Summary
The mammary stem cell and its local microenvironment are central for the maintenance of proper tissue homeostasis during normal development. Defining the hierarchical organization of the epithelial subtypes in the mammary gland and the molecular pathways guiding their development has begun to provide a framework for understanding how cancer stem cells sustain the progression and heterogeneity of breast cancers. The Wnt pathway plays a fundamental role in multiple adult stem cells, as well as in orchestrating proper mammary gland development and maintenance. These processes are intricately guided by the influence of systemic hormones and local factors. Alterations in Wnt signaling can skew the homeostatic balance of the mammary epithelium to drive malignant progression; however, complexities of Wnt pathway components present a challenge in understanding their physiological function.
Introduction
Both systemic hormones and local factors are essential for orchestrating the dynamic changes that occur throughout stages of mammary development, particularly during puberty, estrous cycling, and pregnancy. While systemic hormones act as global mediators within the mammary gland, they rely on the production of local factors through paracrine interactions for the coordination of proper morphogenesis and differentiation [1]. Mammary stem cells (MaSCs) are the key drivers of self-renewal and differentiation throughout development, particularly in active growth phases, but these cells are also essential for the maintenance of tissue homeostasis [2]. The niche, hypothesized as the local tissue microenvironment, is essential to maintain and regulate stem cells within the mammary gland [3]. The existence of MaSCs was demonstrated many decades ago by reconstitution of the mammary gland after transplantation of a mammary epithelial fragment into the cleared fat pad [4]. Recent studies have identified functional MaSCs by surface marker expression followed by mammary reconstitution assays, leading to a preliminary understanding of the hierarchical organization of epithelial subtypes that comprise the mammary ductal tree [2]. This has facilitated the investigation of the molecular signaling pathways regulating MaSC self-renewal and lineage commitment. Of interest, Wingless Related Protein (Wnt) signaling is a likely niche factor and regulator of MaSC dynamics, yet how these signals integrate with systemic hormones and local growth factors remains an unresolved question.
Similar to normal tissues, the cancer stem cell theory suggests the existence of cells within breast cancers that possess “stem-like” qualities in their ability to self-renew and differentiate, albeit in a deregulated manner, ultimately sustaining tumor progression and driving tumor heterogeneity [5]. The fact that tumorigenesis, in many ways, may follow the hierarchical nature of an adult tissue, suggests that similar or related pathways are involved in both MaSC and cancer stem cell (CSC) dynamics. Specifically, Wnt signaling pathways play important roles in multiple aspects of both MaSC and CSC biology. In this review, we will summarize the recent literature on the contribution of Wnt signaling in mammary development and MaSC dynamics, with an emphasis on how these factors are tightly integrated with hormonal cues, as well as the relevance of Wnt pathway activation in breast cancer.
Estrogen(E) Receptor/Progesterone(P) Receptor and Mammary Epithelial Cell Dynamics
The hierarchical model of mammary gland development proposes the existence of stem and progenitor cells, which are under tight control of both cell intrinsic and extrinsic cues, and give rise to the mature mammary epithelium of either the luminal or basal/myoepithelial lineage by a series of lineage-restricted events [7]. The luminal compartment is divided into cells that are either positive or negative for both the Estrogen Receptor α (ERα) and Progesterone Receptor (PR), while the basal/myoepithelial compartment is negative for both ERα and PR. Though ERα positive luminal cells rarely proliferate, they relay proliferative signals via local paracrine factors to ERα negative cells in response to systemic ovarian hormones [8,9]. Currently, significant challenges exist in understanding the complex interactions between MaSCs, their more committed progeny, and differentiated epithelial cells, all of which are required to maintain MaSC activity and the functional integrity of the mammary gland. To date, only a few of the paracrine mediators of hormonal action have been identified, including Amphiregulin [10,11], Receptor Activator for Nuclear Factor κB Ligand (RANKL) [12], and Wnt4 [13,14]. Other Wnt proteins likely represent important mediators of hormone action, yet their specific functional roles have yet to be defined.
