared IFN-γ, perforin, and granzyme B mRNA levels in TLR2-stimulated and non–TLR-stimulated T cells. A represen-tative graph of a RT-PCR reaction shows that IFN-γ mRNA transcripts were higher in TLR2-stimulated T cells than in non–TLR-stimulated T cells, as represented by lower cycle values (Figure 1C). Likewise, TLR2 stimulation increased granzyme B and perforin transcripts (Figure 1C). β-actin levels were similar in TLR2-stimulated and non–TLR-stimulated T cells.
As T-bet and EOMES play a significant role in T-cell activation and because we detected increased effector transcripts, we examined T-bet and EOMES protein levels. TLR2-stimulated cells showed increased T-bet protein levels (Figure 1D; P < .02; data from several experiments). In contrast, EOMES levels remained similar in TLR2-stimulated and non–TLR stimulated T cells (Figure 1D). A polyclonal anti-body against lamin B was used to show equal loading of the nuclear samples.
We used ChIP assays to examine T-bet interaction with the promoters of IFN-γ, perforin, and granzyme B in TLR2-stimulated and non–TLR2-stimulated T cells. DNA/protein complexes immunoprecipitated with a polyclonal anti-T-bet antibody, and gene transcripts were detected by RT-PCR. An increased binding of T-bet to the IFN-γ, perforin, and granzyme B, but not β-actin, promoters was observed in TLR2-ligated T cells (Figure 1E). The amplification of effectors genes was similar when DNA/protein complexes were immunoprecipitated with anti-RNA–pol II antibody, whereas no amplification was detected when using an isotype antibody (Figure 1E).
We next examined the requirement for T-bet in the TLR2-MyD88–mediated up-regulation of effector gene transcription. T-bet−/−OT-1 cells showed similar IFN-γ, granzyme B, and perforin transcript numbers with or without TLR2 ligand (Figure 1F). Consistent with previous reports, the level of effectors molecules in T-bet−/− T cells were reduced, compared with WT T cells.16,17 These data indicated that T-bet played a critical role in the TLR2-mediated enhancement of IFN-γ, granzyme B, and perforin expression.
We conclude that TLR2 engagement on CD8 T cells augments the production of IFN-γ, perforin, and granzyme B, in part, by enhancing T-bet expression levels and, consequently, increasing the transcription of target effector genes.
TLR2 signals enhance T-bet protein, but not gene-transcript, levels
We examined whether increased T-bet protein levels in TLR2-stimulated T cells was associated with enhanced T-bet transcription. The data in Figure 2A (left panel) show similar T-bet mRNA levels in TLR2-stimulated and non–TLR-stimulated CD8 T cells throughout the time course evaluated. In contrast, IFN-γ and granzyme B transcript levels increased in response to TLR2 stimulation (Figure 2A inset panels). We did not detect differences in T-bet mRNA stability between TLR2-stimulated and non–TLR-stimulated T cells (data not shown). Despite similar T-bet transcript numbers, T-bet, but not EOMES, protein levels were significantly elevated in TLR2-stimulated T cells, as examined throughout the course of 96 hours (Figure 2B).
Figure 2.
T-bet gene- and protein-expression levels after TLR2 stimulation. (A) Purified CD8 T cells were activated with plate-bound anti-CD3 antibody (1.5 μg/mL) in the presence or absence of TLR2 agonist (10 μg/mL) for the indicated time points. After these times, the mRNA levels of T-bet, IFN-γ, and granzyme B (+ SD) were determined by RT-PCR. (B) The protein levels of T-bet and EOMES in the nucleus were determined by Western blot analysis. The data shown in panel A are representative of 2 independent experiments, while the data shown in panel B are representative of 3 experiments, with each experiment showing identical trends.
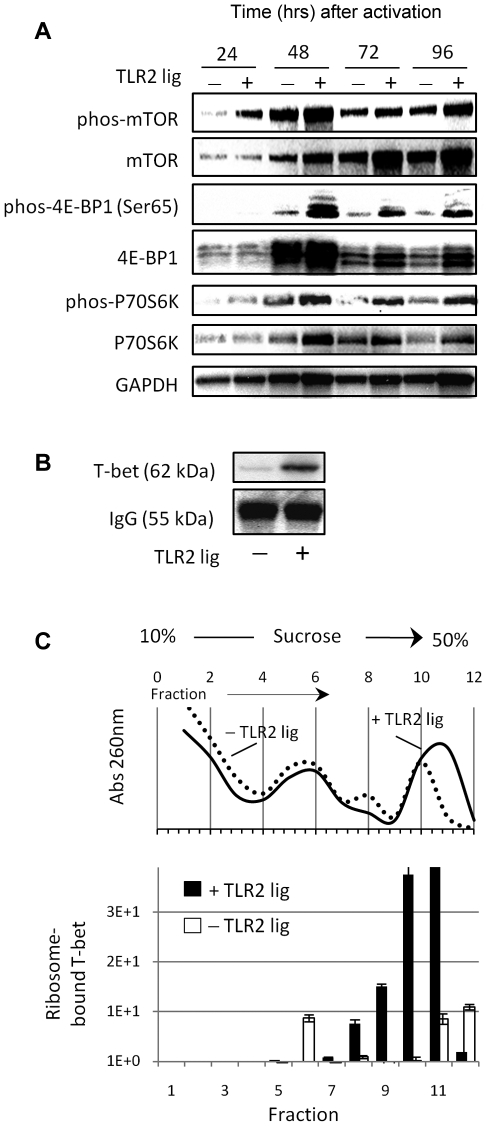
TLR2-stimulated CD8 T cells show enhanced activation of the mTOR pathway and increased T-bet biosynthesis
