Abstract
Eosinophils are granulocytic leukocytes implicated in numerous aspects of immunity and disease. The precise functions of eosinophils, however, remain enigmatic. Alternative models to study eosinophil biology may thus yield novel insights into their function. Eosinophilic cells have been observed in zebrafish but have not been thoroughly characterized. We used a gata2:eGFP transgenic animal to enable prospective isolation and characterization of zebrafish eosinophils, and demonstrate that all gata2hi cells in adult hematopoietic tissues are eosinophils. Although eosinophils are rare in most organs, they are readily isolated from whole kidney marrow and abundant within the peritoneal cavity. Molecular analyses demonstrate that zebrafish eosinophils express genes important for the activities of mammalian eosinophils. In addition, gata2hi cells degranulate in response to helminth extract. Chronic exposure to helminth- related allergens resulted in profound eosinophilia, demonstrating that eosinophil responses to allergens have been conserved over evolution. Importantly, infection of adult zebrafish with Pseudocapillaria tomentosa, a natural nematode pathogen of teleosts, caused marked increases in eosinophil number within the intestine. Together, these observations support a conserved role for eosinophils in the response to helminth antigens or infection and provide a new model to better understand how parasitic worms activate, co-opt, or evade the vertebrate immune response.
Introduction
Eosinophils constitute a distinct lineage of granulocytes that provide innate immune surveillance, assistance with T lymphocyte–mediated humoral immune responses, and tissue remodeling via release of granule components.1 In particular, eosinophils have long been thought to act as the primary sentinels against helminth infection.2 Recent studies in the mouse, however, have demonstrated that sustained helminth infections can require the presence of eosinophils.3 In addition, further studies in the mouse where eosinophils were specifically ablated using genetic techniques have yielded conflicting phenotypes that differ based on the background strain.4,5 It has therefore been difficult to determine the precise and specific roles of eosinophils in the immune response. Because most studies of eosinophil function have been performed using the mouse model, we sought an alternative model in which to query eosinophil function.
In this report, we identify and characterize eosinophils in the zebrafish, a small cyprinid teleost that features an immune system similar to that of mammals.6 Critical for their use in immunologic investigation is the fact that both the innate and adaptive immune systems appear to be highly conserved from zebrafish to mammals in terms of gene function, humoral factors, and effector cell lineages.6 Recent studies have demonstrated that the zebrafish is a suitable host for a variety of infectious diseases, including Gram-positive and Gram-negative bacteria, viruses, and parasites.7–9 In some cases, disease modeling in the zebrafish has proven to more accurately recapitulate human disease than in rodents. For example, Mycobacterium marinum, a close relative to M tuberculosis, causes granuloma formation in the zebrafish, a hallmark of human M tuberculosis disease that is lacking in the mouse model.10 Furthermore, infection using fluorescently labeled bacteria allows disease dissemination to be imaged directly in translucent zebrafish embryos.11 Given that teleosts in general are susceptible to infection by helminths,12,13 we set out to describe and delineate the features and functions of zebrafish eosinophils to assess their participation in immune responses known to involve eosinophils in mammals. Here we show that expression of a gata2:eGFP transgene, in combination with light-scatter characteristics, can be used to isolate pure populations of zebrafish eosinophils. Within whole kidney marrow (WKM), approximately 5% of leukocytes are gata2hi, whereas more than 50% of peritoneal leukocytes are gata2hi eosinophils under steady-state conditions. By contrast, there exist very few neutrophils in the peritoneal cavity, suggesting that eosinophils serve as a key mediator of innate immune defense in this peripheral site. Morphologic analyses indicate that zebrafish eosinophils strongly resemble mammalian eosinophils. Purified gata2hi cells display a gene expression profile consistent with that shown to be important for mammalian eosinophil development, maintenance, and activation. We show that zebrafish eosinophils degranulate in response to Heligmosomoides polygyrus extract (HpAg) in vitro, suggesting that a common response to helminth infection may be a shared feature of vertebrate eosinophils. Furthermore, we show that sustained exposure to helminthic determinants, including HpAg and papain, induces marked eosinophilia in adult zebrafish in vivo. Finally, we show that live infection with Pseudocapillaria tomentosa leads to significant gastrointestinal eosinophilia. Together, these studies provide the first precise purification and thorough characterization of zebrafish eosinophils, enabling further studies to better determine their functions within the vertebrate immune system.
Methods
Zebrafish stocks and embryos
Zebrafish were mated, staged, and raised as previously described14 and maintained in accordance with University of California, San Diego Institutional Animal Care and Use Committee or Oregon State University Institutional Animal Care and Use Committee guidelines. The transgenic lines Tg(gata2:eGFP)la3 15 and Tg(mpx:eGFP)i113 16 were used.
Cell preparation
Single-cell suspensions from WKM and spleen were prepared from adult zebrafish as described.15 Blood was obtained by heart puncture with heparinized tips after death in tricaine. Intraperitoneal exudate (IPEX) cells were collected via lavage with phosphate-buffered saline (PBS; Mediatech). Four sequential washes using 5 μL of PBS were performed using a 10-μL syringe (Hamilton) for a total volume of 20 μL. Single-cell suspensions from other organs were prepared by manual trituration and filtration. Cell counts were performed using a hemacytometer (Hausser Scientific), using Trypan Blue (Invitrogen).
Flow cytometry
Cells were examined using a LSRII flow cytometer (BD Biosciences) as previously described.15,17 Data were analyzed using FlowJo software Version 9.0.2 (TreeStar). Cells were purified using a FACSAria I (BD Biosciences).
Cytology
Cytospins were performed as described15 using a Shandon Cytospin-4 (Thermo Fisher Scientific). Cells were fixed and stained with hematoxylin and eosin (Thermo Fisher Scientific), Wright-Giemsa (WG), myeloperoxidase (MPX), periodic acid–Schiff (PAS), or Toluidine blue (TB) according to the manufacturer's instructions (Sigma-Aldrich). Blood smears were stained with PAS, with hematoxylin as a counterstain.
Microscopy
Cytospins were imaged using a DP70 microscope (Olympus America). For transmission electron microscopy (TEM), cells were fixed in 3% glutaraldehyde in 0.1M cacodylate buffer. Cells were embedded in 12% gelatin/PBS and pelleted. Gelatin pellets were fixed in buffered 1% osmium tetroxide, washed, and dehydrated in a graded ethanol series and transitioned through propylene oxide. Samples were embedded in Embed 812/Araldite (Electron Microscopy Sciences). Thin (60-nm) sections were cut and mounted on parlodion-coated grids and stained with uranyl acetate and lead citrate for examination on a Philips-CM100 electron microscope (Philips/FEI) at 80 kV. Images were obtained using a Megaview-III camera (Olympus America) and processed using Adobe Photoshop CS.
Real-time quantitative polymerase chain reaction
RNA was obtained using Trizol (Invitrogen) according to the manufacturer's instructions. Genomic contamination was prevented by DNAse treatment (Roche Diagnostics). cDNA was prepared using a Superscript-III First-Strand kit (Invitrogen) with a separate control reaction with no reverse transcriptase. Quantitative polymerase chain reaction was performed using Brilliant SYBR Green master mix (Stratagene) and an Mx3000P System (Stratagene). Samples were amplified in triplicate. Reaction product size was confirmed by gel electrophoresis. Primers were designed with Primer3 software Version 0.4.018 or as indicated (ef1α,19 gata2,19 and mpx19). dr-rnasel2: forward, TTCTGGGCTTTATTCACAAC; reverse, TGAAAGAGAAGCTGAAGACC; gcsfr, forward, TGAAGGATCTTCAACCACAC; reverse, GGGAATTATAGGCCACAAAC; ccr9, forward, AACCTCACTCACTCCTCAAAC; reverse, CAGACCACCAGAGTGTTACC; mhc2dab, forward, CAGGCCTACTTGCATCAATTG; reverse, CAGACCAGATGCTCCGATG.