Wnt Signaling
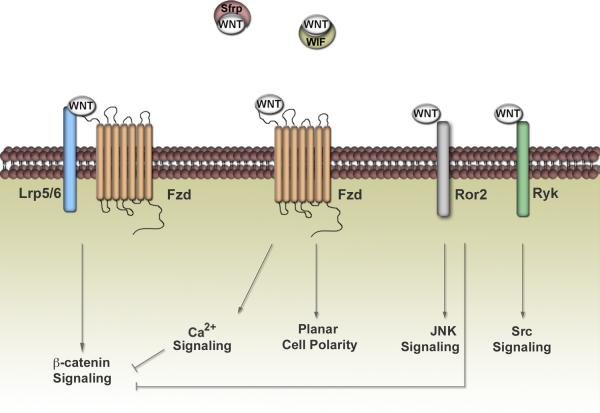
Wnts represent a family of secreted glycoproteins with diverse expression patterns and functions. The best-characterized Wnt pathway is Wnt/ß-catenin-dependent signaling (Figure 1) (reviewed in [15]). This so-called “canonical” pathway signals through seven-pass-transmembrane-spanning receptors, designated Frizzled (Fzd), in addition to co-receptors that belong to the LDL-receptor-related protein family (Lrp5/6). When Wnts bind to this receptor complex, the destruction of a multiprotein complex containing gycogen synthase kinase-ß (GSK-3ß), axin, and adenomatous polyposis coli (APC) occurs, which stabilizes ß-catenin and allows it to interact with the nuclear Lymphoid Enhancer Factor/T-Cell-Specific Transcription Factor (LEF/TCF) family of transcription factors and activate downstream target genes. The Wnt/ß-catenin-independent pathway, on the other hand, involves multiple receptors and downstream signals distinct from ß-catenin (Figure 1) (reviewed in [15]). These pathways can signal through Fzd receptors, independent of Lrp5/6, to mediate planar cell polarity or calcium fluxes. In addition, the single-pass transmembrane receptor tyrosine kinases of the Ror and Ryk families represent newly discovered Wnt/ß-catenin-independent receptors. Under cell-type specific and context-dependent circumstances, Wnt/ß-catenin-independent pathways can inhibit the Wnt/ß-catenin-dependent pathway. Moreover, secreted Wnt inhibitors such as Wnt inhibitory factors (Wifs) and secreted frizzled-related proteins (Sfrps) can modulate Wnt signaling by sequestering Wnts from their receptors. The large number of Wnt ligands, Fzd receptors, co-receptors, co-mediators, and downstream effectors indicates the enormous complexity of Wnt signaling. Wnt/ß-catenin-dependent and -independent signaling pathways are most likely highly integrated within any given context, dictated not only by the ligand-receptor interaction, but the downstream effectors engaged [16].
Figure 1. Wnt Signaling: A Basic View.
Wnt signaling is broadly divided into ß-catenin-dependent and ß-catenin-independent pathways. The ß-catenin-dependent pathway is involved in the stabilization of ß-catenin by the dissociation of a destruction complex that normally phosphorylates and targets ß-catenin for degradation. These signals require Fzd receptors to recruit the LRP co-receptor. ß-catenin-independent pathways encompass a broad range of outputs, dictated by the receptor context and downstream signals chosen. The newly discovered receptor tyrosine kinases Ror and Ryk provide another level of Wnt regulation in addition to Fzd. Within the Wnt family, there are 19 individual Wnt genes along with 10 different Frizzled receptors, illustrating the complexity governing this pathway and the multitude of Wnt-receptor combinations. The secreted Wnt inhibitors, Sfrps and Wifs, can regulate signaling in the extracellular space by sequestering Wnts away from their receptors. Wnt/ß-catenin-independent pathways can antagonize the ß-catenin-dependent pathway, revealing that these pathways are far more integrated than originally thought.
