Abstract
Estrogen depletion following menopause has been correlated with an increased risk of developing Alzheimer’s disease (AD). We previously explored the beneficial effect of (-)-epigallocatechin-3-gallate (EGCG) on AD mice and found increased non-amyloidogenic processing of amyloid precursor protein (APP) through the α-secretase a-disintegrin-and-metallopeptidase-domain 10 (ADAM10). Our results in this study suggest that EGCG-mediated enhancement of non-amyloidogenic processing of APP is mediated by the maturation of ADAM10 via an estrogen receptor-α (ERα)/PI3K/Akt dependent mechanism, independent of furin-mediated ADAM10 activation. These data support prior assertions that central selective estrogen receptor modulation could be a therapeutic target for AD and support the use of EGCG as a well-tolerated alternative to estrogen therapy in the prophylaxis and treatment of this disease.
Keywords: Alzheimer’s disease, Estrogen, Amyloid precursor protein, PI3K, Furin, Epigallocatechin gallate, ADAM10
Introduction
Over the past decade, intense focus has been given to investigating amyloid precursor protein (APP) processing and Aβ metabolism as potential therapeutic targets for Alzheimer s disease (AD).[1] More recently, attention has turned toward the α-secretase/non-amyloidogenic pathway of APP metabolism,[2,3] although its role in AD and potential as a diagnostic marker have been considered for some time.[4–7] Because of the limited amount of APP in the cell, it is believed that the amyloidogenic and non-amyloidogenic pathways compete for substrate in the process of APP proteolysis.[8] Since α-secretase cleaves within the Aβ peptide domain, its activation has the added advantage of precluding neurotoxic Aβ peptide formation.
According to prevalence studies, women have a higher risk of developing AD than men.[9,10] Following menopause, this increased risk of developing AD can be partially attributed to estrogen depletion.[11] In vitro, 17β-estradiol is associated with accumulation of a soluble fragment of APP resulting from α-secretase cleavage (sAPPα)[12] and reduced Aβ generation.[13] In vivo, selective estrogen receptor (ER) modulators reduce Aβ accumulation and improve behavioral performance.[14] [15] Despite these promising results, the efficacy of hormone replacement therapy (HRT) in preventing AD in women has remained controversial.[16,17] While some report that postmenopausal women taking HRT have both a decreased risk and delayed onset of developing AD,[18] others have found that HRT may result in an increased dementia risk; either directly or due to an elevation of other risk factors.[10,19] Given this debate, the fact that APP processing, ER activity, and the risk of AD are interrelated is not surprising. (reviewed by [20]) Because of this, investigators have attempted to explain the mechanistic underpinnings by which estrogen-mediated signaling affects Aβ accumulation. (reviewed by[21]and [22])
Green tea compounds have been analyzed for their efficacy in the modulation of APP processing. Arguably one of the most promising green tea compounds being studied is (−)-epigallocatechin-3-gallate (EGCG), which has gained increasing attention due in part to its reported anti-carcinogenic effects. [23,24] One theory is that EGCG may act on the ER via its gallate group, thereby mimicking the 7α-position of 17β-estradiol.[25] Previous reports suggest that EGCG regulates the production of sAPPα through modulation of protein tyrosine phosphatases [26,27] and protein kinase C-dependent mechanisms.[28,29] Additionally, EGCG has been shown to inhibit the activities of pro-inflammatory cytokines [30–32] and a multitude of cellular signaling pathways[31,33,34]; including those involving the phosphatidylinositide 3 -OH kinase (PI3K)/Akt cascade.[35]
We have previously shown that EGCG reduces Aβ generation in N2a cells overexpressing Swedish mutant APP (SweAPP N2a).[36] In concert with these observations, we found that EGCG promotes α-site cleavage of APP to enhance formation of α-carboxyl terminal fragment of APP (α-CTF) and sAPPα. These events are associated with elevated α-secretase cleavage activity and enhanced activation of ADAM metallopeptidase domain 10 (ADAM10). [37]
In an effort to further characterize the manner in which stimulation of the non-amyloidogenic/α-secretase pathway leads to reductions in Aβ, our current investigation focuses on mechanisms by which EGCG alters APP processing. In the present study, we show that EGCG promotes α-secretase-mediated APP metabolism through both ERα and furin dependent mechanisms. Specifically, EGCG enhanced maturation of ADAM10 via an ERα/PI3K/Akt dependent mechanism, distinct from EGCG-mediated furin upregulation.
Materials and Methods
Reagents
Green tea-derived EGCG (95% purity by HPLC) was purchased from Sigma Chemical Co. (St Louis, Missouri), wortmannin (PI3K inhibitor) was obtained from Calbiochem (San Diego, CA, USA), and the highly selective cell permeable PI3K inhibitor, LY294002, was purchased from Sigma. The selective ERα agonist 1,3,5-Tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole (PPT) was obtained from Sigma and the selective ERα antagonist methyl-piperidino-pyrazole (MPP) and selective ERβ antagonist 4-[2-Phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyri midin-3-yl]phenol (PHTPP) were purchased from Tocris Bioscience (Ellisville, MO). The Akt inhibitor triciribine hydrate (TCN), was obtained from Sigma.
ELISA
Conditioned media were collected and analyzed at a 1:1 dilution using the method as previously described [38] and values were reported as percentage of Aβ1-x secreted relative to control. Quantitation of total Aβ species was performed according to published methods (31). Briefly, 6E10 (capture antibody) was coated at 2 μg/mL in PBS into 96-well immunoassay plates overnight at 4°C. The plates were washed with 0.05% Tween-20 in PBS five times and blocked with blocking buffer (PBS with 1% BSA, 5% horse serum) for 2 hours at room temperature. Conditioned medium or Aβ standards were added to the plates and incubated overnight at 4°C. Following 3 washes, biotinylated antibody, 4G8 (0.5 μg/mL in PBS with 1% BSA) was added to the plates and incubated for 2 hours at room temperature. After 5 washes, streptavidin-horseradish peroxidase (1:200 dilutions in PBS with 1% BSA) was added to the 96-wells for 30 min at room temperature. Tetramethylbenzidine (TMB) substrate was added to the plates and incubated for 15 min at room temperature. 50 μL of stop solution (2 N N2SO4) was added to each well of the plates. The optical density of each well was immediately determined by a microplate reader at 450 nm. In addition, Aβ1-40, or Aβ1-42 was separately quantified in these samples using the Aβ1-40 Aβ1-42 ELISA kits (IBL-America, Minneapolis, MN) in accordance with the manufacturer s instructions. In all cases, Aβ levels were expressed as a percentage of control (i.e., conditioned medium from untreated SweAPP N2a cells).
