Introduction
Our understanding of the molecular mechanisms underlying the pharmacological actions of estrogen receptor (ER) ligands has evolved considerably in recent years. Much of this knowledge has come from a detailed dissection of the mechanism(s) of action of the Selective Estrogen Receptor Modulators (SERMs) tamoxifen and raloxifene, so called for their ability to function as ER agonists or antagonists depending on the tissue in which they operate. These mechanistic insights have had a significant impact on the discovery of second generation SERMs, some of which are in late stage clinical development for the treatment/prevention of breast cancer as well as other estrogenopathies. In addition to the SERMs, however, have emerged the Selective Estrogen Degraders (SERDs), which as their name suggests, interact with and facilitate ER turnover in cells. One drug of this class, fulvestrant, has been approved as a third line treatment for ER-positive metastatic breast cancer. Whereas the first generation SERMs/SERDs were discovered in a serendipitous manner, this review will highlight how our understanding of the molecular pharmacology of ER ligands has been utilized in the development of the next generation of SERMs/SERDs, some of which are likely to have a major impact on the pharmacotherapy of breast cancer.
The evolution in our understanding of ER pharmacology
Drugs like tamoxifen and raloxifene were initially classified as antagonists and were developed as agents that could competitively displace estradiol from ER and inhibit its mitogenic actions in breast cancer cells [1,2]. However, it was apparent even from the earliest studies of these drugs in animals that their pharmacology was substantially more complex and that they were capable of exhibiting agonist, partial agonist or antagonist activities in different tissues [3–6]. Regardless, it was not until much later that it became clear that tamoxifen and raloxifene were more appropriately classified as Selective Estrogen Receptor Modulators (SERMs) [7]. One of the most important paradigm shifting experiments was that performed by Gottardis and Jordan in the late 1980s when they showed, in xenograft models of breast cancer, that whereas tamoxifen initially functioned as an ER antagonist, over time the tumors developed resistance to the drug and eventually switched to recognizing it as an agonist [8]. These data indicated that the pharmacology of tamoxifen was not stable and that over the course of chronic treatment something changes within the target cell that enables it to recognize the ER-tamoxifen complex as transcriptionally “active”. There were also anecdotal reports in the clinical literature of withdrawal responses in patients who progressed while on tamoxifen, a result that supported the observation that even in breast cancer cells tamoxifen could exhibit both antagonist and agonist activities [9]. It was also of significance that clinical studies revealed that both raloxifene and tamoxifen exhibited robust ER agonist activities in bone, and that tamoxifen and structurally related molecules manifested estrogenic activity in the uterus [10,11]. Taken together these data indicated that (a) a given compound, acting through the same receptor, can manifest different activities in different cells, (b) subtle differences in the structure of ER ligands can result in significantly different phenotypic responses, and (c) alterations in the cellular environment in which ER operates can dramatically alter the pharmacology of its bound ligands. The importance of these observations in understanding the pharmacological actions of existing ligands as well as in developing approaches for new ligand discovery has driven efforts directed at defining the cellular mechanisms that enable cells to distinguish between different ER-ligand complexes.
