Abstract
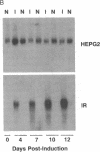
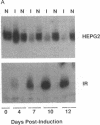
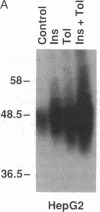
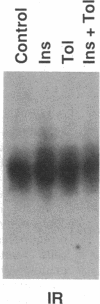
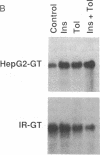
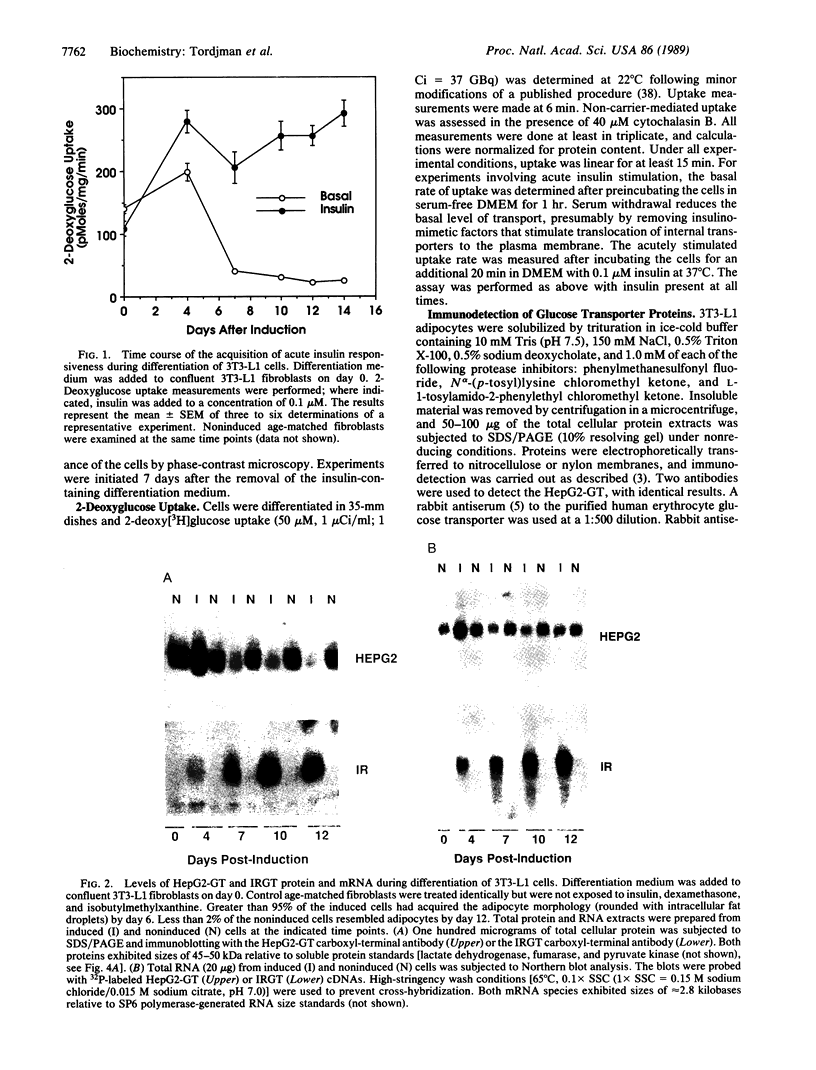
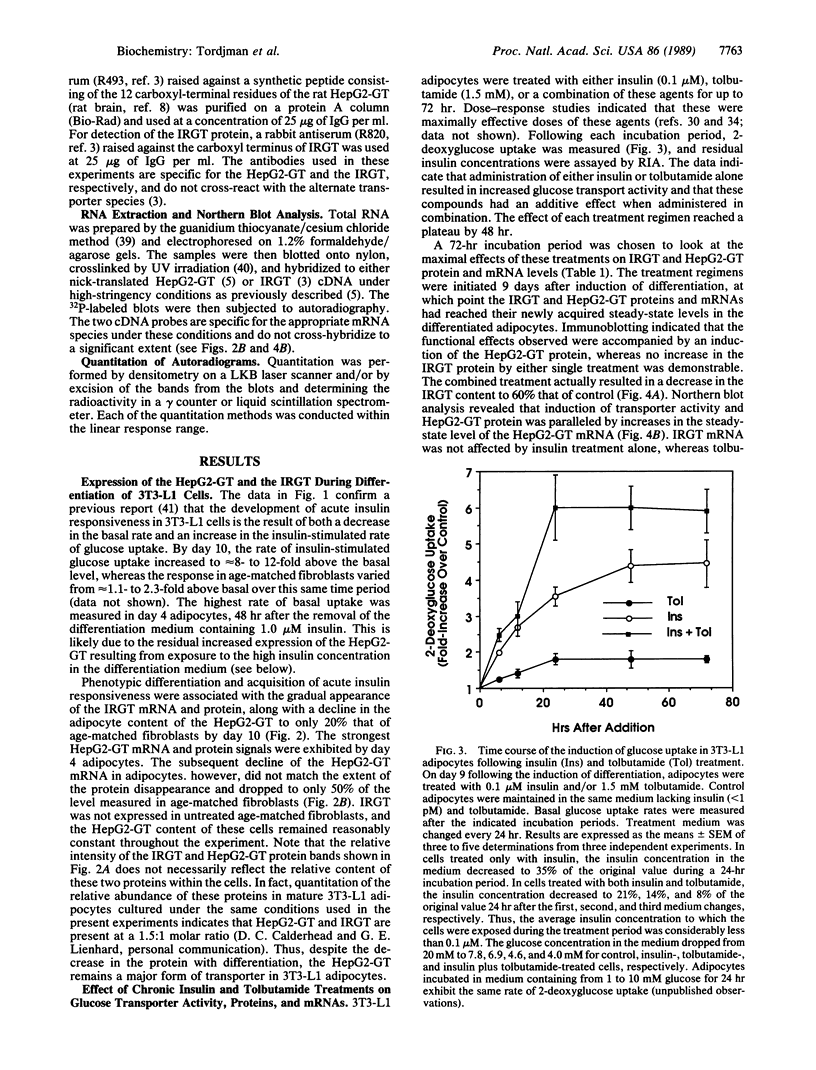
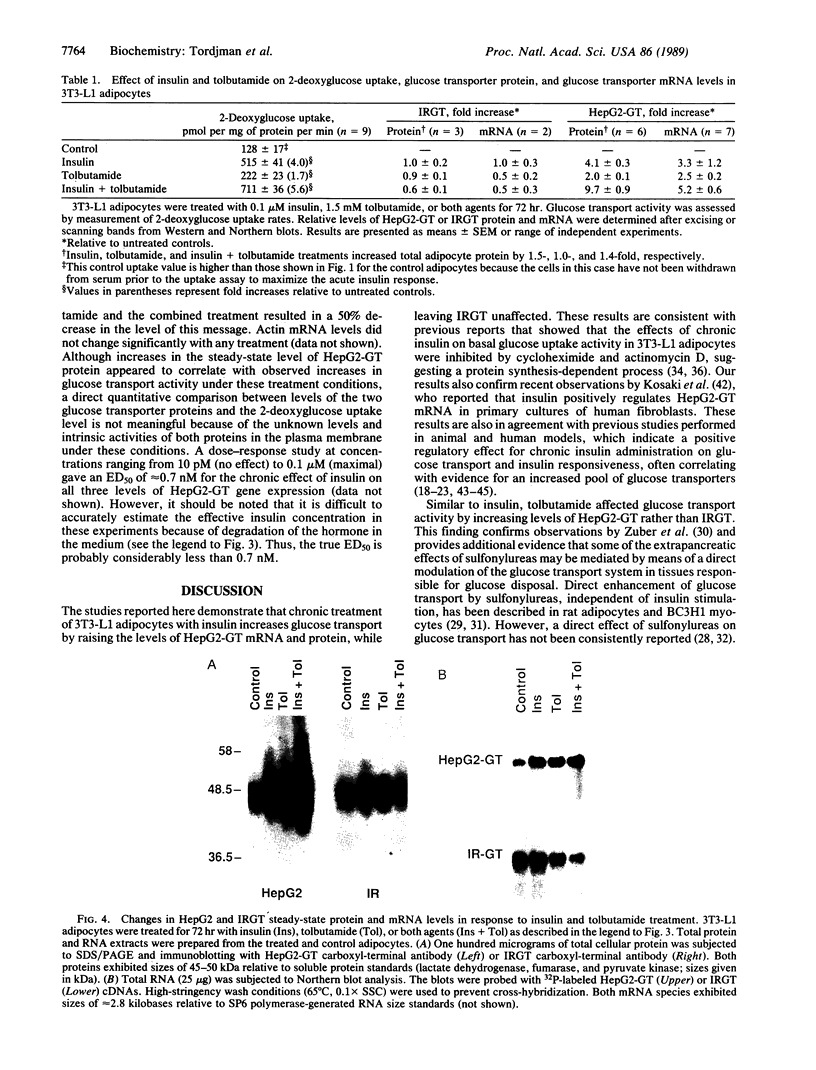
The HepG2-type glucose transporter (HepG2-GT) is expressed in 3T3-L1 fibroblasts and adipocytes. In contrast, the acutely insulin-regulatable glucose transporter (IRGT) is expressed only in the adipocytes. In the present study, the expression of the IRGT was shown to increase in parallel with the acquisition of acutely insulin-stimulated glucose uptake during differentiation of these cells, whereas the level of the HepG2-GT decreased during the course of differentiation in parallel with a decline in basal glucose uptake. We examined the effects of chronic insulin and tolbutamide treatment on glucose transporter activity in conjunction with the expression of these two glucose transporter species in 3T3-L1 adipocytes. Treatment of adipocytes with insulin, tolbutamide, or both agents in combination increased 2-deoxyglucose uptake, HepG2-GT protein, and HepG2-GT mRNA levels in parallel. The effect of combined insulin/tolbutamide administration on these three parameters was greater than the effect of either treatment alone. In contrast, these treatments either had no significant effect or decreased levels of IRGT protein and mRNA. We conclude that chronic treatment of 3T3-L1 adipocytes with insulin or tolbutamide increases glucose uptake primarily by means of a selective increase in the expression of the HepG2-GT. We suggest that part of the in vivo hypoglycemic effect of insulin and sulfonylureas may involve an increased expression of the HepG2-GT.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews W. J., Vasquez B., Nagulesparan M., Klimes I., Foley J., Unger R., Reaven G. M. Insulin therapy in obese, non-insulin-dependent diabetes induces improvements in insulin action and secretion that are maintained for two weeks after insulin withdrawal. Diabetes. 1984 Jul;33(7):634–642. doi: 10.2337/diab.33.7.634. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Sato Y., Felig P., Wahren J. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J Clin Invest. 