Abstract
Osteocytes are derived from osteoblasts and make up over ninety percent of the cells in bone. However, the mechanisms that control the differentiation of osteoblasts into osteocytes embedded in bone matrix are not well understood. With the recent development of transgenic models for manipulating gene expression in osteocytes and transgenic mice carrying lineage reporters for osteoblasts and osteocytes, unprecedented new insights are becoming possible. This article will review recent advances, such as comparative gene and protein expression studies that are delineating the changes in gene and protein expression that accompany osteocyte differentiation. It will also review recent studies in which time lapse dynamic imaging approaches have been used to visualize osteoblast and osteocyte populations within bone. These approaches reveal the key role of cell motility in bone cell function and highlight the dynamic nature of mineralized tissues. Changes in motile properties of the cell may be key in the transition from osteoblast to osteocyte, as reflected in the altered expression of many molecules involved in cytoskeletal function.
Keywords: osteocyte, osteoblast, differentiation, cell motility, bone
Osteoblast to Osteocyte Transition
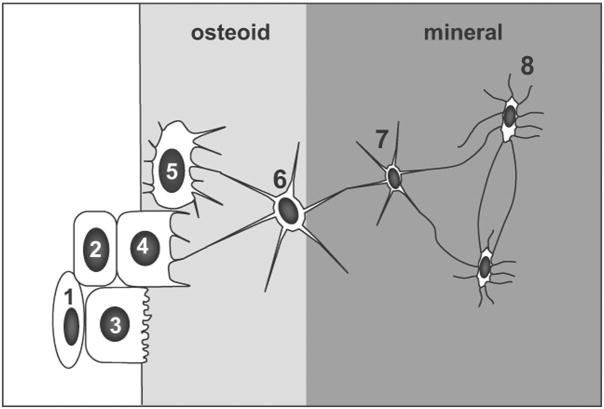
It has been known for over a century that osteocytes are derived from osteoblasts1. However, the precise mechanisms by which an osteoblast becomes buried in bone matrix to take on a new life as an osteocyte remain elusive (reviewed in2). At the end of the bone formation phase, osteoblasts can either become embedded in bone as osteocytes, become inactive osteoblasts or bone lining cells3 or undergo programmed cell death (apoptosis)3, 4. Under certain conditions they may be able to differentiate into cells that produce chondroid bone5. The proportion of osteoblasts that follow each of these fates may be dependant on the animal species, the age and type of bone (reviewed in2) and on hormonal or disease status4, 6. Several transitional stages between osteoblasts and osteocytes have been identified by various authors. In their review article, Franz-Odendaal et al2 combined these observations to propose eight recognizable transitional stages from the osteoblast to the osteocyte (see figure 1). These authors favor an embedding mechanism in which a subpopulation of osteoblasts on the bone surface slows down their matrix production relative to adjacent cells. These osteoblasts then become “buried alive” under the matrix produced by neighboring osteoblasts.
Figure 1.
Schematic diagram illustrating the proposed transitional stages from osteoblast to mature osteocyte. During this process, the volume of the cell body and the number of cell organelles decreases. 1= proliferating preosteoblast, 2= preosteoblastic osteoblast, 3= osteoblast, 4= osteoblastic osteocyte (type I preosteocyte), 5= osteoid osteocyte (type II preosteocyte), 6= type III preosteocyte, 7= young osteocyte, 8= old osteocyte (Modified from Franz-Odendaal et al2)
Although these different maturation stages can be recognized morphologically, there are many unanswered questions about the differentiation process. For example, it remains unclear whether the decision for an osteoblast to become an osteocyte is dictated by a specific pattern of gene expression in a subset of osteoblasts and also whether this is a cell autonomous response or one that is controlled via the surface cells receiving signals from already embedded osteocytes. It is also not known whether every osteoblast has an equal chance of becoming an osteocyte or whether there are specific subpopulations with predefined fates. Another unanswered question is whether osteocytic differentiation is an irreversible process or whether osteocytes have the capacity to revert to being an osteoblast or even give rise to other cell lineages, such as adipocytes and chondrocytes. With the recent generation of transgenic mouse lines in which GFP variants are expressed in osteoblasts and osteocytes7–9, and the generation of transgenic tools for the manipulation of gene expression in osteocytes10, 11 it is becoming possible to address some of these unresolved questions and to gain new insight into the process of osteoblast to osteocyte transition.
