Abstract
Objective
Sleep problems are common, costly, and potentially fatal in older adults. Sleep problems are also commonly associated with alcoholism. Yet, few studies have examined the combined effects of alcoholism and aging on sleep. The purpose of this study was to investigate the main and interactive effects of diagnostic group and age group on sleep.
Method
Male and female alcohol-dependent patients (N=139) and non-alcoholic controls (N=87) completed full-montage polysomnography, structured psychiatric diagnostic interviews, validated rating scales, and alcohol histories at the University of Michigan between 1989 and 1996. The sample was divided for analytic purposes into older (55+ yr) and younger (<55 yr) subgroups.
Results
After controlling for gender, race, body mass index, and psychiatric-related sleep symptoms, alcoholics and older adults had significantly decreased total sleep time and increased stage 1 sleep percentage, respiratory distress, and periodic limb movements. Older adults also had decreased delta sleep percentage and shorter rapid eye movement (REM) sleep latencies. Significant interactions were found between alcoholism and age group for stage 1 sleep percentage, sleep-disordered breathing, and periodic limb movements with older alcoholics having the most disturbances. Older alcoholics also had the highest mean values for sleep latency and the lowest mean values for sleep efficiency and delta sleep percentage when compared to the other three groups.
Conclusions
Older alcoholics have increased sleep disturbances when compared to younger alcoholics and non-alcoholics of both age groups. Care providers should screen for sleep problems among older adults with alcohol problems.
Introduction
Sleep disorders are especially common in older adults. Insomnia affects 25% to 45% of older adults (≥65 yr) (Mellinger et al., 1985), sleep apnea affects 24% to 42% (Ancoli-Israel and Kripke, 1991), and periodic limb movement (PLM) disorder, 45% of older adults (Ancoli-Israel et al., 1991). Characteristics of the sleep electroencephalogram (EEG) also change with age. In general, total nocturnal sleep time, sleep efficiency and delta (slow wave) sleep decrease, whereas light sleep (stage 1) increases (Aldrich, 1998; Benca et al., 1992; Bliwise, 2000). The causes and clinical significance of these changes in the sleep EEG are not entirely clear, but medical illness, medication use, and sleep disorders – all common in older adults – undoubtedly contribute to some disturbances in the sleep EEG (Bliwise, 2000).
Sleep problems are also common in individuals with alcoholism (Aldrich, 1998; Ehlers, 2000; National Institute on Alcohol Abuse and Alcoholism, 1998; Vitiello, 1997). Among alcoholic patients in treatment, rates of symptomatic insomnia range from 36% to 72% (Baekeland et al., 1974; Brower et al., 2001; Caetano et al., 1998; Feuerlein, 1974; Foster et al., 2000), although some of these studies did not control for psychiatric and withdrawal severity and none included a control group of non-alcoholic subjects. In the general population, 18.5% of people with a current diagnosis of alcoholism met criteria for insomnia vs. 10.1% of non-alcoholic respondents (Brower et al., 2000). However, when individuals with psychiatric co-morbidity were excluded, 11.2% of current alcoholics vs. 9.0% of non-alcoholics met criteria for insomnia.
Sleep apnea, characterized by episodes of breathing cessation during sleep, is diagnosed in part by recording the number of apneas (lasting ≥10 seconds) per hour of sleep (apnea index). An apnea index of >5, although not pathognomonic, is included in the diagnostic criteria for obstructive sleep apnea (American Sleep Disorders Association, 1997). Three studies of recently sober alcoholics in combination found that 34 (29.3%) of 116 patients had an apnea index > 5 (Le Bon et al., 1997; Mamdani et al., 1989; Tan et al. 1985). Unfortunately, only one of the three studies included a control group (Tan et al., 1985), which was too small to provide an adequate comparison across studies (0 of 12 control subjects had an apnea index >5).
Whether PLMs and alcoholism are associated is unknown. One study suggested that alcohol use and PLMs are associated (Aldrich and Shipley, 1993), but only two studies to date compared alcoholic patients and non-alcoholic controls. Schiavi et al. (1995) found that PLMs were significantly increased in alcoholic men compared to controls, but Le Bon et al. (1997) found an absence of PLMs in both alcoholic and non-alcoholic subjects. Therefore, further study of the relationship between alcoholism and PLMs during sleep is warranted.
Recently sober alcoholics (who have been abstinent for 2 to 4 wk) also manifest differences in the sleep EEG when compared to non-alcoholic controls (Aldrich et al., 1999; Benca et al., 1992; Gillin et al. 1990b; Le Bon et al., 1997; Snyder and Karacan 1985; Williams and Rundell, 1981). As with older adults, total sleep time, sleep efficiency, and delta sleep are generally decreased, whereas stage 1 sleep is usually increased (Aldrich, 1998; Le Bon et al. 1997). Increases in sleep latency are also noted in most studies of alcoholics (Benca et al., 1992). Finally, recently sober alcoholics may manifest increases in rapid eye movement (REM) sleep and decreases in REM sleep latency, although these REM sleep findings are less consistent across studies. Whether sleep disturbances in alcoholics are premorbid occurrences or whether they are consequences of prolonged, heavy drinking is unknown. In either case, a number of studies indicate that sleep EEG disturbances during early recovery from alcoholism have clinical significance by predicting relapse rates (Brower et al., 1998; Clark et al., 1998; Drummond et al., 1998; Gillin et al., 1994).
Although both aging and alcoholism are associated with an increase in sleep disturbances, few controlled studies have examined the combined effects of age and alcoholism on sleep (Aldrich et al., 1999; Le Bon et al., 1997; Gillin et al., 1990b; Williams and Rundell, 1981). Two of those studies included age-related analyses for single variables only. In the first one, Williams & Rundell (1981) compared 46 male alcoholics (between the ages of 23 and 63 yr) to 20 age-matched controls, and found a negative correlation between age and SWS% that was stronger in alcoholics than in controls. In the second, Le Bon et al. (1997) studied 14 male and 6 female alcoholic patients between the ages of 25 and 58 yr, but did not find a correlation between age and sleep-related respiratory disturbances. By contrast, Aldrich et al. (1999) found an interaction between age and diagnostic group for sleep-disordered breathing in a sample of 139 alcoholics (of which 29 were aged 50 yr and older) and 87 non-alcoholic subjects. However, age-related differences in other sleep measures were analyzed by dividing alcoholics and non-alcoholics into age groups of > 40 yr and ≤ 40 yr. None of the controlled studies examined age by diagnostic group interactions for PLMs.
Gillin et al. (1990b) reviewed evidence and reported new data demonstrating similar sleep patterns among younger alcoholics and older healthy controls. Both younger alcoholics and older controls exhibited decreased total sleep time, decreased sleep efficiency, increased stage 1 sleep percentage (stage 1%), and decreased delta sleep percentage (delta%). Although early investigators attributed the similarity in sleep patterns to premature aging in the alcoholic group, the mechanisms underlying the sleep changes observed with aging and alcoholism remain unknown and could be very different.