Western blot
Cultured cells were lysed in ice-cold lysis buffer described above, and an aliquot corresponding to 20–50 μg of total protein was electrophoretically separated using 10 or 12% Tris-glycine gels. Electrophoresed proteins were then transferred to PVDF membranes (Bio-Rad, Richmond, California), washed in ddH2O, and blocked for 1 hour at ambient temperature in Tris-buffered saline (TBS; Bio-Rad) containing 5% (w/v) non-fat dry milk. After blocking, membranes were hybridized for 1 hour at ambient temperature with various primary antibodies. Membranes were then washed 3 times for 5 min each in ddH2O and incubated for 1 hour at ambient temperature with the appropriate HRP-conjugated secondary antibody (1:1,000, Pierce Biotechnology, Inc. Rockford, Illinois). All antibodies were diluted in TBS containing 5% (w/v) of non-fat dry milk. Blots were developed using the luminol reagent (Pierce Biotechnology). Densitometric analysis was done using the Fluor-S MultiImagerTM with Quantity OneTM software (Bio-Rad). For examining sAPPα, conditioned medium was collected following treatment according to a modified protocol from Chen and Fernandez.[39] sAPPα was extracted using 3K Nanosep centrifugal filters (Pall Life Sciences, Ann Arbor, Michigan) and protein concentrate was prepared for the aforementioned electrophoresis. Antibodies used for western blot included: ADAM10 antibodies (1:1000; Calbiochem and Chemicon), Furin antibody (1:1000; Biomol Intl., Plymouth Meeting, PA), PC7 antibodies (1:1000; Abcam, Cambridge, MA), phospho-Tyr p85 PI3K binding motif, phospho-Akt (Ser 473), total Akt antibodies (1:1000, Cell Signaling Technology, Danvers, MA, USA), or actin antibody (1:1500; as an internal reference control; Roche).
Densitometric analysis was conducted using the Fluor-S MultiImager with Quantity One software (Bio-Rad) or ImageJ software (NIH). Images were scanned, protein bands were captured, and a threshold optical density was obtained that discriminated bands from background. Densitometric values were reported as area of positive pixels in reference to an internal control.
Statistical analysis
All data were normally distributed; therefore, in instances of single mean comparisons, Levene s test for equality of variances followed by t-test for independent samples was used to assess significance. In instances of multiple mean comparisons, analysis of variance (ANOVA) was used, followed by post-hoc comparison using Bonferonni s method as appropriate. Alpha levels were set at 0.05 for all analyses. The statistical package for the social sciences release 18 (SPSS Inc., Chicago, Illinois) or StatPlus®:mac (AnalystSoft, Inc., Vancouver, British Columbia, CA) was used for all data analysis.
Results
The presence of estrogen enhances non-amyloidogenic APP α-secretase cleavage as evidenced by increased sAPPα and decreased Aβ production.[40,41] Thus, the selective actions of estrogen may represent a therapeutic target for the prevention of toxic Aβ species and subsequent neurodegeneration. Our prior investigations suggest a similar mechanism may be involved in EGCG s promotion of sAPPα production. [36] Taken together with studies that implicate ER modulation after treatment with EGCG, [42,43] [25] we set out to explore whether EGCG could act fully or partially through the ER to exert its effect on APP processing.
Estrogen receptor (ERα) inhibition mitigates EGCG-induced ADAM10 activation and non-amyloidogenic APP processing in SweAPP N2a cells
Using similar conditions as in our prior investigations, APP (Swedish mutant APP 695aa isoform) overexpressing murine neuroblastoma N2a cells (SweAPP N2a), known to primarily express ERα [44], were treated with EGCG at 20 μM [36] in the presence of various concentrations (0–2.5μM and 50-200μM) of highly selective ERα antagonist MPP [45,46] or highly selective ERβ antagonist PHTPP, as a structurally related control compound, for 12 hours (Fig. 1). Aβ1-40, 42 peptides were analyzed in conditioned media from these cells by ELISA. Consistent with our central hypothesis, data reveal significant increases in Aβ1-40, 42 peptide production by greater than 100% with co-treatment with EGCG and MPP compared to treatment with EGCG alone. No significant (P>0.05) changes in Aβ peptide production were found with control compound and EGCG cotreatment, thereby suggesting that EGCG promotes non-amyloidogenic processing though ERα modulation.
Figure 1. Estrogen receptor (ERα) inhibition mitigates EGCG-induced ADAM10 activation and non-amyloidogenic APP processing in SweAPP N2a cells.
SweAPP N2a cells (murine neuroblastoma cells overexpressing Swedish mutant 695aa isoform of APP) were treated with EGCG at 20 μM in the presence of estrogen inhibitor (MPP, an antagonist at ERα receptor displaying > 200-fold selectivity for ERα over ERβ) or a control compound lacking estrogen receptor modulation properties at various doses as indicated for 12 hours. Aβ1-40, 42 peptides were analyzed in conditioned media from these cells by ELISA. Data are represented as Aβ1-40, 42 (pg) in total cellular protein (mg) secreted 12 hours after co-treatment as indicated below the figure. Cell lysates were prepared and subjected to western analysis of ADAM10 maturation. Densitometric analysis shows the ratio of active mature (mADAM10) to proform (pro-ADAM10) as indicated below the figure. One-way ANOVA followed by post-hoc comparison revealed significant differences between MPP doses (**P <0.005 with n = 3 for each condition), but not control inhibitor (P > 0.05), for Aβ generation and ratio of mADAM10 to pro-ADAM10.
As our prior investigations demonstrated the requirement of ADAM10 in EGCG promotion of non-amyloidogenic APP metabolism[37], cell lysates from the same SweAPP N2a cells were prepared and subjected to western analysis for ADAM10. Densitometry ratios of mature (mADAM10) to the proform of ADAM10 (pro-ADAM10) band densities at various doses of MPP or control compound treated SweAPP N2a cells show that MPP, but not control compound, significantly inhibits ADAM10 maturation by greater than 70% (Fig. 1). This effect of ERα antagonism correlates with increased production of Aβ peptides thereby indicating downregulation of non-amyloidogenic APP processing.
Conversely, highly selective ERα agonist, PPT (50-200μM), was utilized to compare the effects of ERα activation on downstream signaling and ADAM10 maturation with the previously observed effects of EGCG. SweAPP N2a cells were treated for 12 hours. Similar to EGCG, western analysis for ADAM10 revealed enhanced ADAM10 maturation with PPT although this effect did not reach statistical significance (Supplemental Fig. 1). Interestingly, PPT and EGCG revealed no additive maturation of ADAM10 possibly secondary to ERα saturation at trialed doses of these compounds. Moreover, PPT treatment seemed to attenuate ERα stimulation by EGCG, perhaps alluding to some form of competition between the two agonists. (Supplemental Fig. 1). However, taken together with the MPP results, these results suggest that mechanistically, EGCG promotion of non-amyloidogenic APP processing mediated by ADAM10 may require the activity of ERα in these cells.
EGCG failed to directly promote ADAM10 activation in broken cell preparations
Having shown that EGCG markedly enhances ADAM10 maturation via ERα-mediated signaling in SweAPP N2a cells, we next set out to characterize downstream pathway effectors. We hypothesized that EGCG promotion of non-amyloidogenic APP processing requires structured cellular functions, such as indirect signal transduction cascades or gene regulation, rather than direct activation of ADAM10 by EGCG. To help rule out the possibility of direct activation, broken cell preparations from untreated SweAPP N2a cells were treated with EGCG (10 μM) or PBS (Fig. 2). One hour later, these cell lysates were subjected to western analysis for ADAM10. Densitometric analysis indicates that ratios of mADAM10 to pro-ADAM10 did not vary significantly (P>0.05) suggesting that ADAM10 activation by EGCG is not mediated through a direct cytosolic or molecular interaction with EGCG. These findings provide evidence of the requirement for signal transduction pathways and/or gene regulation in EGCG-mediated ADAM10 activation.