Osteocyte-Specific Genes
Several investigators have reported heterogeneity in osteoblast gene and protein expression patterns12–17, raising the possibility that subpopulations of osteoblasts may be destined for different fates, based on their gene expression. Within the past two decades, more and more markers of the osteocyte have been identified (reviewed in18, 19) and the recent use of transgenic mouse lines in which GFP variants are expressed in osteoblasts and osteocytes7–9, has enabled comparisons of gene and protein expression between osteoblasts and osteocytes. Such comparative studies can provide clues as to which molecules are the key regulators of osteocyte function and potentially identify previously unknown functions for these cells. As we learn more about the osteocyte, it is becoming clear that there is a similar heterogeneity in gene expression in osteocytes within bone. For example, early embedding osteoid osteocytes and young osteocytes are high expressers of E11/gp38 (also known as podoplanin), the significance of which is discussed later in this article. In contrast, more mature, deeply embedded osteocytes express high levels of sclerostin, the protein product of the SOST gene (for review see20). Mutation of SOST causes high bone mass in humans and deletion results in high bone mass in mice21. Therefore, Sclerostin is a negative regulator of bone formation, most likely through antagonizing the actions of Lrp5, a key activator of the Wnt/β-catenin signaling pathway. These observations emphasize the importance of the osteocyte in the regulation of bone mass and the osteocyte is now being targeted for development of therapies for treatment of osteoporosis.
Several proteins that are osteocyte specific or selectively expressed in osteocytes play critical roles in phosphate homeostasis. These include phosphate-regulating gene with homologies to endopeptidases on the X chromosome (PHEX), matrix extracellular phosphoglycoprotein (MEPE), dentin matrix protein 1 (DMP1), and fibroblast growth factor-23 (FGF23) (for review see18, 19). Phex is a marker for avian osteocytes and Dmp1 and MEPE are highly expressed in osteocytes compared to osteoblasts or other cell types. We have observed considerable heterogeneity in Dmp1 expression among osteocytes and have found using transgenic mice expressing GFP driven by the Dmp1 promoter7 that only about fifty percent of the osteocytes in neonatal calvaria express Dmp1 at any given time (SLD, unpublished observation). Deletion or mutation of either Phex or DMP1 results in hypophosphatemic rickets, which is associated with a dramatic increase in FGF23 expression in osteocytes and elevated systemic levels. Therefore, the osteocyte network appears to be able to function as an important endocrine system that regulates phosphate homeostasis and mineral metabolism22
Compared to osteoblasts, osteocytes also appear to be enriched in proteins associated with resistance to hypoxia23, as one would expect, due to their location embedded within bone and the potential for a restricted oxygen supply. Oxygen tension may regulate the differentiation of osteoblasts into osteocytes24 and osteocyte hypoxia may also play a role in disuse-mediated bone resorption25. Another important category of molecules whose expression is altered in osteocytes compared to osteoblasts are molecules involved in cytoskeletal function and cell motility, as will be discussed later in this article.
Osteocytogenesis as an Active Rather than a Passive Process
Osteocytogenesis has been viewed as a passive process whereby some osteoblasts become trapped or “buried” in osteoid, which then passively mineralizes. However, there are several considerations that suggest osteocytogenesis may actually be an active rather than a passive process. One of the first changes to take place in the embedding cell is the formation of dendritic processes, concomitant with a reduction in cytoplasmic volume. Generation of cell processes such as lamellipodia and pseudopodia is a dynamic event, and osteocyte dendrite formation is no exception. The cell undergoes a dramatic transformation from a polygonal cell to a cell extending dendrites in a polarized manner towards the mineralizing front, which is followed by dendrites extending towards the vascular space or bone surface. Once embedded, the osteocyte appears to maintain its polarity both with regard to the directionality of its dendrites and the directionality of mineral formation, with mineral being deposited on one side of the embedding cell rather than equally all around the cell. We have proposed that it is the osteoid osteocyte, i.e. the cell that is undergoing the transition to becoming an osteocyte, that controls and regulates mineralization and not osteoblasts on the bone surface26, 27.
