Abstract
The role of neural stem cells (NSCs) in both the physiological and pathological processes in the brain has been refined through recent studies within the neuro-oncological field. Alterations in NSC regulatory mechanisms may be fundamental for the development and progression of malignant gliomas. A subpopulation of cells within the tumor known as brain tumor stem cells (BTSCs) have been shown to share key properties with NSCs. The BTSC hypothesis has significantly contributed to a potential understanding as to why brain tumors hold such dismal prognosis. On the other hand, the normal NSCs possess the capacity to migrate extensively towards the tumor bulk as well as to lingering neoplastic regions of the brain. The tropism of NSCs towards brain tumors may provide an additional tool for the treatment of brain cancer. The creation of potential therapies through the use of NSCs has been studied and includes the delivery of gene products to specific locations of the central nervous system selectively targeting malignant brain tumor cells and maximizing the efficiency of their delivery. Here, the proposed mechanisms of how brain tumors emerge, the molecular pathways interrupted in NSC pathogenesis and the most recent preclinical results in the use of NSCs for glioma treatment are reviewed.
Keywords: Neural stem cells, Brain tumors, Gliomagenesis, Glioma treatment, Brain tumor stem cells, Cell migration
INTRODUCTION
Malignant gliomas are the most common primary brain tumors in adult humans [1]. Despite advances in treatment including surgery, chemotherapy, radiotherapy and immunotherapy, patients with malignant glial tumors still have a very poor prognosis and a median survival of 14 months [1–4]. The failure of current treatments is also due to a lack of in-depth understanding of the cellular origin of brain tumors. Recent studies on human brain tumors show the co-existence of a heterogeneous population of cells [5–8]. A small subset of cells within this population known as brain tumor stem cells (BTSCs) are found to be resistant to current treatments and capable of maintaining and propagating these tumors [9–13]. BTSCs are thought to arise from aberrant neural stem cells (NSCs) or dedifferentiated mature cells that have undergone mutations [14–16]. BTSCs share characteristics with NSCs in terms of self-renewal capacity, multi-potentiality, tumorigenecity as well as migratory capabilities [15]. These characteristics favor the possibility that the transformation of NSCs and their progenitor cells may lead to the formation of BTSCs and subsequent tumors (Fig. 1) [17–19]. While the origin of BTSCs in brain tumors is still uncertain, NSC tropism towards brain tumors has led to the pursuit of viewing stem cells as promising vehicles for the tracking and suppression of malignant gliomas [20]. In this review, we discuss the potential role that NSCs play in the origin of brain cancer, the molecular pathways underlying the malignant transformation of NSCs into BTSCs as well as the prospective use of normal NSCs as delivery tools for therapeutic agents that may target brain tumors.
Fig. (1).
Normal NSCs self-renew and give rise to neural progenitor cells which differentiate into three neuronal lineages of the brain – neurons, astrocytes and oligodendrocytes (shown by black arrows). Epigenetic and/or genetic alterations in NSCs, progenitor cells, or mature cells through a dedifferentiation process may lead to transformation into BTSCs that are capable of self-renewing and of giving rise to brain tumors (shown by red arrows).
NEURAL STEM CELLS AND GLIOMAGENESIS
The discovery of BTSCs has led to the hypothesis that brain tumors may arise from aberrant NSCs. NSCs have been recognized as the most primordial cells of the nervous system possessing a self-renewing capacity of giving rise to neurons, astrocytes and oligodendroglia in the mature nervous system (Fig. 1) [21–23]. The subventricular zone (SVZ) in the rodent brain is the largest source of NSCs [21, 24]. The SVZ appears to be a vulnerable site for tumor formation, not only because it harbors potentially transformable NSCs, but also because it may provide a perivascular niche that supports tumorigenesis [16, 25, 26]. Clinical studies show that GBMs contacting the SVZ tend to be multifocal and show a higher recurrence incidence at locations distant to the initial lesion, whereas GBMs not involving the SVZ are less multifocal and their recurrence is confined to the resection margin [27]. Furthermore, patients with GBM lesions adjacent to a ventricle involving the SVZ are associated with increased tumor size and decreased survival [28, 29]. Experimental studies on animal models have shown evidence of brain tumors originating from NSCs in the SVZ [30] or from committed glial cells [31–35]. Nonetheless, the acquisition of oncogenic mutations that allow the committed cells to reprogram their self-renewal and proliferation capacities (i.e; “stem cell” characteristics) may also contribute to glioma formation [31–35].
Normal NSCs, which have a greater proliferative potential than their normal differentiated counterparts, may accumulate mutations at a low frequency, leading to cancer over time when exposed to genotoxic stresses [5, 36, 37]. Mutations in genes of normal NSCs cause abnormalities in cell cycle and growth factor regulated signaling pathways acting via receptor tyrosine kinases that lead to gliomagenesis [38]. These genetic changes in NSCs may result in tumorigenesis by activation of oncogenes and/or inactivation of tumor suppressor genes [39, 40]. The following sub-sections enumerate the findings of the epigenetic and/or genetic alterations in NSCs that may promote brain tumor formation.
EPIGENETIC MODIFICATIONS IN NSCS
Epigenetic alterations have been shown to change the expression of molecular targets without modifying their DNA sequence in brain tumors [41]. In particular, studies have shown how DNA methylation and histone modifications alter the gene expression of molecular targets resulting in the formation of tumors [39]. Cytosine methylation at CpG dinucleotides of a signal transducer and activator of transcription (STAT)-binding site results in the differentiation of normal NSCs to neurons, whereas demethylation at that site results in astrocytes [42, 43]. Abnormalities of DNA methylation due to alterations in DNA methyltransferase enzymes in NSCs have been associated with tumorigenesis [42]. Studies have demonstrated that DNA hypermethylation in CpG islands, the promoter region of tumor suppressor genes such as retinoblastoma (RB), tumor protein 53 (p53), phosphatase and tensin homolog deleted from chromosome 10 (PTEN), cyclin-dependent kinase 2 inhibitor (CDKN2/p16) and its alternative reading frame p14-Arf, and O6-methyl guanine methyltransferase (MGMT) promote the formation of brain tumors [41]. On the other hand, histone modifications caused by increased methylation or loss of acetylation in lysine residue of histone 3 lysine 9 (H3K9) in stem cells incline tumor suppressor genes to DNA hypermethylation and heritable gene silencing resulting in tumors [44]. Epigenetic silencing of the tumor suppressor gene lysine deficient protein kinase 2 (WNK2) has been shown to promote cell proliferation through activation of epidermal growth factor receptor (EGFR) [45, 46]. These studies suggest that epigenetic alterations in NSCs play a key role in the initial formation of gliomas. Reversing the promoter methylation of tumor suppressor genes or use of histone deacetylase inhibitors to reactivate the silenced genes may be useful for the treatment of brain tumors.
CHANGES WITHIN CELL CYCLE REGULATORY PATHWAYS IN NSCS AND CANCER-TARGETING THERAPIES
Alterations in cyclins and cyclin-dependent kinases (CDKs), p53 mutations and PTEN deletion are the most common changes in cell cycle regulation reported in astrocytomas [3, 47]. Deletions in CDK inhibitors (CDKIs), PTEN mutations and EGFR amplification are genetic alterations typical of primary GBMs, whereas early and frequent p53 mutations and G:C to A:T mutations at CpG sites are more common in secondary GBMs [47–49].