Wnts in Mammary Development
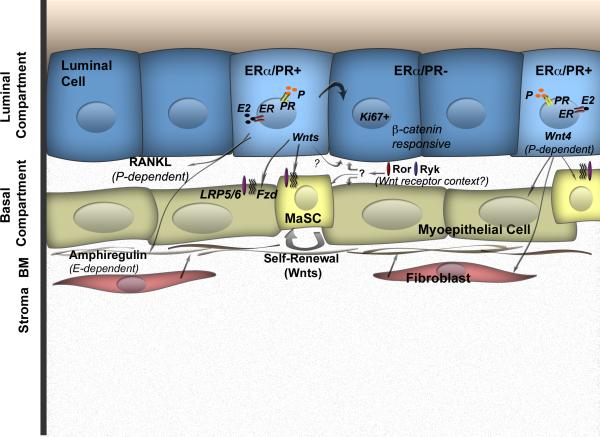
Evidence for a role of Wnt signaling in mammary morphogenesis was first suggested by the differential expression patterns of Wnt4, Wnt5a, Wnt5b, Wnt6, and Wnt7b at different stages of development [17,18]. The temporal and spatial expression patterns of multiple Wnt ligands highlight their potential non-redundant functions in patterning and tissue homeostasis. To identify candidate regulators of branching morphogenesis, Werb and colleagues used genome-wide transcript analysis to identify the spatial localization of individual Wnt proteins within the mammary ductal tree [19], complementing earlier studies [20]. In situ hybridization revealed specific enrichment and localization of Wnt2, Wnt5a, and Wnt7b to the terminal end bud (TEB) microenvironment, while other Wnts (Wnt4, Wnt5b, Wnt6) were localized to both the TEB microenvironment and mature ducts. More recently, Smalley and colleagues demonstrated that Wnt4, Wnt5a, and Wnt7b were enriched within the luminal ERα positive fraction of the mammary epithelium by transcriptome analysis of mammary epithelial subpopulations [21], in agreement with another study by Visvader and colleagues [22]. These data suggest a scenario where the ERα positive sensor cells [1] secrete specific Wnt ligands, either directly or indirectly, in response to reproductive hormones (Figure 2). The identification of the receiving cell within the epithelial hierarchy, along with the specific receptor milieu present in these cells will be a critical first step to unraveling the possible Wnt/ß-catenin-dependent and -independent interactions. Given the evidence for a role of Wnt signaling in the development of many complex tissues [23], the involvement of individual Wnts in the patterning of the mammary gland is highly likely.
Figure 2. Wnts as Paracrine Mediators.
ERα/PR positive cells provide extrinsic cues to ERα/PR negative cells to allow them to proliferate (Ki67). In addition to known paracrine mediators of hormonal action in the mammary gland, such as amphiregulin downstream of estrogen (E-dependent) and RANKL downstream of progesterone (P-dependent), Wnt ligands represent candidate mediators of mammary development. Wnt4 is the best-characterized Wnt, known to facilitate progesterone actions. The role of other Wnts remains unclear. The biological output of secreted Wnts depends on the receptor repertoire of the receiving cell along with the given developmental context. Lrp5/6 resides in the basal cell layer and is a marker of MaSCs, indicating the presence of Wnt/ß-catenin-dependent signaling in this compartment. Secreted inhibitors like Sfrps and Wifs can also modulate Wnt signaling by sequestering Wnts from their receptors (not shown).
Of the Wnt ligands expressed in the mammary gland, roles for Wnt4 and Wnt5a have been assigned in directing branching morphogenesis during distinct developmental periods. During pubertal development, Wnt5a regulates branching morphogenesis and terminal end bud proliferation [24]. Historically a noncanonical Wnt, Wnt5a might function to negatively modulate Wnt/ß-catenin activity and localization within the mammary gland. Wnt4, on the other hand, is required downstream of progesterone to mediate the side branching that occurs during early to mid pregnancy. Ectopic expression of Wnt4 via retroviral delivery is able to induce precocious side branching in the virgin, similar to that seen in early pregnancy [25]. Moreover, Wnt4-/- mammary glands exhibit defects in branching morphogenesis in early pregnancy, analogous to the PR knockout. Interestingly, both Wnt4 and PR colocalize to the luminal compartment of the ductal epithelium [13].
Wnt Modulation of Hormonal Function
Developmental regulation of the mammary gland can be partitioned into stages that are either estrogen-dependent (puberty) or those that are progesterone-dependent (pregnancy). Expression of multiple Wnts during both puberty and pregnancy clearly suggest that they mediate functional development, in some capacity, during these stages; however, Wnt signals seem to primarily regulate progesterone function rather than estrogen function [13,14,26]. For example, ectopic expression of Wnt1 is able to rescue the side-branching defect in PR-/- mammary glands through a paracrine-mediated mechanism. Wnt4 is recognized as the physiological Wnt involved in mediating progesterone action. Wnt4-/- glands early in pregnancy are characterized by an absence of branching. Moreover, within the luminal compartment, PR and Wnt4 are colocalized, and Wnt4 is induced by exogenous progesterone stimulation [13,14,26].