It has been proposed by Karsdal and colleagues that osteocytogenesis is an active invasive process requiring cleavage of collagen and potentially other matrix molecules28. Recent experimental evidence supports this concept, at least in relation to the formation of dendrites, since osteocytes in mice null for the metalloproteinase MT1-MMP that cleaves collagens and other matrix proteins have a significantly reduced number and length of dendritic processes29. These investigators also showed an increase in the dendricity of osteocytes in normal mice between 3 and 4 months of age, supporting earlier observations made by Okada and colleagues in young and old rats30 that the number of dendrites/canaliculi increases with age. It is not clear if the embedded osteocyte can generate new dendrites/canaliculi via an invasive process, or if this increase in dendrite number occurs after remodeling because the newly formed bone contains osteocytes with increased numbers of dendrites/canaliculi. Ongoing studies in our laboratory suggest that the formation of dendrites is a highly dynamic process, as will be discussed in the following sections of this review.
Osteocytes Show Differential Expression of Cytoskeletal Components and Molecules Involved in Cell Motility
Of the various categories of molecules that are differentially expressed in osteocytes compared to osteoblasts, components of the cytoskeleton and/or molecules involved with cell motility are of particular interest. Recent studies in our laboratory using time lapse fluorescence microscopy approaches have suggested that osteoblasts move over the bone surface with average velocities of 4–5μm per hour (see below). In order for these cells to become embedded and differentiate into an osteocyte, they would presumably first need to arrest their motion on the bone surface and then adopt a dendritic morphology. It is therefore easy to see how the regulation of cytoskeletal proteins and molecules involved in cell motility would be important in the differentiation process and especially in controlling dendrite formation. Actin dynamics are likely central to this process and Tanaka and Kamioka were the first to show that there are differences in the localization of actin binding proteins, such as fimbrin, filamin and α-actinin in osteocytes compared to osteoblasts31. Based on their observations, they proposed that organized expression of tubulin, vimentin and actin in the cell bodies and dendrites of osteocytes are crucial to maintain their unique cytoskeletal shape32.
More recently, comparisons of osteoblasts and osteocytes using gene array analysis or proteomics approaches have shown that osteocytes are enriched in a number of proteins important in cytoskeletal function, including destrin, CapG, cdc42 and E11/gp3823, 33–36. Paic and colleagues used gene expression profiling to compare osteoblasts and osteocytes that were harvested by FACS sorting from transgenic mice expressing GFPcyan in osteoblasts and GFPtopaz in osteocytes34. Their study suggested that osteocytes are also enriched in molecules involved in muscle contractile function, including myosin heavy and light chains, α-actin, troponins, tropomyosins and α-actinin. They were also enriched in the actin binding protein, Capzb.
CapG and Capzb are members of the gelsolin family that are important in regulating actin filament length by capping their barbed positive ends. Destrin is a member of the actin depolymerizing factor/cofilin family that regulates assembly and disassembly of actin filaments. These proteins therefore interact with actin and regulate actin polymerization and depolymerization dynamics, which is critical for functions such as cell motility, membrane ruffling and extension of cell processes. E11, also called podoplanin, OTS-8, gp38 or PA2.25, based on its identification in different tissues, is highly expressed in osteocytes that are in the process of embedding or have recently embedded37. It is also expressed in several other cell types with a dendritic morphology, including kidney podocytes, type II lung alveolar cells and cells of the choroid plexus (reviewed in19). E11/gp38 is thought to interact with ezrin-radixin-moiesin (ERM) complexes, which triggers downstream activation of RhoA GTPase to regulate actin cytoskeleton dynamics. Because of this and the dendritic morphology of many of the cell types in which it is expressed, E11/gp38 has been implicated in dendrite formation. Overexpression of E11/gp38 in HeLa cells and HaCaT keratinocytes resulted in redistribution of ezrin to the cell margins and the formation of cell surface protrusions38. Conversely, inhibition of E11/gp38 in osteocyte-like MLO-Y4 cells blocks dendrite elongation in response to fluid flow shear stress37. These data suggest that E11 may play a role in dendrite formation in osteocytes. If so, the fact that E11 expression is mainly restricted to cells that are embedding or have recently embedded, suggests that E11 may be important for the initial formation of dendrites but not for the subsequent maintenance of dendritic morphology.