Cyclins and CDKs regulate the progression of cells through the cell cycle. CDKIs such as p16(INK4A), p18(INK4C), p19(INK4D), p21(WAF1/CIP1) and p27(KIP1), modulate the activity of cyclins and CDKs. Aberrant expression of cyclins and CDKs, and loss of CDKIs have been found in malignant astrocytomas [50]. Dedifferentiated astrocytes from Ink4a/Arf knockout mice have been shown to transform into a malignant phenotype with characteristic NSC markers and self-renewal activity [51]. Cyclin E overexpression in these Ink4a/Arf−/− astrocytes resulted in in vivo tumor formation [51]. These studies indicate that dedifferentiation of mature cells into stem cells may become cancerous through deregulated cell cycle.
p53 regulates the proliferation and differentiation of NSCs in SVZ [52]. Genetic mutations in p53 appear to be essential for tumor formation in mouse models [3, 53, 54]. They have been identified as one of the earliest transformations of NSCs into malignant astrocytoma [55–58]. Most recent studies have proved the accumulation of mutant p53 proteins in NSCs, where subsequent expansion of these mutant p53-expressing cells in the SVZ-associated areas initiates glioma formation [44, 59]. Further mutations in p53 and neurofibromatosis type I (NF1) in mice developed tumors in brain regions associated with the SVZ [60, 61]. Early tumor cells in these p53/NF1 mutant mice shared phenotypic characteristics with normal NSCs, and were highly infiltrative penetrating into white and gray matter [62]. Furthermore, inactivation of p53 followed by Ras activation via NF1 loss has been found to induce aggressive astrocytoma, suggesting a sequential timing within the occurrence of these genetic mutations and the transformation of NSCs into BTSCs [63].
PTEN is a tumor suppressor gene that antagonizes the phosphoinositide 3-kinase (PI3K) pathway and has been implicated in the normal development of the brain as well as in the molecular pathogenesis of human gliomas [64–66]. The SVZ-NSCs in PTEN conditional knockout mice show increased proliferation and self-renewal, decreased cell death, enhancement in G0 cell cycle exit, and growth factor independence that can initiate and sustain the malignant phenotype [66–68]. p53 and PTEN knockout mice show elevated levels of c-myc in NSCs affecting their self-renewal and differentiation potential and giving rise to brain tumors [69, 70].
These studies suggest that genetic changes in cell cycle regulatory proteins in dividing NSCs may lead to abnormal proliferation and result in malignant glioma formation. Current knowledge on cell cycle regulatory pathways in NSCs may be applied to target brain tumors by overexpressing or deleting the critical cell cycle pathway genes.
ALTERATIONS IN GROWTH FACTOR REGULATORY PATHWAYS VIA RECEPTOR TYROSINE KINASES (RTKS) IN NSCS AND CANCER-TARGETING THERAPIES
Constitutive activation of RTKs through growth factors such as epidermal growth factor (EGF) and platelet derived growth factor (PDGF) activate downstream signal transduction pathways including PI3k/AKT, RAS/MAP kinases, and phospholipase C and protein kinase C (PLC/PKC) pathways [49, 71–73]. These pathways regulate proliferation, migration and brain tumor formation.
EGFR is the most frequently activated receptor in brain tumors [74]. The amplification and over-expression of EGFR is present in ~50% of gliomas and results in the inhibition of apoptosis, and the promotion of proliferation, migration, and invasion of brain tumor cells [3, 49, 75–77]. In fact, tumor cells require EGFR for their maintenance and its inhibition has been found to suppress the proliferation and self-renewal of these cells [78]. EGFR stimulation in SVZ cells induces them to become highly proliferative and invasive, leading to periventricular tumor-like formations with broad cellular distribution in the adjacent parenchyma [79–81]. NSCs with PTEN loss and EGFR activation transform into tumors with increased cell proliferation, centrosome amplification, and resistance to oxidative stress as well as ionizing radiation [30]. Loss of Ink4a-Arf and PTEN tumor suppressor genes with concomitant activation of wild-type and/or mutant (vIII) EGFR in the adult mouse brain has been found to generate a highly invasive, rapid-onset high-grade malignant glioma phenotype with prominent pathological and molecular resemblance to GBM in humans [61].
As such the use of EGFR kinase inhibitors such as erlotinib and gefitinib has already been established in glioma treatment with significant success [82, 83]. In spite of these efforts, studies have shown that only 10–20 percent of these patients respond to EGFR kinase inhibitors [62]. This clinical responsiveness was associated with the co-expression of EGFRvIII and PTEN by glioma cells [62]. These results provide evidence of the importance of the EGFR/PTEN pathway as a determinant of GBM tumorigenesis.
PDGF has also been shown to play a major role in the abnormal proliferation and dispersion that drives the formation of malignant gliomas [84]. Low-grade gliomas possess over-expression of PDGF ligand and its receptor (PDGFR) as one of their earliest alterations [85]. PDGF stimulation in adult human and rodent SVZ cells formed atypical hyperplasias [86]. Exposure of myelinating oligodendrocyte precursor cells (OPC) to PDGF-B induced gliomas in mouse brain, indicating that the cell of origin for glioma may be a committed glial progenitor cell [87]. Further, these gliomas resembled human WHO grade II oligodendroglioma based on close similarities in histopathology and expression of cellular markers [87]. Activation of Ras and Akt through PDGF stimulation in NSCs but not in differentiated astrocytes induced tumors that resemble high-grade gliomas [88]. Autocrine stimulation of PDGF-B induced gliomas from nestin or glial fibrillary acidic protein (GFAP)-expressing cells in mouse brain model [53, 89]. PDGF-induced gliomas combined with Ink4a–Arf gene mutation shortened latency and enhanced malignancy of gliomas [53].
Selective interruption of signaling pathways which may be aberrant in brain tumor cells may provide more effective treatment of malignant gliomas [90]. The understanding of the critical pathways known to be involved in glioma formation such as growth signaling and cell cycle control is important for formulating targeted therapy in brain tumors.
CHANGES IN THE DEVELOPMENTAL SIGNALING PATHWAYS OF NSCS AND CANCER-TARGETING THERAPIES
In addition to alterations in cell cycle and growth factor signaling mechanisms, NSCs with changes in Notch and sonic hedgehog (Shh) developmental signaling pathways also appear to give rise to brain tumors.
Recent evidence has shown that the Notch cascade plays a prominent role in the development of stem cells and in neural stem cell regulation [91]. Notch 1 is overexpressed primarily in human gliomas, suggesting its possible contribution to tumor formation [92–95]. The presence of Notch signaling within tumors has also been linked to predictive values representing a hallmark of poor prognosis [96]. Further, Notch blockade in medulloblastoma and glioma cell lines induced apoptosis, differentiation and depletion of BTSCs as well as decreased proliferation [92, 93]. These studies point towards the importance of the Notch signaling pathway within glial tumor formation and how the inhibition of Notch-1 and its ligands could serve as a potential therapeutic target in the treatment of malignant gliomas.
The Shh pathway has been shown to play a fundamental role during the development of the central nervous system and in the survival and proliferation of NSCs [97, 98]. The Shh pathway has been linked to the modulation of NSC proliferation, survival, self-renewal and tumorigenicity. Blockade of this pathway through the aggregation of cyclopamine exhibited synergistic therapeutic effects with temozolamide resulting in the depletion of the brain tumor cell population [99, 100]. Brain tumor cells, like postnatal NSCs are synchronized by the Shh-Gli pathway [101]. However, only normal stem cells exhibit proliferative restrictions and apoptosis with overexpression of Shh as opposed to the tumor cells [101]. Clinically, these findings are of extreme significance as they provide differences between these two cell populations that can be used to devise therapies targeted exclusively to eliminate brain tumor cells without damaging normal NSCs.
While more studies have been underway to confirm the NSC population as the origin of brain tumors, interest has also grown in the potential use of NSCs as an alternate treatment modality to target brain tumors due to their migratory properties.
NEURAL STEM CELL TROPISM TOWARDS BRAIN TUMORS AND USE AS DELIVERY VEHICLES FOR GLIOMA TREATMENT
The capacity of NSCs to mobilize and juxtapose themselves along aggressively advancing tumor cells has been observed by several groups [102–104]. It has been shown that NSCs exhibit extensive tropism toward the site of the tumor and infiltrate tumor foci when implanted intraventricularly, intracranially within normal tissue or into the contralateral hemisphere, as well as through the peripheral intravascular circulation [102]. NSC movement towards the brain tumor was equally pronounced irrespective of the initial implantation site [102]. Glass et al. demonstrated migration of endogenous neural precursors from the SVZ towards a tumor mass and surrounding the bulk of the tumor in an established intracranial glioma mouse model [105]. Furthermore, endogenous neural precursor cells did not manifest a pathotropic movement when implanted with other non-neoplastic lesions suggesting a specific tropism to the brain tumors [105]. In addition, studies have proposed that NSCs may possess a natural ability to suppress tumor growth [105]. Unmodified NSCs co-cultured with GBM cells showed both a suppression of tumor growth and an induction of tumor cell apoptosis in mice, improving their survival [105]. Previous studies have provided evidence of NSCs' anti-tumor activity and migration towards tumor cells [106, 107]. Furthermore, exogenously administered unmodified NSCs may independently inhibit glioma formation in vivo, re-iterating the notion that they may possess inherent oncostatic action [108].