The molecular mechanisms underlying the pharmacological actions of ER ligands
In the absence of ligand ER associates with a large heat shock protein complex in either the nucleus or cytoplasm of target cells. Upon binding an agonist, the receptor undergoes a conformational change that initiates a cascade of events that enables its interaction with the regulatory regions of target genes. Interestingly, although ER has a well defined DNA binding domain and can interact with specific estrogen response elements (EREs) within target genes, it also uses the same domain to interact with DNA in an indirect manner by tethering to proteins such as AP1 or Runx1 that are bound to their cognate response elements [12,13].One of the major changes in our understanding of ER action, however, is that we no longer consider the receptor itself as directly impacting the activity of the transcriptional apparatus. Rather, it serves as a nucleating point for transcriptional coregulators, proteins with different enzymatic activities that can directly modify chromatin structure and/or impact the activity of the general transcription apparatus. Thus, it is the activity of the coregulators recruited by ER, rather than the receptor itself, that is the determinant of the response of the target gene to a specific receptor-ligand complex. There are over 300 proteins that have been shown to interact with one or more members of the nuclear receptor superfamily, a significant number of which can interact with ER.Not surprisingly, there has been a tremendous amount of work performed at a genetic and biochemical level to study the function and mechanism of action of these coregulators in ER pharmacology/physiology. Emerging from these studies are important “tenets” that describe how information is transferred from different ER ligand complexes to the transcription apparatus. Specifically it has been demonstrated that (1) the overall shape of ER is determined by the nature of the ligand with which it is bound, (2) receptor conformation regulates the differential interaction of the receptor with functionally distinct transcriptional coregulators, and (3) the relative and absolute levels of transcriptional coregulators differ between cells [14]. Furthermore, it is now clear that post-translational modifications of the receptors and/or the coregulators with which they interact can also impact the activity of the receptor-ligand complex at target genes. Additional complexity is introduced when one considers that there exist two genetically distinct estrogen receptors, ERα and ERβ that have independent activities when acting as homodimers or can modulate each other’s activity through heterodimerization in cells where they are both expressed [15,16]. While the ERα and ERβ DNA binding domains share high homology, it has been demonstrated by several investigators that in addition to being able to regulate the expression of similar target genes, each receptor sub-type also exhibits distinct target gene repertoires [17,18]. The molecular basis for this discrimination is not known, but undoubtedly it will be found to involve the differential actions of attendant cofactors. Interestingly, the two ER subtypes also exhibit significant differences in their response to pharmacological agents [19]. Notable was the observation that whereas tamoxifen inhibits transactivation by ERα, it can function as an ERβ agonist when analyzed on the same target gene under the same conditions [18]. Furthermore, while the SERD ICI 182,780 interacts with both ERα and ERβ with high affinity, it only induces turnover of ERα within cells [20]. It is worth noting that the expression of ERβ in ERα-positive breast cancer cells and xenograft tumors decreases ERα transcriptional activity and reduces the proliferative response to estrogens. However, the expression of ERβ in ERα-negative models of breast cancer increases cell proliferation [21,22]. Clearly, the important differences in ERα and ERβ pharmacology need to be considered in the design of the next generation of ER-modulators for the treatment and prevention of breast cancer.
Another important advance in our understanding of ER action comes from a reassessment of the definition of a “ligand”, historically considered a small lipophilic molecule that interacts with the ligand-binding domain of the receptor. It is now appropriate to consider that in addition to steroidal agonists, the DNA sequence and the coregulators with which the receptor interacts can impact its overall structure and modulate its biochemical properties [23**–25]. The relevance of these alternate modes of activation was reinforced in studies where it was shown that overexpression of a positive coregulator alone was sufficient to enable ER transcriptional activity. Furthermore, it was demonstrated that the binding kinetics of ER and estradiol were influenced by coregulator induced allosteric changes in the structure of the receptor ligand binding domain [23**]. Cumulatively these findings suggest that in addition to the classical ligand-binding pocket, it may be possible to target additional regulatory surfaces on ER in the search for new classes of receptor modulators.
Given our current understanding of the factors/processes that impact ER function, it is now clear how subtle differences in the structure of a ligand can have profound effects on its pharmacological activity. Furthermore, given the relationship between receptor conformation and activity, it is not surprising that molecular screens capable of detecting specific conformational states of ERα were used in the identification of lasofoxifene and bazedoxifene, second generation SERMs that exhibit distinct biological activities [7,26,27]. This and other information highlight the importance of receptor conformation in determining the pharmacological activity of different compounds and provide a mechanism to achieve functional diversity in ligands. On the other hand, the complexity of these pathways has also highlighted why it has been and will continue to be difficult to develop ER ligands whose pharmacological properties remain stable over time and which can be used for the chronic treatment of breast cancer.
The impact of clinical research on our understanding of ER pharmacology
The majority of breast tumors express ERα and thus tamoxifen (or related SERMs) or aromatase inhibitors have become frontline therapeutic interventions in this disease. Consequently, there is a tremendous amount of clinical information on the performance of ER signaling modulators in breast cancer. Consideration of this data, together with what is known about the mechanism of action of these agents, is instructive with respect to pharmacological characteristics required of the next generation of therapeutics that target this receptor. Whereas it is also likely that ERβ has a role to play in opposing ERα action in the breast, it is likely that it is the targeting of ERα that provides the majority of the therapeutic benefit [28].