With one exception (Aldrich et al., 1999), studies investigating the combined effects of alcoholism and age on sleep (Gillin et al, 1990b; Le Bon et al., 1997; Williams and Rundell, 1981) were limited by small sample sizes (ranging from 20 to 46 alcoholic subjects), few women (6.2% of 97 subjects were women), and no subjects over the age of 65 yr. For example, the study by Gillin et al (1990b) included 13 male alcoholics between the ages of 51 and 61 yr, and no alcoholics over age 61 yr.
The purpose of this study was to investigate the main and interactive effects of age group (<55 yr vs. 55+ yr) and diagnostic group (patients with alcoholism vs. non-alcoholic controls) on sleep EEG measures, sleep-disordered breathing, and PLMs. We hypothesized that older adults with alcoholism would manifest the worst disturbances of sleep across a variety of objective measures.
Method
Subjects
Two groups of subjects were compared in this study. One hundred and thirty-nine subjects (118 males and 21 females) who met DSM-III-R criteria for alcohol dependence (American Psychiatric Association, 1987) were recruited from inpatient and outpatient alcohol treatment programs at the University of Michigan Health System, Chelsea Arbor Treatment Center, and the Ann Arbor Veterans Affairs Medical Center. A comparison group of 87 healthy non-alcoholic subjects (57 males and 30 females) was recruited from the community to study the effects of alcohol administration on sleep. Preliminary results from the alcohol administration studies were published previously (Aldrich et al., 1995), and are not a focus of this report. However, the baseline sleep studies of the control group did not involve alcohol administration, and were used for comparison in this study. Sleep comparisons between the two sample groups have been previously published, where a detailed description of methods may be found (Aldrich et al., 1999). That report focused on sleep-disordered breathing. This report differs from the earlier one by dividing alcoholic and non-alcoholic subjects into age groups of <55 yr and ≥55 yr, and by analyzing groups for main and interactive effects on selected indices of sleep continuity, sleep architecture, REM sleep, and PLMs as well as sleep-disordered breathing. Fifty-five years was chosen for age grouping, because 55 is the lower age limit for admitting patients to the specialized older adult programs at Chelsea Arbor Treatment Center (Blow et al., 2000). Published studies of “older” patient groups have employed different age cutoffs ranging from 45 to 65 yr (Atkinson, 1995). The 55-yr age cutoff lies at the midpoint of this range, and is consistent with several other studies of alcohol and aging (Atkinson et al., 1993; Gomberg, 1995; Kofoed et al., 1987; Moos et al, 1994).
All subjects were screened using the Sleep Disorders Questionnaire (SDQ) (Douglass et al., 1994). The SDQ is a 175-item, validated, self-administered instrument that assesses sleep disturbances during the previous six months and yields four clinical-diagnostic scales: (1) sleep apnea, (2) periodic leg movement disorder, (3) psychiatric sleep disorder, (4) and narcolepsy. To be included in the non-patient control group, subjects had to score below the 70th percentile on all four scales of the SDQ. The reason for this cutoff was to select control subjects who were relatively good sleepers, because some control subjects also participated in studies of nocturnal alcohol administration and sleep. (These data are not reported here.) In order to enhance comparability between the two study groups in terms of symptoms, alcoholic subjects also had to score below the 70th percentile on all four scales of the SDQ. Thus, subjects with strongly symptomatic sleep disturbances and psychiatric-related sleep symptoms were excluded from study. A likely consequence of screening out highly symptomatic individuals would be a minimization of sleep-related differences between the groups. Thus, any sleep-related differences found in this study most likely represent conservative estimates.
The following medical and psychiatric conditions were cause for exclusion from the study, because they can adversely affect sleep: current major depression, psychosis, bipolar disorder, gastrointestinal disease, respiratory disease, heart disease, severe liver disease (such as cirrhosis, serum transaminases > 3 times normal, or total bilirubin > 2 times normal), seizure disorder (except seizures related to alcohol withdrawal in the alcoholic group), degenerative central nervous system disease, cerebrovascular disease, or recent loss of consciousness due to head trauma. Patients with borderline personality disorder were excluded, because of their potential for poor cooperation with study procedures. A past history of major depression, current dysthymic disorder, or anxiety disorders were not cause for exclusion. Psychiatric and substance use diagnoses were determined according to DSM-III-R criteria (American Psychiatric Association, 1987) using the revised Diagnostic Interview Schedule (DIS-III-R) (Robins et al., 1981). Subjects who used medications that affect sleep (e.g., psychotropic medications, centrally acting antihypertensives, oral corticosteroids, theophylline) or addictive substances (except for nicotine) within the two weeks prior to their sleep study were excluded. Urine toxicology was used in addition to self-report to rule out recent drug use. Finally, subjects were excluded if they worked night shifts or intentionally stayed awake during usual bedtime hours.
The protocol for this study was approved by the Institutional Review Boards at University of Michigan Health System and the Ann Arbor Veterans Affairs Medical Center. All subjects were studied after obtaining written informed consent.
Procedures
Prior to polysomnography (PSG), subjects completed a comprehensive medical history, physical examination, psychiatric diagnostic interview, and a sleep evaluation.
Subjects underwent one or more nights of nocturnal polysomnography (PSG) in the sleep laboratory at the University of Michigan. In some cases, patients were studied from their hospital rooms, which were connected by telemetry to the University of Michigan Sleep Laboratory. Only data from the first night of sleep monitoring were used for this report, because not all subjects completed more than one night of study. For the alcoholic group, PSG was performed a minimum of two weeks after admission to a treatment center. The mean time period between polysomnography and the patient's last drink was 31.4 (S.D. 14.4) days. The time period between the last drink and polysomnography did not differ significantly between older and younger alcoholics (28.8 [S.D. 13.1] d vs. 31.7 [14.5] d respectively, t=0.67, p=0.5). For the control groups, subjects were asked to abstain from alcohol for two weeks prior to polysomnography. Drinking history was determined by self-report using the time-line follow-back method (Sobell et al., 1979, 1988).
Sleep was monitored using Sleep Analyzing Computers (Oxford/Microtronics) and standard polysomnographic techniques. Recordings included an electroencephalogram (EEG: C3/A2), electrooculogram (EOG), submental and anterior tibialis electromyogram (EMG), electrocardiogram, abdominal and chest strain gauges to measure respiratory effort, and nose and mouth thermistors to measure airflow. Data were digitized using a bedside portable computer and then transmitted to Sleep Analyzing Computers in the control room. The EEG channel was digitally filtered to yield a nominal band pass of 0.1 to 30 Hz. Data were displayed at a rate of 10mm/second as a virtual polygraph page on a high-resolution monitor and stored to a hard disk at 250 samples/second.