1981 Dec;68(6):1468–1474. doi: 10.1172/JCI110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinglos M. N., Lebovitz H. E. Sulphonylureas increase the number of insulin receptors. Nature. 1978 Nov 9;276(5684):184–185. doi: 10.1038/276184a0. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Mueckler M., McCall A. L., Lodish H. F. Distribution of glucose transporter messenger RNA transcripts in tissues of rat and man. J Clin Invest. 1987 Feb;79(2):657–661. doi: 10.1172/JCI112864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J. E., Kashiwagi A., Verso M. A., Reaven G., Andrews J. Improvement in in vitro insulin action after one month of insulin therapy in obese noninsulin-dependent diabetics. Measurements of glucose transport and metabolism, insulin binding, and lipolysis in isolated adipocytes. J Clin Invest. 1983 Dec;72(6):1901–1909. doi: 10.1172/JCI111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost S. C., Lane M. D. Evidence for the involvement of vicinal sulfhydryl groups in insulin-activated hexose transport by 3T3-L1 adipocytes. J Biol Chem. 1985 Mar 10;260(5):2646–2652. [PubMed] [Google Scholar]
- Fukumoto H., Seino S., Imura H., Seino Y., Bell G. I. Characterization and expression of human HepG2/erythrocyte glucose-transporter gene. Diabetes. 1988 May;37(5):657–661. doi: 10.2337/diab.37.5.657. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Olefsky J. M., Marshall S. Insulin induces progressive insulin resistance in cultured rat adipocytes. Sequential effects at receptor and multiple postreceptor sites. Diabetes. 1986 Mar;35(3):258–267. doi: 10.2337/diab.35.3.258. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Olefsky J. M., Matthaei S., Marshall S. Glucose and insulin co-regulate the glucose transport system in primary cultured adipocytes. A new mechanism of insulin resistance. J Biol Chem. 1987 Jan 5;262(1):189–197. [PubMed] [Google Scholar]
- Gould G. W., Derechin V., James D. E., Tordjman K., Ahern S., Gibbs E. M., Lienhard G. E., Mueckler M. Insulin-stimulated translocation of the HepG2/erythrocyte-type glucose transporter expressed in 3T3-L1 adipocytes. J Biol Chem. 1989 Feb 5;264(4):2180–2184. [PubMed] [Google Scholar]
- Guerre-Millo M., Lavau M., Horne J. S., Wardzala L. J. Proposed mechanism for increased insulin-mediated glucose transport in adipose cells from young, obese Zucker rats. Large intracellular pool of glucose transporters. J Biol Chem. 1985 Feb 25;260(4):2197–2201. [PubMed] [Google Scholar]
