Abstract
Aims: To assess the effectiveness of procysteine (PRO) supplementation provided during a period of abstinence (ABS) on alcohol-induced skeletal muscle atrophy and oxidant stress. Methods: Age- and gender-matched Sprague–Dawley rats were fed the Lieber–DeCarli liquid diet containing either alcohol or an isocaloric substitution (control diet) for 12 week. Next, subgroups of alcohol-fed rats were fed the control diet for 2 week (ABS) supplemented with either PRO (0.35%, w/v) or vehicle. Plantaris morphology was assessed by hematoxylin and eosin staining. Total, reduced and oxidized glutathione (GSH) levels and total antioxidant potential were determined by commercially available assay kits. Antibody arrays were used to determine cytokine levels. Real-time polymerase chain reaction was used to determine gene expressions of two E3 ubiquitin ligases, atrogin-1 and muscle ring finger protein-1 (MuRF-1). Results: Plantaris muscles from alcohol-fed rats displayed extensive atrophy, as well as decreased GSH levels, a trend for decreased total antioxidant potential and elevated atrogin-1 and MuRF-1 mRNA levels. GSH levels and total antioxidant potential continued to decrease during 2 weeks of ABS from alcohol, which were normalized in abstinent rats provided PRO. Gene levels of both E3 ligases returned to baseline during ABS. In parallel, plantaris cross-sectional area increased in both groups during ABS. Conclusions: PRO supplementation during ABS significantly attenuated alcohol-induced redox stress compared with untreated abstinent rats. Thus, our data may suggest that GSH restoration therapy may provide therapeutic benefits to the overall antioxidant state of skeletal muscle when prescribed in conjunction with an established detoxification program for recovering alcoholics.
INTRODUCTION
Chronic alcohol abuse may lead to a host of skeletal muscle complications, including soreness and atrophy with concomitant losses in strength, altered gait and impaired mobility. These derangements, clinically classified as alcoholic myopathy, are likely caused by severe metabolic, physiological and structural alterations to skeletal muscle (Fernandez-Sola et al., 2007; Preedy et al., 2003). For example, development of alcoholic myopathy has been attributed in part to altered redox states and antioxidant imbalance (Adachi et al., 2000; Fernandez-Sola et al., 2007; Otis and Guidot, 2009; Otis et al., 2007), disparities between protein catabolism and protein synthesis (Kumar et al., 2002; Lang et al., 1999; Otis and Guidot, 2009) and acetaldehyde–protein adduct formation (Worrall et al., 2001).
In general, most research has suggested that chronic alcoholic myopathy is reversible with abstinence (ABS; Martin and Peters, 1985; Vary et al., 2004). For example, Vary et al. (2004) have shown that alcohol-induced deficits in protein synthesis were normalized following 72 h of alcohol withdrawal due, in part, to restoration of initiation and elongation factors. However, some case reports have suggested that muscle weakness and atrophy may endure for years (Ekbom et al., 1964; Rossouw et al., 1976), suggesting that long-term alcohol abuse may produce irreversible molecular or structural changes (Fernandez-Sola et al., 2007).
Intriguingly, a recent report has suggested that supplementing the diets of abstinent rats with N-acetylcysteine (NAC), a glutathione (GSH) precursor, significantly increased myocardial GSH peroxidase and citrate synthase activities compared with ABS alone (Seiva et al., 2009). These data suggested that dietary GSH precursors may improve antioxidant defense systems and energy metabolism in abstinent rats; however, it is unclear whether similar benefits of GSH restoration extend to skeletal muscles. Encouragingly, when the GSH precursor procysteine (PRO) is provided concurrently with alcohol, alcohol-induced oxidant stress was abated and components of several anabolic pathways were induced, thereby mitigating alcoholic myopathy (Otis and Guidot, 2009). On the basis of this work, we hypothesized that muscle dysfunction reported by a subset of abstinent patients (Ekbom et al., 1964; Rossouw et al., 1976) may be due in part to alterations in the GSH antioxidant system and persistent oxidant stress. We further hypothesized that PRO supplementation provided concurrently with 2 weeks of ABS would alleviate these derangements to a greater degree than ABS alone.