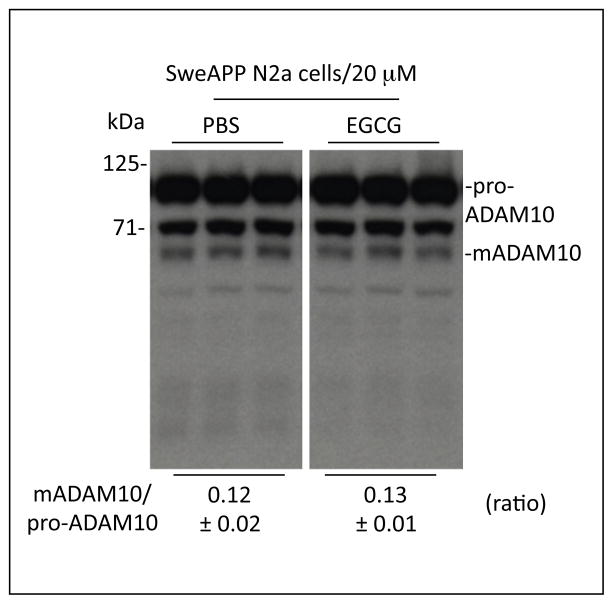
Figure 2. EGCG failed to directly promote ADAM10 activation in broken SweAPP N2a cell preparations.
Cell lysates from untreated SweAPP N2a cells and subsequently treated these lysates with EGCG (10 μM) or PBS. One hour later, these cell lysates were subjected to western analysis of ADAM10. Densitometric analysis shows the ratio of mADAM10 to pro-ADAM10 as indicated below the figure. One-way ANOVA followed by post-hoc comparison revealed no significant differences between the treated conditions (P >0.05 with n = 4 for each condition) for the ratio of mADAM10 to pro-ADAM10.
PI3K/Akt signaling is involved in EGCG-mediated ADAM10 activation and promotion of non-amyloidogenic processing of APP
The involvement of PI3K signaling in the non-genomic activities of estrogen receptors and related downstream signaling events is well known. Comparatively, EGCG is also known to activate PI3K in various cell types. [35,47] Gandy and colleagues had previously supported a role for PI3K activation in promoting sAPPα release from SweAPP N2a cells.[48] Thus, we next investigated the contribution of PI3K to EGCG-mediated ADAM10 activation in SweAPP N2a cells. As shown in Figure 3, SweAPP N2a cells were treated with EGCG (20μM) for 12 hours and sAPPα secretion was quantified by ELISA after varying treatment concentrations (0–50μM) of PI3K inhibitor (wortmannin). Our data show that the PI3K inhibition results in a dose-dependent decrease in sAPPα release by SweAPP N2a cells suggesting inhibition of α-secretase activity (Fig. 3A). LDH release did not vary between control treatment doses of wortmannin (data not shown). Based on these findings we hypothesized that PI3K activation regulates ADAM10 activation in these cells.
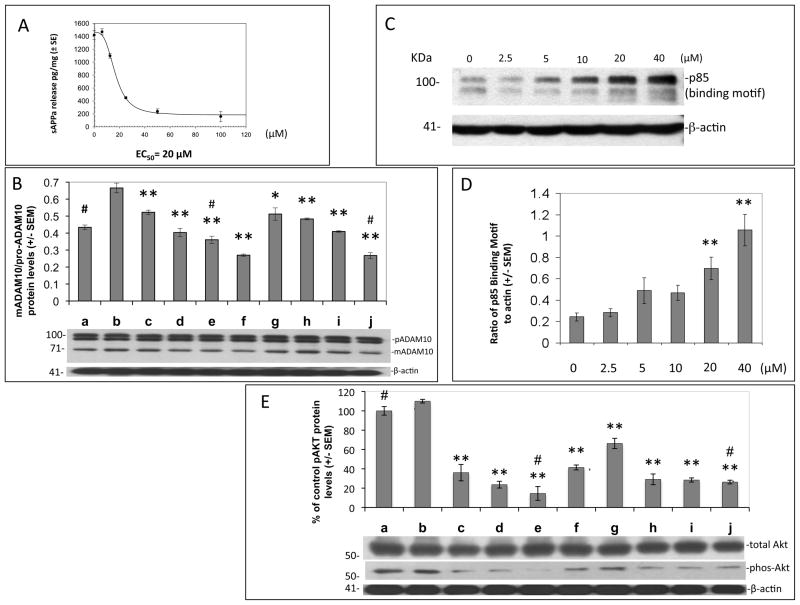
Figure 3. PI3K/Akt signaling is involved in EGCG-mediated ADAM10 activation and promotion of non-amyloidogenic processing of APP.
Various treatment conditions in SweAPP N2a cells are denoted as a-j and correspond to the following: a. no treatment, b. EGCG, c. wortmannin (WM) 200 nM + EGCG, d. WM 400 nM + EGCG, e. WM 10 μM + EGCG, f. WM 400nM, g. LY294002 10μM + EGCG, h. LY294002 50 μM+ EGCG, i. LY294002 100 μM + EGCG, j. LY294002 50 μM. EGCG was used at a concentration of 20 μM for all conditions unless otherwise indicated. (A) SweAPP N2a cells were treated with EGCG and sAPPα release was quantitated by ELISA after varying treatment concentrations of PI3K inhibitor (WM). (B) pro-ADAM10 and mADAM10 following treatment with the PI3K inhibitors, WM and LY294002 were analyzed in cell lysates from SweAPP N2a cells by western blot. Densitometry analysis results are represented as band density ratio means ± SEM (n = 3), * P<0.05, ** P<0.01 of protein of interest compared to EGCG control, # represents the protein of interest compared to control without EGCG treatment. (C, D) SweAPP N2a cell lysates were collected following treatment with various concentrations of EGCG as indicated and analyzed by western analysis for the p85 binding motif of PI3K. Densitometry analysis results are represented as band density ratio of p85 to β-actin (± SEM, n = 3, ** P<0.01). (E) total Akt and phospho-Akt were assessed as percent of associated control following treatment with the PI3K inhibitors and were analyzed in cell lysates from SweAPP N2a cells by western blot. Densitometry analysis results are represented as band density means ± SEM (n = 3), ** P<0.01 of protein of interest compared to EGCG control, # represents the protein of interest compared to control without EGCG treatment.
In order to test this hypothesis, SweAPP N2a cells were treated for 12 hours with 20μM EGCG and varying doses of the PI3K inhibitors, wortmannin (0.2–10μM) and LY294002 (10–100μM),[49] lysates were prepared and subjected to western analysis for ADAM10 (Fig. 3B). Consistent with our sAPPα ELISA findings, ratios of mADAM10/pro-ADAM10 show a decrease following treatment with both PI3K inhibitors; suggesting likely involvement of PI3K in EGCG-mediated ADAM10 activation.