When used in the metastatic setting tamoxifen effectively halts breast tumor progression with a duration of response in the range of 18–24 months.As described above, it is believed that treatment failure represents a switch in the environment of ERα that enables the cell to recognize tamoxifen as an agonist. This hypothesis is supported by data from studies of tamoxifen use in the adjuvant setting, which indicated that patients treated for 5 years with tamoxifen did significantly better than patients treated for longer periods (up to 10 years) [29]. Thus, it appears that rather than simply having no added benefit there was something about longer duration of exposure to tamoxifen that actually caused it to do harm.Given what is now known about ER signaling and coregulator biology, it has been proposed that chronic exposure to tamoxifen, either in the metastatic or adjuvant setting, induces resistance by (a) selecting for a population of cells within the tumor that has in place proteins and processes that enable this drug to manifest agonist activity, or (b) inducing an epigenetic change that results in the expression of components of the transcription apparatus that permits tamoxifen to function as an agonist.Regardless of which of these very similar mechanisms is correct, it is inferred that since ERα is a transcription factor, resistance represents a gain of function activity that results from the ability of the tamoxifen ER-complex to interact with a transcriptional coregulator(s) that enables it to manifest agonist activity. Thus, differences in the expression and/or activity of coregulators are now considered to be primary determinants of tamoxifen agonist/antagonist activity.
There is a substantial amount of experimental evidence that points to the primacy of coregulators in determining ER-ligand pharmacology. One particularly important finding came from the work of Smith et al which demonstrated that overexpression of the transcriptional coregulator SRC-1 alone was sufficient to confer upon cells the ability to recognize tamoxifen as an agonist [30*]. This suggested that although tamoxifen induces a conformational change in ERα that dramatically reduces its ability to interact with coactivators, the impact of this disruptive conformational change can be overcome by increasing the cellular concentration of a specific coactivator. This, coupled with the fact that tamoxifen enables efficient delivery of the receptor to DNA and that it also significantly reduces ERα turnover, explains how this drug can induce significant activation of ER target gene transcription [31]. It is noteworthy, in this regard, that it has been shown that elevated expression of SRC-1 and/or SRC-3 is associated with tamoxifen resistance and that the locus encoding SRC-3 is amplified in a large number of breast cancers [32–34*].
Growth factor receptor signaling modulates ER activity and ligand pharmacology
There is a considerable amount of data to indicate that the development of resistance to tamoxifen is associated with an increase in Her2 expression in breast cancer cells [35]. This compensatory response has been shown to occur in both cellular and animal models of this disease.It has also been shown that treatment of Her2-overexpressing, tamoxifen-resistant breast cancer cells with either the Her2 antagonist trastuzumab or with the dual Her2/EGFR tyrosine kinase inhibitor Lapatinib, is sufficient to restore tamoxifen sensitivity [36]. Furthermore, IGF-1R/EGFR mediated activation of PKA or MAPK signaling pathways in vitro has been shown to result in ligand-independent activation of ERα transcriptional activity and also to increase the efficacy/potency of agonists and partial agonists like tamoxifen [37,38]. Thus, aberrant activation of several different signaling pathways can impact ER pharmacology. One possible explanation for these responses is that Her2/IGF1R activation results in increased phosphorylation of sites on ERα that are associated with ligand activation and that this facilitates coregulator recruitment. Indeed, Her2 and IGF1R expression and phosphorylation, as well as phosphorylation of ER, were found to be prominent in tumors exhibiting de novo or acquired resistance to tamoxifen [39]. However, although there are several studies that highlight a very good correlation between increased phosphorylation and ERα transcriptional activity, a definitive cause and effect relationship remains to be established. Indeed, it has been shown recently that the dramatic effects of PKA on ERα transcriptional activity are likely due to phosphorylation of the coregulator CARM1 rather than direct receptor phosphorylation [40]. Furthermore, a careful analysis of the stoichiometry of phosphorylation following MAPK activation failed to demonstrate a significant increase in receptor phosphorylation, but rather revealed that phosphorylation of its attendant cofactor, SRC-3, was significantly increased [41]. Thus, it appears that activation of Her2 (or IGF1R) results in increased SRC-3 activity and subsequent increases in the agonist efficacy of estradiol and tamoxifen. It is interesting to note that patients with ER-positive tumors that express elevated Her2 and SRC-3 have the poorest overall survival and are least likely to respond to tamoxifen [33].