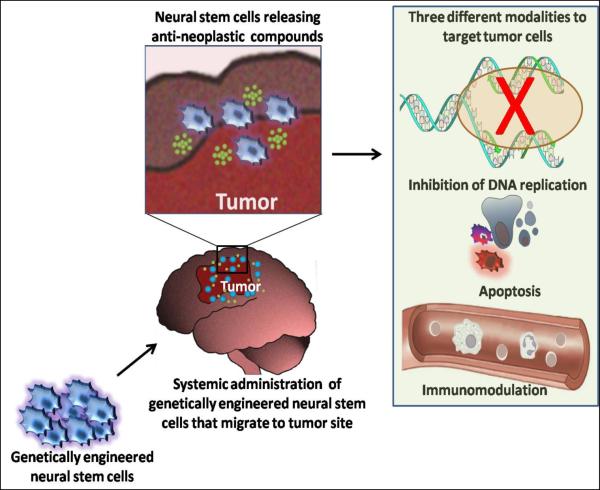
By genetically modifying NSCs to secrete anti-neoplastic compounds it may be possible to achieve high local drug levels at the site of the malignancy (Fig. 2) [103, 108, 109]. Anti-neoplastic compounds that have been evaluated in such a manner fall into three categories: pro-drug converting enzymes, viral vectors and immune response modulators (Table 1).
Fig. (2).
Systemic injections of genetically engineered NSCs migrate to the tumor site and secrete anti-neoplastic compounds such as pro-drug activating enzymes, viral vectors and immune response modulators. These compounds may target brain tumors by blocking DNA replication, by boosting an immune response or by inducing apoptosis of tumor cells.
Table 1.
Neural Stem Cell Mediated Strategies for Glioma Therapy
| Factors delivered | Administration route | Source of NSCs | Result | |
|---|---|---|---|---|
| Pro-drug converting enzymes | Cytosine deaminasea | Intracranial, intravascular | NSC clonal line C17.2 | Reduction in tumor burden |
| HSV- thymidine kinaseb | Intracranial, intratumoral | Embryonic rat NSCs | Increased survival time | |
|
| ||||
| Viral vectors | Replication-conditional HSV-1 vectorc | Intratumoral | NSC clonal line C17.2 | Effective delivery of mutant HSV-1 vectors |
| Adenoviral vectord | Intracranial | Embryonic stem cell derived NSCs | Effective delivery of adenoviral vector | |
|
| ||||
| Immune response modulators | IL-4e | Intracranial, intratumoral | Mouse neural progenitor cells | Increased survival time |
| IL-12f | Intracranial | Embryonic murine NSCs | Increased survival time | |
| TRAILg | Intratumoral | Embryonic murine NSCs | Induction of apoptosis in tumors and inhibition of tumor growth | |
[102] Aboody, K.S., Brown, A.; Rainov, N.G.; Bower, K.A.; Liu, S.; Yang, W.; Small, J.E.; Herrlinger, U.; Ourednik, V.; Black, P.M.; Breakefield, X.O.; Snyder, E.Y., Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc. Natl. Acad. Sci. U.S.A., 2000, 12846–12851, [109] Barresi, V.; Belluardo, N.; Sipione, S.; Mudò, G.; Cattaneo, E.; Condorelli, D.F. Transplantation of prodrug-converting neural progenitor cells for brain tumor therapy. Cancer Gene Ther., 2003, 5(10), 396–402.
[111] Li, S., Tokuyama, T.; Yamamoto, J.; Koide, M.; Yokota, N.; Namba, H. Bystander effect-mediated gene therapy of gliomas using genetically engineered neural stem cells. Cancer Gene Ther., 2005, 7(12), 600–607.
[113] Herrlinger, U.; Woiciechowski, C.; Sena-Esteves, M.; Aboody, K.S.; Jacobs, A.H.; Rainov, N.G.; Snyder, E.Y.; Breakefield, X.O. Neural precursor cells for delivery of replication-conditional HSV-1 vectors to intracerebral gliomas. Mol. Ther., 2000, 4(1), 347–357.,
[117] Arnhold, S.; Hilgers, M.; Lenartz, D.; Semkova, I.; Kochanek, S.; Voges, J.; Andressen, C.; Addicks, K., Neural precursor cells as carriers for a gene therapeutical approach in tumor therapy. Cell Transplant, 2003, 8(12), 827–837.
[108] Benedetti, S., Pirola, B.; Pollo, B.; Magrassi, L.; Bruzzone, M.G.; Rigamonti, D.; Galli, R.; Selleri, S.; Di Meco, F.; De Fraja, C.; Vescovi, A.; Cattaneo, E.; Finocchiaro, G. Gene therapy of experimental brain tumors using neural progenitor cells. Nat. Med., 2000, 4(6), 447–450.
[119] Ehtesham M, Kabos, P.; Kabosova, A.; Neuman, T.; Black, K.L.; Yu, J.S. The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res., 2002, 20(62), 5657–5663.
[124] Ehtesham, M.; Kabos, P.; Gutierrez, M.A.; Chung, N.H.; Griffith, T.S.; Black, K.L.; Yu, J.S. Indcution of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis-inducing ligand. Ibid.24, 7170–7174.
PRO-DRUG AND VIRAL VECTOR DELIVERY VEHICLES FOR GLIOMA TREATMENT
One way that NSCs have been used as therapeutic vehicles has been through the incorporation of pro-drug activating enzymes [103, 109]. These enzymes convert systemically administered pro-drugs into toxic metabolites that then selectively target glioma cells. As such, stem cells are capable of enhancing an inherently low toxic pro-drug into a toxic which can kill tumor cells within their surroundings [110]. Two such systems have been tested using NSCs: cytosine deaminase (CD) [103] and the herpes simplex virus thymidine kinase (HSV-tk) ganciclovir system [111]. A third potential pro-drug system not yet assessed in stem cell therapies includes the delivery of deoxycytidine kinase [112].
The use of CD was effectively demonstrated by intracranially inoculating tumor-bearing mice with CD-expressing NSCs [103]. The CD within the NSCs allowed for the conversion of systemically administered nontoxic pro-drug 5-fluorocytosine (5-FC) to the cytotoxic 5-fluoracil (5-FU) [103]. 5-FU, a chemotherapeutic agent, caused DNA chain termination and subsequent chain death in neoplastic cells [103]. High concentrations of these CD-expressing NSCs were confined in close proximity to the tumor cells, resulting in a significant reduction of tumor burden and increased survival benefit compared to the controls [103]. A significant decrease in the tumor mass of glioma bearing rats with transplanted CD-expressing neural progenitor cells has also been corroborated [109]. Significant tumor regression and prolonged survival has been demonstrated in animals exposed to the herpes simplex virus thymidine kinase (HSV-tk) ganciclovir system [111]. Genetically transduced HSV-Tk-NSCs were injected into tumor-bearing rats followed by systemic ganciclovir treatment [111]. This allowed for the phosphorylation of the systemically processed ganciclovir and subsequent restriction of DNA replication within tumor cells [111].