Preparation of the HpAg
Six female Swiss Webster mice (20 g, 6-8 weeks old) were gavaged with 150 H polygyrus third stage larva (L3) in 0.1-μm–filtered double-distilled water (ddH2O). At day 22 after infection, mice were killed and the small intestines were removed and cut into 3 sections in 0.15M NaCl, and incubated at 37° for 2 hours. Adult H polygyrus (> 200) were transferred with an eyelash pick into ddH2O in a microcentrifuge tube, pelleted for 45 seconds at 300g, washed, and repelleted. Adults were snap-frozen in liquid nitrogen and stored at −80°C. Extracts were prepared using a sonicator (Sonifier 450; Branson), and resulting suspensions were centrifuged at 4000g and sterile 0.22-μm filtered (Millipore). Protein concentration was determined using the bicinchoninic acid protein assay (Pierce Chemical). Extracts were stored at −80°C.
Degranulation assay
Cells were distributed in a 96-well plate and incubated for 2 hours at 32°C and 5% CO2 with media20 containing HpAg or PBS, or lysed with 0.2% Triton-X gata2:eGFPhi cells were plated at 1 × 105 cells/mL. Cell-free supernatants were collected and added to 500 μL of o-phenylenediamine (OPD) (Sigma-Aldrich) in 0.05M phosphate-citrate buffer and 30% hydrogen peroxide to assess degranulation.21 Reactions were stopped after 10 minutes with 4M H2SO4, and assayed by spectrophotometry (SPECTRAmax PLUS384) at 492 nm.
Sensitization assay
Transgenic gata2:eGFP animals were immunized weekly by intraperitoneal injection with 2 μg of papain (Sigma-Aldrich), or 0.5 μg of HpAg, emulsified in incomplete Freund adjuvant (IFA; Sigma-Aldrich), followed by another intraperitoneal injection with emulsified antigen a day before the collection of tissues, after 1 or 4 weeks. Control fish were either treated by intraperitoneal injection with PBS in IFA (vehicle) or left untreated (uninjected). Single-cell suspensions from spleen and blood of treated and untreated fish were prepared as described in “Cell preparation.”
P tomentosa infection assay
P tomentosa infection of adult zebrafish was performed as described previously.8 Fish were demineralized, and histologic sections of individual fish were prepared, with slides being stained with PAS to identify eosinophils. Infection was confirmed by observing the presence of developing larvae in the gut epithelia and lumen. Eosinophils were quantified by counting all nucleated PAS+ cells visible in 25 individual high-power fields (1000× magnifications) of the gastrointestinal tracts of both infected and uninfected animals.
Statistics
Data were analyzed with 2-tailed Student t tests. Differences were deemed significant at P values less than .05.
Results
Distribution of gata2hi cells in tissues of the adult zebrafish
Although considerable progress in the characterization of neutrophils (also termed heterophils) has been made in teleosts, the presence and function of other granulocyte lineages remain unclear.22 Eosinophils were previously identified in zebrafish based on their morphology and ultrastructural characteristics.23,24 In addition, our previous results suggested that eosinophils could be isolated from adult WKM using a gata2:eGFP transgene.15 Because the GATA2 transcription factor is critical for eosinophil differentiation and function,25,26 we reasoned that this transgenic line would enable eosinophil purification from adult zebrafish tissues in a fashion comparable to the purification of neutrophils by use of the mpx:eGFP transgenic line.16
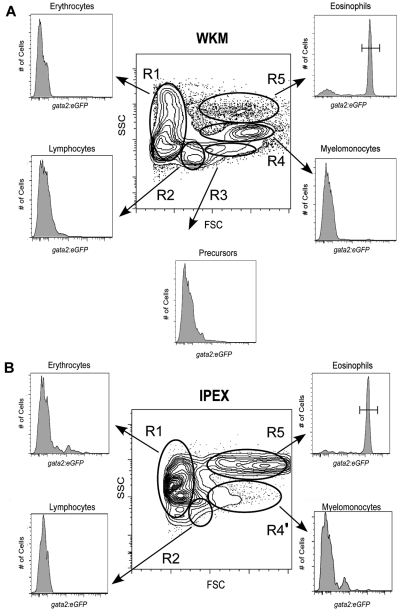
We first analyzed several adult organs for the presence of gata2hi cells, previously observed within the forward scatter (FSC)hi side scatter (SSC)int “myeloid” gate of WKM.6 Compared with cells of other hematopoietic lineages within WKM, we found that gata2hi cells were composed a distinct scatter population that was FSChiSSChi, appearing more granular than other myelomonocytes (Figure 1A, R5), as previously reported by McReynolds et al.17 This region contained fewer cells (3.0% ± 1.3%, n = 26) relative to the conventional myelomonocyte gate (R4, 24% ± 6.9%, n = 26). In gata2:eGFP animals, the FSChiSSChi gate was composed of 74% plus or minus 10% (n = 26) eGFP+ cells. By contrast, all eGFPhi neutrophils in the WKM of mpx:eGFP transgenic animals16 were observed within the conventional FSChiSSCint myeloid gate (supplemental Figure 1A, R4; available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Examination of peripheral blood and additional adult zebrafish tissues, including the spleen, gills, gut, skin, and peritoneal cavity, showed gata2hi cells to be relatively scarce, with the exception of the peritoneal cavity. In IPEX, gata2hi cells were the predominant cell type (Figure 1B; R5, 46.0% ± 18%, n = 16), with other myeloid cells making up a relatively minor population (Figure 1B; R4′, 7.0% ± 2.4%, n = 16). Within the FSChiSSChi fraction, 91% plus or minus 9.5% (n = 16) of cells expressed the gata2:eGFP transgene. By contrast, analysis of mpx:eGFP animals showed that neutrophils were rare in the IPEX (supplemental Figure 1B, R4′). Collectively, these findings suggest that eosinophils are distinct from neutrophils and other hematopoietic lineages, both by expression of gata2:eGFP and by their light scatter characteristics. Together, these parameters provide a reliable technique for the prospective isolation of zebrafish eosinophils.
Figure 1.
Analysis of hematopoietic cells from gata2eGFP transgenic fish by flow cytometry. Contour plots show the regional separation of major blood cell lineages by their light-scatter characteristics: FSCloSSCint-hi (R1, erythroid gate), FSCintSSClo (R2, lymphoid gate), FSChiSSClo (R3, precursor gate), FSChiSSCint (R4, myeloid gate), and FSChiSSChi (R5, eosinophil gate). Histograms indicate the abundance of GFP+ cells in each gate. (A) A representative scatter profile for WKM. Cells within each gate were quantified using the mean ± SD: R1, 37% ± 8.1%; R2, 17% ± 3.95%; R3, 6% ± 1.4%; R4, 24% ± 6.9%; and R5, 3% ± 1.3%. gata2hi cells were only present in the R5 gate: 74% ± 10%; n = 26. (B) A representative scatter profile for IPEX: R1, 9% ± 4.9%; R2, 10% ± 5.2%; R4', 7% ± 2.4%; and R5, 46% ± 18%. gata2hi cells were only present in the R5 gate: 91% ± 9.5%; n = 16.