In addition to signaling through Wnt4, PR seems to control the pattern of ß-catenin response in the mammary epithelium. ß-catenin functions as a key effector of canonical Wnt signaling. While ectopic expression of a constitutively active ΔN89-ß-catenin induces precocious alveolar development in the virgin, absence of PR restricts alveolar expansion to the ductal tips [27,28]. Progesterone, therefore, dictates at least a subset of the ß-catenin response. From these data, PR might orchestrate the patterning of ß-catenin responsive progenitor populations along the lateral ducts (Figure 2) [27]. In the MMTV-ΔN89-ß-catenin model, activity of the canonical Wnt reporter Conductin/Axin2-LacZ is observed solely within a hormone receptor negative luminal population, presumably representing the alveolar progenitors [29]. Additionally, PR regulates another factor downstream of ß-catenin, pygopus homolog 2 (Pygo2), which is involved in controlling ß-catenin response in the nucleus [27,30]. Indeed, other studies highlight ß-catenin as a necessary effector for alveolar development during pregnancy, independent of side-branching. For example, inhibition of ß-catenin, either through tetracyclineinducible expression of the negative regulator Axin, or a dominant-negative form of ß-catenin, results in the inhibition of lobulo-alveolar development during pregnancy [31,32]. Taken together, these results demonstrate the essential role for Wnt/ß-catenin dependent signaling during pregnancy; however, the contribution of individual Wnts during pregnancy is still not well defined.
Lrp5/6: Canonical Wnt in MaSC Self-Renewal
Perhaps the most definitive evidence for a role of Wnt/ß-catenin in MaSC dynamics comes from loss-of-function studies of the Wnt co-receptors Lrp5 and Lrp6. Canonical Wnt signaling requires the presence of Lrp5/6 in conjunction with Frizzled receptors, unlike Wnt/ß-catenin-independent mechanisms. Lrp5-/- mammary glands exhibit fewer TEBs and diminished side branching, presumably as a result of dampened Wnt/ß-catenin activity within MaSCs or progenitors residing in the cap cell layer of the TEB [33]. Accordingly, Lrp5-/- cells display a significant loss in repopulating ability by limiting dilution transplantation. Lrp6, like Lrp5, is required for normal development, yet only TEB number and branching defects are evident in Lrp6 deficient mammary glands [34]. Intriguingly, both Lrp5 and Lrp6 expression reside in the basal compartment of the mammary epithelium, suggested to be the location of the MaSC (Figure 2) [34,35]. When separated by the degree of Lrp5 expression, Lrp5 high cells exhibit enriched MaSC activity [35], indicating that the Lrp5 high population represents the most competent Wnt responsive cell fraction with increased repopulating ability due to its enhanced self-renewing capacity. Moreover, ablation of Lrp5 significantly reduces the percentage of cells within the basal compartment of the mammary gland, suggesting that Wnt/ß-catenin-dependent signaling might also direct cell fate decisions in addition to conferring self-renewal properties [35].
Hormones Modulate Stem Cells: Wnt Participation
Two recent studies have highlighted the direct influence of ovarian hormones on MaSC function and the potential implications of this interaction on breast cancer risk. In a study by Visvader and colleagues, ovariectomy drastically impaired MaSC activity, while E plus P treatment were able to augment this activity [36]. Intriguingly, while ovariectomy reduced the size of the luminal compartment (CD29loCD24+), the MaSC-enriched compartment (CD29hiCD24+) remained unchanged. Upon transplantation of the MaSC-enriched populations from ovariectomized and control mice into the cleared mammary fat pads of host mice, the re-populating ability of the ovariectomized MaSC-enriched population decreased 4.3-fold relative to controls. While the MaSC fraction lacked ER/PR, this compartment was highly responsive to alterations in hormone status, highlighting the need to identify the key paracrine mediators of this regulation.
Similarly, a separate study evaluated MaSC changes throughout stages of estrous in the mouse [37]. In particular, a 14-fold increase in the MaSC pool was observed at diestrous, when P levels peak. In agreement with previous studies [36], RANKL was identified as a putative paracrine mediator of MaSC expansion in response to progesterone; however, Wnt effectors were also identified. In particular, when sorted luminal and basal cell fractions from estrous-staged or hormone-treated (E and P) mice were analyzed by quantitative gene analysis, Wnt4 and RANKL were induced solely in the luminal cell fraction, while Lrp5 was induced primarily in the basal fraction. Moreover, the Wnt target Axin2 was induced in both luminal and basal fractions with E and P treatment. Together, the data emphasize that hormones strikingly modulate MaSC dynamics, and though indirect, RANKL and Wnt effectors act as primary mediators of steroid hormone signals [37]. Since Wnts are implicated in this response, this suggests that sustained exposure to hormones over repeated estrous cycles, or in the short-term during pregnancy, might confer an increased breast cancer risk as a result of deregulation in self-renewal pathways over time.