MATERIALS AND METHODS
Animals and diet
Male Sprague–Dawley rats (200–250 g) were purchased from Charles River (Wilmington, MA, USA) and housed in pairs under a 12:12 light–dark cycle. All procedures were approved by the Emory University Institutional Animal Care and Use Committee (protocol 043-2010). Rats were randomly organized into one of four groups (six per group): (a) isocaloric-fed, alcohol-naïve controls, (b) alcohol-fed for 12 week (EtOH), (c) alcohol-fed for 12 week followed by a 2-week ABS period and (d) alcohol-fed for 12 week followed by a 2-week ABS period in which animals were provided PRO (0.35% w/v, Sigma, St. Louis, MO, USA; ABS + PRO) as described previously (Bechara et al., 2005; Otis and Guidot, 2009; Otis et al., 2007).
Rats were fed the Lieber–DeCarli liquid diet (Research Diets, New Brunswick, NJ, USA) containing either alcohol or an isocaloric substitution with Maltin–Dextrin (control diet) for 12 weeks as described previously (Otis and Guidot, 2009; Otis et al., 2007). Alcohol was added gradually to acclimatize the rats to the diet. Alcohol was added as 18% of total calories for 1 week, then 27% of total calories for 1 week and finally as 36% of total calories for 10 weeks. Rats were anesthetized with sodium pentobarbital (60 mg/kg i.p.). All plantaris muscles were removed in the morning, blotted dry, weighed and mounted in HistoPrep for histochemical analysis or flash frozen in liquid nitrogen for other analyses as described subsequently. Animals were sacrificed by removal of the diaphragm muscle.
Cross-sectional area measurements
Plantaris muscles were embedded in OCT and immediately frozen in isopentane cooled in liquid nitrogen as described previously (Otis and Guidot, 2009; Otis et al., 2007, 2008). Serial sections from the mid-belly of the plantaris muscle were cut at 14 μm and adhered to superfrost slides. Plantaris sections were processed for hematoxylin and eosin staining, dehydrated, mounted and visualized with a Leica microscope. Approximately 125 fibers per muscle were analyzed and cross-sectional areas (CSAs) were determined using ImageJ software (NIH, Bethesda, MD, USA).
GSH and GSH disulfide levels
GSH and GSH disulfide levels were quantified in fresh plantaris homogenates via commercially available kits according to the manufacturer's instructions (Arbor Assay Systems, Ann Arbor, MI, USA). Briefly, total and oxidized GSH levels were determined using a colorimetric substrate that reacted with the free thiol group on GSH. Absorbance at 405 nm was measured on a Multilabel counter (PerkinElmer, Waltham, MA, USA). Free (reduced) GSH was then calculated as the difference between total and oxidized GSH.
Total antioxidant potential
Total antioxidant potential (e.g. GSH, albumin, ascorbic acid and α-tocopherol) was determined in fresh plantaris homogenates via commercially available kits (OxisResearch, Foster City, CA, USA). Briefly, the reduction potential of the homogenates converted Cu2+ to Cu1+. This reduced form of copper creates a stable 2:1 complex with the chromogenic reagent with a maximum absorption at 450 nm. Absorbance was measured on a Multilabel counter (PerkinElmer), and antioxidant potentials were calculated according to the manufacturer's instructions.
Real-time polymerase chain reaction
Plantaris muscles were collected, immediately frozen in liquid nitrogen and stored at −80°C until processed for real-time polymerase chain reaction (PCR) analyses as describedpreviously (Otis and Guidot, 2009; Otis et al., 2007, 2008). Trizol was added to the tissues (1 ml/100 mg tissue) that were then homogenized using an electric tissue homogenizer. Total RNA (2.5 μg) was reverse transcribed in a 40-μl final reaction volume, using random primers and Moloney Murine Leukemia Virus reverse transcriptase (Invitrogen, Carlsbad, CA, USA). The reverse transcription reaction was incubated at 65°C for 10 min, 80°C for 3 min and 42°C for 60 min, respectively. Real-time PCR products were analyzed using the iCycler iQ system (Bio-Rad, Hercules, CA, USA). cDNA (5 μl of a 1:10 dilution) was amplified in a 25-μl reaction, containing 400 nm gene-specific primer pair and iQ Sybr Green Supermix (Bio-Rad). Primer sequences of superoxide dismutase 1–3 (SOD1–3), catalase, atrogin-1 and muscle ring finger protein-1 (MuRF-1) were previously designed using Primer3 (Otis and Guidot, 2009) and ordered from Sigma-Genosys (The Woodlands, TX, USA). Samples were incubated at 95°C for 15 min, followed by 40 cycles of denaturation, annealing and extension at 95, 60 and 72°C, respectively, with fluorescence recorded at the end of each annealing and extension step. As a control, real-time PCR was also performed on 2 μl of each RNA sample to confirm the absence of contaminating genomic DNA. All reactions were performed in triplicate, and the starting quantities of the genes of interest were normalized to 18S rRNA (primers supplied by Ambion, Austin, TX, USA). The 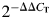 method was used to analyze alterations in gene expression, and values were expressed as fold changes relative to control (Otis and Guidot, 2009; Otis et al., 2007, 2008).