As PI3K has multiple downstream effectors, we next analyzed phosphorylation of PI3K s regulatory subunit p85 in the context of increasing doses of EGCG. SweAPP N2a cells were treated with varying concentration of EGCG (0-40μM) for 4 hours, lysed then subjected to western analysis for PI3K-phospho-p85. Our data indicate a dramatic dose-dependent increase in phosphorylation at this principle activation site with EGCG treatments above 5μM (Fig. 3C & D), altogether suggesting EGCG promotion of ADAM10 activation and non-amyloidogenic APP processing involves active PI3K signaling.
To further characterize downstream effectors involved in EGCG-mediated ADAM10 activation, we examined Akt and phospho-Akt expression following treatment of SweAPP N2a cells with PI3K inhibitors (wortmannin and LY294002) in the presence of 20μM EGCG after 4 hours. Importantly phospho-Akt increased with EGCG (20μM) as compared to untreated SweAPP N2a cells, whereas the addition of PI3K inhibitors, dose-dependently reduced phospho-Akt (Fig. 3E). Significant group differences were not observed between total Akt or actin (data not shown). However, in similar treatment conditions, the Akt inhibitor, TCN, was able to decrease ADAM10 activation, total and phospho-Akt, but not actin, in the presence of EGCG (Fig. 4). These data culminate to support the hypothesis that EGCG may effect ADAM10 activation, and subsequent non-amyloidogenic processing of APP, via involvement of ERα/PI3K/Akt dependent signaling mechanisms.
Figure 4. Akt inhibition by triciribine hydrate (TCN) reduces ADAM10 activation, total Akt, and phosphorylated Akt, in the presence of EGCG.
Various treatment conditions in SweAPP N2a cells are denoted as a-f and correspond to the following: a. no treatment, b. EGCG, c. TCN 5μM, d. TCN 1 μM + EGCG, e. TCN 5 μM + EGCG, f. TCN 50 μM + EGCG. EGCG was used at a concentration of 20 μM for all conditions unless otherwise indicated. (A) SweAPP N2a cells were treated with varying concentrations of Akt inhibitor (TCN) in the presence and absence of EGCG. Cell lysates were prepared and subjected to western analysis of ADAM10 maturation, total Akt, phospho-Akt (Ser473), and β-actin (internal control). (B) Densitometric analysis shows the ratio of active mature (mADAM10) to proform (pro-ADAM10) as indicated below the figure. One-way ANOVA revealed significant differences between TCN treated and control cells at concentrations of 5 and 10 μM (**P<0.01 with n = 3 for each condition) in the presence of EGCG. # represents the protein of interest compared to control without EGCG treatment. (C, D) Densitometric analysis was performed on total Akt and phospho-Akt (Ser473) and represented as percent of associated control (SweAPP N2a +/- EGCG) following treatment with TCN ± SEM (n = 3), * P<0.05 and ** P<0.01 of protein of interest compared to EGCG control, # represents the protein of interest compared to control without EGCG treatment. Significant differences between treated and control were present at concentrations of 5 and 10 μM for total Akt in the presence of EGCG only, while phospo-Akt was significantly reduced for all TCN concentrations in the presence and absence of EGCG.
EGCG enhances the ADAM10 activating enzyme furin independent of PI3K activation in SweAPP N2a cells
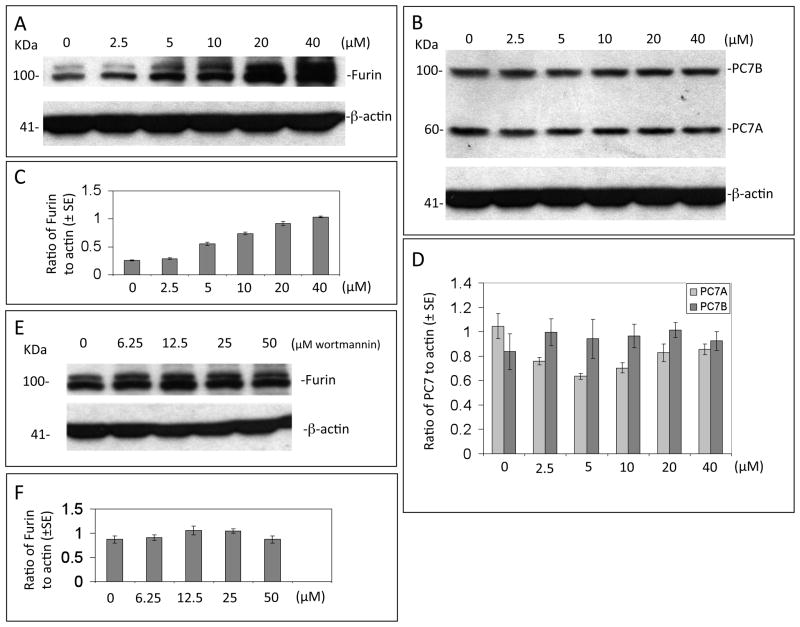
ADAM10 is known to be regulated by proprotein convertases, especially PC7 and furin.[50] To determine whether EGCG enhances activation of ADAM10 through the upstream regulation of proprotein convertases, SweAPP N2a cells were treated with varying concentrations of EGCG (0–40μM) for 4 hours, lysed and subjected to western analysis for furin or PC7. Results indicated that expression of furin but not PC7 isoforms were increased nearly 4-5 fold compared with controls (Fig. 5A-D).
Figure 5. EGCG enhances ADAM10 activating enzyme furin independent of PI3K activation in SweAPP N2a cells.
(A, B) Expression of furin and PC7 was analyzed in lysates from SweAPP N2a cells treated with EGCG at concentrations indicated for 4 hours by western blot. (C, D) Densitometric analysis reveals the band density ratio of furin or PC7 isoforms to β-actin (internal reference control). One-way ANOVA followed by post-hoc comparison revealed significant differences (P < 0.01, n=3, data presented as ±SEM) when comparing each concentration of EGCG and respective furin to actin ratio either to control (PBS) or vs. other EGCG dose. Interestingly EGCG treatments did not significantly affect PC7 isoforms expression (B, D). (E) Expression of furin was analyzed in lysates from SweAPP N2a cells treated with EGCG (20μM) in the presence of PI3K inhibitor (wortmannin) at concentrations indicated for 4 hours by western blot. (F) Densitometric analysis reveals the band density ratio of furin to β-actin (internal reference control). One-way ANOVA followed by post-hoc comparison revealed no significant differences (n=3, data presented as ±SEM) when comparing each concentration of PI3K inhibitor and respective furin to β-actin ratio.