- Haspel H. C., Rosenfeld M. G., Rosen O. M. Characterization of antisera to a synthetic carboxyl-terminal peptide of the glucose transporter protein. J Biol Chem. 1988 Jan 5;263(1):398–403. [PubMed] [Google Scholar]
- Jacobs D. B., Hayes G. R., Lockwood D. H. Effect of chlorpropamide on glucose transport in rat adipocytes in the absence of changes in insulin binding and receptor-associated tyrosine kinase activity. Metabolism. 1987 Jun;36(6):548–554. doi: 10.1016/0026-0495(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Jacobs D. B., Jung C. Y. Sulfonylurea potentiates insulin-induced recruitment of glucose transport carrier in rat adipocytes. J Biol Chem. 1985 Mar 10;260(5):2593–2596. [PubMed] [Google Scholar]
- James D. E., Brown R., Navarro J., Pilch P. F. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988 May 12;333(6169):183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- James D. E., Jenkins A. B., Kraegen E. W. Heterogeneity of insulin action in individual muscles in vivo: euglycemic clamp studies in rats. Am J Physiol. 1985 May;248(5 Pt 1):E567–E574. doi: 10.1152/ajpendo.1985.248.5.E567. [DOI] [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Cushman S. W. Mechanism for markedly hyperresponsive insulin-stimulated glucose transport activity in adipose cells from insulin-treated streptozotocin diabetic rats. Evidence for increased glucose transporter intrinsic activity. J Biol Chem. 1987 Apr 15;262(11):5118–5124. [PubMed] [Google Scholar]
- Kahn B. B., Horton E. S., Cushman S. W. Mechanism for enhanced glucose transport response to insulin in adipose cells from chronically hyperinsulinemic rats. Increased translocation of glucose transporters from an enlarged intracellular pool. J Clin Invest. 1987 Mar;79(3):853–858. doi: 10.1172/JCI112894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnieli E., Armoni M., Cohen P., Kanter Y., Rafaeloff R. Reversal of insulin resistance in diabetic rat adipocytes by insulin therapy. Restoration of pool of glucose transporters and enhancement of glucose-transport activity. Diabetes. 1987 Aug;36(8):925–931. doi: 10.2337/diab.36.8.925. [DOI] [PubMed] [Google Scholar]