method was used to analyze alterations in gene expression, and values were expressed as fold changes relative to control (Otis and Guidot, 2009; Otis et al., 2007, 2008).
Statistics
One-way analyses of variance were performed followed by Student–Newman–Keuls post hoc tests using SigmaStat v2.0 software. Significance was accepted at P ≤ 0.05.
RESULTS
Body weight and plantaris morphology
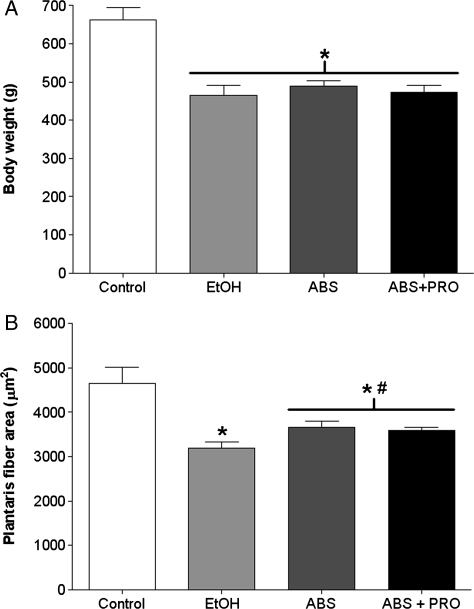
Rats fed alcohol for 12 weeks had a 29% decrease in body weight compared with control-fed rats (Fig. 1A). In parallel, plantaris fiber CSA from alcohol-fed rats displayed a significant reduction in average CSA compared with controls (Fig. 1B). Although 2 weeks of ABS was insufficient to restore body weight, both groups of abstinent rats had increased plantaris CSAs compared with muscles from alcohol-fed rats (P ≤ 0.05).
Fig. 1.
Body weights and plantaris fiber areas. (A) Body weights were significantly reduced in alcohol-fed, abstinent and abstinent rats provided PRO compared with controls. (B) Plantaris muscles from rats chronically fed alcohol (EtOH) for 12 weeks had a 32% reduction in CSA compared with controls. Two weeks of ABS was sufficient to improve alcohol-induced plantaris CSA with no additional benefits conferred by PRO supplementation. Values are expressed as means + SEM (n = 6–7 rats/group). Significance was accepted at P ≤ 0.05. *, compared with control group. #, compared with EtOH group.
Markers of oxidant stress
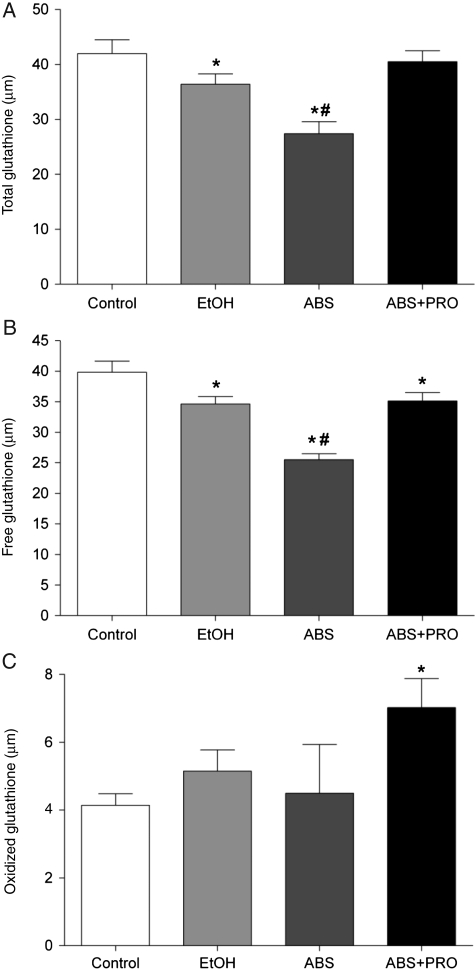
We next determined the levels of total GSH, free GSH (reduced form) and GSH disulfide (GSSG, oxidized form). Chronic alcohol ingestion reduced total and free GSH levels (Fig. 2A and B, respectively; P ≤ 0.05). Interestingly, total and free GSH levels continued to plunge during this 2-week ABS period, but were improved by PRO supplementation (P ≤ 0.05). GSSG levels were increased in abstinent rats supplemented with PRO (Fig. 2C).