The Motile Properties of Bone Cells and Implications for Osteoblast to Osteocyte Transition
Recently, our laboratory has applied time lapse fluorescence dynamic imaging approaches to study the motile properties of bone cells and the osteoblast to osteocyte transition27, 39, 40. These studies have been performed using neonatal calvarial explants or primary bone cell cultures from transgenic mice expressing fluorescent osteocyte and osteoblast lineage reporters. This dynamic imaging approach has provided surprising new insights into the function and interactions of bone cells and has revealed the dynamic nature of skeletal tissues.
Our studies have revealed that osteoblasts move over the bone surface in an apparently random fashion, with average velocities in the range of 4–5μm/hour. This implies that they can move more than 100μm (equivalent to about five cell diameters) within a twenty four hour period40 (and SLD, manuscript in preparation). These observations challenge the concept that osteocytes form passively when subpopulations of stationary osteoblasts on the bone surface slow down their matrix production relative to adjacent cells and then become “buried alive” under the matrix produced by neighboring osteoblasts. The motile properties of surface osteoblasts suggest that the transition to an embedding cell must be associated with an arrest in cell motility prior to embedding, together with altered function of proteins involved in cytoskeletal dynamics.
Another surprising finding from our dynamic imaging studies was that the dendritic connections between osteocytes do not appear to be permanent but rather the dendrites are repeatedly extended and retracted40 (and SLD manuscript in preparation). Osteocytes also appear to show undulating motions of their cell bodies within their lacunae, suggesting that they are not inactive cells, as previously thought and that, even though they are “entombed” within a lacuna, they can still exhibit motile properties. As our work has been limited to examining neonatal mouse calvaria, in which all the osteocytes can be viewed as being relatively young, it remains to be determined whether these properties of dendrite motility and cell body motion are also a characteristic of mature, deeply embedded osteocytes and whether the same motile properties occur in osteocytes in adult bones. Figure 2 summarizes the motile properties that we have observed at various stages during the transition from osteoblast to osteocyte and illustrates the expression patterns of some of the key marker genes that are expressed during this transition.
Figure 2.
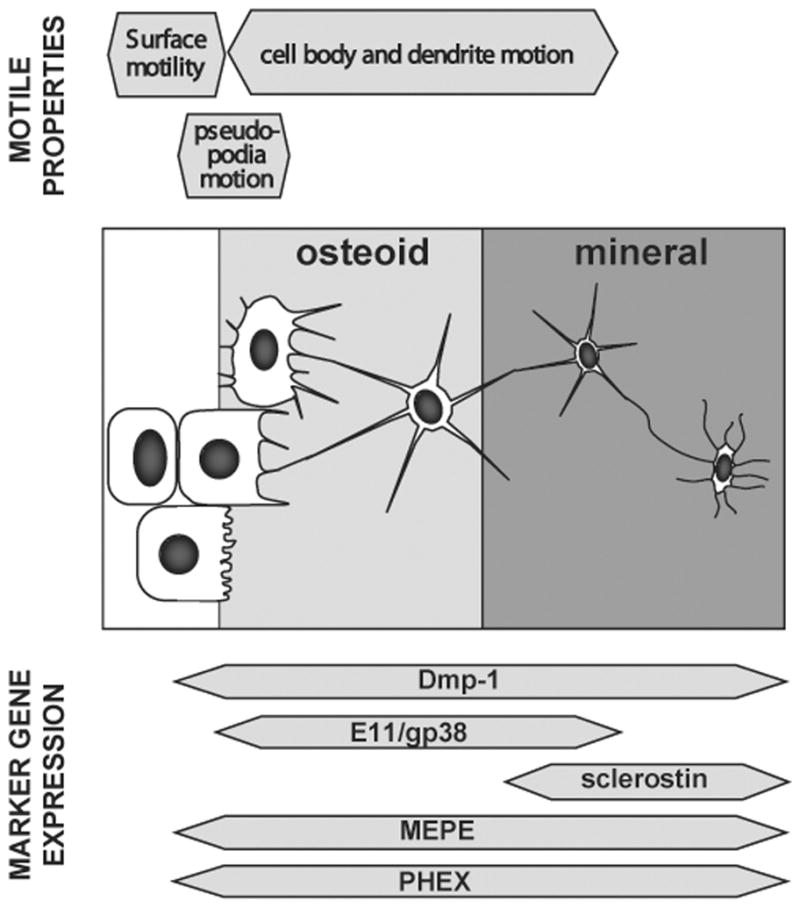
Schematic diagram summarizing the motile properties of the cells at different stages during the transition from osteoblast to mature osteocyte and illustrating the changes in expression of key osteocyte marker genes at these different transitional stages.