Records were masked to remove identifying information so that raters would be blind to diagnostic status and age, then manually scored using 1-minute epochs (Rechtschaffen and Kales, 1968). A large number of sleep variables can be generated with PSG. To avoid statistical problems arising from multiple comparisons and highly correlated variables, representative sleep variables were selected based on a literature review of sleep changes associated with alcoholism or aging (Aldrich, 1998; Benca et al., 1992; Bliwise, 2000; Gillin et al., 1990b; Le Bon et al., 1997; Snyder and Karacan, 1985; Williams and Rundell, 1981). Selected variables for sleep continuity included total sleep time, sleep efficiency (ratio of time spent asleep to time spent in bed × 100%), and sleep latency. Scoring for sleep latency was defined as the time from start of recording to onset of Stage 2, 3, 4 or REM sleep with a duration of at least 10 minutes, and with no more than two minutes of Stage 1 or one minute of Stage 1 plus one minute of wakefulness. Other sleep variables were percentages of net sleep time spent in stage 1 sleep (stage 1%), slow wave sleep (delta%) or REM sleep (REM%), and REM sleep latency. Delta sleep was calculated by adding stage 3 and stage 4 sleep. Sleep disordered breathing was measured by the respiratory distress index (RDI), which is the mean number of apneas plus hypopneas per hour of sleep. The PLM Index is the mean number of PLMs per hour of sleep.
Data analyses
The effects of age group, diagnostic group, and the interaction of age and diagnosis on sleep variables were determined using a general linear model multivariate analysis (SPSS Inc., 1999). Before performing the multivariate analysis, several preparatory statistical procedures were performed. First, continuous variables were tested for normality and transformed as necessary. Only the RDI required normalization, which was accomplished by transforming it to the natural log of (RDI+1). Second, sleep EEG variables (total sleep time, sleep latency, sleep efficiency, stage 1%, delta%, REM%, and REM sleep latency) were tested for correlations using two-tailed Pearson r correlation tests. In the event of two highly correlated sleep variables (r > 0.70), one was selected for entry into the multivariate analysis to reduce collinearity. Third, the four study groups were compared on selected demographic and clinical characteristics that could influence sleep and confound the results, including gender, race, body mass index, psychiatric-related sleep symptoms, and cigarette smoking. The potentially confounding variables were entered into the multivariate model.
Results
A correlation matrix of the seven sleep EEG variables revealed that sleep efficiency correlated highly with both total sleep time (r = .87, p < .001) and sleep latency (r = −.72, p < .001). These statistics remained significant after controlling for the number of correlation tests performed (0.05/21 = .002). Although total sleep time and sleep latency were also significantly correlated (r = −.65, p < .001), their correlation was less strong than either variable’s correlation with sleep efficiency. Therefore, sleep efficiency was not included in the multivariate analysis. The absolute values of all other correlation coefficients in the matrix were < .50.
General characteristics of the four subject groups are shown in Table 1. The two younger groups did not differ in age, nor did the two older groups. However, the mean age of the two older groups was <65 yr. Both control groups had no years of heavy drinking. The older and younger alcoholic groups did not differ in years of heavy drinking (t = −.52, 12.8 df, p = .61), suggesting that they were comparable in terms of lifetime exposure to alcohol. There were more men in the alcoholic groups than in the control groups (F = 4.10, 3 df, p = .007), so analyses of sleep variables were controlled for gender. Similarly, there were more black subjects in the alcoholic groups than in the control groups. Because black alcoholic patients may have higher rates of sleep disturbance than white alcoholic patients may (Irwin et al., 2000), sleep analyses were controlled for race (coded as “black” or “not black”). The four groups did not differ significantly in terms of body mass index. Nevertheless, body mass index was entered as a covariate, because of its well-established association with sleep-disordered breathing (Aldrich et al., 1999). The control groups scored significantly lower than the alcoholic groups on the psychiatric subscale of the SDQ (F = 22.7, 3 df, p < .001). Because psychiatric severity can influence sleep, the SDQ psychiatric subscale was used as a statistical control in the sleep analyses for psychiatric-related sleep symptoms. Finally, few control subjects smoked cigarettes, whereas most alcoholic subjects did. Adjustments for smoking are addressed later in this section.
Table 1.
Descriptive characteristics of the four study groupsa
| Variable | Younger Controls (N=58) |
Older Controls (N=29) |
Younger Alcoholics (N=123) |
Older Alcoholics (N=16) |
|---|---|---|---|---|
| Age (yr) | 38.4 (8.5) | 64.5 (6.1) | 37.7 (7.9) | 64.2 (6.1) |
| Years of heavy drinking | 0.0 | 0.0 | 11.5 (8.4) | 13.9 (16.5) |
| Males (%) | 67.2 | 62.1 | 84.6 | 87.5 |
| Race/Ethnicity | ||||
| Black (%) | 0.0 | 0.0 | 15.4 | 18.8 |
| White (%) | 94.8 | 96.6 | 82.1 | 81.3 |
| Other (%) | 5.2 | 3.4 | 2.4 | 0.0 |
| Body Mass Index | 24.6 (3.5) | 26.8 (5.0) | 25.6 (4.1) | 25.4 (5.2) |
| SDQ Psychiatric Scale (percentile) | 11.8 (9.6) | 10.1 (10.3) | 29.5 (18.2) | 29.1 (20.8) |
| Current Cigarette Smokers (%) | 1.7 | 3.4 | 81.3 | 93.8 |
Continuous variables are expressed as means (S.D.).