The morphologic properties of gata2hi cells are those of zebrafish eosinophils
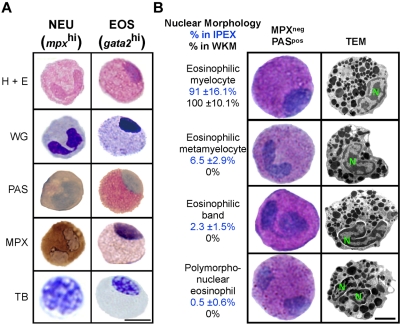
Granulocyte subsets often appear similar to one another by light microscopy but can be distinguished by differential cytochemical staining. Neutrophils, mast cells, and eosinophils have been identified in the zebrafish based on their specific staining for MPX,23,24 TB,27 and PAS,23,24 respectively. GFPhi cells from gata2:eGFP WKM were previously suggested to be eosinophils based on WG staining.6 We sought confirmation of this classification by performing an array of cytochemical stains on cells isolated from 2 different sites. Both gata2hi and mpxhi cells were purified from WKM and IPEX and then subjected to hematoxylin and eosin, WG, PAS, MPX, or TB staining (Figure 2A). Purified gata2hi cells showed pink granule staining with hematoxylin and eosin, purple granule staining with WG, and bright pink cytoplasm with PAS. By contrast, gata2hi cells were negative for MPX or TB stains. As expected, mpxhi cells stained positively for MPX but negatively for PAS or TB stains. These data thus support the designation of mpxhi cells as neutrophils and gata2hi cells as eosinophils.
Figure 2.
gata2hi cells display the morphologic, histochemical, and ultrastructural properties of eosinophils. gata2hi and mpxhi cells were FACS sorted (purity ≥ 95%) from WKM (A-B) or IPEX (B). (A) Purified gata2hi (EOS) and mpxhi (NEU) cells were stained with hematoxylin and eosin, WG, PAS (red precipitate), MPX (brown precipitate), and TB (purple precipitate). Bar represents 5 μm. (B) Purified gata2hi cells were subjected to double staining with MPX and PAS or TEM analysis. A representation of each nuclear morphology (myelocyte, metamyelocyte, band, and polymorphonuclear) is shown as observed by cytospin (left) or TEM (right) analysis. Based on cytochemical analyses, the relative abundance (percentage) in either IPEX (top value) or WKM (bottom value) is provided for each morphologic class. Bar represents 2 μm. N indicates nucleus. Data are mean ± SD (n = 10).
As previously reported, zebrafish mast cells are strongly positive for the TB histologic stain.27 We detected TB+ cells specifically and only within the myelomonocyte gate (FSChiSSCint) of the gill and intestine. Confirming the original report,27 we demonstrated that TB+ cells in these tissues displayed mast cell morphologies characterized by ovoid, eccentric, unsegmented nuclei, high cytoplasmic-to-nuclear ratios, and large, irregular shapes (supplemental Figure 2A). TEM analysis of cells isolated from the myelomonocyte gate of the zebrafish intestine confirmed these morphologic characteristics for mast cells (supplemental Figure 2B). By contrast, gata2hi cells, almost exclusively localized to the eosinophil scatter gate (FSChiSSChi) of WKM and IPEX (Figure 1A-B), displayed round cell shape with lower cytoplasmic-to-nuclear ratios and were negative for TB (Figure 2). Collectively, the staining properties of gata2hi cells distinguish them from neutrophils and mast cells and further support our designation of these cells as eosinophils.
In mammals, both neutrophils and eosinophils undergo a maturation process that involves nuclear segmentation.28,29 Various stages of nuclear remodeling have been observed in zebrafish neutrophils, but only one nuclear morphology has been described for eosinophils in zebrafish and other cyprinid fish.23,24,30 Within WKM, gata2hi cells exhibit the previously described nuclear morphology: a round to oval shaped nucleus typically located peripherally (Figure 2A-B). By contrast, gata2hi cells from the peritoneal cavity displayed a range of nuclear morphologies that appeared similar to those observed in higher vertebrates (Figure 2B). We performed manual counts to evaluate the relative abundance of each form found in WKM and IPEX. The nuclear morphologies of eosinophils in WKM are primarily eosinophilic myelocytes in appearance (Figure 2B). In IPEX, however, gata2hi cells displayed a range of nuclear morphologies. The majority of gata2hi cells were eosinophilic myelocytes in appearance (91% ± 16.1%), with the remaining cells showing nuclear morphologies consistent with those observed in mature eosinophils of higher vertebrates, including eosinophilic metamyelocytes (6.5% ± 2.9%), eosinophilic bands (2.3% ± 1.5%), and polymorphonuclear forms (0.5% ± 0.6%). We observed a similar range in morphologies in gata2hi cells isolated from the skin (data not shown). TEM analysis of gata2hi cells from either WKM or IPEX confirmed the range of nuclear morphologies noted in peritoneal eosinophils, as well as spherical, electron-dense granules23,24 that were similar regardless of the cell's nuclear morphology (Figure 2B).
Next, we wanted to compare the abundance of eosinophils using differential cell counts by histochemical means with that obtained by fluorescence-activated cell sorter (FACS) expression of gata2:eGFP. For differential counts, we used combined staining with MPX and PAS to quantitate and distinguish between neutrophils (MPX+PAS−)23 and eosinophils (MPX−PAS+) in adult tissues (Table 1). By this methodology, approximately half of kidney leukocytes were neutrophils and approximately 6% were eosinophils. Within the peritoneal cavity, these ratios were inverted, with just more than 3% of IPEX cells being neutrophils and more than 56% eosinophils. Overall, our manual counts of eosinophil percentages closely matched those performed by FACS in either tissue (Table 1). Thus, enumeration and isolation of eosinophils from zebrafish tissues are precisely attained using gata2:eGFP expression and light scatter characteristics by flow cytometry.
Table 1.
Eosinophil abundance relative to other leukocytes in adult zebrafish
| Organ | n | Leukocytes,* % (SD) |
||
|---|---|---|---|---|
| Manual count, neutrophils (MPX+ PAS−) | Manual count, eosinophils (MPX− PAS+) | Flow cytometry, gata2hi cells | ||
| Kidney | 26 | 50.2 (10.0) | 5.9 (2.2) | 4.9 (1.6) |
| IPEX | 10 | 3.4 (3.1) | 56.7 (6.2) | 51.5 (6.0) |
Data are shown as mean ± SD. Average kidney (WKM) cell counts were 9.0 ± 1.8 × 105 (n = 5), and IPEX counts were 1.1 ± 0.3 × 105 (n = 5).
IPEX indicates intraperitoneal exudate; MPX, myeloperoxidase; and PAS, periodic acid–Schiff.
Manual count percentages were determined from single-cell suspensions of different organs stained with both MPX and PAS (500 leukocytes per cytospin); flow cytometry percentages were obtained by calculating the percentage of GFPhi cells among leukocytes.
Zebrafish eosinophils and neutrophils display differential gene expression consistent with their mammalian orthologs
Although zebrafish eosinophils have been retrospectively identified based on morphology, the lack of specific isolation techniques has prevented elucidation of eosinophil function. We sought insight into the potential functions of prospectively isolated zebrafish eosinophils by first analyzing the expression profiles of eosinophil-associated genes by quantitative polymerase chain reaction. Using mpxhi neutrophil gene expression as a reference, we determined that gata2hi granulocytes expressed genes thought to be important for eosinophil development and function. Furthermore, gata2hi cells did not express genes important for neutrophil development and function.
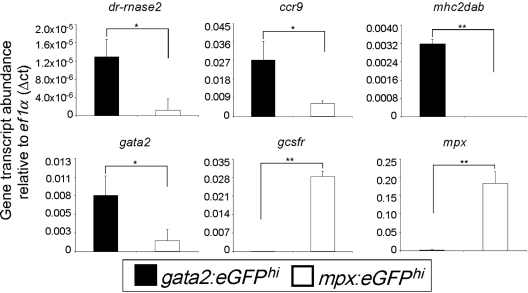
The Gata2 transcription factor plays an essential role in eosinophil development. We confirmed expression of gata2 in zebrafish eosinophils and found significantly fewer transcripts in mpxhi neutrophils (Figure 3). Our morphologic investigations confirmed previous reports that zebrafish eosinophils are negative for MPX cytochemical staining. Correspondingly, we found that gata2hi cells did not express the mpx gene. Neutrophils, by contrast, robustly expressed mpx. The receptor for granulocyte colony-stimulating factor is essential for neutrophil but not eosinophil development, as granulocyte colony-stimulating factor receptor−/− knockout mice have severe neutropenia without differences in eosinophil number.31 Other reports have verified the importance of gcsfr for zebrafish neutrophil biology.32 We confirmed the differential requirement of this receptor by zebrafish granulocytes in our observation that gcsfr was expressed highly by mpxhi cells but not significantly expressed by gata2hi cells.