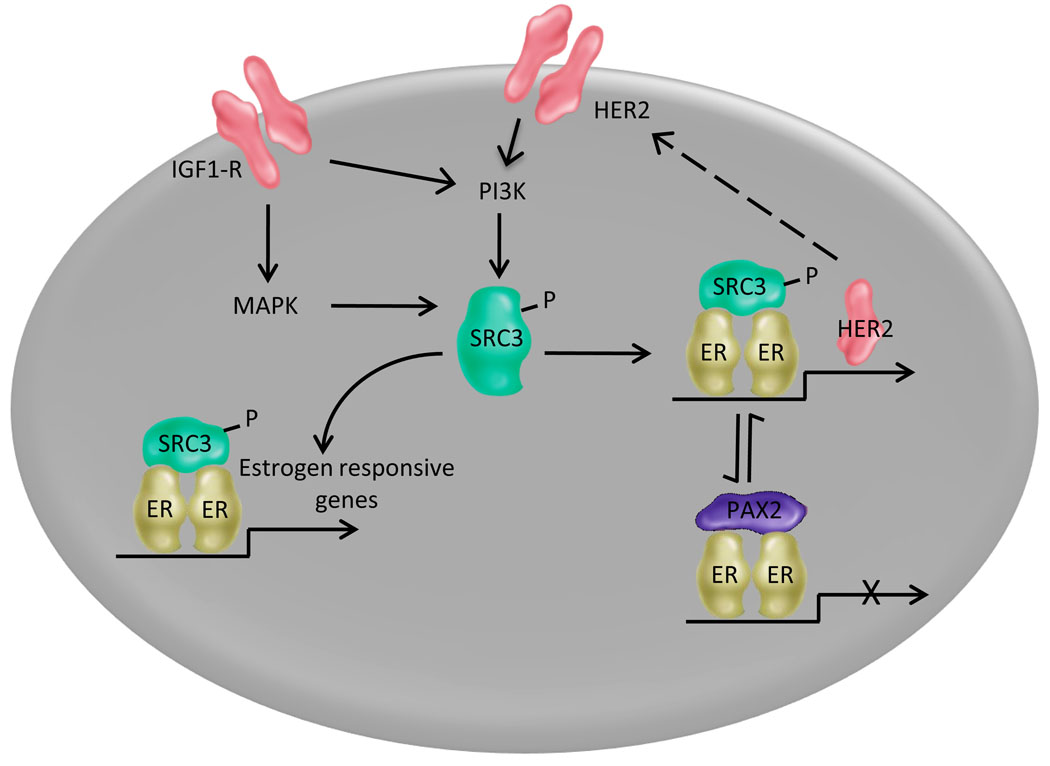
Whereas a functional relationship between increased expression and/or activity of SRC-3, activation of Her2 and alterations in ERα pharmacology has been appreciated for some time, it was not until recently that the biochemical basis for these interactions became apparent. Notable in this regard was the observation that resistance to the dual Her2/EGFR antagonist (Lapatinib) was associated with increased expression of ERα and that sensitivity to this inhibitor could be restored by treating the cells with the pure antiestrogen Fulvestrant [42*]. Equally important was the identification of an ERE within the regulatory region of the Her2 gene, through which the estradiol or tamoxifen occupied ERα repressed transcription of the Her2 gene by recruiting the transcriptional repressor PAX2 [43**]. However, it was observed that when SRC-3 expression and/or activity increased in cells, it was able to out compete PAX2 for ERα binding and repression of Her2 transcription was relieved. Interestingly, it has been shown recently that increased SRC-3 expression is an early response to tamoxifen administration [44]. Together these findings highlight the central role of SRC-3 in ERα signaling in breast tumors and demonstrate how differences in the expression and/or activity of coregulators can dramatically alter the pharmacology of ER ligands. A model describing what we know about ER/Her2 crosstalk and its ability to influence ER pharmacology is detailed in Figure 1.