The HSV-Tk system has also been transported by NSCs to serve as viral vector replication factories, transducing oncolytic genes within the tumor cell population [20, 113]. This type of viral-mediated gene delivery has clinically failed in the past due to the low transduction efficacy that is obtained when the virus is directly injected into the tumor bulk [114–116]. However, by harnessing the migratory capacity of NSCs, a more extensive delivery of the virus may be achieved [20]. In 2000, Herrlinger et al. engineered replication-conditional HSV type 1 vector-bearing NSCs capable of multiplying in rapidly proliferating cell populations, such as the one present within the tumor environment [113]. Effective surrounding of both the tumor and escaping tumor outgrowths by replication-conditional HSV-1 NSCs was observed in nude mice harboring intracranial gliomas [113]. Later studies also demonstrated the efficacy of NSCs as viral packaging lines by transducing precursor cells with a high capacity HC-AdFK7 adenoviral vector [117]. These studies provide evidence of how NSCs can be manipulated to deliver pro-drug activating enzymes as well as viral vectors to the tumor site representing an alternative approach for the treatment of brain tumors.
IMMUNE RESPONSE MODULATORS FOR GLIOMA TREATMENT
NSC mediated glioma therapy can also be used to modulate the host immune response against brain tumors via direct release of cytokines to the tumor tissue. NSC-mediated modulation of immune response has the ability to target independent neoplastic pockets by boosting tumoricidal cell-mediated immunity against tumor-specific antigens [118–120]. Benedetti's group reported that NSCs engineered to express the immunomodulatory cytokine interleukin 4 (IL-4) extended the survival of glioblastoma-bearing rats [108]. IL-4 has been shown to facilitate an immune response against glioblastoma tumor cells by increasing the recruitable precursor T-cell frequency [121]. Ehthesham et al. generated interleukin 12-expressing (IL-12) NSCs through the use of a replication-defective adenoviral vector and implanted them into an established intracranial murine glioma resulting in robust T-cell aggregation along the tumor/normal tissue boundary as well as within tumor microsatellites [119]. Intracranial glioma-bearing mice treated with the tumor-tropic IL-12 cells showed a reduction in tumor burden and improved survival [119]. IL-12 induces cellular immunity through the production and proliferation of interferon gamma (IFN-gamma) as well as through the induction of the cytolytic activity of natural killer cells and T-cells [122]. Localized induction of tumor immunity with IL-12 has also been observed by other groups [120, 123]. The specific homing ability of NSCs allows them to achieve a high concentration of therapeutic compounds within the tumor populated areas. This is further exploited in the delivery of tumor necrosis factor-related apoptosis inducing ligand (TRAIL) [124]. This pro-apoptotic protein belongs to the tumor necrosis factor family and induces its effects by binding to its death domain receptor DR4 and DR5 [125]. TRAIL has been shown to have powerful anticancer activity without exhibiting significant toxicity toward normal tissue [126, 127]. By inoculating TRAIL-secreting NSCs in athymic T-cell incompetent mice, bearing intracranial human glioblastoma xenografts, a dramatic induction of apoptosis in treated tumors and tumor satellites as well a significant inhibition of tumor growth was documented [119]. The use of a secreted form, S-TRAIL, has also been combined with alternate therapeutic modalities such as down-regulation of Bcl2 through the use of short hairpin RNA (shRNA) or inhibition of micro-RNA-21 in order to enhance the eradication of gliomas [128, 129]. The effective targeting of S-TRAIL has also resulted in dramatic reduction of glioma burden in mouse models [130]. Furthermore, Hingten et al. have combined S-TRAIL with the chemotherapeutic agent temozolomide and observed increased tumor cell death, upregulation of proapoptotic proteins, and attenuation of tumor progression in glioma cell lines that had previously exhibited resistance to TRAIL monotherapy [131]. The above discussed findings present promising data on the potential use of NSCs as vehicles for the delivery of immune system modulators such as IL-4, IL-12 as well as TRAIL and S-TRAIL to treat cancer cells.
NEURAL STEM CELLS AS IMAGING TOOLS FOR TUMOR LOCALIZATION
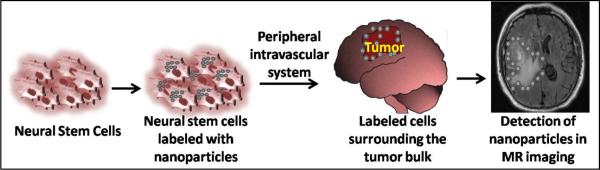
Exploiting the tendency of NSCs to track tumor bulk and outgrowths allows them to be labeled and used as diagnostic imaging tools that can serve to identify the extent of tumor infiltration into normal brain parenchyma as well as the exact positioning of the therapeutic stem cells (Fig. 3). Lewin et al. exploited the sensitivity of magnetic resonance imaging (MRI) to detect ferromagnetic particles conjugated via tat-peptide-derivitisation into hematopoietic and neural progenitor cells [132]. In 2004, MRI was utilized to noninvasively track NSCs and bone marrow stem cells labeled with lipophilic dye-coated superparamagnetic particles and injected to rats via the cisterna magna and a tail vein, respectively [123]. Migration of both NSCs and bone marrow stem cell populations towards the tumor mass and the infiltrating tumor cells was observed [123]. Previously, studies were also able to explore the macroscopic migratory capabilities of NSCs through the transfection of NPCs with the firefly luiferase gene [133]. Studies of implantion of NSCs expressing luciferase into glioma-bearing mice through intracranial, intravenous, intraperitoneal and intraventricular routes have found that the intraventricular path allows for a greater number of NSCs to migrate to the brain tumor [133]. These luciferase-bearing NPCs were later engineered to deliver S-TRAIL, allowing for the simultaneous monitoring of the migration of NSCs and their delivery of the tumoricidal agent to glioma cells [130]. Several groups have also presented the use of semiconductor quantum dots (fluorescent nanoparticles) as an alternative to luciferase expression in labeling NSCs [134, 135]. In particular, it was demonstrated that mammalian neural and progenitor stem cells could be effectively tagged with these dots [136]. Metal nanoshells represent a novel class of clinically promising nanoparticles with both diagnostic and therapeutic capabilities [137, 138]. This technology was used to design immunotargeted nanoshells that could both scatter light, enabling optical molecular cancer imaging, and absorb light, allowing for selective destruction of targeted carcinoma cells through photothermal therapy [138]. These studies provide important tools to further evaluate the temporospatial migration of NPCs, their location, differentiation fate and cell distribution within the intracranial tumor [139]. More importantly, these tools allow for a non-invasive location surveillance of exogenously implanted NSCs at a single cell level permitting both quantitative and qualitative observations on a high resolution scale, granting the clinicians complete visualization of these cells.
Fig. (3).
Intravascular injection in glioma patients of NSCs labeled with nanoparticles in glioma patients are capable of migrating to the site of tumor. These cells can be visualized by MRI and may potentially be used to demarcate the periphery of the tumor. This allows not only for better distinction of the tumor margins but also provides surveillance of the engineered NSCs that are being used to deliver a given therapeutic agent.
CONCLUSIONS
The identification of a tumor-initiating subpopulation of cells will represent a significant improvement in the understanding of human brain tumor formation and its progression. The resistance of brain tumors to clinical treatment has encouraged researchers to consider the tumor-initiating population in the development of therapeutic strategies against gliomas. Moreover, the use of NSCs as vehicles for the delivery of therapeutic genes represents a critical highlight in the treatment of malignant brain tumors. Nevertheless, further studies are required to exploit the endogenous migratory ability of NSCs. A complete understanding of the molecular signaling and interactions that promote NSC tropism towards brain tumors is needed. This will help us gain the necessary insight to engineer NSCs accordingly, increasing their tumor-toxic effect and creating the optimal scenario for their use as treatment delivery vehicles. In the future, NSCs as delivery tools can be further exploited by also taking advantage of the imaging modality they may offer providing physicians with radiological localization of NSCs as they distribute medicinal agents.
However, additional research on NSCs must be performed before any potential translation into the clinical scenario is explored. A number of concerns involving stem cell-based therapy exist and call for further understanding of the biology and safety of stem cells. The role of NSCs within the tumor microenvironment and specifically whether or not they are recruited to form part of the actual tumor must be determined. The development, characterization and isolation of reliable cell lines as probable sources for the use of such therapy are indispensable for the prospective improvement of this therapeutic alternative. A continued study of both the NSCs and tumor-initiating cell populations will certainly provide promising avenues for the formulation of more effective and specific treatment strategies offering new hope for patients in the future.