Figure 3.
gata2hi and mpxhi granulocytes differentially express genes that are important for eosinophil or neutrophil development and function, respectively. gata2hi and mpxhi cells were isolated by FACS (purity ≥ 95%) from WKM. Transcript abundance was measured from either gata2hi (black bars) or mpxhi cells (white bars) by quantitative polymerase chain reaction analysis. Expression of the gene of interest was normalized to that of ef1α. Data are mean ± SD (n = 3 or 4). *P < .05. **P < .001.
The primary effector function of eosinophils is presumed to derive from their granule contents after degranulation. RNases compose 2 of the eosinophil cationic granule proteins: EDN/RNase2 (eosinophil-derived neurotoxin) and ECP/RNase3 (eosinophil cationic protein). Three RNases (dr-rnase1, dr-rnase2, and dr-rnase3) have been identified in zebrafish as being cationic and possessing bactericidal and ribonucleolytic activity,33 with dr-rnase1 and dr-rnase2 being expressed in immune-related tissues, such as the peritoneal cavity, skin, and thymus (not shown). We measured similar levels of dr-rnase1 transcript in mpxhi cells compared with gata2hi cells, indicating that the function of this RNase may be shared with other leukocytes (not shown). Strikingly, we observed a high enrichment of dr-rnase2 transcripts in gata2hi cells compared with mpxhi cells, suggesting that this gene may be a key mediator of zebrafish eosinophil function (Figure 3). dr-rnase3 expression was not detected in either granulocyte subset. We also searched for additional genes encoding eosinophil-derived granule proteins, such as MBP (major basic protein) or EPX (eosinophil peroxidase), but failed to find clear homologs.
In addition to degranulation, eosinophils have been demonstrated to mediate immunity by a variety of other mechanisms. Chemokines and their receptors function through diverse cell signaling pathways to regulate leukocyte polarity, migration, growth, differentiation, and death.34,35 In zebrafish, there are 17 C-C chemokine receptor genes, including homologs for mammalian Ccr6, 7, 8, 9, and Ccrl1 genes.36 ccr9 was previously shown to be expressed in immune-related tissues of the zebrafish,36 and we found it to be expressed selectively by gata2hi cells compared with mpxhi cells (Figure 3). Class II major histocompatibility complex (MHC) proteins are expressed by human airway eosinophils but have not been observed to be expressed by neutrophils.37 Correspondingly, we found that gata2hi cells expressed MHC class II transcripts, but mpxhi cells did not.
In summary, the gene expression profiles of gata2hi and mpxhi granulocyte subsets demonstrate that these populations are distinct. Furthermore, the conservation in gene expression programs within each subset further supports the designation of these cells as zebrafish equivalents of mammalian eosinophils and neutrophils, respectively.
Degranulation of gata2hi cells in response to H polygyrus helminth extracts
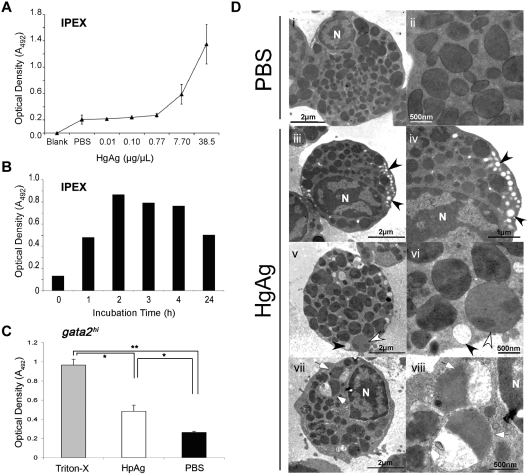
Our morphologic, histochemical, and gene expression analyses indicate that gata2hi cells are eosinophils and suggest that the functions of these cells may be conserved with their mammalian counterparts. A primary role of eosinophils within the mammalian immune system is thought to be the attack of helminthic parasites via degranulation of cytotoxic cationic proteins during infection.2 In higher vertebrates, granules in eosinophils are noted for their bactericidal, ribonucleolytic, cytotoxic, helminthotoxic, and peroxidasic activities.1 Although both cytochemical staining and TEM analyses have demonstrated the presence of granules in zebrafish eosinophils,23,24 there is little information regarding their contents. Our results presented in Figure 3 suggest that eosinophil RNAses might provide some granule defense activity. Although gata2hi cells did not express mpx (Figure 3), zebrafish eosinophils have previously been identified as containing peroxidasic granules.24 Because other eosinophilic peroxidases such as EPX have yet to be identified, we sought alternate means to measure peroxidase activity. In mammals, peroxidase activity after eosinophil degranulation can be measured using the OPD assay.21 Here, we used this assay to measure the response of zebrafish eosinophils to H polygyrus helminth extracts in vitro. After the addition of HpAg to cultures containing IPEX cells, we found that the supernatant contained peroxidase activity (Figure 4). Furthermore, the release of peroxidase from IPEX cells was dependent on the concentration of HpAg and the incubation time (Figure 4A-B). To ensure that eosinophils contributed to the peroxidase release in response to HpAg, we performed the same assay with purified gata2hi cells. HpAg induced gata2hi cells to release approximately 2-fold more peroxidase than those incubated in media alone (Figure 4C). Maximal peroxidase release was gauged by detergent lysis using Triton-X.
Figure 4.
gata2hi eosinophils degranulate in response to H polygyrus helminth extracts. (A) IPEX from 3 WT fish was cultured in media containing either HpAg over a concentration gradient or in PBS alone for 4 hours. Cell-free supernatants were collected and assayed for peroxidase activity using the OPD assay. An average of the optical density (A492) for each dose was taken from 3 independent experiments. Error bars represent SD. (B) Time course of degranulation as measured by OPD assay. IPEX was cultured in media with 10 μg HpAg; then cell-free supernatants were assayed for degranulation. (C) Purified gata2hi cells (∼ 5 × 104) from IPEX were lysed with 0.2% Triton-X or cultured for 2 hours in media with either 10 μg of HpAg or PBS. Cell-free supernatants were then collected and assayed for peroxidase activity using the OPD assay. Bars represent the mean optical density (A492) measured from 3 independent experiments with SD. *P < .05. **P < .001. (D) TEM photomicrographs show purified gata2hi cells (∼ 2.5 × 105) cultured for 2 hours in media with either 12 μg of HpAg (iii-viii) or PBS (i-ii). Note heterogeneous populations of granules that display a range of electron lucency; granules are completely lucent (black arrowheads), marbled (white arrowheads), or variegated (white arrows). Electron-lucent granules were localized along cell peripheries (iii-vi). N indicates nucleus. Images are representative of 2 independent experiments.