Regulation of furin is complex and appears to involve both adaptor proteins and autoactivation depending on the environment of its cellular compartment,[51] however, given our findings of PI3K/Akt involvement in EGCG promotion of ADAM10 activation via ERα, we explored the ability of varying doses of PI3K inhibitor to affect EGCG-mediated furin upregulation. SweAPP N2a cells were treated with EGCG (20μM) in the presence of PI3K inhibitor (wortmannin; 0-50μM) for 4 hours, lysates were prepared and analyzed by western blot (Fig. 5E & F). Interestingly, PI3K inhibition failed to inhibit EGCG-associated furin activation; implicating a divergent, non-PI3K-mediated pathway for EGCG-induced furin activation.
To investigate whether EGCG-mediated furin upregulation could result from non-PI3K dependent activities of ERα, SweAPP N2a cells were treated for overnight in the presence of EGCG (20μM) and either ERα antagonist (MPP) or agonist (PPT) (Supplemental Fig. 1). Results showed no significant changes in furin protein levels between EGCG and either MPP or PPT thereby altogether implicating an alternative ERα- and PI3K-independent mechanism associated with EGCG-induced furin upregulation.
Discussion
Therapeutic modalities that oppose cleavage of APP into Aβ peptides and attenuate resultant cerebral amyloidosis have become a primary focus in the last decade. The main targets have been β- and γ-secretases, the two proteases that cleave APP at the N- and C-terminus of the Aβ peptide and are thus directly responsible for Aβ peptide generation. Although mechanistically promising, early clinical studies aimed at treating amyloid-associated neurodegenerative disease by modulating these proteases have been disappointing, likely because adequate doses for treatment are limited by clinical toxicity.[52] A different strategy, namely the activation of α-secretase, has only recently begun to be evaluated for its therapeutic potential despite the fact that it cleaves within the Aβ peptide domain and thus precludes Aβ peptide generation.[2–4,7]
Although estrogen replacement therapy remains controversial due largely to adverse effects reported in clinical studies looking at its use in postmenopausal women, the development of selective estrogen modulators for AD continues to be pursued vigorously [14,22]. EGCG, a known modulator of APP processing with function at the ER, is believed to be responsible for the health benefits associated with the consumption of green tea, and has been shown by pharmacokinetic and safety studies to be generally well tolerated.[53] Our laboratory has previously shown that EGCG can increase non-amyloidogenic processing of APP through promotion of the α-secretase ADAM10, which consequently reduced Aβ deposition and improved cognition in AD mice.[36,37] In the present study, we further characterize the mechanisms responsible for EGCG s stimulation of ADAM10 in SweAPP N2a cells by elucidating the involvement of key effectors including ERα, PI3K and Akt (Fig. 1–5). In addition, we corroborate a role for furin in ADAM10 activation and present evidence suggesting that EGCG upregulates furin by mechanisms independent from the ERα/PI3K/ADAM10 pathway proposed here (Fig 5 and Supplemental Fig. 1).
As suggested by our prior studies, SweAPP N2a cells treated with EGCG displayed a dramatic increase in the mature active form of ADAM10, associated with enhanced metabolites indicative of non-amyloidogenic APP processing (Fig 1). When these cells were treated concurrently with the selective ERα antagonist MPP and EGCG, dose-dependent reductions in ADAM10 maturation were observed. The ERα agonist PPT appeared to increase ADAM10 maturation alone, but also possibly compete for ER binding in the presence of EGCG; results which further support the involvement of ERα in EGCG s promotion of non-amyloidogenic processing of APP (Fig. 1 and Supplemental Fig. 1). As suggested by other groups, this effect may represent direct activation of membrane associated ERs.[42] Consistent with our and others findings implicating regulated signal transduction mechanisms mediating EGCG s non-amyloidogenic properties, EGCG failed to show a significant ability to enhance activation of ADAM10 in broken cell preparations (Fig.2).
Our finding that EGCG may act through the estrogen receptor is consistent with previous oncologic findings in which EGCG was capable of binding to and downregulating ERα and ERβ. [42,54] [55] Taken together, these data suggest a mechanistic clue as to why gender differences and estrogen depletion have been described in AD and other APP related disorders[56–59]; including multiple studies which support an association between certain polymorphisms of the ESR1 gene and the risk of developing AD.[60–63].
Although several signal transduction pathways have been implicated in ER and EGCG-mediated pathways, we decided to focus on the PI3K second messenger system as this system canonically involves modulation of GSK-3; a molecule considered to play a key role in AD via the regulation of presenilins and tau,[64–66] and ERα has been shown to interact with PI3K/Akt/GSK3 signaling in neuronal cells.[44] However, controversy exists over whether ER-mediated neuroprotection is dependent on PI3K/Akt activation, but not MAPK/ERK signaling[67,68] or whether the coordinated activity of Akt and ERK is responsible for signaling via the ER.[69,70] Whereas the involvement of PI3K signaling subsequent to activation of ERs is well known, comparatively fewer studies have shown EGCG s capacity to activate PI3K in various cell type.[35,47] We find here that SweAPP N2a cells treated with the PI3K inhibitors and EGCG display lower concentrations of sAPPα in media, less activated ADAM10, and less phospho-Akt. Accordingly, PI3K-phospho-p85 was upregulated after EGCG treatment (Fig. 3D); implicating involvement of PI3K in EGCG-mediated ADAM10 activation. Despite these findings, involvement of ERα/MAPK/ERK signaling in EGCG-mediated ADAM10 activation cannot be ruled out.
ADAM10 is known to be regulated by proprotein convertases, especially PC7 and furin.[50] Subsequent experiments aimed at determining whether the EGCG-mediated ERα-PI3K/Akt/ADAM10 pathway involved PC7 or furin upregulation yielded surprising results. As seen in Figure 5, EGCG enhanced ADAM10 activation was associated with dramatic elevations in furin protein, however this furin upregulation was neither affected by PI3K inhibition nor altered by ERα modulation (Supplemental Fig. 1). Collectively it appears that EGCG enhances ADAM10 activation in both a furin-independent manner via the ERα/PI3K pathway and in a divergent furin dependent manner. Furthermore, PI3K-independent, ERα/MAPK/ERK signaling could also be responsible for furin upregulation in the context of ECGC treatment in these cells. Taken with other reports, this suggests that tight cellular control of this α-secretase is maintained by multiple independently regulated mechanisms, and speaks to the importance of ADAM10 s role in cellular function.[71,72](reviewed by [73])
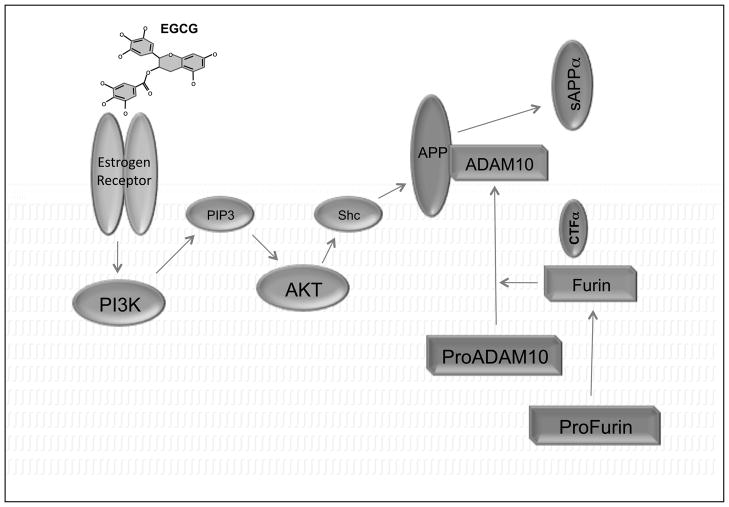
In summary, a possible model for activation of ADAM10 by EGCG in SweAPP N2a cells is represented in Figure 6 (see figure legend for details). Altogether the events depicted, individually or synergistically, lead to enhanced ADAM10 maturation and promotion of non-amyloidogenic processing of APP following EGCG treatment.