The data presented in Figure 2A indicated that the increased T-bet protein levels in TLR2-stimulated T cells was not regulated at the level of RNA, but likely resulted from improved protein synthesis and/or stability. To determine whether TLR2 signals influenced the protein-synthesis machinery, we tested for the phosphorylated (activated) mammalian target of mTOR, which regulates ribosome biogenesis and translation, in extracts of TLR2-stimulated and nonstimulated T cells. Elevated levels of phosphorylated mTOR in TLR2-stimulated T cells were detected as early as 24 hours after activation (Figure 3A). mTOR activation leads to the phosphorylation (inactivation) of the repressor of mRNA translation eukaryotic initiation 4E-BP1 and the phosphorylation (activation) of p70S6K, which enhances protein translation.21 TLR2-stimulated CD8 T cells showed enhanced phosphorylation of both p70S6K and 4E-BP1 (Figure 3A). Increased levels of phosphorylated p70S6K and 4E-BP1 in TLR2-ligated cells persisted over the evaluated time course. T-bet protein synthesis in TLR2-stimulated and nonstimulated CD8 T cells was evaluated by [35S]methionine incorporation in pulse-chase assays. Analysis by SDS-PAGE indicated that T-bet protein synthesis in T cells increased in response to TLR2 stimulation (Figure 3B). A polyclonal antibody against anti-IgG was used to show equal loading of the samples.
Figure 3.
TLR2 signals in CD8 T cells activate the mTOR pathway and are associated with increased T-bet biosynthesis. (A) CD8 T cells were activated with plate-bound anti-CD3 antibody (1.5 μg/mL) in the presence or absence of TLR2 ligand (10 μg/mL) for the time points indicated. The levels of phosphorylated or nonphosphorylated mTOR, 4E-EBP1, and P70S6K protein were determined by Western blot. (B) Forty-eight hours after activation, the levels of newly synthesized T-bet protein were determined in pulse-chase experiments. (C) Alternatively, the levels of newly synthesized T-bet RNA in TLR2-stimulated and nonstimulated T cells were determined in the different ribosomal fractions and quantified using RT-PCR. The data shown are representative of 3 independent experiments.
P70S6K can influence protein synthesis rates by activating the translation-initiation factor eIF4B whereas phosphorylated 4E-BP1 promotes translation by releasing the cap-binding protein eIF4E. Because the distribution of transcripts in polyribosomes correlates with the rate of synthesis of the corresponding protein, we examined the distribution of T-bet mRNA in ribosome-free and polysome-bound fractions. The associated RNAs were extracted using sucrose-gradient fractionation and, ultimately, quantified using RT-PCR. The non-TLR2-stimulated fractions 5-7 contained higher levels of T-bet RNA (Figure 3B). In contrast, the absorbance profile of TLR2-stimulated T cells displayed a stronger peak at fractions 9-12 and expressed significantly higher levels of newly synthesized T-bet RNA (Figure 3C). Collectively, these data indicate that the activating TLR2-MyD88 signals within CD8 T cells augments T-bet biosynthesis by enhancing mTOR activation.
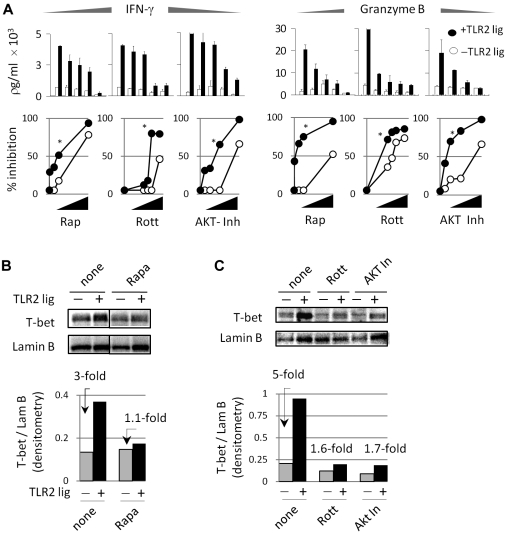
Inhibiting mTOR, PKC, or PI3K reduces the costimulatory effects of TLR2 stimulation
To assess the involvement of mTOR in TLR2-ligated CD8 T-cell activation, we used the highly specific mTOR inhibitor, rapamycin. TLR2-stimulated T cells were more sensitive to rapamycin than non–TLR-stimulated T cells (Figure 4A top and bottom panels; P < .001), as determined by IFN-γ and granzyme B production. TLR2 signals are also known to activate multiple signal-transduction pathways simultaneously in different T-cell types. These signals include p38, PI3K, AKT, and PKC isoforms, which have been shown to activate or enhance the effects of mTOR in different T-cell types.22 To determine which signaling pathway(s) downstream of TLR2 contributed to the enhanced production of IFN-γ and granzyme B, we blocked each of these molecules using pharmacologic inhibitors. The PKCθ inhibitor, rottlerin, significantly reduced the costimulatory effects of TLR2 ligand, as demonstrated by a greater reduction of IFN-γ and granzyme B production than non–TLR-stimulated T cells (Figure 4A top and bottom panels). Similarly, TLR2-stimulated T cells were more sensitive to AKT inhibition (Figure 4A). PI3K inhibitors also reduced the costimulatory effects of TLR2 agonist, whereas p38 inhibitors did not appear to decrease the costimulatory effects of TLR2 agonists (data not shown). We excluded the possibility that these effects were caused by T-cell death by examining the apoptotic sensitivity of T cells to inhibitors, as determined by labeling with propidium iodide and annexin V (data not shown). These data indicated that TLR2 signals in CD8 T cells increased IFN-γ and granzyme B production in a manner that depended predominantly on the enhanced activation of mTOR, as well as the activation of AKT and PKC.