Eosinophils exhibit variable degrees of degranulation, ranging from partial to nearly complete.38 We analyzed the ultrastructure of gata2hi cells after incubation with HpAg to survey for morphologic changes indicative of degranulation. The majority of gata2hi cells incubated in media with PBS contained fully intact, spherical electron-dense granules (Figure 4Di-ii), with few cells containing electron-lucent, peripheral granules. The frequency of cells containing electron-lucent granules at the periphery increased at least 20-fold (data not shown) in gata2hi cells incubated in media with HpAg (Figure 4Diii-viii). In addition to the greater frequency of electron-lucent granules, the granules of HpAg-stimulated eosinophils had a highly heterogeneous appearance. Granules appeared marbled in electron density, and some contained both electron-lucent and electron-dense regions (Figure 4Dv-viii). Another striking difference was an increase in the size and number of lipid bodies in cells stimulated with HpAg (not shown), consistent with the reported induction of lipid body formation in mammalian eosinophils after exposure to inflammatory stimuli.39 Taken together with the results from the OPD assay, the ultrastructural properties of stimulated gata2hi cells suggest that zebrafish eosinophils degranulate in response to helminth extract.
Chronic exposure to helminth-related antigens results in pronounced eosinophilia
During homeostatic conditions, eosinophils are rare in blood and most tissues. However, various inflammatory insults and hematologic malignancies can result in large increases in eosinophil number that are collectively termed eosinophilia.40 Allergenic sensitization and parasitic helminth infections are potent inducers of peripheral tissue eosinophilia and have been well studied in human and murine models.41,42 Despite numerous accounts of eosinophilia during allergic and helminth-related immune responses, the relationships between these pathologies and eosinophil function remain poorly understood.3,41,42 We were interested in determining the in vivo response of zebrafish gata2hi cells to helminth components to determine the efficacy of using the zebrafish model to better understand the role of eosinophils in immunity.
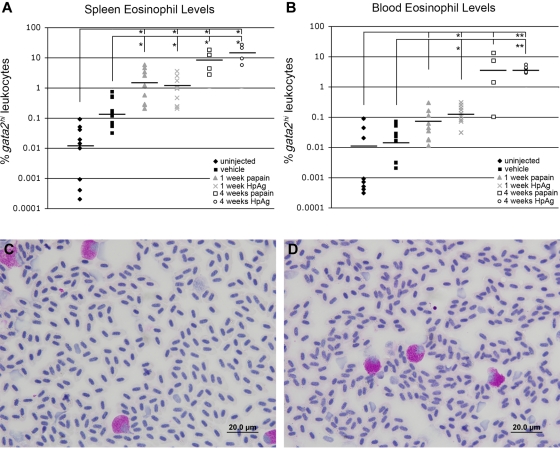
We used a series of injections using components of helminth infection to elicit an eosinophil response. Papain-like cysteine proteases are released by helminth parasites to facilitate skin and intestinal infection, tissue migration, and feeding.43 Furthermore, in vitro studies have shown that human eosinophils are activated by papain and are induced to release inflammatory mediators via degranulation.44 We found that the spleens of gata2:eGFP zebrafish immunized with papain for one or 4 weeks were enlarged and contained approximately 100- or 1000-fold increases in eosinophil percentages from steady-state levels, respectively (Figure 5A). The percentage of eosinophils within peripheral blood leukocytes from the same animals exhibited approximately 10- or 100-fold increases, respectively (Figure 5B). Consistent with our in vitro findings using HpAg helminth extract, we observed an approximate 1000-fold increase in splenic eosinophil frequency after repeated injections of extract over 4 weeks (Figure 5A) and an approximate 500-fold increase in peripheral blood eosinophil frequency (Figure 5B). Flow cytometric increases of gata2hi cells were confirmed by dramatic increases in PAS+ eosinophils in peripheral blood smears from animals injected with papain (Figure 5C) or HpAg (Figure 5D) for 4 weeks. Collectively, the development of eosinophilia after systemic immunization with helminth-related antigens indicates that the response of zebrafish eosinophils to allergens has been conserved over evolution.
Figure 5.
Sustained exposure to papain or H polygyrus extract induces splenic and peripheral blood eosinophilia. (A) Spleens and (B) blood from treated and untreated adult gata2:eGFP+ zebrafish were analyzed for gata2hi leukocyte levels by FACS. Treated groups were injected weekly with papain or HpAg emulsified in IFA and tissues collected after one or 4 weeks. Control animals were either mock treated or uninjected. Bars represent means. *P < .05. **P < .001. Blood smears from fish immunized for 4 weeks with papain (C) or HpAg (D) showed elevated frequencies of PAS+ eosinophils. Images in panels C and D were acquired on an Olympus DP70 microscope with a 100× oil objective stained with PAS and hematoxylin. Images were collected with an Olympus DP Controller Version 2.1.1.183 and processed with Adobe Photoshop CS.
Parasitic helminth infection induces marked gastrointestinal eosinophilia in zebrafish
As sensitization with helminthic antigens resulted in eosinophilia, we were interested in determining the participation of zebrafish eosinophils in the host immune response to live helminth infection. Eosinophils are relatively rare in the gastrointestinal tract of healthy mice, localized within the lamina propria.45 After infection by helminths, however, murine gastrointestinal eosinophils dramatically increase in number.46 We used P tomentosa, a natural gastrointestinal pathogen of zebrafish, to measure eosinophil abundance during infection.8
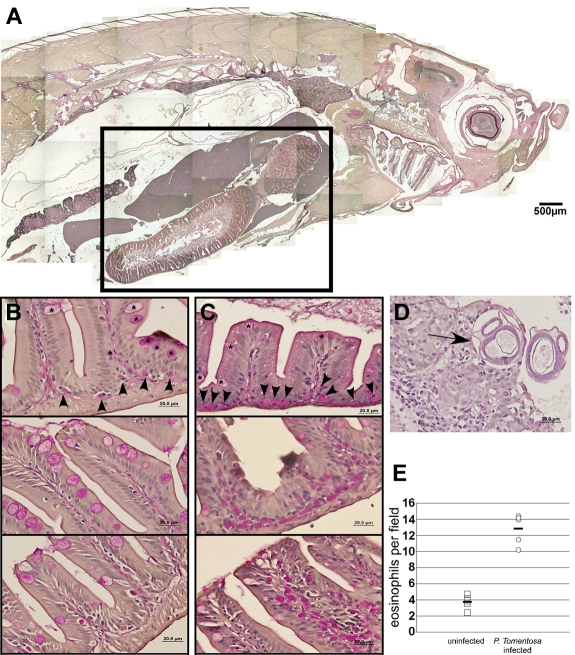
Helminth-infected and uninfected adult zebrafish were embedded in paraffin, sectioned sagittally, and stained with PAS to observe eosinophil distribution (Figure 6A). Uninfected fish exhibited sparsely distributed eosinophils along the base of the intestinal lamina propria (Figure 6B). By contrast, infected animals showed increased concentration of PAS+ eosinophils along the lamina propria, with considerable infiltration into the luminal projections of the gut (Figure 6C). Infection by P tomentosa was confirmed by the presence of larvae in the gastrointestinal epithelia and lumen (Figure 6D). Compared with uninfected animals, the gastrointestinal eosinophil number in infected animals showed an approximate 3-fold increase (Figure 6E). Taken together with the sensitization data, these findings strongly support the conservation of eosinophil-mediated immune responses in zebrafish to those in mammals.
Figure 6.
In vivo infection by P tomentosa leads to colonization of the gut and peritoneal cavity and an increase in local eosinophil number. (A) Composite image of sagittal section of adult zebrafish, stained with PAS. Inset: Area including intestines, shown in close-up views in panels B and C. (B) Imaging of the gut in uninfected animals shows nucleated PAS+ eosinophils interspersed along the basal lamina propria (arrowheads). Intestinal goblet cells (asterisks) are also PAS+, but distinct from eosinophils. (C) Infected animals show an increased number of PAS+ eosinophils along the basal lamina propria and also distally localized into villi. (D) P tomentosa larvae are observed within gut tissue (arrow). (E) Counts of eosinophils increase approximately 3-fold in intestines of infected animals. Images in panels A through D were acquired with an Olympus DP70 microscope and collected with Olympus DP Controller Version 2.1.1.183 and processed with Adobe Photoshop CS. Panel A is a composite image of a PAS and hematoxylin-stained section taken with a 10× dry objective. Images in panels B through D were taken with a 100× oil immersion objective and stained with PAS and hematoxylin.
