Abstract
Introduction
Cannabis dependence is a common but poorly understood condition in adolescents. Marijuana craving has been posited as a potential contributing factor to continued use and relapse, but relatively few studies have focused on the measurement of craving and reactivity to marijuana cues. The present work sought to explore reactivity to marijuana cues within this age group.
Methods
Thirty treatment-seeking cannabis dependent adolescents (age 13–20) completed a cue reactivity session, consisting of exposure to and manipulation of in vivo marijuana cues (“joint” and lighter) and matching neutral cues (pencil and eraser), in counterbalanced order. Subjective craving and physiological reactivity were assessed.
Results
Participants demonstrated increased craving and skin conductance reactivity in response to marijuana cues, relative to neutral cues.
Conclusion
In vivo marijuana cues appear to elicit significant subjective and physiological reactivity among treatment-seeking cannabis dependent adolescents. Further work is needed with a larger sample and with a wider variety of cues.
Keywords: Marijuana, Cannabis, Cue Reactivity, Craving, Youth, Adolescent
1. Introduction
The cue reactivity paradigm, while well established as a laboratory method for investigating craving for a number of substances (e.g., cocaine, nicotine, alcohol) (for review, see Carter & Tiffany, 1999), has only recently been used to investigate marijuana craving. Studies to date have indicated that marijuana-related cues elicit reactivity among marijuana users, but broad interpretation is limited by inconsistent methodology. First, cue types have varied across studies, from marijuana-related words (Field et al., 2004), to marijuana-related pictures (Field et al., 2006; Filbey et al., 2009a; Wölfling et al., 2008), to virtual reality marijuana use situations (Bordnick et al., 2009), to in vivo exposure to marijuana-related items (Gray et al., 2008; Haughey et al., 2008). Second, studies that assess marijuana cue reactivity have relied heavily on subjective self-report measures, though notable exceptions include the use of physiological measures (Gray et al., 2008), evoked potential measurement (Wölfling et al., 2008) and functional brain imaging (Filbey et al., 2009a). Third, inclusion criteria have varied in terms of severity/frequency of marijuana use and participants’ ages.
Despite the prevalence of marijuana use among adolescents, little is known about basic biobehavioral mechanisms involved in those who use marijuana chronically. To address this knowledge gap, we recently conducted a preliminary investigation that examined reactivity to a set of marijuana cues in adolescents with cannabis use disorders (Gray et al., 2008). Marijuana cues were provided in three formats: imagery scripts, videos, and an in vivo presentation of paraphernalia (e.g., a joint and lighter). Matching neutral control cues for each format were presented as well. Participants demonstrated increased subjective craving and skin conductance reactivity to marijuana cues, relative to neutral cues, with in vivo cues producing the greatest level of reactivity.
While results of this preliminary investigation were encouraging, the study was limited because cues were presented in a fixed order, with neutral cues preceding marijuana cues. Notably, the in vivo cues, which produced the greatest level of reactivity, were presented last. This raised the possibility that the observed reactivity to these cues may have been an artifact of time elapsed rather than cue type or format (i.e., participants may have reported greater craving due to time elapsed since last use). A counterbalanced design would have made it possible to rule out this potential confound. We thus designed the present follow-up study to evaluate subjective craving and physiological reactivity to in vivo marijuana and matched neutral cues, presented in counterbalanced order, among treatment-seeking cannabis dependent adolescents. We hypothesized that participants would exhibit greater craving and reactivity in response to marijuana cues versus neutral cues.
2. Methods
2.1 Participants
Adolescent marijuana users (ages 13–20) interested in cutting down or quitting marijuana use were recruited from the general community via print and online advertisements. Upon completion of a brief phone screen, potentially eligible individuals presented to the clinic for informed consent and final assessment of eligibility, including comprehensive medical, laboratory, psychiatric, and substance use evaluation (Sheehan et al., 1998; Sheehan et al., 2010). In order to meet eligibility criteria, participants were required to use marijuana on average at least three times weekly for the last year, and meet criteria for current cannabis dependence. Exclusion criteria included having an unstable medical or psychiatric condition or current use of medications that might interfere with heart rate or skin conductance. Parents/guardians provided consent (with participant assent) for individuals under age 18. Participants were compensated $50 for participation in the laboratory session. At the conclusion of laboratory procedures, participants enrolled in a 4-week cannabis dependence treatment trial (data not reported here).