Figure 1. The physical and functional interaction between the ER and Her2/IGFR signaling pathways in breast cancer cells influences the pharmacology of ER ligands.
The transcriptional activity of ERα and its pharmacological response to endogenous and exogenous ligands is determined in large part by the repertoire of coregulators expressed in a given cell and the impact of activated signaling pathways on the activity of receptor:coregulator complexes. Whereas there are a large number of coregulators, each of which may have a different effect on receptor activity, a model highlighting the interactions between the ERα-SRC-3/Her2 regulatory axis is presented for illustrative purposes. The complete details supporting this model are presented in the text. In short, however, it is now clear that differences in SRC-3 expression or activity can result in differential activation of the ER target genes. Of particular importance is the observation that increases in SRC-3 activity and or expression can relieve ER-mediated repression of Her2 expression. This initiates a positive feed forward loop that results in increased Her2 signaling and subsequently increased ER signaling. Under these conditions the biocharacter of tamoxifen has been shown to switch from that of an antagonist to an agonist.
Transcriptional coregulators as determinants of the pharmacological activity of ER ligands in breast cancer
Whereas the discussion above has focused on SRC-3, it is clear that there are many additional coregulators, the dysregulated expression and/or activity of which are likely to impact endocrine treatment of breast tumors. For instance, it has been shown that the NR coregulator HoxB13 is overexpressed in tamoxifen-resistant breast cancers [45,46]. Interestingly, diagnostic tests that measure HoxB13 expression are routinely used to predict the likelihood of response to tamoxifen [47]. However, the mechanism(s) by which this coregulator impacts ER signaling and pharmacology in breast cancer remains unclear. Another interesting coregulator is RTA-1/Fox2, a regulator of RNA splicing whose expression level alters tamoxifen pharmacology [48]. Interestingly, many known coregulators are among the known targets of this splicing regulator, although the precise mechanism by which differential splicing impacts ERα pharmacology remains to be determined [49].
It should be apparent from this discussion that it is differences in coregulator activity and expression, rather than biochemical changes in ERα itself, that determine the activity of receptor bound ligands. It is not surprising, therefore, that there is a considerable amount of interest in targeting the ERα-coregulator interface directly, using molecules that bind to the coregulator binding pockets on the receptor, or indirectly, using molecules that inhibit the activity and/or expression of coregulators [50,51]. Although protein-protein interaction surfaces are generally large and difficult to target with small molecules, the results of efforts in this direction suggest that this general approach may be feasible.
Exploitation of the mechanistic complexity in ER signaling for new drug development
Two recent findings from studies of the mechanism of action of ER that bear significance with respect to new drug discovery are (a) ligands induce different conformational changes in receptor structure which then engender different cofactor interactions and (b) there are mechanisms other than occupancy of the receptor ligand binding pocket by a small molecule agonist that can result in ER transcriptional activation. Considerable progress has been made in exploiting these findings in the development of new ER modulators. Two specific examples of the progress made in this regard will serve to illustrate this point.