Figure 4.
Suppressing the mTOR pathway inhibits the costimulatory effects of TLR2 in CD8 T cells. (A) CD8 T cells were activated as described in Figure 2. Briefly, purified T cells were activated in each of the absence or presence of TLR2 ligand for 72 hours. Various concentrations of rapamycin were added for the last 6 hours of culture. Alternatively, rottlerin and AKT inhibitor were added with or without the TLR2 ligand for the initial 18 hours of activation. After this time point, inhibitors and TLR2 ligand were washed off and T cells were cultured for an additional 54 hours. After 72 hours, the levels of IFN-γ and granzyme B were determined by ELISA. The top panels show the amount of IFN-γ and granzyme B, whereas the bottom panels show the percentage inhibition after normalizing levels to cultures that did not receive inhibitors. (B) Alternatively, T-bet protein levels were determined in the presence of rapamycin, rottlerin, or Akt inhibitor by Western blot. The fold changes in T-bet expression levels in TLR2-stimulated over non–TLR-stimulated T cells are shown.
We next examined T-bet protein levels in TLR2-stimulated and non–TLR-stimulated T cells with or without rapamycin. In the absence of rapamycin, TLR stimulation increased T-bet levels (Figure 4B top and bottom panels). In contrast, rapamycin reduced T-bet protein levels in TLR2-stimulated to those similar of non–TLR-stimulated T cells (Figure 4B). PKC and AKT inhibitors also reduced T-bet protein levels in TLR2-stimulated T cells (Figure 4C). Granted, both TLR2-stimulated and non–TLR-stimulated T cells showed reduced T-bet levels in the presence of these inhibitors, however, TLR2-stimulated cells demonstrated a higher degree of sensitivity. Altogether, these data indicated that the TLR2-mediated up-regulation of T-bet is highly sensitive to the blockade of the mTOR pathway and the suppression AKT and PKC signaling.
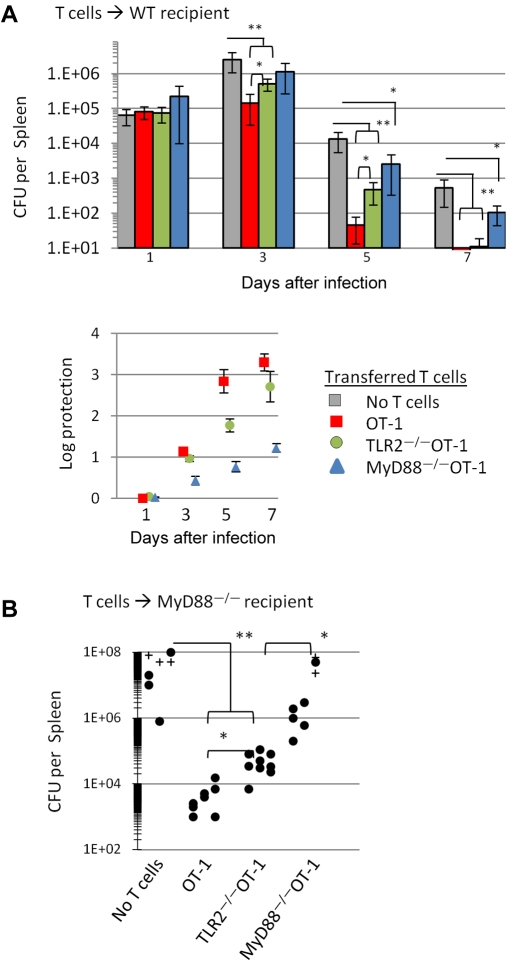
TLR2-MyD88 signals within CD8 T cells contribute to the efficient clearance of an intracellular pathogen
CD8 T cells, as well as TLR2 and MyD88 signals, have been shown to play a critical role in host resistance to various pathogens, including L monocytogenes (LMs).23–27 We examined whether activating TLR2-MyD88 signals in CD8 T cells contributed to the clearance of LMs in vivo. Naive OT-1, TLR2−/−OT-1, or MyD88−/−OT-1 T cells were injected intravenously into B6 mice and challenged with LM expressing ovalbumin (LM-Ova) 1 day later. The number of CFUs in the spleens was determined at various time points after LM-Ova challenge.