When Wnt Signaling Goes Awry: Implications in Breast Cancer
In many malignancies, mutations in Wnt pathway mediators like Adenomatous polyposis coli (APC), Ctnnb1(ß-catenin), and Axin, are frequently observed and play a critical role in their pathogenesis; however, these overt Wnt pathway mutations are rarely observed in breast cancer. Instead, excessive Wnt pathway activation is likely to contribute to the pathogenesis of breast cancer. For example, the downregulation of the secreted Wnt inhibitors, secreted frizzled-related proteins (Sfrps), are observed in breast cancer [38-40]. Mouse models expressing both Wnt1 and stabilized ß-catenin (ΔN89-ß-catenin) in the mammary gland have provided strong evidence for the implications of excessive Wnt pathway activation in breast cancer. In particular, while these models seem to target two distinct progenitor compartments and activate the Wnt/ß-catenin pathway in each [29], they both exhibit an enrichment in stem/progenitor cell fractions, likely by altering self-renewal and differentiation networks within these populations [29,41-44]. From a therapeutic standpoint, there is also ample evidence that demonstrates Wnt/ß-catenin signaling mediates the radiation-resistance of mammary progenitor cell populations [45,46].
Interestingly, Lrp6 expression is now recognized to be higher in triple-negative breast cancers, lacking ER, PR and HER2 [47]. The inhibition or overexpression of Lrp6 attenuates or augments, respectively, Wnt activation, proliferation, and in vivo tumor growth, emphasizing the potential therapeutic benefit of targeting Lrp6 in triple-negative forms of breast cancer. An independent study also has documented the elevated expression of Lrp6 particularly within basal-like/triple-negative breast cancers, with Lrp5 showing a similar correlation [34]. Moreover, a Wnt signature derived from lung metastases in an orthotopic model of human breast cancer strongly overlaps with the same breast cancer subtype [48]. These data suggest that Lrp6, in particular, might promote the de-regulated self-renewal and differentiation of tumors categorized within the basal-like/triple-negative group. Nuclear and cytoplasmic accumulation of the Wnt effector ß-catenin is also preferentially associated with basal-like breast cancers [49]. Both cytoplasmic and nuclear localization of ß-catenin were able to predict poor survival. Moreover, simultaneous expression of nuclear/cytoplasmic ß-catenin along with CD44+/CD24- cell populations indicates that these tumors are also enriched for CSCs. While ß-catenin localization alone does not necessarily dictate Wnt/ß-catenin activity, together with Lrp6 expression in basal-like/triple-negative breast cancers, these data strongly suggest the importance of Wnt/ß-catenin activity within this breast cancer subtype. This is consistent with the hypothesis that Wnt signals support the growth of hormone-independent breast cancers and may provide a therapeutic opportunity specifically for the basal-like/triple-negative breast cancers, where steroid hormone receptor and HER2-based therapies are ineffective.
Conclusions
MaSC and CSC populations exhibit striking parallels in their self-renewal and differentiation capabilities. Just like the MaSC is able to give rise to more differentiated progeny that comprises the ductal, alveolar, and myoepithelial lineages, CSCs differentiate into phenotypically diverse populations with limited proliferative potential that comprise the tumor bulk. Wnt signaling clearly represents a pathway that regulates normal MaSC dynamics, and its dysregulation results in neoplastic transformation. We have provided a picture where Wnt signals, at multiple levels, seem to be intricately involved in the hormonal cues governing mammary development and tumorigenesis. Importantly, not all Wnts and their receptors are created equal. Current characterization of Wnts in MaSC and CSC biology largely describes the Wnt/ß-catenin-dependent arm of signaling; however, the identification and characterization of ß-catenin-independent components is still evolving. Unraveling the complexities of Wnt pathway components in both MaSC and CSC dynamics should shed some light on potential therapeutic applications of targeting this important pathway in breast cancer.