Note: SDQ = Sleep Disorders Questionnaire (Douglass et al., 1994)
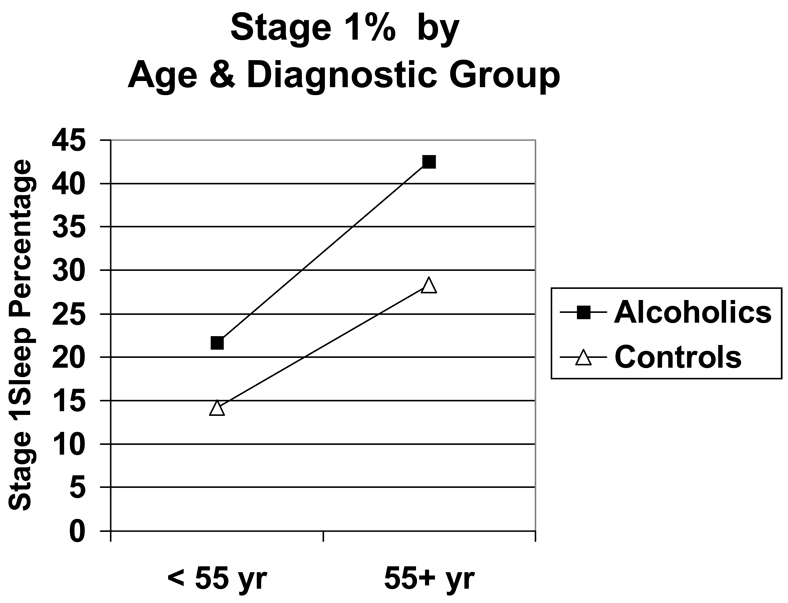
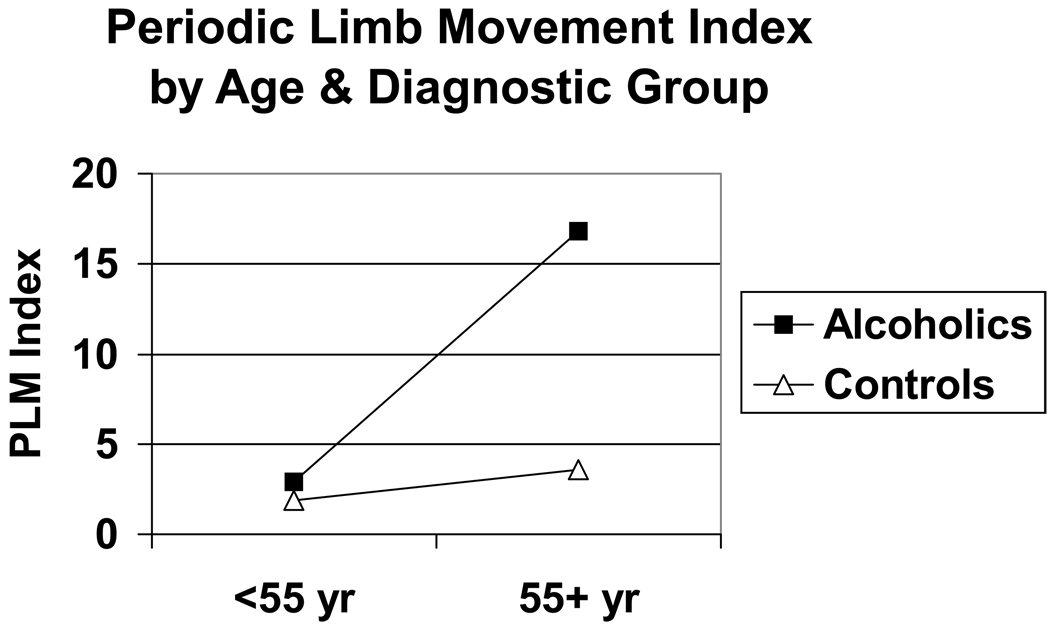
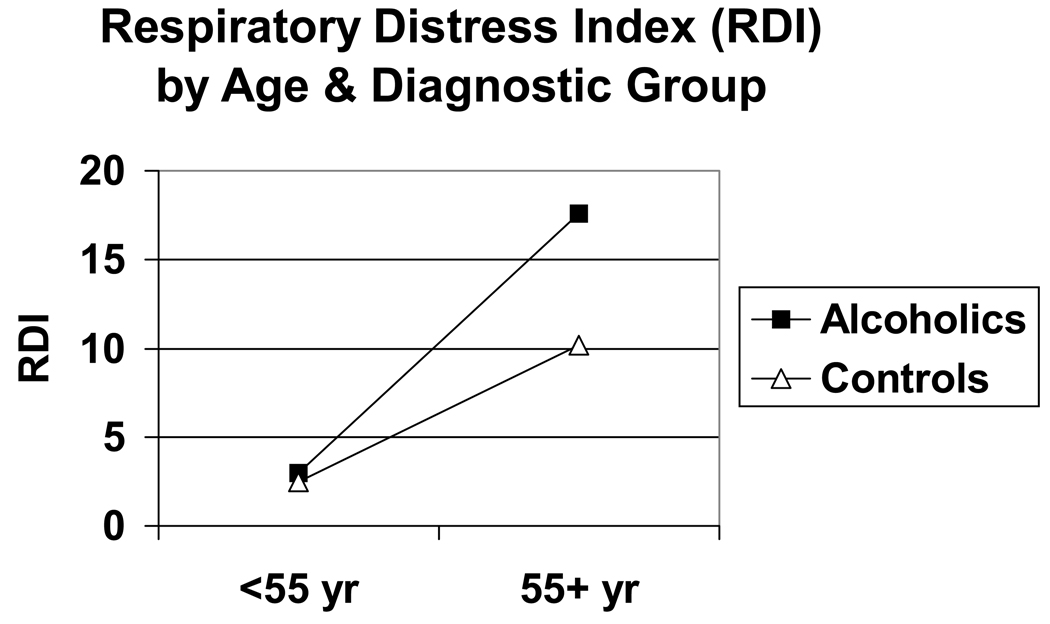
Overall, older adults had greater sleep disturbances than younger adults, and alcoholics had greater sleep disturbances than non-alcoholics (Table 2). Alcoholics slept significantly worse than non-alcoholics as measured by total sleep time, stage 1%, RDI, and PLM index. Similarly, older subjects slept significantly worse than younger subjects in terms of decreased total sleep time and increased stage 1%, RDI and PLM index. In addition, older subjects had significantly lower delta% values and shorter REM sleep latencies than younger subjects had. Significant interactions between age and diagnostic group for stage 1%, RDI, and PLM index were obtained with older alcoholics having the highest values for stage 1%, RDI, and PLM index (Figure 1–Figure 3).
Table 2.
Effects of age and diagnostic group on objective sleep measuresa
| Variable | Younger Controls (N=58) |
Older Controls (N=29) |
Younger Alcoholics (N=123) |
Older Alcoholics (N=16) |
|---|---|---|---|---|
| RDI b,c,d | 2.5 (13.1) | 10.2 (25.8) | 3.0 (7.3) | 17.6 (24.2) |
| PLM Index b,c,d | 1.9 (8.8) | 3.6 (5.4) | 2.9 (7.6) | 16.8 (40.0) |
| Total Sleep Time (min)b,c | 357.1 (54.4) | 316.0 (49.0) | 313.9 (48.4) | 277.7 (58.7) |
| Sleep Latency (min) | 26.5 (36.5) | 31.7 (32.9) | 34.6 (31.7) | 43.5 (43.9) |
| Sleep Efficiency (%) e | 87.2 (12.2) | 78.0 (11.5) | 81.2 (10.8) | 71.6 (15.0) |
| Stage 1 Sleep (%) b,c,d | 14.2 (10.5) | 28.3 (18.3) | 21.7 (10.4) | 42.5 (23.2) |
| Delta Sleep (%) c | 13.1 (9.8) | 8.1 (10.0) | 8.5 (8.8) | 4.3 (7.8) |
| REM Sleep Latency (min) c | 77.4 (45.3) | 63.7 (38.3) | 79.7 (52.7) | 48.6 (57.3) |
| REM Sleep (%) | 19.8 (5.5) | 18.5 (7.2) | 20.0 (6.2) | 20.4 (8.3) |
Variables are expressed as means (S.D.).
Significant diagnostic group effect (alcoholics vs. controls) after controlling for age group, gender, race, body mass index, psychiatric-related sleep symptoms, and other sleep variables.
Significant age effect (<55 vs. 55+ yr) after controlling for diagnostic group, gender, race, body mass index, psychiatric-related sleep symptoms, and other sleep variables.
Significant age by diagnostic group interaction after controlling for gender, race, body mass index, psychiatric-related sleep symptoms, and other sleep variables.