The CFUs in the spleens of control and treated mice were similar 1 day after infection. The data in the bottom panel of Figure 5A show the log protection. Three days after infection, mice receiving OT-1 or TLR2−/−OT-1 T cells showed significantly (P < .001) lower CFUs than mice receiving MyD88−/−OT-1 or no T cells. OT-1 T cells were also more effective at clearing LM-Ova than TLR2−/−OT-1 on day 3 (P < .05). By day 5, mice receiving OT-1 cells showed significantly lower CFUs than mice receiving MyD88−/−OT-1 (P < .001) or TLR2−/−OT-1 T cells (P < .01). However, TLR2−/−OT-1 T cells cleared LMs more efficiently than MyD88−/−OT-1 T cells. MyD88−/−OT-1 showed more enhanced LM-Ova clearance than mice not receiving T cells (day 5). By day 7, all T-cell groups showed greater control of infection over mice that did not receive T cells (P < .001); however, MyD88−/−OT-1 responses lagged behind those of OT-1 or TLR2−/−OT-1.
Figure 5.
TLR2- or MyD88-signaling–impaired OT-1 T cells show a diminished ability to clear a L monocytogenes infection. (A) Purified OT-1, TLR2−/−OT-1, and MyD88−/−OT-1 CD8 T cells (2.5 × 105) were intravenously injected into WT mice, followed by intravenous injection with 1 × 104 CFU LM-OVA 1 day later. For the determination of bacterial burdens in mice, spleens were collected at various time points after infection and homogenized in distilled water, and serial dilutions of homogenates were cultured on BHI agar. (B) MyD88−/− mice were injected intravenously with OT-1, TLR2−/−OT-1, and MyD88−/−OT-1 CD8 T cells, followed by infection with LM-Ova 1 day later. Five days later, spleens were collected, and the bacterial plaques were counted. Circles represent CFUs per spleen of individual mice. The results shown are the combination of 3 independent experiments, with 3-4 mice per group. Crosses represent mice that succumbed to bacterial challenge before assessing bacterial load. Changes in CFUs were analyzed by ANOVA; *P < .05, **P < .001.
We also examined the ability of OT-1, TLR2−/−OT-1, or MyD88−/−OT-1 CD8 T cells to resolve an LM-Ova infection in MyD88−/−mice. Mice receiving OT-1 or TLR2−/−OT-1 demonstrated an enhanced clearance of LM-Ova than did untreated mice (P < .0001) or mice injected with MyD88−/−OT-1 T cells (Figure 5B; P < .001) 5 days after infection. We detected a significant difference (P < .05) in the level of protection offered by OT-1 cells, compared with TLR2−/−OT-1, suggesting that TLR2 expression on OT-1 T cells contributed, to some extent, to the clearance of LM-Ova. Mice receiving MyD88−/−OT-1 T cells also offered a small degree of protection, compared with mice that did not receive T cells. However, the level of protection was not as pronounced as mice receiving OT-1 or TLR2−/−OT-1 T cells, indicating that the lack of MyD88 in CD8 T cells was critical for the effective clearance of LM-Ova.
It is worth noting that the CFUs in untreated MyD88−/− mice was significantly higher than the number of LM-Ova initially injected into mice, indicating that substantial bacterial replication had occurred after 5 days. Furthermore, the CFUs in MyD88−/− mice that did not receive T cells were substantially higher than in WT mice, indicating that the expression of MyD88 on cells other than T cells played a critical role in reducing bacterial load (Figure 5A-B). Collectively, these data demonstrate that activating TLR2-MyD88 signals in CD8 T cells plays an important role in resolving an LM infection.
Activating TLR2-MyD88 signals in OT-1 CD8 T cells in vivo augments effector molecule expression
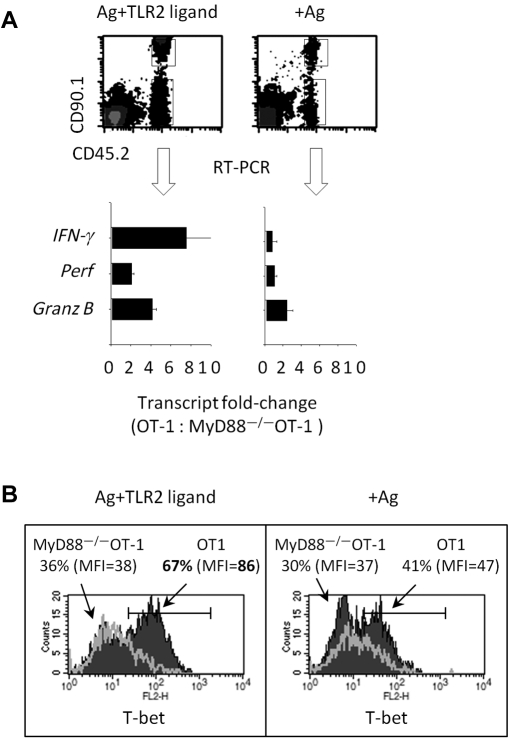
We examined the physiologic significance of activating TLR2-MyD88 signals in CD8 T cells in vivo. OT-1 (expressing the congenic markers, CD90.1+and CD45.2+) and MyD88−/−OT-1 T cells (CD90.2+ CD45.2+) were mixed at an equal ratio and intravenously injected into CD90.2+ CD45.1+ WT mice. One day later, antigen-pulsed splenocytes were coinjected (intravenously) with or without the TLR2 ligand. Five days later, transferred T cells were purified using FACS, and RNA was extracted, reverse transcribed to cDNA, and followed by the quantification of gene transcripts by RT-PCR (Figure 6A top panel).
Figure 6.