- Klip A., Ramlal T., Young D. A., Holloszy J. O. Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett. 1987 Nov 16;224(1):224–230. doi: 10.1016/0014-5793(87)80452-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Olefsky J. M. Long-term regulation of adipocyte glucose transport capacity by circulating insulin in rats. J Clin Invest. 1978 Jul;62(1):73–81. doi: 10.1172/JCI109116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolterman O. G., Prince M. J., Olefsky J. M. Insulin resistance in noninsulin-dependent diabetes mellitus: impact of sulfonylurea agents in vivo and in vitro. Am J Med. 1983 Jan 17;74(1A):82–101. doi: 10.1016/0002-9343(83)90655-1. [DOI] [PubMed] [Google Scholar]
- Kosaki A., Kuzuya H., Yoshimasa Y., Yamada K., Okamoto M., Nishimura H., Kakehi T., Takeda J., Seino Y., Imura H. Regulation of glucose-transporter gene expression by insulin in cultured human fibroblasts. Diabetes. 1988 Nov;37(11):1583–1586. doi: 10.2337/diab.37.11.1583. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Przybyla A. E., Chirgwin J. M. Isolation of RNA using guanidinium salts. Methods Enzymol. 1987;152:219–227. doi: 10.1016/0076-6879(87)52023-7. [DOI] [PubMed] [Google Scholar]
- Maloff B. L., Drake L., Riedy D. K., Lockwood D. H. Effects of sulfonylureas on the actions of insulin and insulin-mimickers: potentiation of stimulated hexose transport in adipocytes. Eur J Pharmacol. 1984 Sep 17;104(3-4):319–326. doi: 10.1016/0014-2999(84)90408-4. [DOI] [PubMed] [Google Scholar]
- Maloff B. L., Lockwood D. H. In vitro effects of a sulfonylurea on insulin action in adipocytes. Potentiation of insulin-stimulated hexose transport. J Clin Invest. 1981 Jul;68(1):85–90. doi: 10.1172/JCI110257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Prince M. J., Olefsky J. M. Direct in vitro effect of a sulfonylurea to increase human fibroblast insulin receptors. J Clin Invest. 1980 Sep;66(3):608–611. doi: 10.1172/JCI109894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M. D. Development of insulin responsiveness of the glucose transporter and the (Na+,K+)-adenosine triphosphatase during in vitro adipocyte differentiation. J Biol Chem. 1982 Jun 25;257(12):6978–6986. [PubMed] [Google Scholar]
- Rogers B. J., Standaert M. L., Pollet R. J. Direct effects of sulfonylurea agents on glucose transport in the BC3H-1 myocyte. Diabetes. 1987 Nov;36(11):1292–1296. doi: 10.2337/diab.36.11.1292. [DOI] [PubMed] [Google Scholar]