Fig. 2.
GSH and GSH disulfide levels in rat plantaris. (A) Total GSH levels (i.e. the sum of free and oxidized GSH pools) were reduced in plantaris muscles from rats chronically fed alcohol (EtOH) for 12 weeks. This trend continued during a 2-week period of ABS that was normalized in rats receiving PRO. (B) The available pool of free (reduced) GSH was decreased in plantaris muscles from alcohol-fed rats and continued to decrease during ABS. PRO supplementation effectively restored the GSH pool. (C) GSH disulfide (oxidized) was increased in plantaris muscles from ABS + PRO rats. Values are expressed as means + SEM (n = 6–7 rats/group). Significance was accepted at P ≤ 0.05. *, compared with control group. #, compared with EtOH group.
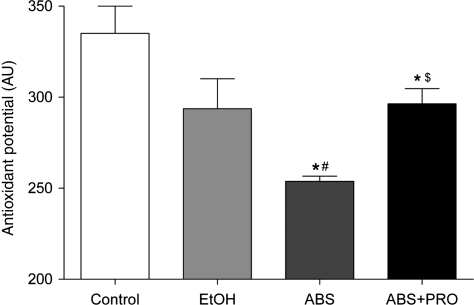
We also determined the total antioxidant potential of plantaris muscles. This assay measures the activities of several antioxidant mechanisms, including enzymatic systems such as GSH peroxidase and catalase, large molecule systems such as albumin and small molecule systems such as uric acid, α-tocopherol and ascorbic acid. Although not statistically significant (P = 0.06), there was a trend for a decreased total antioxidant potential in plantaris muscles from alcohol-fed rats compared with controls (Fig. 3). Total antioxidant potential continued to decrease during this 2-week ABS period, which mirrored the decrements in the GSH system. PRO supplementation improved total antioxidant potential compared with ABS alone.
Fig. 3.
Antioxidant potential in rat plantaris. There was a trend (P = 0.06) for reduced total antioxidant potential in plantaris muscles from rats fed alcohol (EtOH) for 12 weeks. Total antioxidant potential continued to decrease during ABS, but was increased due to PRO supplementation. Values are expressed as means + SEM (n = 6–7 rats/group). Significance was accepted at P ≤ 0.05. *, compared with control group. #, compared with EtOH group, $, compared with ABS group.
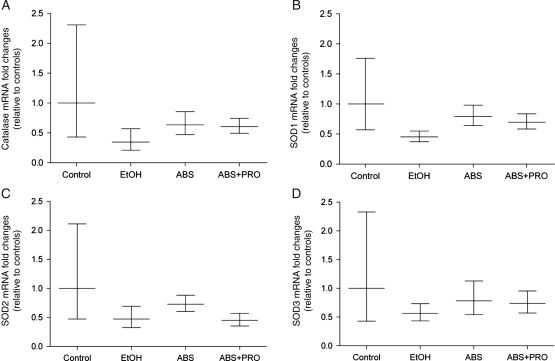
To determine whether the reduced total antioxidant capacity was also due in part to decreased antioxidant enzyme levels, we next quantified gene expressions of catalase, SOD-1, SOD-2 and SOD-3. However, the mRNA levels of these enzymes were unchanged by chronic alcohol ingestion or during the ABS period (Fig. 4).
Fig. 4.
Catalase and SOD isozymes gene expressions in rat plantaris. Gene expressions of (A) catalase and (B–D) three SOD isozymes (i.e. Cu/Zn-SOD1, Mn-SOD2 and Cu/Zn-SOD3) were unchanged in any group. Data are represented as means ± range of potential values based on the 2−ΔΔCT method (Otis et al., 2007; Otis et al., 2008) and expressed as fold changes relative to controls (n = 6–7 rats/group). Significance was accepted at P ≤ 0.05.