TLR2 stimulation on CD8 T cells in vivo augments the number of effectors gene transcripts and increases T-bet expression. (A) Purified OT-1 (CD90.1+ CD45.2+) and MyD88−/−OT-1 (CD90.2+ CD45.2+) CD8 T cells were mixed at a 1:1 ratio, and 1 × 106 T cells were injected intravenously into CD45.1+CD90.2+ mice. One day later, mice were injected intravenously with Ova157-164–pulsed CD45.1 splenocytes plus TLR2 agonist (10 μg) or Ova157-164–pulsed splenocytes alone. Five days after adoptive T-cell transfer, cells were sorted by flow cytometry, and the levels of effectors gene transcripts were determined by RT-PCR. The numbers of each transcript in OT-1 and MyD88−/−OT-1 T cells were normalized to β-actin, and fold changes of gene transcripts are shown. Error bars represent the SD from the mean of 3 PCR reactions. (B) Alternatively, intracellular T-bet protein levels in transferred T cells were determined via intracellular staining and flow cytometry. The data shown are representative of 2 independent experiments, each yielding identical trends. The fold changes in gene expression between were analyzed by ANOVA; *P < .05, **P < .001.
In mice injected with antigen and TLR2 agonist, we detected increased IFN-γ (7-fold), perforin (3-fold), and granzyme B (4-fold) transcripts in OT-1 T cells, compared with MyD88−/−OT-1 T cells (Figure 6A bottom panel). In contrast, the ratio of effector transcripts in OT-1 versus MyD88−/−OT-1 T cells was similar. Furthermore, a larger frequency of OT-1 T cells expressed higher T-bet protein levels than did MyD88−/−OT-1 T cells, as determined by comparing mean fluorescent intensities (Figure 6B). Because OT-1 and MyD88−/−OT-1 were injected into the same host and we detected increased expression levels of effector molecules in OT-1 T cells after injection with TLR2 ligand, we conclude that these differences arose from the activation of MyD88 via TLR2 engagement on CD8 T cells. This conclusion is further supported by the data in Figure 1 showing increased levels of effectors gene transcripts and T-bet protein levels (Figures 1–4) after TLR2 engagement. These results indicate that (1) TLR2-MyD88 activation in CD8 T cells occurs in vivo; (2) activation of TLR2-MyD88 signals within T cells enhances their response to antigenic stimulation; and (3) the loss of MyD88 in CD8 T cells reduces their ability to efficiently respond to antigenic activation.
Discussion
Understanding the signals that contribute to the generation of productive T-cell responses is critical for enhancing the efficacy of vaccines and developing effective immunotherapies for cancer patients. The engagement of TLRs on APCs is a critical step for the optimal activation and expansion of T cells. However, several reports indicate that the ligation of specific TLRs directly on primary CD4 and CD8 T cells6,7,11–13,28,29 or T-cell lines30 can enhance their responses. The study reported here was aimed at gaining a better understanding of the molecular mechanisms involved in the costimulatory effects of TLR-MyD88 activation in CD8 T cells.
We showed that TLR2 engagement directly on WT or TCR transgenic CD8 T cells increased the expression of IFN-γ, granzyme B, and perforin at both the RNA and protein level (Figures 1–4). We confirmed these effects occurred in a TLR2- and MyD88-dependent manner, as TLR2 agonists did not stimulate TLR2−/−OT-1 or MyD88−/−OT-1 T-cell responses. The increased production of effector molecules correlated with higher T-bet protein levels and increased binding to IFN-γ, granzyme B, and perforin promoters. The biologic importance of activating TLR2-MyD88 signals within T cells in vivo is underscored in experiments showing that OT-1 T cells cleared an intracellular pathogen more effectively than did TLR2−/−OT-1 or MyD88−/−OT-1 T cells, and in experiments showing that OT-1 T cells expressed elevated levels of effectors gene transcripts after injection with TLR2 ligand and TLR2 agonist (Figure 5).
Several aspects of our study merit additional comment. The first point relates to the signaling pathway by which TLR2 signals increase T-bet protein levels. Recent studies conducted in B cells and DCs confirm a connection with the TLR-induced expression of T-bet31,32; however, the molecular mechanisms are unknown. Our present data show that elevated T-bet levels in TLR2-stimulated CD8 T cells was, in part, due to the enhanced activation of the mTOR pathway, as depicted in Figure 7. Accordingly, enhanced mTOR activation correlated with increased phosphorylation of 2 key substrates involved in protein synthesis: p70S6K and 4E-BP1. That TLR2 stimulation in CD8 T cells increased T-bet biosynthesis is further supported by pulse-chase experiments and, in studies in which we detected elevated levels of newly synthesized T-bet RNA, within sucrose gradient fractions containing polyribosomes (Figure 3). The precise signaling network by which TLR2 signals activate the mTOR pathway is not yet fully defined. However, the TLR stimulation on cells of the innate immune system leads to the activation of PI3K and activates Akt.33 Our data show that blocking Akt (in CD8 T cells) impeded the TLR2 ligand–induced production of effectors molecules and reduced T-bet protein levels to levels comparable to non–TLR-stimulatedT cells (Figure 4). These findings are also consistent with a recent study reporting that direct TLR2 stimulation on CD8 T cells enhanced their survival in vitro in a manner dependent on the PI3K-Akt pathway.14 Interestingly, we also found that inhibitingPKCθ, a member of the Ca2+-independent, novel PKC subfamily, reduced the costimulatory effects of TLR2 agonists. A recent study suggests that there is, indeed, interplay between the PI3K/mTOR PKC-θ pathway in T cells34 and that PKC-θ plays a critical role in cytokine production (ie, TNF-α and IFN-γ).35–37 Furthermore, Solomou and colleagues found that blocking PKC-θ decreased T-bet protein and IFN-γ levels in T cells from patients with aplastic anemia.38 Collectively, these data support a signaling pathway in which TLR engagement amplifies Akt and PKC signals, which, in turn, enhance protein biosynthesis by augmenting mTOR activation, ultimately augmenting T-bet expression and effector gene transcription.
