Abstract
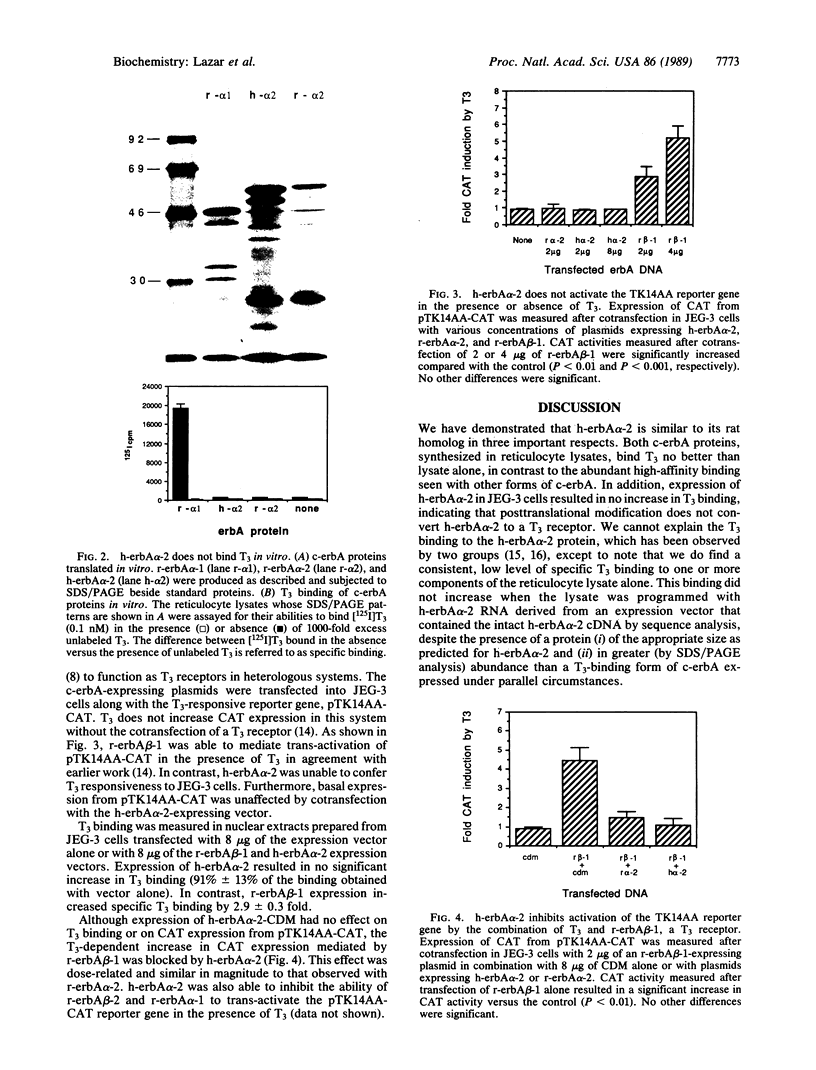
Multiple thyroid hormone (T3; L-3,3',5-triiodothyronine) receptors related to the viral oncogene v-erbA have been identified in mammalian tissues and have been shown to mediate certain actions of T3. The role of a carboxyl-terminal variant of alpha-type c-erbA (c-erbA alpha-2) is controversial, since the human form has been reported to be a T3 receptor, while the rat form has been shown to not bind T3. In fact, the rat homolog of c-erbA alpha-2 has been reported to be an inhibitor of T3 action. We have compared the properties of human c-erbA alpha-2 with those of its rat homolog and with other forms of c-erbA. Neither form of c-erbA alpha-2 binds T3 with high affinity whether synthesized in reticulocyte lysates or by transient expression in mammalian cells. Also, neither form increases the expression of a T3-responsive gene. However, human c-erbA alpha-2 inhibits T3 action mediated by a T3-receptor form of c-erbA to an extent similar to that of rat c-erbA alpha-2. Our data strongly suggest that human c-erbA alpha-2 has a biological function similar to that of its rat homolog. Thus, the modulation of T3 action by an endogenous inhibitor related to T3 receptors is likely a general regulatory mechanism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benbrook D., Pfahl M. A novel thyroid hormone receptor encoded by a cDNA clone from a human testis library. Science. 1987 Nov 6;238(4828):788–791. doi: 10.1126/science.3672126. [DOI] [PubMed] [Google Scholar]
- Forman B. M., Yang C. R., Stanley F., Casanova J., Samuels H. H. c-erbA protooncogenes mediate thyroid hormone-dependent and independent regulation of the rat growth hormone and prolactin genes. Mol Endocrinol. 1988 Oct;2(10):902–911. doi: 10.1210/mend-2-10-902. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. A superfamily of potentially oncogenic hormone receptors. Nature. 1986 Dec 18;324(6098):615–617. doi: 10.1038/324615a0. [DOI] [PubMed] [Google Scholar]
- Izumo S., Mahdavi V. Thyroid hormone receptor alpha isoforms generated by alternative splicing differentially activate myosin HC gene transcription. Nature. 1988 Aug 11;334(6182):539–542. doi: 10.1038/334539a0. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Lazar M. A., Hodin R. A., Brent G. A., Larsen P. R., Chin W. W., Moore D. D. Inhibition of thyroid hormone action by a non-hormone binding c-erbA protein generated by alternative mRNA splicing. Nature. 1989 Feb 16;337(6208):659–661. doi: 10.1038/337659a0. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Warne R. L., Brent G. A., Harney J. W., Larsen P. R., Moore D. D. Isolation of a cDNA clone encoding a biologically active thyroid hormone receptor. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5031–5035. doi: 10.1073/pnas.85.14.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar M. A., Hodin R. A., Darling D. S., Chin W. W. Identification of a rat c-erbA alpha-related protein which binds deoxyribonucleic acid but does not bind thyroid hormone. Mol Endocrinol. 1988 Oct;2(10):893–901. doi: 10.1210/mend-2-10-893. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi T., Tennyson G. E., Nikodem V. M. Alternative splicing generates messages encoding rat c-erbA proteins that do not bind thyroid hormone. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5804–5808. doi: 10.1073/pnas.85.16.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai A., Sakurai A., Bell G. I., DeGroot L. J. Characterization of a third human thyroid hormone receptor coexpressed with other thyroid hormone receptors in several tissues. Mol Endocrinol. 1988 Nov;2(11):1087–1092. doi: 10.1210/mend-2-11-1087. [DOI] [PubMed] [Google Scholar]
- Nakai A., Seino S., Sakurai A., Szilak I., Bell G. I., DeGroot L. J. Characterization of a thyroid hormone receptor expressed in human kidney and other tissues. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2781–2785. doi: 10.1073/pnas.85.8.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Casanova J. Depletion of L-3,5,3'-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology. 1979 Jul;105(1):80–85. doi: 10.1210/endo-105-1-80. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Thompson C. C., Weinberger C., Lebo R., Evans R. M. Identification of a novel thyroid hormone receptor expressed in the mammalian central nervous system. Science. 1987 Sep 25;237(4822):1610–1614. doi: 10.1126/science.3629259. [DOI] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]