Atrogin-1 and MuRF-1 gene expressions
Twelve weeks of daily alcohol ingestion induced mRNA expressions of the muscle-specific E3 ubiquitin ligases, atrogin-1 and MuRF-1 (Fig. 5). Regardless of nutritional intervention, a 2-week period of ABS was sufficient to abrogate both gene levels compared with plantaris muscles from alcohol-fed rats (P ≤ 0.05).
Fig. 5.
Atrogin-1 and MuRF-1 gene expressions in rat plantaris. Real-time PCR analyses were performed for atrogin-1 and MuRF-1in plantaris muscles from rats chronically fed alcohol (EtOH) for 12 weeks. Alcohol ingestion dramatically increased mRNA levels of both E3 ligases. Two weeks of ABS was sufficient to normalize atrogin-1 and MuRF-1 inductions due to alcohol ingestion with no additional benefits conferred by PRO supplementation. Data are represented as means ± range of potential values based on the 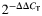 method (Otis et al., 2007; Otis et al., 2008) and expressed as fold changes relative to controls (n = 6–7 rats/group). Significance was accepted at P ≤ 0.05. *, compared with control group. #, compared with EtOH group.
method (Otis et al., 2007; Otis et al., 2008) and expressed as fold changes relative to controls (n = 6–7 rats/group). Significance was accepted at P ≤ 0.05. *, compared with control group. #, compared with EtOH group.
DISCUSSION
In this study, we attempted to enhance muscle recovery during a period of ABS with GSH precursor supplementation. As expected, 12 weeks of daily alcohol ingestion caused significant oxidant stress and plantaris atrophy. In parallel, we showed that chronic alcohol ingestion strongly induced atrogin-1 and MuRF-1, E3 ubiquitin ligases implicated in skeletal muscle atrophy (Attaix et al., 2005; Bodine et al., 2001; Glass, 2005; Gomes et al., 2001). Two weeks of ABS was sufficient to abate expressions of the E3 ligases and minimize the extent of alcohol-induced muscle atrophy. Despite these improvements, several markers of oxidant stress continued to decrease during ABS. Importantly, abstinent rats provided PRO displayed marked improvements in GSH availability and total antioxidant potential compared with ABS alone. Together, these data suggest that antioxidant therapy using GSH precursors such as PRO, NAC or S-adenosyl-l-methionine may provide additional therapeutic benefits to muscle oxidant states when prescribed in conjunction with an appropriate detoxification program.
Reduced levels of skeletal muscle GSH have been reported in a variety of disease states, including HIV infection (Droge et al., 1994), critical illnesses that may result in prolonged bed rest and reduced muscle activity (Burnham et al., 2005; Servais et al., 2007) and chronic obstructive pulmonary disease (Rabinovich et al., 2006). Similarly, we and others have shown that chronic alcohol ingestion altered normal GSH metabolism in skeletal muscle (Fernandez-Sola et al., 2002; Otis and Guidot, 2009; Otis et al., 2007). We now suggest that the deleterious effects of long-term alcohol ingestion on GSH metabolism and antioxidant capacity continued for at least 2 weeks of ABS. Persistent oxidant stress in muscles from abstinent rats is likely not due to defects in the SOD1–3 or catalase scavenging systems and may suggest oxidant stress may normalize with longer durations of ABS. Nevertheless, oxidant stress and reduced GSH levels did not affect muscle hypertrophy during ABS, but GSH deficiency may have significant impacts on multiple biological mechanisms. For example, GSH is the principal nucleophilic scavenger of free radicals in skeletal muscle, stabilizes other antioxidant systems, reduces proteins and helps to maintain their function, attenuates redox-sensitive catabolic factors and has salutary effects on membranes through the reduction of alcohol-induced lipid peroxidation (Jackson, 2008; Otis et al., 2007; Wu et al., 2004). Thus, this enduring oxidant stress may provide a partial reason why muscle dysfunction persists in small subsets of abstinent patients (Ekbom et al., 1964; Rossouw et al., 1976).