Not entered into the multivariate analysis due to its high correlations with both total sleep time and sleep latency.
Figure 1.
Stage 1% as a function of age group and diagnostic group. Significant effects were obtained for age (F = 57.7, 1 df, p <.001), diagnosis (F = 26.7, 1 df, p < .001) and age × diagnosis interaction (F = 4.3, 1 df, p = .038). Effects were significant after controlling for gender, race, body mass index, psychiatric-related sleep symptoms, and other sleep variables.
Figure 3.
PLM index as a function of age group and diagnostic group. Significant effects were obtained for age (F = 11.7, 1 df, p = .001), diagnosis (F = 10.2, 1 df, p = .002), and age × diagnosis interaction (F = 8.1, 1 df, p = .005). Effects were significant after controlling for gender, race, body mass index, psychiatric-related sleep symptoms, and other sleep variables.
Because only two control subjects were smokers and only one older alcoholic was not a smoker, it was not possible to adjust statistically for smoking between alcoholic and non-alcoholic subjects. However, it was possible to analyze the effects of current smoking status (smoker vs. non-smoker) on sleep measures among the alcoholic groups. In this second multivariate analysis, the following variables were included: age group, gender, race, body mass index, and SDQ psychiatric subscale score. After entering these variables, there were no main effects of smoking status on total sleep time, sleep latency, stage 1%, delta%, REM%, REM sleep latency, RDI, or PLM index.
Discussion
The main findings of the study were (1) recently abstinent alcoholics had more disturbed sleep than non-alcoholics in terms of decreased total sleep time and increased stage 1%, RDI, and PLM index; (2) older adults had more disturbed sleep than younger adults in terms of decreased sleep total sleep time, increased stage 1%, decreased delta%, decreased REM sleep latency, increased RDI and increased PLM index; and (3) alcoholism and aging interacted to manifest increased stage 1%, sleep-disordered breathing (RDI), and PLM index in older alcoholics. Results were significant after controlling for other variables that can influence sleep; i.e., gender, race, body mass index, and psychiatric scores. Although other indicators of sleep disturbance did not significantly differ between groups, older alcoholics had the lowest mean values for sleep efficiency, delta%, and REM sleep latency and the highest mean value for sleep latency (Table 2). The interactive effect between alcoholism and aging on the RDI was reported previously (Aldrich et al., 1999), and will not be discussed in further detail here.
A decrease in total sleep time with either aging or alcoholism is consistent with several previous reports (Aldrich, 1998; Gillin et al., 1990b; Le Bon et al., 1997; Snyder and Karacan, 1985). This study extends those findings by suggesting that alcoholism and older age together result in an additive, not interactive, effect in decreasing total sleep time. (An additive effect indicates that both age and alcoholism had independent main effects on total sleep time without a significant interaction between them.) An increase in stage 1% with age or alcoholism is also consistent with previous reports (Aldrich, 1998; Gillin et al., 1990b; Le Bon et al., 1997; Williams and Rundell, 1981). In contrast to total sleep time, alcoholism and older age interacted to increase stage 1%. As expected, delta% was significantly lower in older subjects than in younger subjects. Unexpectedly, delta% did not differ significantly between alcoholics and controls, although the difference approached significance (F = 2.92, 1 df, p = .09). The data are consistent with one other study that found no significant difference in delta% between alcoholics and controls (Snyder and Karacan, 1985), although most controlled studies have found less delta sleep in alcoholics than in non-alcoholic controls (Gillin et al., 1990b; Le Bon et al., 1997; Williams and Rundell, 1981). Abnormalities in stage 1%, total sleep time, and delta sleep may explain in part the frequent, subjective complaints of insomnia among older adults and alcoholics.
Some controlled studies have found increased REM sleep percent and shortened REM sleep latency during the post-acute alcohol withdrawal period (Gillin et al., 1990a; Moeller et al., 1993; Williams and Rundell, 1981), while other controlled studies have not (Gillin et al., 1990b; Le Bon et al., 1997; Snyder and Karacan, 1985). No significant differences between the alcoholic and control groups were found for either REM sleep percent or REM sleep latency in this study. The discrepant findings across studies suggest that REM sleep abnormalities in alcoholics may vary by alcoholic subtypes. For example, alcoholic patients who are comorbidly depressed (Gillin et al., 1990a; Moeller et al., 1993) and those who subsequently relapse (Brower et al., 1998; Gillin et al., 1994) may have the highest likelihood of manifesting REM sleep abnormalities. Further studies are needed to confirm this hypothesis.
Although multivariate analyses controlled for race, there were no black subjects in the control group. The literature is both sparse and unclear about the differences in sleep between black and white people. Irwin et al. (2000) found that black subjects had more objective sleep abnormalities than white subjects did. Moreover, they reported an interaction between race and alcoholism resulting in prolonged sleep latency, loss of delta sleep, and shortened REM sleep latency among black alcoholics. Among older adults in the general population, however, lower prevalence rates of insomnia were found in black than white individuals (Foley et al., 1999). To complicate matters further, black women had the highest incidence rate of insomnia, despite low prevalence rates (Foley et al., 1999). Further research is needed to clarify racial differences in sleep.
Several limitations of the study deserve mention. One limitation was the exclusive use of first-night data from polysomnographic recordings. Subjects generally sleep worse during their first night in a laboratory and, thus, sleep disturbances may have been exaggerated in this study. Therefore, caution is urged when comparing the absolute sleep values reported here to other studies. Nevertheless, the first-night recording bias should have affected all study subjects equally, so the comparisons between study groups should be relatively unbiased by the so-called first-night effect. Another potential limitation was that some patients had their sleep monitored in their hospital rooms rather than in the sleep laboratory, whereas all control subjects were monitored in the sleep laboratory. It is possible that environmental factors such as noise and interruptions by clinical staff or other patients may have contributed to the poor sleep of alcoholic subjects. On the other hand, patients were monitored only in private rooms without roommates, hospital staff was informed of procedures, and a “Do Not Disturb” sign was posted on the door indicating that a sleep study was in progress.
A number of variables may have confounded the results. First, a past history of major depression may have contributed to sleep disturbances in this study. Reduced REM sleep latency and low SWS% are characteristic of major depression and may sometimes persist after depression has remitted (Thase et al., 1998). Because alcoholic patients are more likely than control subjects to have a past history of depression, their sleep could be worse. Likewise, the inclusion of subjects with dysthymia may have contributed to sleep abnormalities such as increased stage 1% and low SWS% (Arriaga et al., 1990, 1995). Second, anxiety disorders and their treatment can affect sleep. Benzodiazepines are commonly prescribed to treat anxiety and can cause rebound insomnia upon discontinuation. Although subjects were free of anti-anxiety medications for at least two weeks prior to study, discontinuation effects may sometimes last longer than that. Third, the amount of drinking in recent months was not specified and may have differed between younger and older alcoholics. Heavy, recent drinking would likely correlate with more sleep disturbance among alcoholic patients. Fourth, the severity of acute alcohol withdrawal, which can differ as a function of age (Brower et al., 1994), may be associated with worse sleep during subacute withdrawal (Gillin et al., 1990b). Because the severity of acute withdrawal was not measured in this study, no conclusions can be drawn regarding the association between withdrawal severity and impaired sleep in the alcoholic groups.