2.2 Measures
Subjective craving was assessed using the Marijuana Craving Questionnaire (MCQ), a 12-item measure exploring four domains of craving: Compulsivity, Emotionality, Expectancy, and Purposefulness (Heishman et al., 2009). Craving was also assessed using a single-item visual analog rating scale (“I have a desire to smoke marijuana”) rated on a 21-point scale (0 – 20). Both the MCQ and single-item rating were administered immediately prior to and following the neutral and marijuana cue presentations.
Skin conductance (SC) and heart rate (HR) were collected following procedures similar to those previously reported (Gray et al. 2008). SC data were recorded in microsiemens and log transformed to normalize the data. Heart rate (HR) data were measured in beats per minute (BPM). Physiological data were collected for 90 seconds during baseline and cue presentation periods. Average SC and HR values were calculated for each 90-second period.
2.3 Procedures
Eligible participants were scheduled to complete a laboratory cue reactivity procedure after abstaining from using marijuana for 24 hours. The procedure consisted of two separate cue types, neutral and marijuana, presented in counterbalanced order and separated by a 10-minute rest period during which a nature slide show was presented. A standardized, matched, strictly timed recording (90 sec) was used to instruct participants to handle the cues. During marijuana cue presentation, participants were instructed to hold and smell a marijuana cigarette (“joint”) and “flick” a lighter. During neutral cue presentation, participants were instructed to hold and similarly manipulate a pencil and eraser.
Prior to the cue handling instructions, the experimenter placed the cues under a lid in front of participants and administered the MCQ. The experimenter then left the room and initiated baseline data collection (baseline physiology and the pre-cue single-item craving rating). Next, the cue handling instructions were administered by computer as physiological measures were collected. Immediately after cue handling, final physiological measures were taken and the post-cue single-item rating was administered by computer. The experimenter re-entered the room to administer the MCQ and remove the cues.
2.4 Statistical Analysis
Analyses were conducted using SPSS 15.0. Repeated-measures ANCOVAs were used, with Time (pre cue versus during/post cue offset) and Cue Type (marijuana versus neutral) included as within-subjects measures. Marijuana use (“hits” per day) within 28 days preceding the cue session was included as a covariate, centered to the overall mean; “hits” were quantified using methods previously reported (Gray et al., 2009). For the MCQ, an additional within-subjects measure, Scale, was included so that all four domains could be assessed simultaneously rather than separately, thus protecting against inflation of Type I errors due to having multiple statistical comparisons.
3. Results
3.1 Participant Demographics and Baseline Marijuana Use
Thirty participants enrolled and completed study procedures (age range 13–20; mean age 18.8 ± SE 0.24). Twenty-two were male; 27 were White, 2 African-American, and 1 Hispanic. During the 28 days preceding enrollment, participants reported using a daily average of 16.6 ± SE 2.2 “hits” of marijuana on 24.3 ± SE 0.7 days. Each “hit” of marijuana was quantified using methods previously reported (Gray et al., 2009). Exploratory Pearson correlations revealed that number of “hits” was generally positively related to self-report ratings (significant correlations [p < .05] with pre-neutral cue MCQ Purposefulness rating and with post-marijuana cue single-item craving, MCQ Purposefulness, and MCQ Compulsivity ratings), and generally negatively (but non-significantly) related to physiological measures.