ACKNOWLEDGMENTS
This work is supported by National Institutes of Health (NIH) grant NIH-K08NS05585, Howard Hughes Medical Institutions - 57005932, Robert Wood Johnson - 63519, Maryland Stem Cell Technology Development Corporation (Tedco)-2007-MSCRFE-0139-00 (Alfredo Quinones-Hinojosa) and Howard Hughes Medical Student Fellowship Grant (Neda Isabel Sedora-Roman). We acknowledge Drs. Hugo Guerrero-Cázares and Tomás Garzon-Muvdi and David Purger for critical comments of the review and artwork. We apologize to authors whose work is not cited in this review because of space constraints.
REFERENCES
- [1].CBTRUS . Primary Brain Tumors in the United States. Central Brain Tumor Registry of the United States; 2007–2008. [Google Scholar]
- [2].Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. (Berl) 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15(11):1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- [4].Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- [5].Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- [6].Quinones-Hinojosa A, Sanai N, Smith JS, McDermott MW. Techniques to assess the proliferative potential of brain tumors. J. Neurooncol. 2005;74(1):19–30. doi: 10.1007/s11060-004-5758-0. [DOI] [PubMed] [Google Scholar]
- [7].Singh S, Dirks PB. Brain tumor stem cells: identification and concepts. Neurosurg. Clin. N. Am. 2007;18(1):31–38. viii. doi: 10.1016/j.nec.2006.10.014. [DOI] [PubMed] [Google Scholar]
- [8].Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat. Rev. Cancer. 2006;6(6):425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- [9].Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64(19):7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- [10].Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39(3):193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- [11].Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- [12].Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- [13].Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board J, Macdonald T, Rutka J, Guha A, Gajjar A, Curran T, Gilbertson RJ. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8(4):323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- [14].Chaichana KL, Guerrero-Cazares H, Capilla-Gonzalez V, Zamora-Berridi G, Achanta P, Gonzalez-Perez O, Jallo GI, Garcia-Verdugo JM, Quinones-Hinojosa A. Intra-operatively obtained human tissue: protocols and techniques for the study of neural stem cells. J. Neurosci. Methods. 2009;180(1):116–125. doi: 10.1016/j.jneumeth.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Quinones-Hinojosa A, Chaichana K. The human subventricular zone: a source of new cells and a potential source of brain tumors. Exp. Neurol. 2007;205(2):313–324. doi: 10.1016/j.expneurol.2007.03.016. [DOI] [PubMed] [Google Scholar]
- [16].Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- [17].Hadjipanayis CG, Van Meir EG. Tumor initiating cells in malignant gliomas: biology and implications for therapy. J. Mol. Med. 2009;87(4):363–374. doi: 10.1007/s00109-009-0440-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat. Rev. Cancer. 2003;3(12):895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- [19].Dietrich J, Imitola J, Kesari S. Mechanisms of Disease: the role of stem cells in the biology and treatment of gliomas. Nat. Clin. Pract. Oncol. 2008;5(7):393–404. doi: 10.1038/ncponc1132. [DOI] [PubMed] [Google Scholar]
- [20].Yip S, Sidman RL, Snyder EY. Neural stem cells as novel cancer therapeutic vehicles. Eur. J. Cancer. 2006;9(42):1298–1308. doi: 10.1016/j.ejca.2006.01.046. [DOI] [PubMed] [Google Scholar]
- [21].Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- [22].Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427(6976):740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- [23].Yu JJ, Sun X, Yuan X, Lee JW, Snyder EY, Yu JS. Immunomodulatory neural stem cells for brain tumour therapy. Expert Opin. Biol. Ther. 2006;6(12):1255–1262. doi: 10.1517/14712598.6.12.1255. [DOI] [PubMed] [Google Scholar]
- [24].Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3(3):265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66(16):7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- [26].Gilbertson RJ, Rich JN. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat. Rev. Cancer. 2007;7(10):733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- [27].Balordi F, Fishell G. Mosaic removal of hedgehog signaling in the adult SVZ reveals that the residual wild-type stem cells have a limited capacity for self-renewal. J. Neurosci. 2007;27(52):14248–14259. doi: 10.1523/JNEUROSCI.4531-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chaichana KL, McGirt MJ, Frazier J, Attenello F, Guerrero-Cazares H, Quinones-Hinojosa A. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J. Neurooncol. 2008;89(2):219–224. doi: 10.1007/s11060-008-9609-2. [DOI] [PubMed] [Google Scholar]
- [29].Mineo JF, Bordron A, Baroncini M, Ramirez C, Maurage CA, Blond S, Dam-Hieu P. Prognosis factors of survival time in patients with glioblastoma multiforme: a multivariate analysis of 340 patients. Acta Neurochir. (Wien) 2007;149(3):245–253. doi: 10.1007/s00701-006-1092-y. [DOI] [PubMed] [Google Scholar]
- [30].Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, Alvarez-Buylla A, Parada LF. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15(1):45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, Rowitch DH, Louis DN, DePinho RA. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1(3):269–277. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- [32].Holland EC, Hively WP, DePinho RA, Varmus HE. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 1998;12:3675–3685. doi: 10.1101/gad.12.23.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rich JN, Guo C, McLendon RE, Bigner DD, Wang XF, Counter CM. A genetically tractable model of human glioma formation. Cancer Res. 2001;61(9):3556–3560. [PubMed] [Google Scholar]
- [34].Xiao A, Yin C, Yang C, Di Cristofano A, Pandolfi PP, Van Dyke T. Somatic induction of Pten loss in a preclinical astrocytoma model reveals major roles in disease progression and avenues for target discovery and validation. Cancer Res. 2005;65:5172–5180. doi: 10.1158/0008-5472.CAN-04-3902. [DOI] [PubMed] [Google Scholar]
- [35].Assanah M, Lochhead R, Ogden A, Bruce J, Goldman J, Canoll P. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J. Neurosci. 2006;26(25):6781–6790. doi: 10.1523/JNEUROSCI.0514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu. Rev. Cell Dev. Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- [37].Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425(6961):968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- [38].Konopka G, Bonni A. Signaling pathways regulating gliomagenesis. Curr. Mol. Med. 2003;3(1):73–84. doi: 10.2174/1566524033361609. [DOI] [PubMed] [Google Scholar]
- [39].Nagarajan RP, Costello JF. Molecular epigenetics and genetics in neuro-oncology. Neurotherapeutics. 2009;6(3):436–446. doi: 10.1016/j.nurt.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ohgaki H, Kleihues P. Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci. 2009 doi: 10.1111/j.1349-7006.2009.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nagarajan RP, Costello JF. Epigenetic mechanisms in glioblastoma multiforme. Semin. Cancer Biol. 2009;19(3):188–197. doi: 10.1016/j.semcancer.2009.02.005. [DOI] [PubMed] [Google Scholar]