Figure 6. Working model of effects of EGCG on APP processing.
In this model, EGCG activates membrane associated estrogen receptors in a ligand dependent, non-genomic, manner setting in motion receptor tyrosine phosphorylation of the p85 regulatory subunit of PI3K. Subsequently PIP2 is converted to PIP3, which, in turn, activates Akt to negatively regulate GSK3 and numerous other downstream effectors required for cellular growth and survival. In our model, Akt may also act directly on APP by phosphorylating C-terminal tyrosine sites or indirectly through the adaptor protein Shc to effect APP phosphorylation.[74] Substrate modifications such as phosphorylation and/or association with adaptor proteins may enhance the binding capacity and substrate-mediated activation of ADAM10 directly[71] or indirectly through autoactivation[51] and enhanced expression of furin, also observed after EGCG treatment.
Our findings support a role for therapeutic selective ERα modulation in the attenuation or prevention of toxic oligomeric Aβ species formation in AD and related disorders. In addition, our data provide a basic mechanistic rationale for previous clinical findings revealing an increased risk of AD in the context of age-dependent estrogen depletion in women. Further exploration of EGCG s effects on estrogen modulation, activation of ADAM10, and promotion of non-amyloidogenic APP processing is warranted to support the use of this compound as a safe alternative to estrogen replacement therapy in the prevention and treatment of AD.
Supplementary Material
Acknowledgments
This work was supported by the NCAAM (R43AT004871, J.T.), NIA (R43AG033417, J.T.), and Veterans Affairs Merit Grant (J.T). N2a cell line stably transfected with the “Swedish” mutant 695 isoform of APP (SweAPP N2a cells; APP695), were kind gifts from S. Gandy. We thank Doug Shytle (University of South Florida) for helpful advice.
Abbreviations
- EGCG
Epigallocatechin 3-gallate
- ER
estrogen receptor
- PI3K
Phosphoinositide 3-kinase
- PIP2
Phosphatidylinositol [3,4]-bisphosphate
- PIP3
Phosphatidylinositol [3,4,5]-triphosphate
- AKT
Ak-transforming
- p85
~85kD regulatory subunit of PI3K
- Shc
Src homology 2 domain-containing
- APP
amyloid precursor protein
- ADAM10
a disintegrin and metalloprotease domain 10
- sAPPα
soluble amyloid precursor protein alpha
- α-CTF
alpha carboxyl terminal fragment
- HRT
hormone replacement therapy
- Aβ
β-amyloid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Jorissen E, et al. The disintegrin/metalloproteinase ADAM10 is essential for the establishment of the brain cortex. J Neurosci. 30:4833–44. doi: 10.1523/JNEUROSCI.5221-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Postina R, et al. A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J Clin Invest. 2004;113:1456–64. doi: 10.1172/JCI20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahrenholz F. Alpha-secretase as a therapeutic target. Curr Alzheimer Res. 2007;4:412–7. doi: 10.2174/156720507781788837. [DOI] [PubMed] [Google Scholar]
- 5.Fahrenholz F, Tippmann F, Endres K. Retinoids as a perspective in treatment of Alzheimer's disease. Neurodegener Dis. 7:190–2. doi: 10.1159/000295662. [DOI] [PubMed] [Google Scholar]
- 6.Lannfelt L, Basun H, Vigo-Pelfrey C, Wahlund LO, Winblad B, Lieberburg I, Schenk D. Amyloid beta-peptide in cerebrospinal fluid in individuals with the Swedish Alzheimer amyloid precursor protein mutation. Neurosci Lett. 1995;199:203–6. doi: 10.1016/0304-3940(95)12059-d. [DOI] [PubMed] [Google Scholar]
- 7.Lammich S, Kojro E, Postina R, Gilbert S, Pfeiffer R, Jasionowski M, Haass C, Fahrenholz F. Constitutive and regulated alpha-secretase cleavage of Alzheimer's amyloid precursor protein by a disintegrin metalloprotease. Proc Natl Acad Sci U S A. 1999;96:3922–7. doi: 10.1073/pnas.96.7.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi S, Refolo LM, Sambamurti K. Amyloid precursor protein compartmentalization restricts beta-amyloid production: therapeutic targets based on BACE compartmentalization. J Mol Neurosci. 2004;24:137–43. doi: 10.1385/JMN:24:1:137. [DOI] [PubMed] [Google Scholar]
- 9.Baum LW. Sex, hormones, and Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 2005;60:736–43. doi: 10.1093/gerona/60.6.736. [DOI] [PubMed] [Google Scholar]
- 10.Musicco M. Gender differences in the occurrence of Alzheimer's disease. Funct Neurol. 2009;24:89–92. [PubMed] [Google Scholar]
- 11.Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer's disease. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaffe AB, Toran-Allerand CD, Greengard P, Gandy SE. Estrogen regulates metabolism of Alzheimer amyloid beta precursor protein. J Biol Chem. 1994;269:13065–8. [PubMed] [Google Scholar]
- 13.Xu H, et al. Estrogen reduces neuronal generation of Alzheimer beta-amyloid peptides. Nat Med. 1998;4:447–51. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
- 14.Carroll JC, Pike CJ. Selective estrogen receptor modulators differentially regulate Alzheimer-like changes in female 3xTg-AD mice. Endocrinology. 2008;149:2607–11. doi: 10.1210/en.2007-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27:13357–65. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honjo H, et al. Progestins and estrogens and Alzheimer's disease. J Steroid Biochem Mol Biol. 2005;93:305–8. doi: 10.1016/j.jsbmb.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Almeida OP, Flicker L. Association between hormone replacement therapy and dementia: is it time to forget? Int Psychogeriatr. 2005;17:155–64. doi: 10.1017/s1041610205001559. [DOI] [PubMed] [Google Scholar]
- 18.Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet. 1996;348:429–32. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 19.Sano M, et al. A multi-center, randomized, double blind placebo-controlled trial of estrogens to prevent Alzheimer's disease and loss of memory in women: design and baseline characteristics. Clin Trials. 2008;5:523–33. doi: 10.1177/1740774508096313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandy S. Neurohormonal Signaling Pathways and the Regulation of Alzheimer beta-Amyloid Precursor Metabolism. Trends Endocrinol Metab. 1999;10:273–279. doi: 10.1016/s1043-2760(99)00166-6. [DOI] [PubMed] [Google Scholar]