Acknowledgements
Grant support: JMR is supported by NIH grant R37 CA16303 from the National Cancer Institute. KR is supported by Department of Defense (DOD) Breast Cancer Research Program (BCRP) Postdoctoral Fellowship BC093440.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Annotated References
- 1.Brisken C, Duss S. Stem cells and the stem cell niche in the breast: an integrated hormonal and developmental perspective. Stem Cell Rev. 2007;3:147–156. doi: 10.1007/s12015-007-0019-1. [DOI] [PubMed] [Google Scholar]
- 2.Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009;23:2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanos T, Brisken C. What signals operate in the mammary niche? Breast Dis. 2008;29:69–82. doi: 10.3233/bd-2008-29108. [DOI] [PubMed] [Google Scholar]
- 4.Deome KB, Faulkin LJ, Jr., Bern HA, Blair PB. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19:515–520. [PubMed] [Google Scholar]
- 5.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 6.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106:13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson CJ, Khaled WT. Mammary development in the embryo and adult: a journey of morphogenesis and commitment. Development. 2008;135:995–1003. doi: 10.1242/dev.005439. [DOI] [PubMed] [Google Scholar]
- 8.LaMarca HL, Rosen JM. Minireview: hormones and mammary cell fate--what will I become when I grow up? Endocrinology. 2008;149:4317–4321. doi: 10.1210/en.2008-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke RB, Howell A, Potten CS, Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57:4987–4991. [PubMed] [Google Scholar]
- 10.Booth BW, Boulanger CA, Anderson LH, Jimenez-Rojo L, Brisken C, Smith GH. Amphiregulin mediates self-renewal in an immortal mammary epithelial cell line with stem cell characteristics. Exp Cell Res. 2010;316:422–432. doi: 10.1016/j.yexcr.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc Natl Acad Sci U S A. 2007;104:5455–5460. doi: 10.1073/pnas.0611647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 12.Beleut M, Rajaram RD, Caikovski M, Ayyanan A, Germano D, Choi Y, Schneider P, Brisken C. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci U S A. 2010;107:2989–2994. doi: 10.1073/pnas.0915148107. [The authors demonstrate that progesterone elicits proliferation in two distinct waves; the first wave involves PR positive cells and requires cyclin D1, while the second, larger wave, consists of mostly PR negative cells and requires receptor activator of NF-κB-ligand (RANKL). Therefore, progesterone mediates its effects by both cell intrinsic and extrinsic cues. Wnt4 might be intricately involved in the paracrine RANKL-mediated effects.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, McMahon JA, McMahon AP, Weinberg RA. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 2000;14:650–654. [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson GW, Hennighausen L, Johnson PF. Side-branching in the mammary gland: the progesterone-Wnt connection. Genes Dev. 2000;14:889–894. [PubMed] [Google Scholar]
- 15.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 16.van Amerongen R, Mikels A, Nusse R. Alternative wnt signaling is initiated by distinct receptors. Sci Signal. 2008;1:re9. doi: 10.1126/scisignal.135re9. [DOI] [PubMed] [Google Scholar]
- 17.Gavin BJ, McMahon AP. Differential regulation of the Wnt gene family during pregnancy and lactation suggests a role in postnatal development of the mammary gland. Mol Cell Biol. 1992;12:2418–2423. doi: 10.1128/mcb.12.5.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buhler TA, Dale TC, Kieback C, Humphreys RC, Rosen JM. Localization and quantification of Wnt-2 gene expression in mouse mammary development. Dev Biol. 1993;155:87–96. doi: 10.1006/dbio.1993.1009. [DOI] [PubMed] [Google Scholar]
- 19.Kouros-Mehr H, Werb Z. Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev Dyn. 2006;235:3404–3412. doi: 10.1002/dvdy.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber-Hall SJ, Phippard DJ, Niemeyer CC, Dale TC. Developmental and hormonal regulation of Wnt gene expression in the mouse mammary gland. Differentiation. 1994;57:205–214. doi: 10.1046/j.1432-0436.1994.5730205.x. [DOI] [PubMed] [Google Scholar]