Another potential confound was the disproportionate number of cigarette smokers in the alcoholic subjects. Cigarette smoking is a known risk factor for sleep-disordered breathing (Wetter and Young, 1994), and could have confounded the RDI comparisons between alcoholic and control subjects. When smoking status was entered as a factor in a multivariate comparison of younger and older alcoholics, however, no main effect of smoking on RDI was found. Cigarette smoking has also been associated with increased sleep latency (Soldatos et al., 1980), although this study did not replicate that finding and no main effects of age group or diagnostic group on sleep latency were revealed in this study. Other drugs of abuse can also interfere with sleep (e.g., Thompson et al., 1995), and alcohol dependence increases the likelihood of using other substances. Thus, other drug use – marijuana, cocaine, other stimulants, sedatives, opiates, hallucinogens, phencyclidine -- may have accounted for some of the differences between alcoholic and control subjects. Among the alcoholic subjects, however, the younger group was more likely to have used drugs in the past 12 months than the older group (30% vs. 7.7%), yet the older group had more sleep disturbance. Therefore, it does not appear that other drug use accounted for more sleep disturbance in older vs. younger alcoholic subjects.
Another limitation was the small number of older alcoholics (N=16), especially older female alcoholics (N=2). Although specific data were not available, older alcoholics may have been more likely than younger ones to be excluded for medical reasons. The exclusion criteria, therefore, while making it easier to attribute sleep disturbances to age and alcoholism, limited the generalization of results to the total population of older alcoholics in treatment. Nevertheless, older alcoholics with more medical problems than those studied here would be expected to have even more disturbances in sleep than those without medical problems. Finally, self-selection bias was a possible limitation, because alcoholic patients with sleep disturbances may be more likely to volunteer for sleep studies than alcoholics who sleep relatively well. We tried to minimize this bias by screening for alcoholic subjects (as well as control subjects) who scored below the 70th percentile on the four subscales of the SDQ. The likely effect of applying the 70th-percentile criterion would be to minimize sleep-related differences between the study groups. Still, self-selection bias may have accentuated the differences in sleep between alcoholic and non-alcoholic subjects.
Sleep disturbances, as well as alcoholism, can result in decreased quality of life, impaired daytime performance (Mendelson et al. 1984), memory dysfunction (Roehrs and Roth 1995), deficits in concentration, and an increased risk for depression (Breslau et al., 1996; Ford and Kamerow, 1989; Weissman et al. 1997). The risk of motor vehicle accidents due to falling asleep while driving is a potentially fatal consequence of sleep disturbances (Aldrich, 1989; Hicks et al., 1998; Roehrs et al., 1994) that is increased further by drinking alcohol (Aldrich and Chervin, 1997). Stoller (1994) estimated that about 10% of alcohol-related costs in this country could be attributed to insomnia (e.g., the costs of falling asleep while driving because of the enhanced sedative effects of alcohol in people with a sleep deficit [(Zwyghuizen-Doorenbos et al., 1988]). Given that the cost of alcohol problems was estimated to be $166.5 billion in 1995 (National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism, 1998), insomnia may have contributed to $16.6 billion of those costs.
Sleep-disordered breathing, as well as alcoholism, has been associated with fatal heart disease and stroke (Strollo and Rogers, 1996). Premature mortality has also been associated with insomnia (Pollak et al., 1990; Wingard and Berkman, 1983) and decreased hours of sleep (Kripke et al., 1979), even after controlling for physical health and other risk factors. More recent studies have failed to show an association between insomnia and mortality (Althuis et al., 1998; Brabbins et al., 1993; Foley et al., 1995), but none of these latter studies adequately controlled for alcohol consumption, which can also impact mortality rates. Although the evidence may be mixed for insomnia, many studies call into question the old clinical adage: “No one ever died from not sleeping well.” Therefore, clinicians should screen for sleep problems among alcoholic patients, especially older ones.
Figure 2.
RDI as a function of age group and diagnostic group. RDI was normally transformed to Natural Log (RDI +1) for statistical analyses. Significant effects were obtained for age (F = 26.2, 1 df, p < .001), diagnosis (F = 14.9, 1 df, p < .001), and age × diagnosis interaction (F = 4.7, 1 df, p = .031). Effects were significant after controlling for gender, race, body mass index, psychiatric-related sleep symptoms, and other sleep variables.
Acknowledgement
The authors gratefully acknowledge the invaluable contributions of Michael S. Aldrich, M.D. to the planning and implementation of this study. Dr. Aldrich died on July 18, 2000.
This research was supported by grants P50 AA07378 and K24 AA00304 from the National Institute on Alcohol Abuse and Alcoholism
References
- Aldrich MS. Automobile accidents in patients with sleep disorders. Sleep. 1989;12:487–494. doi: 10.1093/sleep/12.6.487. [DOI] [PubMed] [Google Scholar]
- Aldrich M. Effects of alcohol on sleep. In: Gomberg ESL, Hegedus AM, Zucker RA, editors. Alcohol Problems and Aging. National Institute on Alcohol Abuse and Alcoholism Research Monograph Series. Vol. 33. Bethesda, MD: National Institutes on Health (NIH Publication No. 98-4163); 1998. pp. 281–300. [Google Scholar]
- Aldrich MS, Brower KJ, Hall JM. Sleep-disordered breathing in alcoholics. Alcohol. Clin. Exp. Res. 1999;23:134–140. [PubMed] [Google Scholar]
- Aldrich MS, Chervin RD. Alcohol use, obstructive sleep apnea, and sleep-related motor vehicle accidents. Sleep Res. 1997;26:308. [Google Scholar]
- Aldrich MS, O'Neal EA, Shipley JE. Sleep and low doses of alcohol: age and gender effects (abstract) Sleep Res. 1995;24:67. [Google Scholar]
- Aldrich MS, Shipley JE. Alcohol use and periodic limb movements of sleep. Alcohol. Clin. Exp. Res. 1993;17:192–196. doi: 10.1111/j.1530-0277.1993.tb00747.x. [DOI] [PubMed] [Google Scholar]