The embedding of an osteocyte is a relatively rare event that has proved difficult to capture by time lapse imaging using the calvarial organ culture explant approach. However, insight into this has come from our studies of primary osteoblast cultures, in which mineralized nodules form in vitro that recapitulate the in vivo bone formation process. Using primary osteoblasts from Dmp1-GFP mice7, we have used time lapse imaging to visualize the process of bone nodule formation in conjunction with using alizarin red as a vital dye for mineral deposition27, 39. These studies have shown that mineralization is associated with induction of GFP expression and that the deposition of mineral is associated with an arrest in motility of the GFP-positive cells and a change from a polygonal to a dendritic morphology27,39. These observations suggest that the processes of osteocyte differentiation and mineral deposition are dynamically integrated and further support the concept that the cell type responsible for mineralization is distinct from other osteoblasts and is part of a subpopulation that is already transitioning to become an osteocyte.
The final question that can be addressed using time lapse imaging approaches in living bones relates to whether differentiation towards an osteocyte is an irreversible process. Can the osteocyte be released from its lacuna, for example during osteoclast resorption?. Once released, can it dedifferentiate to become an osteoblast or even revert to an earlier differentiation stage?. From time lapse fluorescent imaging of neonatal calvaria expressing the Dmp1-GFP transgene, we have on rare occasions, observed the expansion of osteoclastic resorption pits in which we can observe the bone around embedded osteocytes being resorbed. When this happens, the osteocytes either appear to undergo cell death or they appear to be able to crawl out of their lacunae into the resorbed area (SLD, unpublished observations). It is not yet clear whether these cells can revert to becoming an osteoblast, but future studies using these live cell imaging approaches should provide further insight into this unresolved question.
Summary and Perspective
In summary, dynamic imaging of living bone cells in a viable bone organ culture, together with comparative gene and protein expression studies, are revealing unimagined activities and functions for osteocytes. Previously, it was thought that the osteoblast was the dynamic cell while the osteocyte was passive and acted as a ‘placeholder’ in bone. Many important functions were assigned to the osteoblast such as being the prodigious producer of matrix, the regulator of osteoclast recruitment and activation, the sole regulator of biomineralization, etc. Once a surface cell was designated to become an osteocyte, it became a “couch potato” where the still active osteoblasts piled collagen around and on top of it. Supposedly, the osteoblast regulated the initiation of mineralization from the bone surface, past the osteoid front. Dendritic processes were thought to mainly be necessary for viability of the embedded osteocyte. A few pioneers in the osteocyte field did propose functions outside of this dogma, such as osteocytes acting as mechanosensors and having the capacity to remodel their perilacunar matrix. As we learn more about the function of osteocyte enriched genes, it is becoming clear that the osteocyte is an important regulator of bone mass and a key endocrine regulator of phosphate metabolism. New technologies such as dynamic imaging have revealed novel, unimagined properties for osteocytes such as the capacity to extend and retract dendritic processes. As taking a snapshot of a galloping stallion cannot reveal the magnificence and grace of its motion, the same is true for the surprising movements and interactions of osteoblasts and osteocytes and it is clear that we must now view the skeleton as a dynamic interactive tissue.