3.2 Subjective Craving
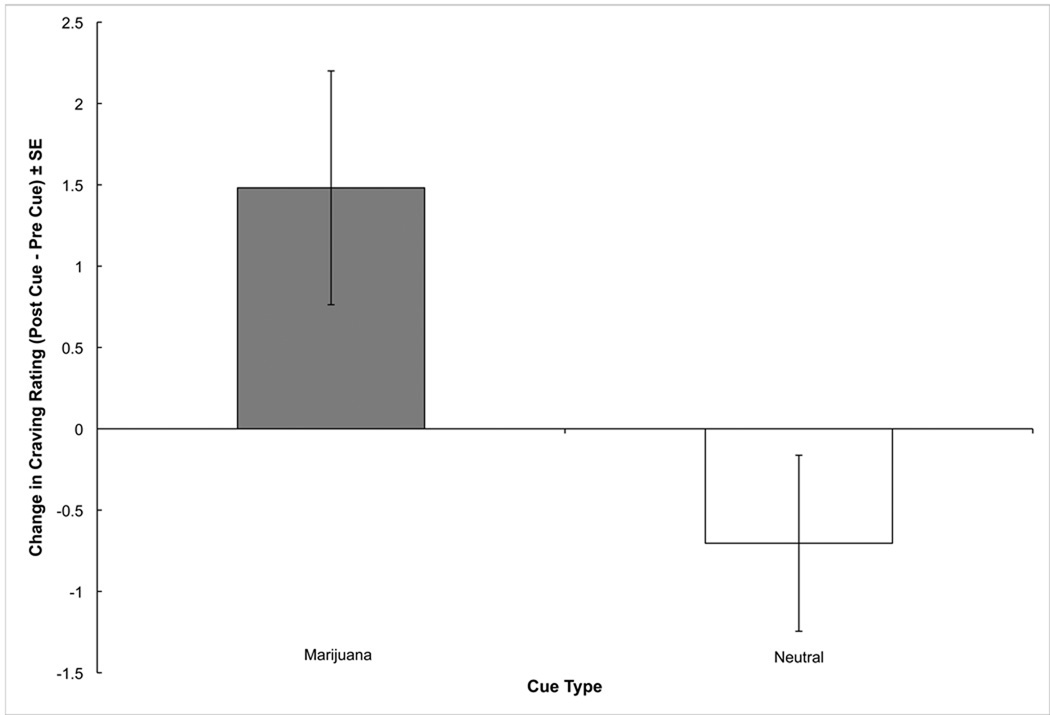
Single item ratings data were available for 27 of the 30 participants. The covariate, marijuana use, reached significance, F(1,25) = 5.94, p < .05. The Time × Cue Type interaction was significant, F(1,25) = 5.79, p < .05 (Figure 1). To further explore the interaction, a post-hoc repeated measures ANCOVA was performed using (pre-post) change scores for craving; this revealed that the change in craving for marijuana cues, M = 1.48 ± SE .72, was significantly greater than the change in craving for the neutral cues, M = −.70 ± SE .54, F(1,25) = 5.79, p < .05. The covariate, marijuana use, was not significant, p < .24, suggesting that mean level of use was associated with overall craving levels, but not levels of reactivity.
Figure 1.
Change (post cue – pre cue) in single item visual analog scale rating (“I have a desire to smoke marijuana” rated on a 21-point scale [0 – 20]), by cue type.
MCQ data were available for 29 of the 30 participants. The three-way 2 × 2 × 4 (Time × Cue Type × Scale) ANCOVA revealed a main effect for Time, F(1,27) = 5.16, p < .05, and a main effect for Scale, F(3,81) = 62.85, p < .001. To further examine these main effects, post-hoc LSD tests were performed, revealing that the overall post-cue mean, 11.34 ± SE .60, was higher than the overall pre-cue mean, 11.00 ± SE .51. In addition, post-hoc LSD tests suggested that the mean value for each individual scale differed from all other values at the .05 level. No main effects or interactive effects for Cue Type were observed. The covariate, marijuana use, did not reach significance, though there was a trend towards significance, p < .09.
The lack of differences in MCQ ratings was in contrast to our prior work that found significant changes on the Purposefulness and Expectancy scales. To determine whether this lack of replication occurred due the counterbalancing strategy used in the present study, the three-way ANCOVA (Time × Cue Type × Scale) was repeated separately for both presentation orders. No significant effects of cue type were observed, nor did cue type interact with any other effect. Thus, the MCQ effects did not replicate within the subgroup of current participants who received cues in the same order as those in the previous study, and the lack of replication is not a function of the current counterbalancing strategy.