- Althuis MD, Fredman L, Langenberg PW, Magaziner J. The relationship between insomnia and mortality among community-dwelling older women. J. Am. Geriatr. Soc. 1998;46:1270–1273. doi: 10.1111/j.1532-5415.1998.tb04544.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. DSM-III-R: Diagnostic and Statistical Manual of Mental Disorders; Washington DC: American Psychiatric Association; 1987. [Google Scholar]
- American Sleep Disorders Association. International Classification of Sleep Disorders, Revised: Diagnostic and Coding Manual; Rochester, MN: American Sleep Disorders Association; 1997. [Google Scholar]
- Ancoli-Israel S, Kripke DF. Prevalent sleep problems in the aged. Biofeedback Self. Regul. 1991;16:349–359. doi: 10.1007/BF00999989. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Periodic limb movements in sleep in community-dwelling elderly. Sleep. 1991;14:496–500. doi: 10.1093/sleep/14.6.496. [DOI] [PubMed] [Google Scholar]
- Arriaga F, Cavaglia F, Matos-Pires A, Lara E, Paiva T. EEG sleep characteristics in dysthymia and major depressive disorder. Neuropsychobiology. 1995;32:128–131. doi: 10.1159/000119225. [DOI] [PubMed] [Google Scholar]
- Arriaga F, Rosado P, Paiva T. The sleep of dysthymic patients: a comparison with normal controls. Biol Psychiatry. 1990;27:649–656. doi: 10.1016/0006-3223(90)90533-8. [DOI] [PubMed] [Google Scholar]
- Atkinson RM. Treatment programs for aging alcoholics. In: Beresford T, Gomberg E, editors. Alcohol and Aging. New York: Oxford University Press; 1995. pp. 186–210. [Google Scholar]
- Atkinson RM, Tolson RL, Turner JA. Factors affecting outpatient treatment compliance of older male problem drinkers. Journal of Studies on Alcohol. 1993;54:102–106. doi: 10.15288/jsa.1993.54.102. [DOI] [PubMed] [Google Scholar]
- Baekeland F, Lundwall L, Shanahan TJ, Kissin B. Clinical correlates of reported sleep disturbance in alcoholics. Q. J. Stud. Alcohol. 1974;35:1230–1241. [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: A meta-analysis. Arch. Gen. Psychiatry. 1992;49:651–668. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Normal aging. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 3rd Edition. Philadelphia: Saunders; 2000. pp. 26–42. [Google Scholar]
- Blow FC, Walton MA, Chermack ST, Mudd SA, Brower KJ. Older adult treatment outcome following elder-specific inpatient alcoholism treatment. J Subst Abuse Treat. 2000;19:67–75. doi: 10.1016/s0740-5472(99)00101-4. [DOI] [PubMed] [Google Scholar]
- Brabbins CJ, Dewey ME, Copeland JRM, Davidson IA, McWilliam C, Saunders P, Sharma VK, Sullivan C. Insomnia in the elderly: prevalence, gender differences and relationships with morbidity and mortality. Int. J. Geriatr. Psychiat. 1993;8:473–480. [Google Scholar]
- Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol. Psychiatry. 1996;39:411–418. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Hall JM. Polysomnographic and subjective sleep predictors of alcoholic relapse. Alcohol. Clin. Exp. Res. 1998;22:1864–1871. [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Robinson EAR, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry. 2001;158 doi: 10.1176/appi.ajp.158.3.399. xxx-xxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Mudd S, Blow FC, Young JP, Hill EM. Severity and treatment of alcohol withdrawal in elderly versus younger patients. Alcohol Clin Exp Res. 1994;18:196–201. doi: 10.1111/j.1530-0277.1994.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Robinson EAR, Zucker RA. Epidemiology of insomnia and alcoholism in the general population. Alcohol. Clin. Exp. Res. 2000;24 suppl 5:43A. [Google Scholar]
- Caetano R, Clark CL, Greenfield TK. Prevalence, trends, and incidence of alcohol withdrawal symptoms: analysis of general population and clinical samples. Alcohol Health Res. World. 1998;22:73–79. [PMC free article] [PubMed] [Google Scholar]
- Clark CP, Gillin JC, Golshan S, Demodena A, Smith TL, Danowski S, Irwin M, Schuckit M. Increased REM sleep density at admission predicts relapse by three months in primary alcoholics with a lifetime diagnosis of secondary depression. Biol. Psychiatry. 1998;43:601–607. doi: 10.1016/s0006-3223(97)00457-5. [DOI] [PubMed] [Google Scholar]
- Douglass AB, Bornstein R, Nino-Murcia G, Keenan S, Miles L, Zarcone V, Jr., Guilleminault C, Dement WC. The Sleep Disorders Questionnaire. I: Creation and multivariate structure of SDQ. Sleep. 1994;17:160–167. doi: 10.1093/sleep/17.2.160. [DOI] [PubMed] [Google Scholar]
- Drummond SPA, Gillin JC, Smith TL, DeModena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol. Clin. Exp. Res. 1998;22:1796–1802. [PubMed] [Google Scholar]
- Ehlers CL. Alcohol and sleep. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA's Neuroscience and Behavioral Research Portfolio. National Institute on Alcohol Abuse and Alcoholism Research Monograph Series. Vol. 34. Bethesda, MD: National Institutes of Health; 2000. pp. 417–433. [Google Scholar]
- Feuerlein W. The acute alcohol withdrawal syndrome: findings and problems. Br. J. Addict. 1974;69:141–148. doi: 10.1111/j.1360-0443.1974.tb01294.x. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Monjan AA, Izmirlian G, Hays JC, Blazer DG. Incidence and remission of insomnia among elderly adults in a biracial cohort. Sleep. 1999;22 Suppl 2:S373–S378. [PubMed] [Google Scholar]
- Ford DE, Kamerow DB. Epidemiologic study of sleep disturbance and psychiatric disorders. JAMA. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Foster JH, Marshall EJ, Peters TJ. Application of a quality of life measure, the life situation survey (LSS), to alcohol-dependent subjects in relapse and remission. Alcohol Clin Exp Res. 2000;24:1687–1692. [PubMed] [Google Scholar]
- Gillin JC, Smith TL, Irwin M, Butters N, Demodena A, Schuckit M. Increased pressure for rapid eye movement sleep at time of hospital admission predicts relapse in nondepressed patients with primary alcoholism at 3-month follow-up. Arch. Gen. Psychiat. 1994;51:189–197. doi: 10.1001/archpsyc.1994.03950030025003. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Smith TL, Irwin M, Kripke DF, Brown S, Schuckit M. Short REM latency in primary alcoholic patients with secondary depression. Am J Psychiatry. 1990a;147:106–109. doi: 10.1176/ajp.147.1.106. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Smith TL, Irwin M, Kripke DF, Schuckit M. EEG sleep studies in "pure" primary alcoholism during subacute withdrawal: relationships to normal controls, age, and other clinical variables. Biol. Psychiatry. 1990b;27:477–488. doi: 10.1016/0006-3223(90)90439-9. [DOI] [PubMed] [Google Scholar]
- Gomberg ESL. Older alcoholics: entry into treatment. In: Beresford T, Gomberg E, editors. Alcohol and Aging. New York: Oxford University Press; 1995. pp. 169–185. [Google Scholar]