Discussion
In recent years, the zebrafish has emerged as a new vertebrate model to study hematopoiesis and immunity from a unique perspective. In evolutionary terms, the zebrafish offers the opportunity to examine the origins of the extant immune system, the core functions conserved within each hematopoietic lineage, and the specific adaptations that have occurred because teleosts and mammals diverged from a common ancestor. In this study, we have isolated zebrafish eosinophils to enable the precise study of this granulocytic lineage and to compare and contrast the biology of teleost eosinophils to their mammalian counterparts. We demonstrate that expression of a gata2:eGFP transgene, combined with light-scatter characteristics, is sufficient to isolate zebrafish eosinophils to purity. This advance has permitted the thorough characterization of zebrafish eosinophils at the morphologic, histochemical, ultrastructural, and functional levels. These findings form the foundation for future studies to better determine the roles played by eosinophils in the vertebrate immune response.
Gata-2 has been reported to play an essential role in both the differentiation and maintenance of murine eosinophils. Gata2 is required for differentiation of murine myeloid progenitors to eosinophils and is expressed in terminally differentiated cells.25 In addition, enforced expression of Gata2 instructs granulocyte/monocyte-restricted progenitors toward the exclusive fate of eosinophils.47 GATA-2 also regulates the expression of the critical eosinophil granule protein RNAse2.26 These observations are consistent with our findings that a gata2:eGFP transgene labels eosinophils in the zebrafish. Expression of gata2, however, is not exclusive to eosinophils. Expression and function of gata2 are also required for the development of other granulocyte lineages, including mast cells and basophils.47 In teleosts, there exist controversy and confusion regarding the presence and function of mast cells, basophils, and eosinophils. Historically, studies in teleosts have described a granulocyte type termed the mast cell/eosinophilic granule cell resulting from staining characteristics similar to both cell types.22 Although the ultrastructure of gata2hi cells resembles that of mast cell/eosinophilic granule cells in other teleosts, we have determined in this study that zebrafish eosinophils have unique characteristics compared with mast cells. To summarize, we detected TB+ mast cells specifically and only within the myelomonocyte gate (FSChiSSCint) of the gill and intestine. By contrast, gata2hi eosinophils almost exclusively localized to the eosinophil scatter gate (FSChiSSChi) in all tissues analyzed and were negative for TB. In addition, the dynamic nuclear morphologies observed within gata2hi cells in peripheral tissues demonstrate that zebrafish eosinophils bear stronger resemblance to the eosinophils of mammals than previously realized.15,23,24 Whether or not the gata2hi population includes basophils or their precursors remains to be determined. Early studies in the zebrafish suggested the presence of a granulocyte type that resembled both eosinophils and basophils.23 It is currently unknown, however, whether a distinct lineage of basophils exists in the zebrafish or in teleosts in general. Resolving this issue will require the development of additional reagents and assays.
In a comprehensive screen for gata2hi cells and PAS+ cells in adult tissues, we found proportionately more eosinophils in the peritoneal cavity than in any other tissue. Furthermore, other granulocytes were virtually absent from this site. At first glance, this is surprising given that the relative abundance of eosinophils in the mammalian peritoneal cavity is low under steady-state conditions.48 In mammals, however, there is a preferential migration to and robust accumulation of eosinophils in the peritoneal cavity during inflammation and helminth infection, indicating that this site may serve as a reservoir for eosinophils under specific inflammatory conditions.48,49 The abundance of eosinophils in the zebrafish peritoneum under steady-state conditions suggests that the peritoneal cavity may have an evolutionarily conserved role in the storage and dissemination of eosinophils but also highlights potential differences of the immune microenvironment of the peritoneal cavity in teleosts. This may be because fish often serve as intermediate hosts for many parasites that use fish-eating birds or mammals as definitive hosts,50,51 and that sites of parasite residence often include the peritoneal cavity.13
Within the peritoneal cavity, we observed morphologic differences in eosinophils compared with those in WKM. From WKM, gata2hi cells uniformly displayed oval, peripherally localized nuclei typical of the eosinophils previously described in zebrafish.23,24 From IPEX, we observed gata2hi cells that had segmented nuclei more typical of those described in the maturation of granulocytes in higher vertebrates. These data suggest that the uniform morphology previously observed in zebrafish described most eosinophils but that the segmentation of the nucleus into a polymorphonuclear form is a conserved feature of the vertebrate eosinophil. It is important to note, however, that although the nuclear morphology of zebrafish eosinophils seems to indicate a similar maturation process as seen in mammals, all eosinophils investigated showed similar appearance and distribution of electron-dense granules, and all showed similar peroxidase activity. In addition, eosinophilic myelocytes were observed in peripheral sites during helminthic sensitization and infection studies. It may be possible to use recent advances in zebrafish cell culture techniques to investigate precursor-progeny relationships within the eosinophilic lineage.20
In this study, we performed the first gene expression analyses in purified zebrafish eosinophils and observed the expression of genes important for mammalian eosinophil development and function. In the case of dr-rnase2, we observed strong expression in gata2hi cells, and at levels significantly higher than in mpxhi neutrophils. RNAses are noted for their ribonucleolytic and antiviral activities, toxicity toward helminthic parasites, and bactericidal properties.1 Although dr-rnase2 is not a clear homolog of the mammalian EDN/RNase2 or ECP/RNase3 genes, this RNase has been shown to have similarly potent bactericidal activities.33 Our results provide evidence that dr-rnase2 may be an eosinophil-specific cationic protein and supports the possibility that eosinophils have provided similar host defense mechanisms from early on in the evolutionary history of the cell.52 Careful analyses of the granule contents of zebrafish eosinophils are required to better elucidate the role of RNAses in the leukocytes of basal vertebrates. In vitro studies have shown that human peripheral blood eosinophils express the CCR9 chemokine receptor and migrate toward its cognate CCL25 ligand after stimulation with interferon-γ, interleukin-3, and granulocyte-macrophage colony-stimulating factor.53 Furthermore, human airway eosinophils have been observed to express elevated levels of CCR9, verifying the in vivo relevance of this chemokine receptor in eosinophil biology.54 In addition to the specific up-regulation of CCR9 on airway eosinophils, MHC class II proteins and costimulatory molecules are likewise up-regulated, compared with peripheral blood eosiniphils.37,55 These observations have led to speculation that CCR9 plays a role in eosinophil trafficking to tissues where they may regulate the adaptive immune response by functioning as antigen-presenting cells during inflammation.53 We found that gata2hi cells expressed MHC class II transcripts, whereas mpxhi cells did not. Whether or not zebrafish eosinophils can traffic and present antigen to lymphocytes remains to be determined.
A previous study suggested that the granules of zebrafish eosinophils have peroxidase activity.24 Our results indicate that zebrafish eosinophils lack expression of the mpx gene, are negative for MPX activity, and exhibit granules with ultrastructural properties distinct from neutrophils. Zebrafish eosinophils therefore probably express a yet to be described ortholog of the eosinophil peroxidase precursor (epx) gene and/or a peroxidase gene not previously described. Functionally, we determined that the granules of zebrafish eosinophils contain peroxidase activity as measured by the OPD assay and that degranulation occurs in response to helminth extracts. These results were confirmed by ultrastructural analyses showing that gata2hi eosinophils partially degranulated in response to HpAg extracts. Although the robustness of this response was similar to that noted previously in assays for mammalian eosinophils,56 it is worth noting that H polygyrus is a pathogen tropic to rodents and thus may not elicit optimal responses from zebrafish eosinophils. Despite these species differences, systemic sensitization with either HpAg extract or papain induced dramatic increases in eosinophil number in vivo. Furthermore, live infection with a natural helminth pathogen also resulted in increased eosinophil number. These results indicate that many of the determinants that elicit eosinophilic immune responses in mammals have been conserved across evolution. Taken together, our results suggest that further zebrafish infection models, both using the assays described here and using additional helminth and fungal pathogens native to teleosts, will serve to better elucidate the roles of eosinophils in the vertebrate immune response.