Figure 7.
Diagram depicting how the TLR and TCR signals influence the expression levels of T-bet and effector molecules. TCR activation allows the transmission of TLR2-MyD88 signals, perhaps, by augmenting the expression levels of TLR-related molecules or TLR2 at the cell surface. The engagement of TLR2 by TLR2 agonists augments Akt and PKC activation, which, in turn, may prolong or enhance mTOR activation. Enhanced mTOR activation results in increased phosphorylation of molecules involved in protein translation, such as p70S6K and 4EBP1. Increased p70S6K and 4EBP1 activity enhances T-bet translation. Increased T-bet protein levels increase the transcription of target genes, such as IFN-γ, perforin, and granzyme B.
It is worth noting that we detected moderate increases in EOMES protein expression, ranging from 1.1 to 1.7, after TLR2 stimulation. We set out to confirm that the increased EOMES expression was associated with increased binding to the promoter regions of effector genes (IFN-γ, perforin, and granzyme B) by ChIP assays. However, we were unable to confirm that these changes in EOMES protein were associated with increased binding to DNA or effector-gene transcription (data not shown).
The present studies also emphasized the physiologic significance of TLR2-MyD88 signals in CD8 T cells. We demonstrated that whereas OT-1 T cells effectively cleared an LM-Ova infection (Figure 5), MyD88−/−OT-1 T cells showed a delayed ability to destroy infected cells. TLR2−/−OT-1 also demonstrated a slightly delayed protective response against LM-Ova, compared with OT-1 T cells, but was more effective than MyD88−/−OT-1 T cells. These data suggest that TLR2 stimulation on CD8 T cells contributes to, but is not absolutely essential for, the elimination of this pathogen. Consistent with our data, Machata et al39 and Torres et al26 demonstrated a critical role for TLR2 in the recognition of LM, although it was not determined which cell types expressed TLR2. Our data suggest that TLR expression on effector CD8 T cells may represent a unique adaptation of these cells to facilitate rapid activation in response against intracellular pathogens. Moreover, studies in which we cotransferred OT-1 and MyD88−/−OT-1 T cells into the same recipient strongly indicated that triggering the TLR2-MyD88 pathway in CD8 T cells enhanced T-cell activation, as determined by increased expression of T bet and effectors genes in OT-1, but not MyD88−/−OT-1 (Figure 6). Although the findings presented here indicate that TLR2-MyD88 stimulation on CD8 T cells contributed to enhanced activation and improved clearance of this LM, we cannot rule out the role for other TLRs on T cells. Nor can we exclude the possibility that other non-TLR, but MyD88-dependent, signals (ie, IL-1/IL-18)40 in T cells contributed to these responses.
A role for TLR2 engagement on CD8 T cells in the clearance of a viral infection is currently under investigation. Quigley and colleagues reported that CD8 T cells lacking TLR2-MyD88 signaling were associated with weak T-cell survival and an impaired ability to differentiate into long-lived memory cells in response to a vaccinia viral (VV) infection.14 In contrast, Zhao and colleagues indicated that TLR2 expression (on a polyclonal population of CD8 T cells) did not contribute to either the expansion or cytokine production by VV-specific CD8 T cells.29 Zhao et al attributed these discrepancies to the 2 different experimental systems used (C57BL/6 mice vs B10.D2). Nevertheless, both studies emphasized the importance of intrinsic MyD88-dependent signaling in CD8 T cells for the generation of effective immunity against this pathogen.
These studies also raise the question where TLRs on T cells localize during T-cell activation. At present, there are no data available demonstrating that TLR2 localizes to the immunologic synapse (IS), and if so, where in the synapse it would be found. We were unsuccessful in detecting TLR2 by immunofluorescence, likely due to the quality of anti-TLR2 antibodies currently available for this particular technique. However, several studies have shown that PKC-θ is recruited to the center of the IS upon TCR/CD28 signaling.35,41,42 Ohnuma et al revealed that, in monocytes, caveolin 1, which is a major component in the IS, binds IRAK-1 (IL-1 receptor–associated serine/threonine kinase 1).43 Given that TLR2 that transduces signals via IRAK-1 and is also enriched in caveolin-1–associated lipid raft microdomains,44 it is conceivable that TCR stimulation promotes the recruitment of TLR2 to the IS, which, in turn, may enhance TCR/CD3 signals by promoting PKC-θ and Akt activation.