- Hicks GJ, Davis JW, Hicks RA. Fatal alcohol-related traffic crashes increase subsequent to changes to and from daylight savings time. Percept. Mot. Skills. 1998;86:879–882. doi: 10.2466/pms.1998.86.3.879. [DOI] [PubMed] [Google Scholar]
- Irwin M, Miller C, Gillin JC, Demodena A, Ehlers CL. Polysomnographic and spectral sleep EEG in primary alcoholics: an interaction between alcohol dependence and African-American ethnicity. Alcohol. Clin. Exp. Res. 2000;24:1376–1384. [PubMed] [Google Scholar]
- Kofoed LL, Tolson RL, Atkinson RM, Toth RL, Turner JA. Treatment compliance of older alcoholics: an elder-specific approach is superior to "mainstreaming". Journal of Studies on Alcohol. 1987;48:47–51. doi: 10.15288/jsa.1987.48.47. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Simons RN, Garfinkel L, Hammond EC. Short and long sleep and sleeping pills. Arch. Gen. Psychiat. 1979;36:103–116. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- Le Bon O, Verbanck P, Hoffmann G, Murphy JR, Staner L, De Groote D, Mampunza S, Den Pulk A, Vacher C, Kornreich Ch, Pelc I. Sleep in detoxified alcoholics: impairment of most standard sleep parameters and increased risk for sleep apnea, but not myoclonias -- a controlled study. J. Stud. Alcohol. 1997;58:30–36. doi: 10.15288/jsa.1997.58.30. [DOI] [PubMed] [Google Scholar]
- Mamdani M, Hollyfield R, Ravi RD, Dorus W, Borge GF. Prevalence of sleep apnea among abstinent chronic alcoholic men [abstract] Sleep Res. 1989;18:349. [Google Scholar]
- Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment: prevalence and correlates. Arch. Gen. Psychiatry. 1985;42:225–232. doi: 10.1001/archpsyc.1985.01790260019002. [DOI] [PubMed] [Google Scholar]
- Mendelson WB, Garnett D, Gillin JC, Weingartner H. The experience of insomnia and daytime and nighttime functioning. Psychiatry Res. 1984;12:235–250. doi: 10.1016/0165-1781(84)90029-5. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Gillin JC, Irwin M, Golshan S, Kripke DF, Schuckit M. A comparison of sleep EEGs in patients with primary major depression and major depression secondary to alcoholism. J Affect Disord. 1993;27(1):39–42. doi: 10.1016/0165-0327(93)90095-2. [DOI] [PubMed] [Google Scholar]
- Moos RH, Mertens JR, Brennan PL. Rates and predictors of four-year readmission among late-middle-aged and older substance abuse patients. Journal of Studies on Alcohol. 1994;55:561–570. doi: 10.15288/jsa.1994.55.561. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Alcohol and sleep. Alcohol Alert. 1998;41:1–4. [Google Scholar]
- National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism. The Economic Costs of Alcohol and Drug Abuse in the United States 1992. Rockville, MD: National Institutes of Health (Publication No. 98-4327); 1998. [Google Scholar]
- Pollak CP, Perlick D, Linsner JP, Wenston J, Hsieh F. Sleep problems in the community elderly as predictors of death and nursing home placement. J. Community Health. 1990;15:123–135. doi: 10.1007/BF01321316. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales AA. Washington, DC: Government Printing Office (NIH Publication No. 204); A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. 1968 doi: 10.1046/j.1440-1819.2001.00810.x. [DOI] [PubMed]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule: its history, characteristics, and validity. Arch. Gen. Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Beare D, Zorick F, Roth T. Sleepiness and ethanol effects on simulated driving. Alcohol. Clin. Exp. Res. 1994;18:154–158. doi: 10.1111/j.1530-0277.1994.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Roth T. Alcohol-induced sleepiness and memory function. Alcohol Health Res. World. 1995;19:30–135. [PMC free article] [PubMed] [Google Scholar]
- Schiavi RC, Stimmel BB, Mandeli J, White D. Chronic alcoholism and male sexual function. Am J Psychiatry. 1995;152:1045–1051. doi: 10.1176/ajp.152.7.1045. [DOI] [PubMed] [Google Scholar]
- Snyder S, Karacan I. Sleep patterns of sober chronic alcoholics. Neuropsychobiology. 1985;13:97–100. doi: 10.1159/000118169. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abuser's self-reports of drinking behavior. Behavioral Research and Therapy. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. Br J Addiction. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Soldatos CR, Kales JD, Scharf MB, Bixler EO, Kales A. Cigarette smoking associated with sleep difficulty. Science. 1980;207:551–553. doi: 10.1126/science.7352268. [DOI] [PubMed] [Google Scholar]
- SPSS, Inc. SPSS Base 10.0 Applications Guide. Chicago, IL: Author; 1999. [Google Scholar]
- Stoller MK. Economic effects of insomnia. Clin. Ther. 1994;16:873–897. [PubMed] [Google Scholar]
- Strollo PJ, Rogers RM. Obstructive sleep apnea. New Engl. J. Med. 1996;334:99–104. doi: 10.1056/NEJM199601113340207. [DOI] [PubMed] [Google Scholar]
- Tan ET, Lambie DG, Johnson RH, Robinson BJ, Whiteside EA. Sleep apnoea in alcoholic patients after withdrawal. Clin. Sci. 1985;69:655–661. doi: 10.1042/cs0690655. [DOI] [PubMed] [Google Scholar]
- Thase ME, Fasiczka AL, Berman SR, Simons AD, Reynolds CF. 3. Electroencephalographic sleep profiles before and after cognitive behavior therapy of depression. Arch Gen Psychiatry. 1988;55:138–144. doi: 10.1001/archpsyc.55.2.138. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Gillin JC, Golshan S, Irwin M. Polygraphic sleep measures differentiate alcoholics and stimulant abusers during short-term abstinence. Biol Psychiatry. 1995;38:831–836. doi: 10.1016/0006-3223(95)00070-4. [DOI] [PubMed] [Google Scholar]
- Vitiello MV. Sleep, alcohol, and alcohol abuse. Addiction Biology. 1997;2:151–158. doi: 10.1080/13556219772697. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Greenwald S, Nino-Murcia G, Dement WC. The morbidity of insomnia uncomplicated by psychiatric disorders. Gen. Hosp. Psychiatry. 1997;19:245–250. doi: 10.1016/s0163-8343(97)00056-x. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Young TB. The relation between cigarette smoking and sleep disturbance. Prev. Med. 1994;23:328–334. doi: 10.1006/pmed.1994.1046. [DOI] [PubMed] [Google Scholar]
- Williams HL, Rundell OH. Altered sleep physiology in chronic alcoholics: reversal with abstinence. Alcohol. Clin. Exp. Res. 1981;2:318–325. doi: 10.1111/j.1530-0277.1981.tb04905.x. [DOI] [PubMed] [Google Scholar]
- Wingard DL, Berkman LF. Mortality risk associated with sleeping patterns among adults. Sleep. 1983;6:102–107. doi: 10.1093/sleep/6.2.102. [DOI] [PubMed] [Google Scholar]
- Zwyghuizen-Doorenbos A, Roehrs T, Lamphere J, Zorick F, Roth T. Increased daytime sleepiness enhances ethanol's sedative effects. Neuropsychopharmacology. 1988;1:279–286. [PubMed] [Google Scholar]