- [42].Kohyama J, Kojima T, Takatsuka E, Yamashita T, Namiki J, Hsieh J, Gage FH, Namihira M, Okano H, Sawamoto K, Nakashima K. Epigenetic regulation of neural cell differentiation plasticity in the adult mammalian brain. Proc. Natl. Acad. Sci. U.S.A. 2008;105(46):18012–18017. doi: 10.1073/pnas.0808417105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hsieh J, Gage FH. Epigenetic control of neural stem cell fate. Curr. Opin. Genet. Dev. 2004;14(5):461–469. doi: 10.1016/j.gde.2004.07.006. [DOI] [PubMed] [Google Scholar]
- [44].Ohm JE, McGarvey KM, Yu X, Cheng L, Schuebel KE, Cope L, Mohammad HP, Chen W, Daniel VC, Yu W, Berman DM, Jenuwein T, Pruitt K, Sharkis SJ, Watkins DN, Herman JG, Baylin SB. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat. Genet. 2007;39(2):237–242. doi: 10.1038/ng1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dey N, Crosswell HE, De P, Parsons R, Peng Q, Su JD, Durden DL. The protein phosphatase activity of PTEN regulates SRC family kinases and controls glioma migration. Cancer Res. 2008;68(6):1862–1871. doi: 10.1158/0008-5472.CAN-07-1182. [DOI] [PubMed] [Google Scholar]
- [46].Moniz S, Verissimo F, Matos P, Brazao R, Silva E, Kotelevets L, Chastre E, Gespach C, Jordan P. Protein kinase WNK2 inhibits cell proliferation by negatively modulating the activation of MEK1/ERK1/2. Oncogene. 2007;26(41):6071–6081. doi: 10.1038/sj.onc.1210706. [DOI] [PubMed] [Google Scholar]
- [47].Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- [48].Ohgaki H. Genetic pathways to glioblastomas. Neuropathology. 2005;25(1):1–7. doi: 10.1111/j.1440-1789.2004.00600.x. [DOI] [PubMed] [Google Scholar]
- [49].Moriya S, Kazlauskas A, Akimoto K, Hirai S, Mizuno K, Takenawa T, Fukui Y, Watanabe Y, Ozaki S, Ohno S. Platelet-derived growth factor activates protein kinase C epsilon through redundant and independent signaling pathways involving phospholipase C gamma or phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. U.S.A. 1996;93(1):151–155. doi: 10.1073/pnas.93.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Dirks PB, Rutka JT. Current concepts in neuro-oncology: the cell cycle--a review. Neurosurgery. 1997;40(5):1000–13. doi: 10.1097/00006123-199705000-00025. discussion 1013–1015. [DOI] [PubMed] [Google Scholar]
- [51].Jeon HM, Jin X, Lee JS, Oh SY, Sohn YW, Park HJ, Joo KM, Park WY, Nam DH, DePinho RA, Chin L, Kim H. Inhibitor of differentiation 4 drives brain tumor-initiating cell genesis through cyclin E and notch signaling. Genes Dev. 2008;22(15):2028–2033. doi: 10.1101/gad.1668708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Armesilla-Diaz A, Bragado P, Del Valle I, Cuevas E, Lazaro I, Martin C, Cigudosa JC, Silva A. p53 regulates the self-renewal and differentiation of neural precursors. Neuroscience. 2009;158(4):1378–1389. doi: 10.1016/j.neuroscience.2008.10.052. [DOI] [PubMed] [Google Scholar]
- [53].Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15(15):1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhu Y, Parada LF. The molecular and genetic basis of neurological tumours. Nat. Rev. Cancer. 2002;2(8):616–626. doi: 10.1038/nrc866. [DOI] [PubMed] [Google Scholar]
- [55].Louis DN, von Deimling A, Chung RY, Rubio MP, Whaley JM, Eibl RH, Ohgaki H, Wiestler OD, Thor AD, Seizinger BR. Comparative study of p53 gene and protein alterations in human astrocytic tumors. J. Neuropathol. Exp. Neurol. 1993;52(1):31–38. doi: 10.1097/00005072-199301000-00005. [DOI] [PubMed] [Google Scholar]
- [56].Rasheed BK, McLendon RE, Herndon JE, Friedman HS, Friedman AH, Bigner DD, Bigner SH. Alterations of the TP53 gene in human gliomas. Cancer Res. 1994;54(5):1324–1330. [PubMed] [Google Scholar]
- [57].von Deimling A, Eibl RH, Ohgaki H, Louis DN, von Ammon K, Petersen I, Kleihues P, Chung RY, Wiestler OD, Seizinger BR. p53 mutations are associated with 17p allelic loss in grade II and grade III astrocytoma. Cancer Res. 1992;52(10):2987–2990. [PubMed] [Google Scholar]
- [58].van Meyel DJ, Ramsay DA, Casson AG, Keeney M, Chambers AF, Cairncross JG. p53 mutation, expression, and DNA ploidy in evolving gliomas: evidence for two pathways of progression. J. Natl. Cancer Inst. 1994;86(13):1011–1017. doi: 10.1093/jnci/86.13.1011. [DOI] [PubMed] [Google Scholar]
- [59].Gil-Perotin S, Marin-Husstege M, Li J, Soriano-Navarro M, Zindy F, Roussel MF, Garcia-Verdugo JM, Casaccia-Bonnefil P. Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors. J. Neurosci. 2006;26(4):1107–1116. doi: 10.1523/JNEUROSCI.3970-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Reilly KM, Loisel DA, Bronson RT, McLaughlin ME, Jacks T. Nf1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects. Nat. Genet. 2000;26(1):109–113. doi: 10.1038/79075. [DOI] [PubMed] [Google Scholar]
- [61].Zhu H, Acquaviva J, Ramachandran P, Boskovitz A, Woolfenden S, Pfannl R, Bronson RT, Chen JW, Weissleder R, Housman DE, Charest A. Oncogenic EGFR signaling cooperates with loss of tumor suppressor gene functions in glioma-genesis. Proc. Natl. Acad. Sci. U.S.A. 2009;106(8):2712–2716. doi: 10.1073/pnas.0813314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Liau LM, Cavenee WK, Rao PN, Beroukhim R, Peck TC, Lee JC, Sellers WR, Stokoe D, Prados M, Cloughesy TF, Sawyers CL, Mischel PS. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 2005;19(353):2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- [63].Zhu Y, Guignard F, Zhao D, Liu L, Burns DK, Mason RP, Messing A, Parada LF. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005;8(2):119–130. doi: 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Stiles B, Groszer M, Wang S, Jiao J, Wu H. PTENless means more. Dev. Biol. 2004;2(273):175–184. doi: 10.1016/j.ydbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- [65].van Diepen MT, Eickholt EB. Function of PTEN during the formation and maintenance of neuronal circuits in the brain. Dev. Neurosci. 2008;1–3(80):59–64. doi: 10.1159/000109852. [DOI] [PubMed] [Google Scholar]
- [66].Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294(5549):2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- [67].Groszer M, Erickson R, Scripture-Adams DD, Dougherty JD, Le Belle J, Zack JA, Geschwind DH, Liu X, Kornblum HI, Wu H. PTEN negatively regulates neural stem cell self-renewal by modulating G0-G1 cell cycle entry. Proc. Natl. Acad. Sci. U.S.A. 2006;103(1):111–116. doi: 10.1073/pnas.0509939103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Gregorian C, Nakashima J, Le Belle J, Ohab J, Kim R, Liu A, Smith KB, Groszer M, Garcia AD, Sofroniew MV, Carmichael ST, Kornblum HI, Liu X, Wu H. Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J. Neurosci. 2009;29(6):1874–1886. doi: 10.1523/JNEUROSCI.3095-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z, Stommel JM, Dunn KL, Wiedemeyer R, You MJ, Brennan C, Wang YA, Ligon KL, Wong WH, Chin L, DePinho RA. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455(7216):1129–1133. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z, Stommel JM, Dunn KL, Wiedemeyer R, You MJ, Brennan C, Wang YA, Ligon KL, Wong WH, Chin L, dePinho RA. Pten and p53 converge on c-Myc to control differentiation, self-renewal, and transformation of normal and neoplastic stem cells in glioblastoma. Cold Spring Harb. Symp. Quant. Biol. 2008;73:427–437. doi: 10.1101/sqb.2008.73.047. [DOI] [PubMed] [Google Scholar]
- [71].de Vries-Smits AM, Burgering BM, Leevers SJ, Marshall CJ, Bos JL. Involvement of p21ras in activation of extracellular signal-regulated kinase 2. Nature. 1992;357(6379):602–604. doi: 10.1038/357602a0. [DOI] [PubMed] [Google Scholar]
- [72].Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81(5):727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- [73].Koul D. PTEN signaling pathways in glioblastoma. Cancer Biol. Ther. 2008;7(9):1321–1325. doi: 10.4161/cbt.7.9.6954. [DOI] [PubMed] [Google Scholar]