3.3 Physiological Reactivity
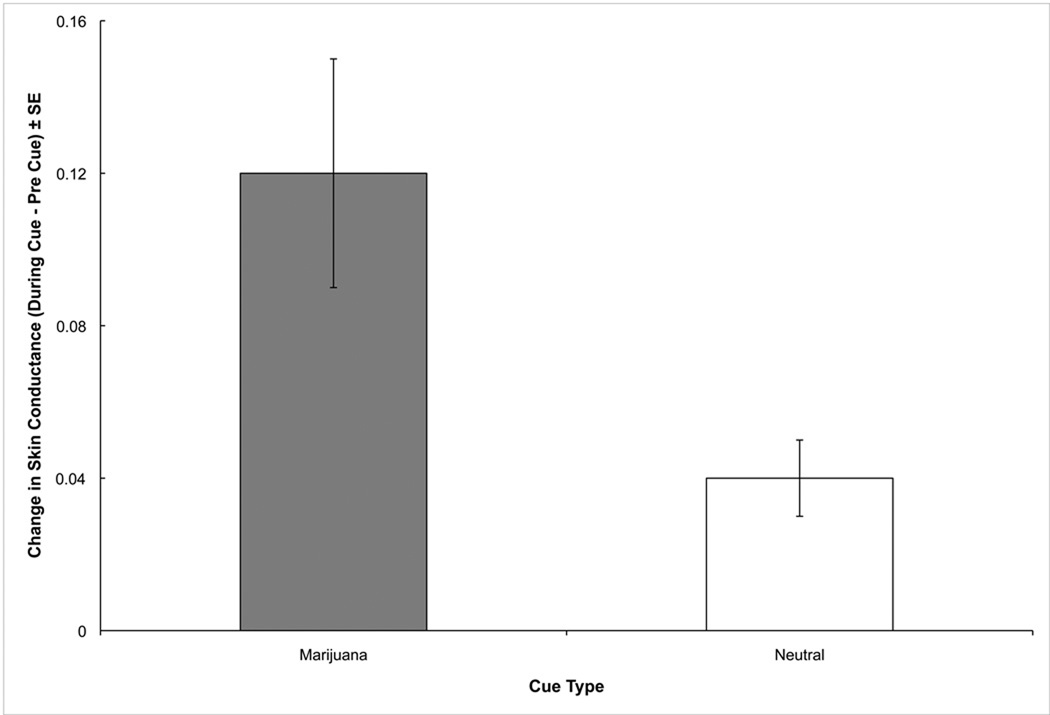
SC data were available for 23 of the 30 participants. Significant effects were noted for Time, F(1,21) = 24.35, p < .001, and the Time × Cue Type interaction, F(1,21) = 9.66, p < .01 (Figure 2). To better understand the nature of this interaction, a follow-up repeated measures ANCOVA examining SC level changes was performed, revealing that the overall increase in SC in response to marijuana cues, M = .12 ± SE .03, was significantly greater than the increase in response to neutral cues, M = .04, SE = .01, F(1,21) = 9.66, p < .01. The covariate was non-significant in all analyses.
Figure 2.
Change (during cue – pre cue) in skin conductance, by cue type.
HR data were available for 24 of the 30 participants. A significant effect was noted for Time, F(1,22) = 18.17, p < .001, and examination of the data patterns suggested HR was higher during cue presentation (when participants were handling cues, irrespective of Type). As with SC, the covariate was non-significant.
4. Discussion and Conclusions
Results indicate that, in accord with our hypotheses, in vivo marijuana cues, relative to neutral cues, elicit significant subjective craving and skin conductance reactivity among treatment-seeking cannabis dependent adolescents. These results are generally consistent with our prior investigation of imagery, video, and in vivo cue reactivity in adolescents (Gray et al., 2008), as there were significantly greater increases in self-reported craving (measured by single-item rating) and skin conductance. One noteworthy difference, however, was the lack of cue-related differences in MCQ ratings.
Although the failure to replicate the MCQ findings was apparently not a function of the counterbalancing scheme of the presented study, it may reflect multiple factors. First, while in vivo cues may be the most robust elicitors of reactivity (Niaura et al., 1998, Shadel et al., 2001; Staiger & White 1991), when presented in isolation, as was done in the present study, they appeared to produce less of a response in MCQ ratings than they did in our previous study (when they were preceded by imagery and video cues). Thus, future studies may benefit from presenting cues of multiple modalities or for longer duration. A second possibility is that the counterbalanced order of cues in the present study may have addressed cumulative effects encountered in the prior, fixed-order study, though this possibility seems unlikely. Had the previous study’s findings been a simple result of a lack of counterbalancing, then a similar pattern of results should have been observed in the present sample among subjects who received the cues in the same order. This pattern, however, was not observed.