- Rosen O. M., Smith C. J., Fung C., Rubin C. S. Development of hormone receptors and hormone responsiveness in vitro. Effect of prolonged insulin treatment on hexose uptake in 3T3-L1 adipocytes. J Biol Chem. 1978 Oct 25;253(20):7579–7583. [PubMed] [Google Scholar]
- Rubin C. S., Hirsch A., Fung C., Rosen O. M. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem. 1978 Oct 25;253(20):7570–7578. [PubMed] [Google Scholar]
- Scarlett J. A., Kolterman O. G., Ciaraldi T. P., Kao M., Olefsky J. M. Insulin treatment reverses the postreceptor defect in adipocyte 3-O-methylglucose transport in type II diabetes mellitus. J Clin Endocrinol Metab. 1983 Jun;56(6):1195–1201. doi: 10.1210/jcem-56-6-1195. [DOI] [PubMed] [Google Scholar]
- Schmid P. C., Zuzarte-Augustin M. L., Schmid H. H. Properties of rat liver N-acylethanolamine amidohydrolase. J Biol Chem. 1985 Nov 15;260(26):14145–14149. [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneri R., Pezzino V., Wong K. Y., Goldfine I. D. Comparison of the in vitro effect of biguanides and sulfonylureas on insulin binding of its receptors in target cells. J Clin Endocrinol Metab. 1982 Jan;54(1):95–100. doi: 10.1210/jcem-54-1-95. [DOI] [PubMed] [Google Scholar]
- Wardzala L. J., Hirshman M., Pofcher E., Horton E. D., Mead P. M., Cushman S. W., Horton E. S. Regulation of glucose utilization in adipose cells and muscle after long-term experimental hyperinsulinemia in rats. J Clin Invest. 1985 Aug;76(2):460–469. doi: 10.1172/JCI111994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardzala L. J., Jeanrenaud B. Potential mechanism of insulin action on glucose transport in the isolated rat diaphragm. Apparent translocation of intracellular transport units to the plasma membrane. J Biol Chem. 1981 Jul 25;256(14):7090–7093. [PubMed] [Google Scholar]
- van Putten J. P., Krans H. M. Characterization of the sulfonylurea-induced potentiation of the insulin response in cultured 3T3 adipocytes. Biochem Pharmacol. 1986 Jul 1;35(13):2141–2144. doi: 10.1016/0006-2952(86)90583-6. [DOI] [PubMed] [Google Scholar]
- van Putten J. P., Wieringa T., Krans H. M. Long-term regulation of hexose transport by insulin in cultured mouse (3T3) adipocytes. Diabetologia. 1985 Jan;28(1):51–56. doi: 10.1007/BF00277000. [DOI] [PubMed] [Google Scholar]