- 21.Gandy S, Petanceska S. Regulation of Alzheimer beta-amyloid precursor trafficking and metabolism. Biochim Biophys Acta. 2000;1502:44–52. doi: 10.1016/s0925-4439(00)00031-4. [DOI] [PubMed] [Google Scholar]
- 22.Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol. 2009;30:239–58. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moyers SB, Kumar NB. Green tea polyphenols and cancer chemoprevention: multiple mechanisms and endpoints for phase II trials. Nutr Rev. 2004;62:204–11. doi: 10.1111/j.1753-4887.2004.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 24.Lin JK, Liang YC. Cancer chemoprevention by tea polyphenols. Proc Natl Sci Counc Repub China B. 2000;24:1–13. [PubMed] [Google Scholar]
- 25.Fang H, et al. Structure-activity relationships for a large diverse set of natural, synthetic, and environmental estrogens. Chem Res Toxicol. 2001;14:280–94. doi: 10.1021/tx000208y. [DOI] [PubMed] [Google Scholar]
- 26.Park JW, Choi YJ, Suh SI, Kwon TK. Involvement of ERK and protein tyrosine phosphatase signaling pathways in EGCG-induced cyclooxygenase-2 expression in Raw 264.7 cells. Biochem Biophys Res Commun. 2001;286:721–5. doi: 10.1006/bbrc.2001.5415. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto M, Leung KP, Ansai T, Sugimoto A, Maeda N. Inhibitory effects of green tea catechins on protein tyrosine phosphatase in Prevotella intermedia. Oral Microbiol Immunol. 2003;18:192–5. doi: 10.1034/j.1399-302x.2003.00056.x. [DOI] [PubMed] [Google Scholar]
- 28.Levites Y, Amit T, Mandel S, Youdim MB. Neuroprotection and neurorescue against Abeta toxicity and PKC-dependent release of nonamyloidogenic soluble precursor protein by green tea polyphenol (-)-epigallocatechin-3-gallate. FASEB J. 2003;17:952–4. doi: 10.1096/fj.02-0881fje. [DOI] [PubMed] [Google Scholar]
- 29.Levites Y, Amit T, Youdim MB, Mandel S. Involvement of protein kinase C activation and cell survival/ cell cycle genes in green tea polyphenol (-)-epigallocatechin 3-gallate neuroprotective action. J Biol Chem. 2002;277:30574–80. doi: 10.1074/jbc.M202832200. [DOI] [PubMed] [Google Scholar]
- 30.Li R, Huang YG, Fang D, Le WD. (-)-Epigallocatechin gallate inhibits lipopolysaccharide-induced microglial activation and protects against inflammation-mediated dopaminergic neuronal injury. J Neurosci Res. 2004;78:723–31. doi: 10.1002/jnr.20315. [DOI] [PubMed] [Google Scholar]
- 31.Han MK. Epigallocatechin gallate, a constituent of green tea, suppresses cytokine-induced pancreatic beta-cell damage. Exp Mol Med. 2003;35:136–9. doi: 10.1038/emm.2003.19. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed S, Rahman A, Hasnain A, Lalonde M, Goldberg VM, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1 beta-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic Biol Med. 2002;33:1097–105. doi: 10.1016/s0891-5849(02)01004-3. [DOI] [PubMed] [Google Scholar]
- 33.Aktas O, et al. Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J Immunol. 2004;173:5794–800. doi: 10.4049/jimmunol.173.9.5794. [DOI] [PubMed] [Google Scholar]
- 34.Lin CL, Chen TF, Chiu MJ, Way TD, Lin JK. Epigallocatechin gallate (EGCG) suppresses beta-amyloid-induced neurotoxicity through inhibiting cAbl/FE65 nuclear translocation and GSK3 beta activation. Neurobiol Aging. 2009;30:81–92. doi: 10.1016/j.neurobiolaging.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Chung JH, Han JH, Hwang EJ, Seo JY, Cho KH, Kim KH, Youn JI, Eun HC. Dual mechanisms of green tea extract (EGCG)-induced cell survival in human epidermal keratinocytes. FASEB J. 2003;17:1913–5. doi: 10.1096/fj.02-0914fje. [DOI] [PubMed] [Google Scholar]
- 36.Rezai-Zadeh K, et al. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci. 2005;25:8807–14. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obregon DF, et al. ADAM10 activation is required for green tea (-)-epigallocatechin-3-gallate-induced alpha-secretase cleavage of amyloid precursor protein. J Biol Chem. 2006;281:16419–27. doi: 10.1074/jbc.M600617200. [DOI] [PubMed] [Google Scholar]
- 38.Tan J, et al. Role of CD40 ligand in amyloidosis in transgenic Alzheimer's mice. Nat Neurosci. 2002;5:1288–93. doi: 10.1038/nn968. [DOI] [PubMed] [Google Scholar]
- 39.Chen M, Fernandez HL. Stimulation of beta-amyloid precursor protein alpha-processing by phorbol ester involves calcium and calpain activation. Biochem Biophys Res Commun. 2004;316:332–40. doi: 10.1016/j.bbrc.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 40.Manthey D, Heck S, Engert S, Behl C. Estrogen induces a rapid secretion of amyloid beta precursor protein via the mitogen-activated protein kinase pathway. Eur J Biochem. 2001;268:4285–91. doi: 10.1046/j.1432-1327.2001.02346.x. [DOI] [PubMed] [Google Scholar]
- 41.Gouras GK, Xu H, Gross RS, Greenfield JP, Hai B, Wang R, Greengard P. Testosterone reduces neuronal secretion of Alzheimer's beta-amyloid peptides. Proc Natl Acad Sci U S A. 2000;97:1202–5. doi: 10.1073/pnas.97.3.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodin MG, Fertuck KC, Zacharewski TR, Rosengren RJ. Estrogen receptor-mediated actions of polyphenolic catechins in vivo and in vitro. Toxicol Sci. 2002;69:354–61. doi: 10.1093/toxsci/69.2.354. [DOI] [PubMed] [Google Scholar]
- 43.Qiao Y, Cao J, Xie L, Shi X. Cell growth inhibition and gene expression regulation by (-)-epigallocatechin-3-gallate in human cervical cancer cells. Arch Pharm Res. 2009;32:1309–15. doi: 10.1007/s12272-009-1917-3. [DOI] [PubMed] [Google Scholar]
- 44.Mendez P, Garcia-Segura LM. Phosphatidylinositol 3-kinase and glycogen synthase kinase 3 regulate estrogen receptor-mediated transcription in neuronal cells. Endocrinology. 2006;147:3027–39. doi: 10.1210/en.2005-1224. [DOI] [PubMed] [Google Scholar]
- 45.Sun J, Huang YR, Harrington WR, Sheng S, Katzenellenbogen JA, Katzenellenbogen BS. Antagonists selective for estrogen receptor alpha. Endocrinology. 2002;143:941–7. doi: 10.1210/endo.143.3.8704. [DOI] [PubMed] [Google Scholar]
- 46.Yi KW, et al. Role of estrogen receptor-alpha and -beta in regulating leptin expression in 3T3-L1 adipocytes. Obesity (Silver Spring) 2008;16:2393–9. doi: 10.1038/oby.2008.389. [DOI] [PubMed] [Google Scholar]