- [74].Cancer genome atlas research network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Shinojima N, Tada K, Shiraishi S, Kamiryo T, Kochi M, Nakamura H, Makino K, Saya H, Hirano H, Kuratsu J, Oka K, Ishimaru Y, Ushio Y. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63(20):6962–6970. [PubMed] [Google Scholar]
- [76].Lund-Johansen M, Bjerkvig R, Humphrey PA, Bigner SH, Bigner DD, Laerum OD. Effect of epidermal growth factor on glioma cell growth, migration, and invasion in vitro. Cancer Res. 1990;50(18):6039–6044. [PubMed] [Google Scholar]
- [77].Wechsler-Reya R, Scott MP. The developmental biology of brain tumors. Annu. Rev. Neurosci. 2001;24:385–428. doi: 10.1146/annurev.neuro.24.1.385. [DOI] [PubMed] [Google Scholar]
- [78].Soeda A, Inagaki A, Oka N, Ikegame Y, Aoki H, Yoshimura S, Nakashima S, Kunisada T, Iwama T. Epidermal growth factor plays a crucial role in mitogenic regulation of human brain tumor stem cells. J. Biol. Chem. 2008;16(283):10958–10966. doi: 10.1074/jbc.M704205200. [DOI] [PubMed] [Google Scholar]
- [79].Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J. Neurosci. 2002;22(3):629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J. Neurosci. 1997;17(15):5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kim SU, Park IH, Kim TH, Kim KS, Choi HB, Hong SH, Bang JH, Lee MA, Joo IS, Lee CS, Kim YS. Brain transplantation of human neural stem cells transduced with tyrosine hydroxylase and GTP cyclohydrolase 1 provides functional improvement in animal models of Parkinson disease. Neuropathology. 2006;26(2):129–140. doi: 10.1111/j.1440-1789.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- [82].Prados MD, Lamborn KR, Chang S, Burton E, Butowski N, Malec M, Kapadia A, Rabbitt J, Page MS, Fedoroff A, Xie D, Kelley SK. Phase 1 study of erlotinib HCl alone and combined with temozolomide in patients with stable or recurrent malignant glioma. Neuro. Oncol. 2006;1(8):67–78. doi: 10.1215/S1522851705000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Rich JN, Reardon DA, Peery T, Dowell JM, Quinn JA, Penne KL, Wikstrand CJ, Van Duyn LB, Dancey JE, McLendon RE, Kao JC, Stenzel TT, Ahmed Rasheed BK, Tourt-Uhlig SE, Herndon JE, 2nd, Vredenburgh JJ, Sampson JH, Friedman AH, Bigner DD, Friedman HS. Phase II trial of gefitinib in recurrent glioblastoma. J. Clin. Oncol. 2004;13(22):133–142. doi: 10.1200/JCO.2004.08.110. [DOI] [PubMed] [Google Scholar]
- [84].Assanah MC, Bruce JN, Suzuki SO, Chen A, Goldman JE, Canoll P. PDGF stimulates the massive expansion of glial progenitors in the neonatal forebrain. Glia. 2009;57(16):1835–1847. doi: 10.1002/glia.20895. [DOI] [PubMed] [Google Scholar]
- [85].Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY. Primary brain tumours in adults. Lancet. 2003;361(9354):323–331. doi: 10.1016/S0140-6736(03)12328-8. [DOI] [PubMed] [Google Scholar]
- [86].Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinojosa A, Vanden Berg S, Alvarez-Buylla A. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51(2):187–199. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- [87].Lindberg N, Kastemar M, Olofsson T, Smits A, Uhrbom L. Oligodendrocyte progenitor cells can act as cell of origin for experimental glioma. Oncogene. 2009;28(23):2266–2275. doi: 10.1038/onc.2009.76. [DOI] [PubMed] [Google Scholar]
- [88].Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat. Genet. 2000;25(1):55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- [89].Shih AH, Dai C, Hu X, Rosenblum MK, Koutcher JA, Holland EC. Dose-dependent effects of platelet-derived growth factor-B on glial tumorigenesis. Cancer Res. 2004;64(14):4783–4789. doi: 10.1158/0008-5472.CAN-03-3831. [DOI] [PubMed] [Google Scholar]
- [90].Mao XG, Zhang X, Zhen HN. Progress on potential strategies to target brain tumor stem cells. Cell Mol. Neurobiol. 2009;29:141–155. doi: 10.1007/s10571-008-9310-1. [DOI] [PubMed] [Google Scholar]
- [91].Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat. Neurosci. 2005;6(8):709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- [92].Kanamori M, Kawaguchi T, Nigro JM, Feuerstein BG, Berger MS, Miele L, Pieper RO. Contribution of Notch signaling activation to human gliolbastoma mutlforme. J. Neurosurg. 2007;3(106):417–427. doi: 10.3171/jns.2007.106.3.417. [DOI] [PubMed] [Google Scholar]
- [93].Purow BW, Haque RM, Noel MW, Su Q, Burdick MJ, Lee J, Sundaresan T, Pastorino S, Park JK, Mikolaenko I, Maric D, Eberhart CG, Fine HA. Expression of Notch-1 and its ligands, Delta-like-1, Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;6(65):2353–2363. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- [94].Shih AH, Holland EC. Notch signaling enhances nestin expression in gliomas. Neoplasia. 2006;12(8):1072–1082. doi: 10.1593/neo.06526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Weijzen S, Rizzo P, Braid M, Vaishnav R, Jonkheer SM, Zlobin A, Osborne BA, Gottipati S, Aster JC, Hahn WC, Rudolf M, Siziopikou K, Kast WM, Miele L. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transforming cells. Nat. Med. 2002;9(8):979–986. doi: 10.1038/nm754. [DOI] [PubMed] [Google Scholar]
- [96].Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, Feuerstein BG, Aldape K. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;3(9):157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- [97].Cai C, Thorne J, Grabel L. Hedgehog serves as a mitogen and survival factor during embryonic stem cell neurogenesis. Stem cells. 2008;5(26):1097–1108. doi: 10.1634/stemcells.2007-0684. [DOI] [PubMed] [Google Scholar]
- [98].Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat. Neurosci. 2003;1(6):21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- [99].Barr EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A, Eberhart CG. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem cells. 2007;10(25):2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr. Biol. 2007;2(17):165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Galvin KE, Ye H, Erstad DJ, Feddersen R, Wetmore C. Gli1 induces G2/M arrest and apoptosis in hippocampal but not tumor-derived neural stem cells. Stem cells. 2008;4(26):1027–1034. doi: 10.1634/stemcells.2007-0879. [DOI] [PubMed] [Google Scholar]
- [102].Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, Breakefield XO, Snyder EY. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc. Natl. Acad. Sci. U.S.A. 2000:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Aboody KS, Najbauer J, Schmidt NO, Yang W, Wu JK, Zhuge Y, Przylecki W, Carroll R, Black PM, Perides G. Targeting of melanoma brain metastases using engineered neural stem/progenitor cells. Neuro. Oncol. 2006;2(8):119–126. doi: 10.1215/15228517-2005-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kim SK, Kim SK, Cargioli TG, Machluf M, Yang W, Sun Y, Al-Hashem R, Kim SU, Black PM, Carroll RS. PEX-producing human neural stem cells inhibit tumor growth in a mouse glioma model. Clin. Cancer Res. 2005;16(11):5965–5970. doi: 10.1158/1078-0432.CCR-05-0371. [DOI] [PubMed] [Google Scholar]
- [105].Glass R, Synowitz M, Kronenberg G, Walzlein JH, Markovic DS, Wang LP, Gast D, Kiwit J, Kempermann G, Kettenmann H. Glioblastoma-induced attraction of endogenous neural precursor cells is associated with improved survival. J. Neurosci. 2005;10(25):2637–2646. doi: 10.1523/JNEUROSCI.5118-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Staflin K, Honeth G, Kalliomäki S, Kjellman C, Edvardsen K, Lindvall M. Neural progenitor cell lines inhibit rat tumor growth in vivo. Cancer Res. 2004;15(64):5347–5354. doi: 10.1158/0008-5472.CAN-03-1246. [DOI] [PubMed] [Google Scholar]