Supplementary Material
Acknowledgments
The authors thank Dawne Page, Miriam Werneck, Valerie Wittamer, and Julien Bertrand for critical evaluation of the manuscript, J. Bertrand and V. Wittamer for ccr9, gcsfr, and mhc2 primer sequences, Malcolm Wood for TEM analyses, Roger Rainville and Lisa Phelps for excellent animal care, and Kerstin Richter for excellent laboratory management.
This work was supported by a National Institute of Diabetes and Digestive and Kidney Diseases research supplement fellowship (3R01DK074482-01; G.L.-V.), Institutional Research and Academic Career Development Award Fellowship GMO68524 (G.L.-V.), University of California, San Diego (CURE 5P30CA23100-22S2; K.M.B.), San Diego State University (MARC 5T34GM08303; O.R.-F.), National Research Service Award T32-HL086344 (D.L.S.), National Institutes of Health grants R01-AI056189 (R.V.A.) and R01-DK074482) (D.T.), the American Society of Hematology (D.T.), the Arnold and Mabel Beckman Foundation (D.T.), and the Senyei Family Foundation (D.T.). Research at Oregon State University was supported by US Public Health Service (National Institutes of Environmental Health Sciences grants ES011587, ES03850, ES00210, and ES013124), the National Center for Research Resources (grant RR12546), from the Center for Fish Disease Research (pilot funding), and the John Fryer Salmon Disease Laboratory at Oregon State University.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.M.B. and G.L.-V. performed the research and analyzed the data; D.L.S., K.B., and O.R.-F. performed aspects of the research; Y.H. and R.V.A. prepared and provided the H polygyrus extracts; J.M.S. prepared and provided sections and paraffin blocks of P tomentosa–infected animals; K.M.B., G.L.-V., and D.T. designed the research; and K.M.B., G.L.-V., D.L.S., and D.T. wrote the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Traver, University of California, San Diego Division of Biological Sciences, Section of Cell and Developmental Biology, 9500 Gilman Dr, La Jolla, CA 92093-0380; e-mail: dtraver@ucsd.edu.
References
- 1.Hogan SP, Rosenberg HF, Moqbel R, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38(5):709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 2.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 3.Fabre V, Beiting DP, Bliss SK, et al. Eosinophil deficiency compromises parasite survival in chronic nematode infection. J Immunol. 2009;182(3):1577–1583. doi: 10.4049/jimmunol.182.3.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humbles AA, Lloyd CM, McMillan SJ, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305(5691):1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 5.Lee JJ, Dimina D, Macias MP, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305(5691):1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 6.Traver D, Herbomel P, Patton EE, et al. The zebrafish as a model organism to study development of the immune system. Adv Immunol. 2003;81:253–330. [PubMed] [Google Scholar]
- 7.Kent ML, Bishop-Stewart JK. Transmission and tissue distribution of Pseudoloma neurophilia (Microsporidia) of zebrafish, Danio rerio (Hamilton). J Fish Dis. 2003;26(7):423–426. doi: 10.1046/j.1365-2761.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- 8.Kent ML, Bishop-Stewart JK, Matthews JL, Spitsbergen JM. Pseudocapillaria tomentosa, a nematode pathogen, and associated neoplasms of zebrafish (Danio rerio) kept in research colonies. Comp Med. 2002;52(4):354–358. [PubMed] [Google Scholar]
- 9.Sullivan C, Kim CH. Zebrafish as a model for infectious disease and immune function. Fish Shellfish Immunol. 2008;25(4):341–350. doi: 10.1016/j.fsi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Lesley R, Ramakrishnan L. Insights into early mycobacterial pathogenesis from the zebrafish. Curr Opin Microbiol. 2008;11(3):277–283. doi: 10.1016/j.mib.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meijer AH, van der Sar AM, Cunha C, et al. Identification and real-time imaging of a myc-expressing neutrophil population involved in inflammation and mycobacterial granuloma formation in zebrafish. Dev Comp Immunol. 2008;32(1):36–49. doi: 10.1016/j.dci.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Dezfuli BS, Lui A, Boldrini P, Pironi F, Giari L. The inflammatory response of fish to helminth parasites. Parasite. 2008;15(3):426–433. doi: 10.1051/parasite/2008153426. [DOI] [PubMed] [Google Scholar]
- 13.Secombes CJ. Fish immune responses to experimental and natural infection with helminth parasites. Annu Rev Fish Dis. 1996;6:167–177. [Google Scholar]
- 14.Reinhard E, Nedivi E, Wegner J, Skene JH, Westerfield M. Neural selective activation and temporal regulation of a mammalian GAP-43 promoter in zebrafish. Development. 1994;120(7):1767–1775. doi: 10.1242/dev.120.7.1767. [DOI] [PubMed] [Google Scholar]
- 15.Traver D, Paw BH, Poss KD, Penberthy WT, Lin S, Zon LI. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat Immunol. 2003;4(12):1238–1246. doi: 10.1038/ni1007. [DOI] [PubMed] [Google Scholar]
- 16.Renshaw SA, Loynes CA, Trushell DM, Elworthy S, Ingham PW, Whyte MK. A transgenic zebrafish model of neutrophilic inflammation. Blood. 2006;108(13):3976–3978. doi: 10.1182/blood-2006-05-024075. [DOI] [PubMed] [Google Scholar]
- 17.McReynolds LJ, Tucker J, Mullins MC, Evans T. Regulation of hematopoiesis by the BMP signaling pathway in adult zebrafish. Exp Hematol. 2008;36(12):1604–1615. doi: 10.1016/j.exphem.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 19.Bertrand JY, Kim AD, Teng S, Traver D. CD41+ cmyb+ precursors colonize the zebrafish pronephros by a novel migration route to initiate adult hematopoiesis. Development. 2008;135(10):1853–1862. doi: 10.1242/dev.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stachura DL, Reyes JR, Bartunek P, Paw BH, Zon LI, Traver D. Zebrafish kidney stromal cell lines support multilineage hematopoiesis. Blood. 2009;114(2):279–289. doi: 10.1182/blood-2009-02-203638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adamko DJ, Wu Y, Gleich GJ, Lacy P, Moqbel R. The induction of eosinophil peroxidase release: improved methods of measurement and stimulation. J Immunol Methods. 2004;291(1):101–108. doi: 10.1016/j.jim.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Reite OB, Evensen O. Inflammatory cells of teleostean fish: a review focusing on mast cells/eosinophilic granule cells and rodlet cells. Fish Shellfish Immunol. 2006;20(2):192–208. doi: 10.1016/j.fsi.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Bennett CM, Kanki JP, Rhodes J, et al. Myelopoiesis in the zebrafish, Danio rerio. Blood. 2001;98(3):643–651. doi: 10.1182/blood.v98.3.643. [DOI] [PubMed] [Google Scholar]
- 24.Lieschke GJ, Oates AC, Crowhurst MO, Ward AC, Layton JE. Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood. 2001;98(10):3087–3096. doi: 10.1182/blood.v98.10.3087. [DOI] [PubMed] [Google Scholar]