In conclusion, our findings reveal a previously unknown link between the TLR2-MyD88 and mTOR pathways and highlight its impact on CD8 T-cell activation. These results indicate that the immunostimulatory effects of various TLR agonists may extend beyond solely activating innate immune cells, but may function to augment T cell–mediated immune responses by stimulating TLR-MyD88 signals directly on activated T cells. These findings present the opportunity for enhancing the efficacy of vaccines and cancer immunotherapies by manipulating TLR-MyD88 signaling within CD8 T cells.
Acknowledgments
This work was supported by a National Cancer Institute grant (1R01CA140917), National Institutes of Health Center for Biomedical Research Center Excellence grant (1P20RR021970), the Louisiana Cancer Research Consortium, and the University of Maryland Greenebaum Cancer Center.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.G., L.Z., R.S., and N.A. performed research; C.V.-G. provided statistical support; and E.D. designed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eduardo Davila, University of Maryland/Greenebaum Cancer Center, Rm 10-041, Bressler Research Bldg, 655 W Baltimore St, Baltimore, MD 21201-1559; e-mail: EDavila@som.umaryland.edu.
References
- 1.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85(2):85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 2.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 3.Bendigs S, Salzer U, Lipford GB, Wagner H, Heeg K. CpG-oligodeoxynucleotides co-stimulate primary T cells in the absence of antigen-presenting cells. Eur J Immunol. 1999;29(4):1209–1218. doi: 10.1002/(SICI)1521-4141(199904)29:04<1209::AID-IMMU1209>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 4.Caron G, Duluc D, Fremaux I, et al. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J Immunol. 2005;175(3):1551–1557. doi: 10.4049/jimmunol.175.3.1551. [DOI] [PubMed] [Google Scholar]
- 5.Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004;172(10):6065–6073. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelman AE, LaRosa DF, Zhang J, et al. The adaptor molecule MyD88 activates PI-3 kinase signaling in CD4+ T cells and enables CpG oligodeoxynucleotide-mediated costimulation. Immunity. 2006;25(5):783–793. doi: 10.1016/j.immuni.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci U S A. 2004;101(9):3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaRosa DF, Stumhofer JS, Gelman AE, et al. T cell expression of MyD88 is required for resistance to Toxoplasma gondii. Proc Natl Acad Sci U S A. 2008;105(10):3855–3860. doi: 10.1073/pnas.0706663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuguchi T, Takagi K, Musikacharoen T, Yoshikai Y. Gene expressions of lipopolysaccharide receptors, toll-like receptors 2 and 4, are differently regulated in mouse T lymphocytes. Blood. 2000;95(4):1378–1385. [PubMed] [Google Scholar]
- 10.Tabiasco J, Devevre E, Rufer N, et al. Human effector CD8+ T lymphocytes express TLR3 as a functional coreceptor. J Immunol. 2006;177(12):8708–8713. doi: 10.4049/jimmunol.177.12.8708. [DOI] [PubMed] [Google Scholar]
- 11.Zheng L, Asprodites N, Keene AH, Rodriguez P, Brown KD, Davila E. TLR9 engagement on CD4 T lymphocytes represses {gamma}-radiation-induced apoptosis through activation of checkpoint kinase response elements. Blood. 2008;111(5):2704–2713. doi: 10.1182/blood-2007-07-104141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asprodites N, Zheng L, Geng D, Velasco-Gonzalez C, Sanchez-Perez L, Davila E. Engagement of Toll-like receptor-2 on cytotoxic T-lymphocytes occurs in vivo and augments antitumor activity. FASEB J. 2008;22:3628–3637. doi: 10.1096/fj.08-108274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cottalorda A, Verschelde C, Marcais A, et al. TLR2 engagement on CD8 T cells lowers the threshold for optimal antigen-induced T cell activation. Eur J Immunol. 2006;36(7):1684–1693. doi: 10.1002/eji.200636181. [DOI] [PubMed] [Google Scholar]
- 14.Quigley M, Martinez J, Huang X, Yang Y. A critical role for direct TLR2-MyD88 signaling in CD8 T-cell clonal expansion and memory formation following vaccinia viral infection. Blood. 2009;113(10):2256–2264. doi: 10.1182/blood-2008-03-148809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol. 2004;4(11):900–911. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- 16.Intlekofer AM, Takemoto N, Wherry EJ, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6(12):1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan BM, Juedes A, Szabo SJ, von HM, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci U S A. 2003;100(26):15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho JY, Grigura V, Murphy TL, Murphy K. Identification of cooperative monomeric Brachyury sites conferring T-bet responsiveness to the proximal IFN-gamma promoter. Int Immunol. 2003;15(10):1149–1160. doi: 10.1093/intimm/dxg113. [DOI] [PubMed] [Google Scholar]
- 19.Buwitt-Beckmann U, Heine H, Wiesmuller KH, et al. TLR1- and TLR6-dependent recognition of bacterial lipopeptides. J Biol Chem. 2006;281(14):9049–9057. doi: 10.1074/jbc.M512525200. [DOI] [PubMed] [Google Scholar]
- 20.Ingalls RR, Lien E, Golenbock DT. Differential roles of TLR2 and TLR4 in the host response to Gram-negative bacteria: lessons from a lipopolysaccharide-deficient mutant of Neisseria meningitidis. J Endotoxin Res. 2000;6(5):411–415. [PubMed] [Google Scholar]