- 47.Lorenz M, Wessler S, Follmann E, Michaelis W, Dusterhoft T, Baumann G, Stangl K, Stangl V. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J Biol Chem. 2004;279:6190–5. doi: 10.1074/jbc.M309114200. [DOI] [PubMed] [Google Scholar]
- 48.Petanceska SS, Gandy S. The phosphatidylinositol 3-kinase inhibitor wortmannin alters the metabolism of the Alzheimer's amyloid precursor protein. J Neurochem. 1999;73:2316–20. doi: 10.1046/j.1471-4159.1999.0732316.x. [DOI] [PubMed] [Google Scholar]
- 49.Cali JJ. Frequently Asked Questions: Kinase Inhibitors and Activators. In Cell Notes (Promega Corp) 2001 [Google Scholar]
- 50.Anders A, Gilbert S, Garten W, Postina R, Fahrenholz F. Regulation of the alpha-secretase ADAM10 by its prodomain and proprotein convertases. FASEB J. 2001;15:1837–9. doi: 10.1096/fj.01-0007fje. [DOI] [PubMed] [Google Scholar]
- 51.Anderson ED, Molloy SS, Jean F, Fei H, Shimamura S, Thomas G. The ordered and compartment-specfific autoproteolytic removal of the furin intramolecular chaperone is required for enzyme activation. J Biol Chem. 2002;277:12879–90. doi: 10.1074/jbc.M108740200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Strooper B, Vassar R, Golde T. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat Rev Neurol. 6:99–107. doi: 10.1038/nrneurol.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chow HH, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–9. [PubMed] [Google Scholar]
- 54.Kuruto-Niwa R, Inoue S, Ogawa S, Muramatsu M, Nozawa R. Effects of tea catechins on the ERE-regulated estrogenic activity. J Agric Food Chem. 2000;48:6355–61. doi: 10.1021/jf0008487. [DOI] [PubMed] [Google Scholar]
- 55.Farabegoli F, Barbi C, Lambertini E, Piva R. (-)-Epigallocatechin-3-gallate downregulates estrogen receptor alpha function in MCF-7 breast carcinoma cells. Cancer Detect Prev. 2007;31:499–504. doi: 10.1016/j.cdp.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 56.Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005;62:685–91. doi: 10.1001/archpsyc.62.6.685. [DOI] [PubMed] [Google Scholar]
- 57.Ruitenberg A, Ott A, van Swieten JC, Hofman A, Breteler MM. Incidence of dementia: does gender make a difference? Neurobiol Aging. 2001;22:575–80. doi: 10.1016/s0197-4580(01)00231-7. [DOI] [PubMed] [Google Scholar]
- 58.Swanwick G, Lawlor BA. Is female gender a risk factor for Alzheimer's disease? Int Psychogeriatr. 1999;11:219–22. doi: 10.1017/s1041610299005785. [DOI] [PubMed] [Google Scholar]
- 59.Schupf N, et al. Estrogen receptor-alpha variants increase risk of Alzheimer's disease in women with Down syndrome. Dement Geriatr Cogn Disord. 2008;25:476–82. doi: 10.1159/000126495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Porrello E, Monti MC, Sinforiani E, Cairati M, Guaita A, Montomoli C, Govoni S, Racchi M. Estrogen receptor alpha and APOEepsilon4 polymorphisms interact to increase risk for sporadic AD in Italian females. Eur J Neurol. 2006;13:639–44. doi: 10.1111/j.1468-1331.2006.01333.x. [DOI] [PubMed] [Google Scholar]
- 61.Mattila KM, et al. Interaction between estrogen receptor 1 and the epsilon4 allele of apolipoprotein E increases the risk of familial Alzheimer's disease in women. Neurosci Lett. 2000;282:45–8. doi: 10.1016/s0304-3940(00)00849-1. [DOI] [PubMed] [Google Scholar]
- 62.Ma SL, Tang NL, Tam CW, Lui VW, Lau ES, Zhang YP, Chiu HF, Lam LC. Polymorphisms of the estrogen receptor alpha (ESR1) gene and the risk of Alzheimer's disease in a southern Chinese community. Int Psychogeriatr. 2009;21:977–86. doi: 10.1017/S1041610209990068. [DOI] [PubMed] [Google Scholar]
- 63.Corbo RM, Gambina G, Ruggeri M, Scacchi R. Association of estrogen receptor alpha (ESR1) PvuII and XbaI polymorphisms with sporadic Alzheimer's disease and their effect on apolipoprotein E concentrations. Dement Geriatr Cogn Disord. 2006;22:67–72. doi: 10.1159/000093315. [DOI] [PubMed] [Google Scholar]
- 64.Rezai-Zadeh K, et al. Flavonoid-mediated presenilin-1 phosphorylation reduces Alzheimer's disease beta-amyloid production. J Cell Mol Med. 2009;13:574–88. doi: 10.1111/j.1582-4934.2008.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer's disease. J Alzheimers Dis. 2006;9:309–17. doi: 10.3233/jad-2006-9s335. [DOI] [PubMed] [Google Scholar]
- 66.Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer's disease amyloid-beta peptides. Nature. 2003;423:435–9. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 67.Yi KD, Cai ZY, Covey DF, Simpkins JW. Estrogen receptor-independent neuroprotection via protein phosphatase preservation and attenuation of persistent extracellular signal-regulated kinase 1/2 activation. J Pharmacol Exp Ther. 2008;324:1188–95. doi: 10.1124/jpet.107.132308. [DOI] [PubMed] [Google Scholar]
- 68.Quesada A, Lee BY, Micevych PE. PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson's disease. Dev Neurobiol. 2008;68:632–44. doi: 10.1002/dneu.20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mannella P, Brinton RD. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action. J Neurosci. 2006;26:9439–47. doi: 10.1523/JNEUROSCI.1443-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao L, Brinton RD. Estrogen receptor alpha and beta differentially regulate intracellular Ca(2+) dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res. 2007;1172:48–59. doi: 10.1016/j.brainres.2007.06.092. [DOI] [PubMed] [Google Scholar]
- 71.Janes PW, et al. Cytoplasmic relaxation of active Eph controls ephrin shedding by ADAM10. PLoS Biol. 2009;7:e1000215. doi: 10.1371/journal.pbio.1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou A, Martin S, Lipkind G, LaMendola J, Steiner DF. Regulatory roles of the P domain of the subtilisin-like prohormone convertases. J Biol Chem. 1998;273:11107–14. doi: 10.1074/jbc.273.18.11107. [DOI] [PubMed] [Google Scholar]
- 73.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3:753–66. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tarr PE, Roncarati R, Pelicci G, Pelicci PG, D'Adamio L. Tyrosine phosphorylation of the beta-amyloid precursor protein cytoplasmic tail promotes interaction with Shc. J Biol Chem. 2002;277:16798–804. doi: 10.1074/jbc.M110286200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.