References
- 1.Gegenbauer C. Carpus und Tarsus. Leipzig; Schultergtel: 1864. Untersuchungen zur vergleichenden Anatomie der Wirbeltiere. Heft 1. [Google Scholar]
- 2.Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Dev Dyn. 2006;235:176–90. doi: 10.1002/dvdy.20603. [DOI] [PubMed] [Google Scholar]
- 3.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–37. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 4.Jilka RL, et al. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res. 1998;13:793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- 5.Li M, et al. Histochemical evidence of the initial chondrogenesis and osteogenesis in the periosteum of a rib fractured model: implications of osteocyte involvement in periosteal chondrogenesis. Microsc Res Tech. 2004;64:330–42. doi: 10.1002/jemt.20088. [DOI] [PubMed] [Google Scholar]
- 6.Jilka RL, et al. Quantifying Osteoblast and Osteocyte Apoptosis: Challenges and Rewards. J Bone Miner Res. 2007 doi: 10.1359/jbmr.070518. [DOI] [PubMed] [Google Scholar]
- 7.Kalajzic I, et al. Dentin matrix protein 1 expression during osteoblastic differentiation, generation of an osteocyte GFP-transgene. Bone. 2004;35:74–82. doi: 10.1016/j.bone.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Kalajzic I, et al. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. J Bone Miner Res. 2002;17:15–25. doi: 10.1359/jbmr.2002.17.1.15. [DOI] [PubMed] [Google Scholar]
- 9.Kalajzic Z, et al. Directing the expression of a green fluorescent protein transgene in differentiated osteoblasts: comparison between rat type I collagen and rat osteocalcin promoters. Bone. 2002;31:654–60. doi: 10.1016/s8756-3282(02)00912-2. [DOI] [PubMed] [Google Scholar]
- 10.Lu Y, et al. DMP1-targeted Cre expression in odontoblasts and osteocytes. J Dent Res. 2007;86:320–5. doi: 10.1177/154405910708600404. [DOI] [PubMed] [Google Scholar]
- 11.Tatsumi S, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–75. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Aubin JE, Bellows CG, Turksen K, Liu F, Heersche JNM. Analysis of the osteoblast lineage and regulation of differentiation. In: Slavkin H, Price P, editors. Chemistry and biology of mineralized tissues. Elsevier Science; Amsterdam: 1992. pp. 267–276. [Google Scholar]
- 13.Aubin JE, Turksen K, Heersche JNM. Osteoblastic cell lineage. In: Noda M, editor. Cellular and molecular biology of bone. Academic Press; San Diego: 1993. pp. 1–45. [Google Scholar]
- 14.Candeliere GA, Liu F, Aubin JE. Individual osteoblasts in the developing calvaria express different gene repertoires. Bone. 2001;28:351–61. doi: 10.1016/s8756-3282(01)00410-0. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, et al. Developmental expression of osteopontin (OPN) mRNA in rat tissues: evidence for a role for OPN in bone formation and resorption. Matrix. 1993;13:113–23. doi: 10.1016/s0934-8832(11)80070-3. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda T, et al. Age-related reduction in bone matrix protein mRNA expression in rat bone tissues: application of histomorphometry to in situ hybridization. Bone. 1995;16:17–23. doi: 10.1016/s8756-3282(94)00002-6. [DOI] [PubMed] [Google Scholar]
- 17.Liu F, Malaval L, Aubin JE. The mature osteoblast phenotype is characterized by extensive plasticity. Exp Cell Res. 1997;232:97–105. doi: 10.1006/excr.1997.3501. [DOI] [PubMed] [Google Scholar]
- 18.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–15. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonewald LF. Osteocytes as dynamic multifunctional cells. Ann N Y Acad Sci. 2007;1116:281–90. doi: 10.1196/annals.1402.018. [DOI] [PubMed] [Google Scholar]
- 20.Kneissel M. The Promise of Sclerostin Inhibitioin for the Treatment of Osteoporosis. IBMS BoneKEy. 2009;6:259–264. [Google Scholar]
- 21.Balemans W, et al. A generalized skeletal hyperostosis in two siblings caused by a novel mutation in the SOST gene. Bone. 2005;36:943–7. doi: 10.1016/j.bone.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Feng JQ, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–5. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo D, et al. Identification of proteins involved in cytoskeletal rearrangement, anti-hypoxia and membrane channels in osteocytes over osteoblasts. J Bone Miner Res. 2006;21:S168. [Google Scholar]