- 25.Hirasawa R, Shimizu R, Takahashi S, et al. Essential and instructive roles of GATA factorsin eosinophil development. J Exp Med. 2002;195(11):1379–1386. doi: 10.1084/jem.20020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu Z, Dyer KD, Xie Z, Radinger M, Rosenberg HF. GATA transcription factors regulate the expression of the human eosinophil-derived neurotoxin (RNase 2) gene. J Biol Chem. 2009;284(19):13099–13109. doi: 10.1074/jbc.M807307200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobson JT, Seibert J, Teh EM, et al. Carboxypeptidase A5 identifies a novel mast cell lineage in the zebrafish providing new insight into mast cell fate determination. Blood. 2008;112(7):2969–2972. doi: 10.1182/blood-2008-03-145011. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez JA, Wangh LJ. New insights into the mechanisms of nuclear segmentation in human neutrophils. J Cell Biochem. 1999;73(1):1–10. doi: 10.1002/(sici)1097-4644(19990401)73:1<1::aid-jcb1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 29.Sparrevohn S, Wulff HR. The nuclear segmentation of eosinophils under normal and pathological conditions. Acta Haematol. 1967;37(2):120–125. doi: 10.1159/000209059. [DOI] [PubMed] [Google Scholar]
- 30.Bielek E. Developmental stages and localization of peroxidatic activity in the leucocytes of three teleost species (Cyprinus carpio L.; Tinca tinca L.; Salmo gairdneri Richardson). Cell Tissue Res. 1981;220(1):163–180. doi: 10.1007/BF00209975. [DOI] [PubMed] [Google Scholar]
- 31.Lieschke GJ, Grail D, Hodgson G, et al. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84(6):1737–1746. [PubMed] [Google Scholar]
- 32.Liongue C, Hall CJ, O'Connell BA, Crosier P, Ward AC. Zebrafish granulocyte colony-stimulating factor receptor signaling promotes myelopoiesis and myeloid cell migration. Blood. 2009;113(11):2535–2546. doi: 10.1182/blood-2008-07-171967. [DOI] [PubMed] [Google Scholar]
- 33.Cho S, Zhang J. Zebrafish ribonucleases are bactericidal: implications for the origin of the vertebrate RNase A superfamily. Mol Biol Evol. 2007;24(5):1259–1268. doi: 10.1093/molbev/msm047. [DOI] [PubMed] [Google Scholar]
- 34.Thelen M. Dancing to the tune of chemokines. Nat Immunol. 2001;2(2):129–134. doi: 10.1038/84224. [DOI] [PubMed] [Google Scholar]
- 35.Thelen M, Stein JV. How chemokines invite leukocytes to dance. Nat Immunol. 2008;9(9):953–959. doi: 10.1038/ni.f.207. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Chang MX, Wu SG, Nie P. Characterization of C-C chemokine receptor subfamily in teleost fish. Mol Immunol. 2009;46(3):498–504. doi: 10.1016/j.molimm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Sedgwick JB, Calhoun WJ, Vrtis RF, Bates ME, McAllister PK, Busse WW. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J Immunol. 1992;149(11):3710–3718. [PubMed] [Google Scholar]
- 38.Capron M, Tomassini M, Torpier G, Kusnierz JP, MacDonald S, Capron A. Selectivity of mediators released by eosinophils. Int Arch Allergy Appl Immunol. 1989;88(1):54–58. doi: 10.1159/000234748. [DOI] [PubMed] [Google Scholar]
- 39.Bozza PT, Yu W, Penrose JF, Morgan ES, Dvorak AM, Weller PF. Eosinophil lipid bodies: specific, inducible intracellular sites for enhanced eicosanoid formation. J Exp Med. 1997;186(6):909–920. doi: 10.1084/jem.186.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valent P. Pathogenesis, classification, and therapy of eosinophilia and eosinophil disorders. Blood Rev. 2009;23(4):157–165. doi: 10.1016/j.blre.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Nials AT, Uddin S. Mouse models of allergic asthma: acute and chronic allergen challenge. Dis Model Mech. 2008;1(4–5):213–220. doi: 10.1242/dmm.000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol. 2004;113(1):30–37. doi: 10.1016/j.jaci.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 43.Robinson MW, Dalton JP, Donnelly S. Helminth pathogen cathepsin proteases: it's a family affair. Trends Biochem Sci. 2008;33(12):601–608. doi: 10.1016/j.tibs.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Miike S, Kita H. Human eosinophils are activated by cysteine proteases and release inflammatory mediators. J Allergy Clin Immunol. 2003;111(4):704–713. doi: 10.1067/mai.2003.1332. [DOI] [PubMed] [Google Scholar]
- 45.Rothenberg ME, Mishra A, Brandt EB, Hogan SP. Gastrointestinal eosinophils. Immunol Rev. 2001;179:139–155. doi: 10.1034/j.1600-065x.2001.790114.x. [DOI] [PubMed] [Google Scholar]
- 46.Friend DS, Gurish MF, Austen KF, Hunt J, Stevens RL. Senescent jejunal mast cells and eosinophils in the mouse preferentially translocate to the spleen and draining lymph node, respectively, during the recovery phase of helminth infection. J Immunol. 2000;165(1):344–352. doi: 10.4049/jimmunol.165.1.344. [DOI] [PubMed] [Google Scholar]
- 47.Iwasaki H, Mizuno S, Arinobu Y, et al. The order of expression of transcription factors directs hierarchical specification of hematopoietic lineages. Genes Dev. 2006;20(21):3010–3021. doi: 10.1101/gad.1493506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melichar B, Freedman RS. Immunology of the peritoneal cavity: relevance for host-tumor relation. Int J Gynecol Cancer. 2002;12(1):3–17. doi: 10.1046/j.1525-1438.2002.01093.x. [DOI] [PubMed] [Google Scholar]
- 49.Ohnmacht C, Pullner A, van Rooijen N, Voehringer D. Analysis of eosinophil turnover in vivo reveals their active recruitment to and prolonged survival in the peritoneal cavity. J Immunol. 2007;179(7):4766–4774. doi: 10.4049/jimmunol.179.7.4766. [DOI] [PubMed] [Google Scholar]
- 50.Chai JY, Darwin Murrell K, Lymbery AJ. Fish-borne parasitic zoonoses: status and issues. Int J Parasitol. 2005;35(11):1233–1254. doi: 10.1016/j.ijpara.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 51.Cross JH. Intestinal capillariasis. Clin Microbiol Rev. 1992;5(2):120–129. doi: 10.1128/cmr.5.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee JJ, Lee NA. Eosinophil degranulation: an evolutionary vestige or a universally destructive effector function? Clin Exp Allergy. 2005;35(8):986–994. doi: 10.1111/j.1365-2222.2005.02302.x. [DOI] [PubMed] [Google Scholar]
- 53.Jung YJ, Woo SY, Jang MH, et al. Human eosinophils show chemotaxis to lymphoid chemokines and exhibit antigen-presenting-cell-like properties upon stimulation with IFN-gamma, IL-3 and GM-CSF. Int Arch Allergy Immunol. 2008;146(3):227–234. doi: 10.1159/000115891. [DOI] [PubMed] [Google Scholar]
- 54.Liu LY, Jarjour NN, Busse WW, Kelly EA. Chemokine receptor expression on human eosinophils from peripheral blood and bronchoalveolar lavage fluid after segmental antigen challenge. J Allergy Clin Immunol. 2003;112(3):556–562. doi: 10.1016/s0091-6749(03)01798-6. [DOI] [PubMed] [Google Scholar]
- 55.Shi HZ, Humbles A, Gerard C, Jin Z, Weller PF. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest. 2000;105(7):945–953. doi: 10.1172/JCI8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLaren DJ, Mackenzie CD, Ramalho-Pinto FJ. Ultrastructural observations on the in vitro interaction between rat eosinophils and some parasitic helminths (Schistosoma mansoni, Trichinella spiralis and Nippostrongylus brasiliensis). Clin Exp Immunol. 1977;30(1):105–118. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.