- [107].Weinstein DE, Shelanski ML, Liem RK. C17, a retrovirally immortalized neuronal cell line, inhibits proliferation of astrocytes and astrocytoma cells by a contact-mediated mechanism. Glia. 1990;2(3):130–139. doi: 10.1002/glia.440030207. [DOI] [PubMed] [Google Scholar]
- [108].Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, Galli R, Selleri S, Di Meco F, De Fraja C, Vescovi A, Cattaneo E, Finocchiaro G. Gene therapy of experimental brain tumors using neural progenitor cells. Nat. Med. 2000;4(6):447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- [109].Barresi V, Belluardo N, Sipione S, Mudò G, Cattaneo E, Condorelli DF. Transplantation of prodrug-converting neural progenitor cells for brain tumor therapy. Cancer Gene Ther. 2003;5(10):396–402. doi: 10.1038/sj.cgt.7700580. [DOI] [PubMed] [Google Scholar]
- [110].Aghi M, Hochberg F, Breakefield XO. Prodrug activation enzymes in cancer gene therapy. J. Gene Med. 2000;3(2):148–164. doi: 10.1002/(SICI)1521-2254(200005/06)2:3<148::AID-JGM105>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- [111].Li S, Tokuyama T, Yamamoto J, Koide M, Yokota N, Namba H. Bystander effect- mediated gene therapy of gliomas using genetically engineered neural stem cells. Cancer Gene Ther. 2005;7(12):600–607. doi: 10.1038/sj.cgt.7700826. [DOI] [PubMed] [Google Scholar]
- [112].Manome Y, Wen PY, Dong Y, Tanakam T, Mitchell BS, Kufe DW, Fine HA. Viral vector transduction of the human deoxycytidine kinase cDNA sensitizes glioma cells to the cytotoxic effects of cytosine arabinoside in vitro and in vivo. Nat. Med. 1996;5(2):567–573. doi: 10.1038/nm0596-567. [DOI] [PubMed] [Google Scholar]
- [113].Herrlinger U, Woiciechowski C, Sena-Esteves M, Aboody KS, Jacobs AH, Rainov NG, Snyder EY, Breakefield XO. Neural precursor cells for delivery of replication-conditional HSV-1 vectors to intracerebral gliomas. Mol. Ther. 2000;4(1):347–57. doi: 10.1006/mthe.2000.0046. [DOI] [PubMed] [Google Scholar]
- [114].Chiocca EA, Aghi M, Fulci G. Viral therapy for glioblastoma. Cancer J. 2003;3(9):167–179. doi: 10.1097/00130404-200305000-00005. [DOI] [PubMed] [Google Scholar]
- [115].Gomez-Manzano C, Yung WK, Alemany R, Fueyo J. Genetically modified adenoviruses against gliomas: from bench to bedside. Neurology. 2004;3(63):418–426. doi: 10.1212/01.wnl.0000133302.15022.7f. [DOI] [PubMed] [Google Scholar]
- [116].Kew Y, Levin VA. Advances in gene therapy and immunotherapy for brain tumors. Curr Opin. Neurol. 2003;6(16):665–670. doi: 10.1097/01.wco.0000102625.38669.62. [DOI] [PubMed] [Google Scholar]
- [117].Arnhold S, Hilgers M, Lenartz D, Semkova I, Kochanek S, Voges J, Andressen C, Addicks K. Neural precursor cells as carriers for a gene therapeutical approach in tumor therapy. Cell Transplant. 2003;8(12):827–837. doi: 10.3727/000000003771000174. [DOI] [PubMed] [Google Scholar]
- [118].Ehtesham M, Stevensonm CB, Thompson RC. Stem cell therapies for malignant glioma. Neurosurg. Focus. 2005;3(19) doi: 10.3171/foc.2005.19.3.6. [DOI] [PubMed] [Google Scholar]
- [119].Ehtesham M, Kabos P, Kabosova A, Neuman T, Black KL, Yu JS. The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. 2002;20(62):5657–5663. [PubMed] [Google Scholar]
- [120].Jean WC, Spellman SR, Wallenfriedman MA, Hall WA, Low WC, Wallenfriedman MA. Interleukin-12 based immuno-therapy against rat 9L glioma. Neurosurgery. 1998;4(24):850–856. doi: 10.1097/00006123-199804000-00097. [DOI] [PubMed] [Google Scholar]
- [121].Faber C, Terao E, Morga E, Heuschling P. Interleukin-4 enhances the in vitro precursor cell recruitment for tumor-specific T lymphocytes in patients with glioblastoma. J. Immunother. 2000;1(23):11–16. doi: 10.1097/00002371-200001000-00003. [DOI] [PubMed] [Google Scholar]
- [122].Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, Anichini A. Interleukin-12: biological properties and clinical application. Clin. Cancer Res. 2007;16(13):4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- [123].Yang SY, Liu H, Zhang JN. Gene therapy of rat malignant gliomas using neural stem cells expressing IL-12. DNA Cell Biol. 2004;6(23):381–389. doi: 10.1089/104454904323145263. [DOI] [PubMed] [Google Scholar]
- [124].Ehtesham M, Kabos P, Gutierrez MA, Chung NH, Griffith TS, Black KL, Yu JS. Indcution of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2002;24(62):7170–7174. [PubMed] [Google Scholar]
- [125].Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;6(3):673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- [126].Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Invest. 1999;2(104):155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat. Med. 1999;2(5):146–147. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- [128].Corsten MF, Miranda R, Kasmieh R, Krichevsky AM, Weissleder R, Shah K. MicroRNA-21 knockdown disrupts glioma growth in vivo and displays synergistic cytotoxicity with neural precursor cells delivered S-TRAIL in human gliomas. Cancer Res. 2007;19(67):8994–9000. doi: 10.1158/0008-5472.CAN-07-1045. [DOI] [PubMed] [Google Scholar]
- [129].Kock N, Kasmieh R, Weissleder R, Shah K. Tumor therapy mediated by lentiviral expression of shBcl-2 and S-TRAIL. Neoplasia. 2007;5(9):435–442. doi: 10.1593/neo.07223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Shah K, Bureau E, Kim DE, Yang K, Tang Y, Weissleder R, Breakefield XO. Glioma therapy and real-time imaging of neural precursor cell migration and tumor regression. Ann. Neurol. 2005;1(57):34–41. doi: 10.1002/ana.20306. [DOI] [PubMed] [Google Scholar]
- [131].Hingtgen S, Ren X, Terwilliger E, Classon M, Weissleder R, Shah K. Targeting mutliple pathways in gliomas with stem cell and viral delivered S-TRAIL and Temozolmide. Mol. Cancer Ther. 2008;11(7):3575–3585. doi: 10.1158/1535-7163.MCT-08-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, Weissleder R. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat. Biotechnol. 2000;18(4):410–414. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- [133].Tang Y, Shah K, Messerli SM, Snyder E, Breakefield X, Weissleder R. In vivo tracking of neural progenitor cell migration to glioblastomas. Hum. Gene Ther. 2003;13(14):1247–1254. doi: 10.1089/104303403767740786. [DOI] [PubMed] [Google Scholar]
- [134].Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004;8(22):959–960. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- [135].Stroh M, Zimmer JP, Duda DG, Levchenko TS, Cohen KS, Brown EB, Scadden DT, Torchilin VP, Bawendi MG, Fukumura D, Jain RK. Quantum dots spectrally distinguish multiple species within the tumor milieu in vivo. Nat. Med. 2005;6(11):678–682. doi: 10.1038/nm1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Slotkin JR, Chakrabarti L, Dai HN, Carney RS, Hirata T, Bregman BS, Gallicano GI, Corbin JG, Haydar TF. In vivo quantum dot labeling of mammalian stem and progenitor cells. Dev. Dyn. 2007;12(263):3393–3401. doi: 10.1002/dvdy.21235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Hirsch LR, Stafford RJ, Bankson JA, Sershenm SR, Riveram B, Price RE, Hazle JD, Halas NJ, West JL. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. U.S.A. 2003;23(100):13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Fischer I, Gagner JP, Law M, Newcomb EW, Zagzag D. Angiogenesis in gliomas: biology and molecular pathophysiology. Brain Pathol. 2005;15(4):297–310. doi: 10.1111/j.1750-3639.2005.tb00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Yip S, Shah K. Stem-cell based therapies for brain tumors. Curr. Opin. Mol. Ther. 2008;4(10):334–342. [PubMed] [Google Scholar]