- * 21.Kendrick H, Regan JL, Magnay FA, Grigoriadis A, Mitsopoulos C, Zvelebil M, Smalley MJ. Transcriptome analysis of mammary epithelial subpopulations identifies novel determinants of lineage commitment and cell fate. BMC Genomics. 2008;9:591. doi: 10.1186/1471-2164-9-591. [The authors perform whole transcriptome analysis of basal, luminal ER positive, and luminal ER negative cells isolated from the virgin mouse mammary gland. These data demonstrate a detailed molecular characterization of these cell types in an effort to understand the regulators of cell lineages and cell fate determination within the mammary gland.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 22.Lim E, Wu D, Pal B, Bouras T, Asselin-Labat ML, Vaillant F, Yagita H, Lindeman GJ, Smyth GK, Visvader JE. Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Res. 2010;12:R21. doi: 10.1186/bcr2560. [The authors provide molecular characterization of four epithelial subtypes from the mouse mammary gland and compare these signatures with mouse models of mammary tumorigenesis to identify conserved gene signatures. Furthermore, the study recognizes conserved genes and networks between murine populations and their human counterparts. Components of the Wnt pathway were conserved between mouse and human cell populations.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller RK, McCrea PD. Wnt to build a tube: contributions of Wnt signaling to epithelial tubulogenesis. Dev Dyn. 239:77–93. doi: 10.1002/dvdy.22059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roarty K, Serra R. Wnt5a is required for proper mammary gland development and TGF-beta-mediated inhibition of ductal growth. Development. 2007;134:3929–3939. doi: 10.1242/dev.008250. [DOI] [PubMed] [Google Scholar]
- 25.Bradbury JM, Edwards PA, Niemeyer CC, Dale TC. Wnt-4 expression induces a pregnancy-like growth pattern in reconstituted mammary glands in virgin mice. Dev Biol. 1995;170:553–563. doi: 10.1006/dbio.1995.1236. [DOI] [PubMed] [Google Scholar]
- 26.Brisken C, Park S, Vass T, Lydon JP, O'Malley BW, Weinberg RA. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci U S A. 1998;95:5076–5081. doi: 10.1073/pnas.95.9.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiremath M, Lydon JP, Cowin P. The pattern of beta-catenin responsiveness within the mammary gland is regulated by progesterone receptor. Development. 2007;134:3703–3712. doi: 10.1242/dev.006585. [DOI] [PubMed] [Google Scholar]
- 28.Imbert A, Eelkema R, Jordan S, Feiner H, Cowin P. Delta N89 beta-catenin induces precocious development, differentiation, and neoplasia in mammary gland. J Cell Biol. 2001;153:555–568. doi: 10.1083/jcb.153.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 29.Teissedre B, Pinderhughes A, Incassati A, Hatsell SJ, Hiremath M, Cowin P. MMTV-Wnt1 and -DeltaN89beta-catenin induce canonical signaling in distinct progenitors and differentially activate Hedgehog signaling within mammary tumors. PLoS One. 2009;4:e4537. doi: 10.1371/journal.pone.0004537. [For years, many studies assumed MMTV-Wnt1 and MMTV-DeltaN89beta-catenin models of mouse mammary tumorigenesis were very similar; however, the authors provide the first evidence that Wnt1 and deltaN89-beta-catenin activate canonical Wnt signaling in distinct epithelial progenitors.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu B, Sun P, Yuan Y, Moraes RC, Li A, Teng A, Agrawal A, Rheaume C, Bilanchone V, Veltmaat JM, et al. Pygo2 expands mammary progenitor cells by facilitating histone H3 K4 methylation. J Cell Biol. 2009;185:811–826. doi: 10.1083/jcb.200810133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu W, Shakya R, Costantini F. Impaired mammary gland and lymphoid development caused by inducible expression of Axin in transgenic mice. J Cell Biol. 2001;155:1055–1064. doi: 10.1083/jcb.200107066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tepera SB, McCrea PD, Rosen JM. A beta-catenin survival signal is required for normal lobular development in the mammary gland. J Cell Sci. 2003;116:1137–1149. doi: 10.1242/jcs.00334. [DOI] [PubMed] [Google Scholar]
- 33.Lindvall C, Evans NC, Zylstra CR, Li Y, Alexander CM, Williams BO. The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis. J Biol Chem. 2006;281:35081–35087. doi: 10.1074/jbc.M607571200. [DOI] [PubMed] [Google Scholar]
- 34.Lindvall C, Zylstra CR, Evans N, West RA, Dykema K, Furge KA, Williams BO. The Wnt co-receptor Lrp6 is required for normal mouse mammary gland development. PLoS One. 2009;4:e5813. doi: 10.1371/journal.pone.0005813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 35.Badders NM, Goel S, Clark RJ, Klos KS, Kim S, Bafico A, Lindvall C, Williams BO, Alexander CM. The Wnt receptor, Lrp5, is expressed by mouse mammary stem cells and is required to maintain the basal lineage. PLoS One. 2009;4:e6594. doi: 10.1371/journal.pone.0006594. [The authors demonstrate that cells expressing Lrp5, along with Lrp6, overlap with the MaSC compartment. When sorted based on Lrp5 expression, the cells with highest Lrp5 expression exhibited enhanced in vivo repopulating ability. Lrp5-/- glands lost stem cell activity, and the basal compartment of the Lrp5-/- mammary gland was significantly reduced. These data demonstrate the requirement for Wnt/ß-catenin activity in mediating MaSC activity and identify Lrp5 as a marker of MaSCs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 36.Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010 doi: 10.1038/nature09027. in press. DOI: 10.1038/nature09027. [This is the first study to definitively show that steroid hormone signaling strikingly modulates MaSC activity. The authors implicate RANKL as a paracrine mediator of MaSC expansion during pregnancy and provide a potential explanation for the transient increase in breast cancer incidence that accompanies pregnancy.] [DOI] [PubMed] [Google Scholar]