Nevertheless, muscle atrophy is often reversible for former alcoholics enrolled in a rehabilitation program that focuses on ABS and nutritional support (Andersen et al., 1998; Fernandez-Sola et al., 2000; Peters et al., 1985; Sestoft et al., 1994; Slavin et al., 1983). Our data may suggest that these gains in muscle fiber size may be due in part to the normal attenuation of catabolic factors such as atrogin-1 and MuRF-1 once the alcohol stimulus has been removed. Unfortunately, complete ABS from alcohol is often difficult to achieve with documented success rates of 33% (Fillmore et al., 1991). Fernandez-Sola et al. (2000) have shown that muscle strength can be improved in recovering alcoholics who adhere to a controlled, low-dose alcohol (≤60 g/day) consumption paradigm. Although these data are encouraging for 67% of recovering alcoholics that struggle to completely abstain from alcohol, the impact of low-dose alcohol consumption on oxidant stress was not considered. In light of our current work, alcohol treatment centers may recommend low-dose alcohol consumption in combination with dietary GSH restoration to maximize muscle recovery and more quickly normalize muscle redox states.
Atrogin-1 and MuRF-1 are E3 ubiquitin ligases implicated in skeletal muscle atrophy (Attaix et al., 2005; Bodine et al., 2001; Gomes et al., 2001; Lang et al., 2007; Mastrocola et al., 2008; Otis et al., 2008) and appear to regulate early stages of alcoholic myopathy. Specifically, atrogin-1 was strongly induced in plantaris muscles from rats chronically fed alcohol for 6 weeks, a duration of abuse that preceded muscle atrophy (Otis et al., 2007). Here, both atrogin-1 and MuRF-1 remained elevated in atrophied plantaris muscles from rats fed alcohol for 12 weeks. Importantly, atrogin-1 was attenuated following GSH restoration during these shorter durations of abuse. Yet, when alcohol abuse continued for longer durations (e.g. 35 weeks), we have shown that atrogin-1 levels become refractory to GSH restoration (Otis and Guidot, 2009), suggesting that early intervention with GSH precursors is integral to combat alcohol-induced catabolic factors. Interestingly, the current work unveiled a cyclical response of atrogin-1 as we have previously shown that 28 weeks of chronic alcohol abuse did not induce expression of this ligase, despite the presence of overt plantaris atrophy (Otis and Guidot, 2009). In support of this notion, several reports suggested that muscle proteolysis or atrophy may occur independently of changes in atrogin-1 mRNA levels (Cai et al., 2004; Fareed et al., 2006; Vary et al., 2008). Together, these data may reveal important temporal associations between alcohol-induced GSH depletion, redox-sensitive induction of E3 ligases and resultant muscle atrophy.
In conclusion, we showed that long-term alcohol ingestion created an overall catabolic state in atrophied rat plantaris muscles, as evidenced by oxidant stress and induction of ubiquitin ligases. Although ABS alone was sufficient to improve skeletal muscle fiber diameter, several markers of oxidant stress persisted. PRO supplementation provided during this abstinent period abrogated oxidant stress, improved muscle antioxidant capacity and attenuated production of the E3 ligase atrogin-1. Thus, our data may suggest that GSH restoration therapy may provide therapeutic benefits to the overall antioxidant state of skeletal muscle when prescribed in conjunction with an established detoxification program for recovering alcoholics.
Funding
This work was supported by grant K01 AA017190-02 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (to J.S.O.) and by grant P-50 AA013757 from the NIAAA and a Veterans’ Affairs Merit Review (to D.M.G.).