- 21.Abraham RT, Wiederrecht GJ. Immunopharmacology of rapamycin. Annu Rev Immunol. 1996;14:483–510. doi: 10.1146/annurev.immunol.14.1.483. [DOI] [PubMed] [Google Scholar]
- 22.Yang Q, Guan KL. Expanding mTOR signaling. Cell Res. 2007;17(8):666–681. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- 23.Seki E, Tsutsui H, Tsuji NM, et al. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J Immunol. 2002;169(7):3863–3868. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- 24.Edelson BT, Unanue ER. MyD88-dependent, but Toll-like receptor 2-independent, innate immunity to Listeria: no role for either in macrophage listericidal activity. J Immunol. 2002;169(7):3869–3875. doi: 10.4049/jimmunol.169.7.3869. [DOI] [PubMed] [Google Scholar]
- 25.Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin, RegIII gamma, and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204(8):1891–1900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres D, Barrier M, Bihl F, et al. Toll-like receptor 2 is required for optimal control of Listeria monocytogenes infection. Infect Immun. 2004;72(4):2131–2139. doi: 10.1128/IAI.72.4.2131-2139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lara-Tejero M, Pamer EG. T cell responses to Listeria monocytogenes. Curr Opin Microbiol. 2004;7(1):45–50. doi: 10.1016/j.mib.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Cottalorda A, Mercier BC, Mbitikon-Kobo FM, et al. TLR2 engagement on memory CD8(+) T cells improves their cytokine-mediated proliferation and IFN-gamma secretion in the absence of Ag. Eur J Immunol. 2009;39(10):2673–2681. doi: 10.1002/eji.200939627. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Y, De TC, Flynn R, Ware CF, Croft M, Salek-Ardakani S. The adaptor molecule MyD88 directly promotes CD8 T cell responses to vaccinia virus. J Immunol. 2009;182(10):6278–6286. doi: 10.4049/jimmunol.0803682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogel SN, Hilfiker ML, Caulfield MJ. Endotoxin-induced T lymphocyte proliferation. J Immunol. 1983;130(4):1774–1779. [PubMed] [Google Scholar]
- 31.Liu N, Ohnishi N, Ni L, Akira S, Bacon KB. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat Immunol. 2003;4(7):687–693. doi: 10.1038/ni941. [DOI] [PubMed] [Google Scholar]
- 32.Lugo-Villarino G, Ito S, Klinman DM, Glimcher LH. The adjuvant activity of CpG DNA requires T-bet expression in dendritic cells. Proc Natl Acad Sci U S A. 2005;102(37):13248–13253. doi: 10.1073/pnas.0506638102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pierre P. Immunity and the regulation of protein synthesis: surprising connections. Curr Opin Immunol. 2009;21(1):70–77. doi: 10.1016/j.coi.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava KK, Batra S, Sassano A, et al. Engagement of protein kinase C-theta in interferon signaling in T cells. J Biol Chem. 2004;279(29):29911–29920. doi: 10.1074/jbc.M401997200. [DOI] [PubMed] [Google Scholar]
- 35.Monks CR, Kupfer H, Tamir I, Barlow A, Kupfer A. Selective modulation of protein kinase C-theta during T-cell activation. Nature. 1997;385(6611):83–86. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- 36.Barouch-Bentov R, Lemmens EE, Hu J, et al. Protein kinase C-theta is an early survival factor required for differentiation of effector CD8+ T cells. J Immunol. 2005;175(8):5126–5134. doi: 10.4049/jimmunol.175.8.5126. [DOI] [PubMed] [Google Scholar]
- 37.Sun Z, Arendt CW, Ellmeier W, et al. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404(6776):402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 38.Solomou EE, Keyvanfar K, Young NS. T-bet, a Th1 transcription factor, is up-regulated in T cells from patients with aplastic anemia. Blood. 2006;107(10):3983–3991. doi: 10.1182/blood-2005-10-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machata S, Tchatalbachev S, Mohamed W, Jansch L, Hain T, Chakraborty T. Lipoproteins of Listeria monocytogenes are critical for virulence and TLR2-mediated immune activation. J Immunol. 2008;181(3):2028–2035. doi: 10.4049/jimmunol.181.3.2028. [DOI] [PubMed] [Google Scholar]
- 40.Adachi O, Kawai T, Takeda K, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9(1):143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 41.Bi K, Tanaka Y, Coudronniere N, et al. Antigen-induced translocation of PKC-theta to membrane rafts is required for T cell activation. Nat Immunol. 2001;2(6):556–563. doi: 10.1038/88765. [DOI] [PubMed] [Google Scholar]
- 42.Huang J, Lo PF, Zal T, et al. CD28 plays a critical role in the segregation of PKC theta within the immunologic synapse. Proc Natl Acad Sci U S A. 2002;99(14):9369–9373. doi: 10.1073/pnas.142298399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okumura S, Kashiwakura J, Tomita H, et al. Identification of specific gene expression profiles in human mast cells mediated by Toll-like receptor 4 and FcepsilonRI. Blood. 2003;102(7):2547–2554. doi: 10.1182/blood-2002-12-3929. [DOI] [PubMed] [Google Scholar]
- 44.Soong G, Reddy B, Sokol S, Adamo R, Prince A. TLR2 is mobilized into an apical lipid raft receptor complex to signal infection in airway epithelial cells. J Clin Invest. 2004;113(10):1482–1489. doi: 10.1172/JCI20773. [DOI] [PMC free article] [PubMed] [Google Scholar]