The current study confirms that reactivity to marijuana cues can be evoked in cannabis dependent adolescents. Subsequent studies should seek to include a larger sample and more comprehensively compare reactivity to a variety of cue types. It would also be of interest to investigate whether cue reactivity is sustained (LaRowe et al., 2007) or extinguished (Price et al., 2010) over multiple exposure sessions, across a spectrum of marijuana use frequency/severity. Additionally, future work is needed to explore the correlation between marijuana cue reactivity and other clinically relevant variables (e.g., subsequent marijuana use and response to treatment) in cannabis dependent adolescents. Other potential lines of research include investigation of genetic underpinnings of marijuana cue reactivity among adolescents, as similar studies have revealed significant associations among adults (Filbey et al., 2009b; Haughey et al., 2008).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bordnick PS, Copp HL, Traylor A, Graap KM, Carter BL, Walton A, Ferrer M. Reactivity to cannabis cues in virtual reality environments. Journal of Psychoactive Drugs. 2009;41:105–112. doi: 10.1080/02791072.2009.10399903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Field M, Eastwood B, Bradley BP, Mogg K. Selective processing of cannabis cues in regular cannabis users. Drug and Alcohol Dependence. 2006;85:75–82. doi: 10.1016/j.drugalcdep.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Bradley BP. Cognitive bias and drug craving in recreational cannabis users. Drug and Alcohol Dependence. 2004;74:105–111. doi: 10.1016/j.drugalcdep.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. PNAS. 2009a;106:13016–13021. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Individual and additive effects of the CNR1 and FAAH genes in brain response to marijuana cues. Neuropsychopharmacology. 2009b doi: 10.1038/npp.2009.200. doi:10.1038/npp.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, LaRowe SD, Upadhyaya HP. Cue reactivity in young marijuana smokers: A preliminary investigation. Psychology of Addictive Behaviors. 2008;22:582–586. doi: 10.1037/a0012985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Watson NL, Christie DK. Challenges in quantifying marijuana use. American Journal on Addictions. 2009;18:178–179. doi: 10.1080/10550490902772579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey HM, Marshall E, Schacht JP, Louis A, Hutchison KE. Marijuana withdrawal and craving: Influence of the cannabinoid receptor 1 (CNR1) and fatty acid amide hydrolase (FAAH) genes. Addiction. 2008;103:1678–1686. doi: 10.1111/j.1360-0443.2008.02292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug and Alcohol Dependence. 2009;102:35–40. doi: 10.1016/j.drugalcdep.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe SD, Saladin ME, Carpenter MJ, Upadhyaya HP. Reactivity to nicotine cues over repeated cue reactivity sessions. Addictive Behaviors. 2007;32:2888–2899. doi: 10.1016/j.addbeh.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykken DT, Venables PH. Direct measurement of skin conductance: a proposal for standardization. Psychophysiology. 1971;8:656–672. doi: 10.1111/j.1469-8986.1971.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Abrams DB, Monti PM, Rohsenow DJ, Sirota A. Individual differences in cue reactivity among smokers trying to quit: effects of gender and cue type. Addictive Behaviors. 1998;23:209–224. doi: 10.1016/s0306-4603(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Price KL, Saladin ME, Baker NL, Tolliver BK, DeSantis SM, McRae-Clark AL, Brady KT. Extinction of drug cue reactivity in methamphetamine-dependent individuals. Behaviour Research and Therapy. 2010 doi: 10.1016/j.brat.2010.05.010. doi:10.1016/j.brat.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadel WG, Niaura R, Abrams DB. Effect of different cue stimulus delivery channels on craving reactivity: comparing in vivo and video cues in regular cigarette smokers. Journal of Behavior Therapy and Experimental Psychiatry. 2001;32:203–209. doi: 10.1016/s0005-7916(01)00035-0. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Harnett-Sheehan K, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar G. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:S22–S33. [PubMed] [Google Scholar]
- Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, Milo KM, Stock SL, Wilkinson B. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID) Journal of Clinical Psychiatry. 2010;71:313–326. doi: 10.4088/JCP.09m05305whi. [DOI] [PubMed] [Google Scholar]
- Staiger PK, White JM. Cue reactivity in alcohol abusers: stimulus specificity and extinction of the responses. Addictive Behaviors. 1991;16:211–221. doi: 10.1016/0306-4603(91)90014-9. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2006 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H-32, DHHS Publication No. SMA 07-4293) Rockville, MD: United States Department of Health and Human Services; 2007. [Google Scholar]
- Wölfling K, Flor H, Grüsser SM. Psychophysiological responses to drug-associated stimuli in chronic heavy cannabis use. European Journal of Neuroscience. 2008;27:976–983. doi: 10.1111/j.1460-9568.2008.06051.x. [DOI] [PubMed] [Google Scholar]