- 24.Hirao M, et al. Oxygen tension is an important mediator of the transformation of osteoblasts to osteocytes. J Bone Miner Metab. 2007;25:266–76. doi: 10.1007/s00774-007-0765-9. [DOI] [PubMed] [Google Scholar]
- 25.Gross TS, et al. Selected Contribution: Osteocytes upregulate HIF-1alpha in response to acute disuse and oxygen deprivation. J Appl Physiol. 2001;90:2514–9. doi: 10.1152/jappl.2001.90.6.2514. [DOI] [PubMed] [Google Scholar]
- 26.Barragan-Adjemian C, et al. Mechanism by which MLO-A5 late osteoblasts/early osteocytes mineralize in culture: similarities with mineralization of lamellar bone. Calcif Tissue Int. 2006;79:340–53. doi: 10.1007/s00223-006-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dallas SL, et al. Dynamic Imaging of Fluorescently Tagged Osteoblast and Osteocyte Populations Integrates Mineralization Dynamics with Osteoblast to Osteocyte Transition. J Bone Miner Res. 2007;22(suppl1):S13. [Google Scholar]
- 28.Karsdal MA, et al. Matrix metalloproteinases (MMPs) safeguard osteoblasts from apoptosis during transdifferentiation into osteocytes: MT1-MMP maintains osteocyte viability. DNA Cell Biol. 2004;23:155–65. doi: 10.1089/104454904322964751. [DOI] [PubMed] [Google Scholar]
- 29.Holmbeck K, et al. The metalloproteinase MT1-MMP is required for normal development and maintenance of osteocyte processes in bone. J Cell Sci. 2005;118:147–56. doi: 10.1242/jcs.01581. [DOI] [PubMed] [Google Scholar]
- 30.Okada S, et al. The canalicular structure of compact bone in the rat at different ages. Microsc Microanal. 2002;8:104–15. doi: 10.1017/s1431927601020037. [DOI] [PubMed] [Google Scholar]
- 31.Kamioka H, et al. Terminal differentiation of osteoblasts to osteocytes is accompanied by dramatic changes in the distribution of actin-binding proteins. J Bone Miner Res. 2004;19:471–8. doi: 10.1359/JBMR.040128. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka-Kamioka K, et al. Osteocyte shape is dependent on actin filaments and osteocyte processes are unique actin-rich projections. J Bone Miner Res. 1998;13:1555–68. doi: 10.1359/jbmr.1998.13.10.1555. [DOI] [PubMed] [Google Scholar]
- 33.Billiard J, et al. Transcriptional profiling of human osteoblast differentiation. J Cell Biochem. 2003;89:389–400. doi: 10.1002/jcb.10514. [DOI] [PubMed] [Google Scholar]
- 34.Paic F, et al. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone. 2009 doi: 10.1016/j.bone.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang W, et al. Gene expression signatures of a fibroblastoid preosteoblast and cuboidal osteoblast cell model compared to the MLO-Y4 osteocyte cell model. Bone. 2009;44:32–45. doi: 10.1016/j.bone.2008.08.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo D, et al. Identification of Osteocyte-Selective Proteins. doi: 10.1002/pmic.201000306. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang K, et al. E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol Cell Biol. 2006;26:4539–52. doi: 10.1128/MCB.02120-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin-Villar E, et al. Characterization of human PA2.26 antigen (T1alpha-2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int J Cancer. 2005;113:899–910. doi: 10.1002/ijc.20656. [DOI] [PubMed] [Google Scholar]
- 39.Dallas SL, et al. Time lapse imaging techniques for comparison of mineralization dynamics in primary murine osteoblasts and the late osteoblast/early osteocyte-like cell line MLO-A5. Cells Tissues Organs. 2009;189:6–11. doi: 10.1159/000151745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veno PA, et al. Dynamic Imaging in Living Calvaria Reveals the Motile Properties of Osteoblasts and Osteocytes and suggests Heterogeneity of Osteoblasts in Bone. 29th Annual Meeting of the American Society of Bone and Mineral Research; Sept, 2007; Honolulu. 2007. [Google Scholar]