- * 37.Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote P, Clarke C, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010 doi: 10.1038/nature09091. in press. DOI:10.1038/nature09091. [This study is the first to investigate MaSC dynamics during estrous and shows that progesterone expands the MaSC pool maximally during diestrous. The authors identify Wnt components and RANKL as mediators of MaSC expansion. Moreover, this study recognizes MaSCs as putative targets for transformation given their dynamic activation during reproductive cycling.] [DOI] [PubMed] [Google Scholar]
- 38.Suzuki H, Toyota M, Carraway H, Gabrielson E, Ohmura T, Fujikane T, Nishikawa N, Sogabe Y, Nojima M, Sonoda T, et al. Frequent epigenetic inactivation of Wnt antagonist genes in breast cancer. Br J Cancer. 2008;98:1147–1156. doi: 10.1038/sj.bjc.6604259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veeck J, Geisler C, Noetzel E, Alkaya S, Hartmann A, Knuchel R, Dahl E. Epigenetic inactivation of the secreted frizzled-related protein-5 (SFRP5) gene in human breast cancer is associated with unfavorable prognosis. Carcinogenesis. 2008;29:991–998. doi: 10.1093/carcin/bgn076. [DOI] [PubMed] [Google Scholar]
- 40.Ugolini F, Charafe-Jauffret E, Bardou VJ, Geneix J, Adelaide J, Labat-Moleur F, Penault-Llorca F, Longy M, Jacquemier J, Birnbaum D, et al. WNT pathway and mammary carcinogenesis: loss of expression of candidate tumor suppressor gene SFRP1 in most invasive carcinomas except of the medullary type. Oncogene. 2001;20:5810–5817. doi: 10.1038/sj.onc.1204706. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci U S A. 2003;100:15853–15858. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu BY, McDermott SP, Khwaja SS, Alexander CM. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc Natl Acad Sci U S A. 2004;101:4158–4163. doi: 10.1073/pnas.0400699101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 44.Vaillant F, Asselin-Labat ML, Shackleton M, Forrest NC, Lindeman GJ, Visvader JE. The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res. 2008;68:7711–7717. doi: 10.1158/0008-5472.CAN-08-1949. [DOI] [PubMed] [Google Scholar]
- 45.Chen MS, Woodward WA, Behbod F, Peddibhotla S, Alfaro MP, Buchholz TA, Rosen JM. Wnt/beta-catenin mediates radiation resistance of Sca1+ progenitors in an immortalized mammary gland cell line. J Cell Sci. 2007;120:468–477. doi: 10.1242/jcs.03348. [DOI] [PubMed] [Google Scholar]
- 46.Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci U S A. 2007;104:618–623. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 47.Liu CC, Prior J, Piwnica-Worms D, Bu G. LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proc Natl Acad Sci U S A. 2010;107:5136–5141. doi: 10.1073/pnas.0911220107. [This study identifies the overexpression of Lrp6 within a subpopulation of human breast cancers, particularly the triple-negative subtype. The authors use an Lrp6 antagonist, Mesd, to successfully suppress tumorigenesis in the MMTV-Wnt1 mouse model and provide evidence for Lrp6 as a therapeutic target.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 48.DiMeo TA, Anderson K, Phadke P, Fan C, Perou CM, Naber S, Kuperwasser C. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res. 2009;69:5364–5373. doi: 10.1158/0008-5472.CAN-08-4135. [The authors demonstrate overexpression of multiple Wnt signaling components in lung metastases of an orthotopic model of human breast cancer. Moreover, the Wnt genes identified within this signature were associated with basal-like breast cancers. Lrp6 was required for the self-renewal and seeding of these tumors in vivo.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 49.Khramtsov AI, Khramtsova GF, Tretiakova M, Huo D, Olopade OI, Goss KH. Wnt/{beta}-Catenin Pathway Activation Is Enriched in Basal-Like Breast Cancers and Predicts Poor Outcome. Am J Pathol. 2010 doi: 10.2353/ajpath.2010.091125. in press. DOI: 10.2353/ajpath.2010.091125. [The authors show that Wnt/β-catenin pathway activation is enriched in basal-like breast cancers and this predicts poor outcome. The study provides evidence for β-catenin cytoplasmic and nuclear staining predominantly within basal-like/triple-negative tumors compared to Luminal A and B subtypes. Additionally, nuclear and cytoplasmic staining for β-catenin overlapped with CD44+/CD24- staining, providing evidence for enriched stem-like populations within these tumors.] [DOI] [PMC free article] [PubMed] [Google Scholar]