REFERENCES
- Adachi J, Asano M, Ueno Y, et al. 7alpha- and 7beta-hydroperoxycholest-5-en-3beta-ol in muscle as indices of oxidative stress: response to ethanol dosage in rats. Alcohol Clin Exp Res. 2000;24:675–81. [PubMed] [Google Scholar]
- Andersen H, Borre M, Jakobsen J, et al. Decreased muscle strength in patients with alcoholic liver cirrhosis in relation to nutritional status, alcohol abstinence, liver function, and neuropathy. Hepatology. 1998;27:1200–6. doi: 10.1002/hep.510270503. doi:10.1002/hep.510270503. [DOI] [PubMed] [Google Scholar]
- Attaix D, Ventadour S, Codran A, et al. The ubiquitin–proteasome system and skeletal muscle wasting. Essays Biochem. 2005;41:173–86. doi: 10.1042/EB0410173. doi:10.1042/EB0410173. [DOI] [PubMed] [Google Scholar]
- Bechara RI, Pelaez A, Palacio A, et al. Angiotensin II mediates glutathione depletion, transforming growth factor-beta1 expression, and epithelial barrier dysfunction in the alcoholic rat lung. Am J Physiol Lung Cell Mol Physiol. 2005;289:L363–70. doi: 10.1152/ajplung.00141.2005. doi:10.1152/ajplung.00141.2005. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Latres E, Baumhueter S, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–8. doi: 10.1126/science.1065874. doi:10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- Burnham EL, Moss M, Ziegler TR. Myopathies in critical illness: characterization and nutritional aspects. J Nutr. 2005;135:1818S–23. doi: 10.1093/jn/135.7.1818S. [DOI] [PubMed] [Google Scholar]
- Cai D, Frantz JD, Tawa NE, Jr., et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–98. doi: 10.1016/j.cell.2004.09.027. doi:10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Droge W, Eck HP, Mihm S, et al. The role of a cysteine and glutathione deficiency in the immunopathology of HIV infection. Acta Microbiol Immunol Hung. 1994;41(Suppl.):17–20. [PubMed] [Google Scholar]
- Ekbom K, Hed R, Kirstein L, et al. Muscular affections in chronic alcoholism. Arch Neurol. 1964;10:449–58. doi: 10.1001/archneur.1964.00460170019003. [DOI] [PubMed] [Google Scholar]
- Fareed MU, Evenson AR, Wei W, et al. Treatment of rats with calpain inhibitors prevents sepsis-induced muscle proteolysis independent of atrogin-1/MAFbx and MuRF1 expression. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1589–97. doi: 10.1152/ajpregu.00668.2005. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sola J, Garcia G, Elena M, et al. Muscle antioxidant status in chronic alcoholism. Alcohol Clin Exp Res. 2002;26:1858–62. doi:10.1111/j.1530-0277.2002.tb02493.x. [PubMed] [Google Scholar]
- Fernandez-Sola J, Nicolas JM, Sacanella E, et al. Low-dose ethanol consumption allows strength recovery in chronic alcoholic myopathy. QJM. 2000;93:35–40. doi: 10.1093/qjmed/93.1.35. doi:10.1093/qjmed/93.1.35. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sola J, Preedy VR, Lang CH, et al. Molecular and cellular events in alcohol-induced muscle disease. Alcohol Clin Exp Res. 2007;31:1953–62. doi: 10.1111/j.1530-0277.2007.00530.x. doi:10.1111/j.1530-0277.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- Fillmore KM, Hartka E, Johnstone BM, et al. Preliminary results from a meta-analysis of drinking behavior in multiple longitudinal studies. Br J Addict. 1991;86:1203–10. doi: 10.1111/j.1360-0443.1991.tb01700.x. doi:10.1111/j.1360-0443.1991.tb01700.x. [DOI] [PubMed] [Google Scholar]
- Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37:1974–84. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Gomes MD, Lecker SH, Jagoe RT, et al. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA. 2001;98:14440–5. doi: 10.1073/pnas.251541198. doi:10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MJ. Redox regulation of skeletal muscle. IUBMB Life. 2008;60:497–501. doi: 10.1002/iub.72. doi:10.1002/iub.72. [DOI] [PubMed] [Google Scholar]
- Kumar V, Frost RA, Lang CH. Alcohol impairs insulin and IGF-I stimulation of S6K1 but not 4E-BP1 in skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283:E917–28. doi: 10.1152/ajpendo.00181.2002. [DOI] [PubMed] [Google Scholar]
- Lang CH, Huber D, Frost RA. Burn-induced increase in atrogin-1 and MuRF-1 in skeletal muscle is glucocorticoid independent but downregulated by IGF-I. Am J Physiol Regul Integr Comp Physiol. 2007;292:R328–36. doi: 10.1152/ajpregu.00561.2006. [DOI] [PubMed] [Google Scholar]
- Lang CH, Wu D, Frost RA, et al. Inhibition of muscle protein synthesis by alcohol is associated with modulation of eIF2B and eIF4E. Am J Physiol. 1999;277:E268–76. doi: 10.1152/ajpendo.1999.277.2.E268. [DOI] [PubMed] [Google Scholar]
- Martin F, Peters TJ. Alcoholic muscle disease. Alcohol Alcohol. 1985;20:125–36. [PubMed] [Google Scholar]
- Mastrocola R, Reffo P, Penna F, et al. Muscle wasting in diabetic and in tumor-bearing rats: role of oxidative stress. Free Radic Biol Med. 2008;44:584–93. doi: 10.1016/j.freeradbiomed.2007.10.047. doi:10.1016/j.freeradbiomed.2007.10.047. [DOI] [PubMed] [Google Scholar]
- Otis JS, Guidot DM. Procysteine stimulates expression of key anabolic factors and reduces plantaris atrophy in alcohol-fed rats. Alcohol Clin Exp Res. 2009;33:1450–9. doi: 10.1111/j.1530-0277.2009.00975.x. doi:10.1111/j.1530-0277.2009.00975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JS, Brown LA, Guidot DM. Oxidant-induced atrogin-1 and transforming growth factor-beta1 precede alcohol-related myopathy in rats. Muscle Nerve. 2007;36:842–8. doi: 10.1002/mus.20883. doi:10.1002/mus.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JS, Ashikhmin YI, Brown LA, et al. Effect of HIV-1-related protein expression on cardiac and skeletal muscles from transgenic rats. AIDS Res Ther. 2008;5:8. doi: 10.1186/1742-6405-5-8. doi:10.1186/1742-6405-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters TJ, Martin F, Ward K. Chronic alcoholic skeletal myopathy—common and reversible. Alcohol. 1985;2:485–9. doi: 10.1016/0741-8329(85)90120-x. doi:10.1016/0741-8329(85)90120-X. [DOI] [PubMed] [Google Scholar]
- Preedy VR, Ohlendieck K, Adachi J, et al. The importance of alcohol-induced muscle disease. J Muscle Res Cell Motil. 2003;24:55–63. doi: 10.1023/a:1024842817060. doi:10.1023/A:1024842817060. [DOI] [PubMed] [Google Scholar]
- Rabinovich RA, Ardite E, Mayer AM, et al. Training depletes muscle glutathione in patients with chronic obstructive pulmonary disease and low body mass index. Respiration. 2006;73:757–61. doi: 10.1159/000094395. doi:10.1159/000094395. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Keeton RG, Hewlett RH. Chronic proximal muscular weakness in alcoholics. S Afr Med J. 1976;50:2095–8. [PubMed] [Google Scholar]
- Seiva FR, Amauchi JF, Rocha KK, et al. Alcoholism and alcohol abstinence: N-acetylcysteine to improve energy expenditure, myocardial oxidative stress, and energy metabolism in alcoholic heart disease. Alcohol. 2009;43:649–56. doi: 10.1016/j.alcohol.2009.09.028. doi:10.1016/j.alcohol.2009.09.028. [DOI] [PubMed] [Google Scholar]
- Servais S, Letexier D, Favier R, et al. Prevention of unloading-induced atrophy by vitamin E supplementation: links between oxidative stress and soleus muscle proteolysis? Free Radic Biol Med. 2007;42:627–35. doi: 10.1016/j.freeradbiomed.2006.12.001. doi:10.1016/j.freeradbiomed.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestoft L, Iversen P, Nordgaard I, et al. Working capacity and expression of myosin heavy chain isoforms in skeletal muscle of chronic alcoholic men without liver disease after 1 day and 4 weeks of alcohol abstinence. Clin Sci (Lond) 1994;86:433–40. doi: 10.1042/cs0860433. [DOI] [PubMed] [Google Scholar]
- Slavin G, Martin F, Ward P, et al. Chronic alcohol excess is associated with selective but reversible injury to type 2B muscle fibres. J Clin Pathol. 1983;36:772–77. doi: 10.1136/jcp.36.7.772. doi:10.1136/jcp.36.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary TC, Nairn AC, Lang CH. Restoration of protein synthesis in heart and skeletal muscle after withdrawal of alcohol. Alcohol Clin Exp Res. 2004;28:517–25. doi: 10.1097/01.alc.0000121653.80502.54. doi:10.1097/01.ALC.0000121653.80502.54. [DOI] [PubMed] [Google Scholar]
- Vary TC, Frost RA, Lang CH. Acute alcohol intoxication increases atrogin-1 and MuRF1 mRNA without increasing proteolysis in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1777–89. doi: 10.1152/ajpregu.00056.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrall S, Niemela O, Parkkila S, et al. Protein adducts in type I and type II fibre predominant muscles of the ethanol-fed rat: preferential localisation in the sarcolemmal and subsarcolemmal region. Eur J Clin Invest. 2001;31:723–30. doi: 10.1046/j.1365-2362.2001.00848.x. doi:10.1046/j.1365-2362.2001.00848.x. [DOI] [PubMed] [Google Scholar]
- Wu G, Fang YZ, Yang S, et al. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–92. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]