(a) Development of molecules that engender different conformational changes in ER structure
One of the first attempts to develop molecules that inhibited tamoxifen-resistant breast cancer led to the identification of keoxifene (now called raloxifene), a high affinity ER ligand that was structurally unrelated to tamoxifen. The primary rationale at the time for this approach was that resistance to tamoxifen was thought to occur either as a consequence of ERα mutations that disrupted tamoxifen binding or because the drug itself was modified in such a way to alter its pharmacological properties. However, keoxifene (raloxifene) was not found to be effective in breast cancer and its development was discontinued [52]. In addition to unfavorable pharmaceutical properties, we now know from an abundance of structural studies that the overall conformation of the ERα-tamoxifen and ERα-raloxifene complexes are extremely similar and it is likely that they would interact with the same cofactors [53–55]. Therefore, the cross-resistance observed in the clinic was not surprising, and neither was their similar efficacy in breast cancer prevention as noted in the Study of Tamoxifen and Raloxifene (STAR) trial [56–58]. It was inferred, however, from the studies of tamoxifen/raloxifene pharmacology that compounds that enabled ERα to adopt a distinctly different conformation and that disrupted specific receptor cofactor interactions may have utility in the treatment of tamoxifen refractory tumors. To test this hypothesis, we developed a series of in vitro screens that facilitated the identification of compounds that enabled ER to adopt a conformational state distinct from those induced by tamoxifen, raloxifene or estradiol. In this manner, GW5638/DPC974 was identified, a compound that was subsequently shown to interfere with ERα action by directly disrupting the folding of the critical helix 12 in the ligand binding domain of the receptor [59*,60]. Importantly, this molecule had excellent pharmaceutical properties and inhibited the growth of both tamoxifen sensitive and resistant tumor xenografts. Most notably, in a small investigator-initiated clinical trial of the drug there was evidence of efficacy in patients with heavily treated metastatic disease. Unfortunately, a victim of corporate mergers and portfolio reviews, this drug was not determined to be a financial winner and was discontinued. Regardless, the work with this drug firmly established the concept that it was possible to manipulate ERα structure and identify compounds that could be used in the pharmacotherapy of tamoxifen-resistant ER-positive tumors. It will be of interest to see whether compounds like bazedoxifene that induce unique structural changes in ERα will be effective in the treatment of tamoxifen refractory breast cancers.
(b) Development of selective estrogen receptor degraders
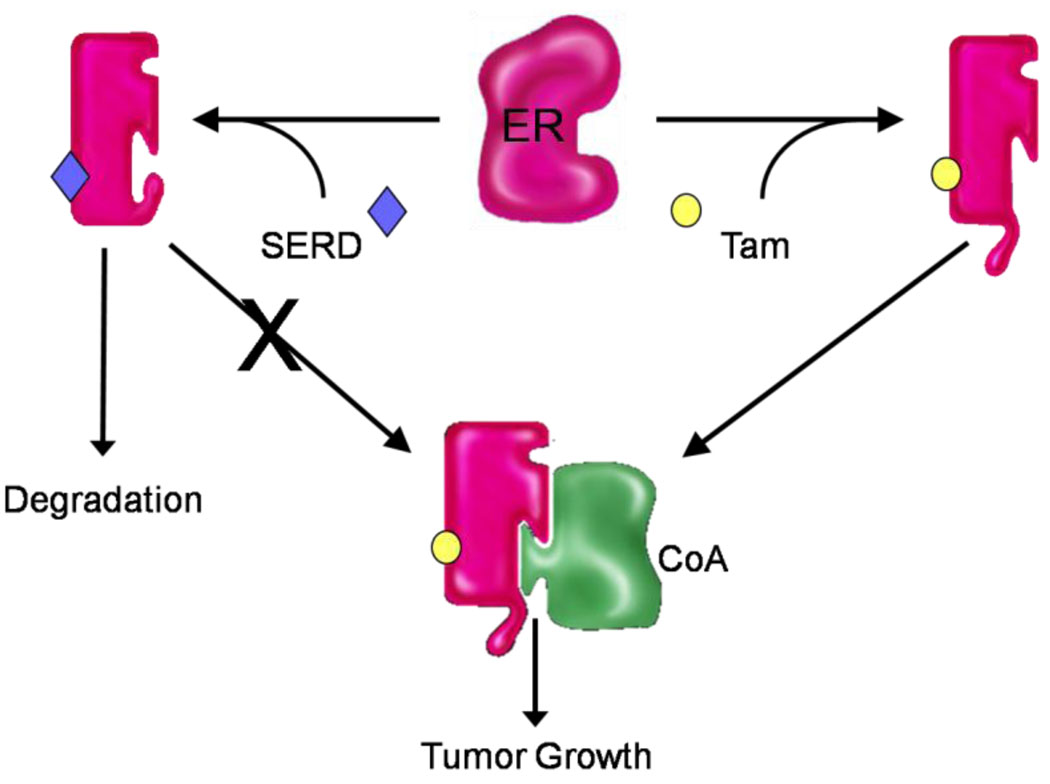
Although it is possible to manipulate ERα structure and regulate its cofactor interaction preferences, it should be apparent from the information presented above that the mere presence of the receptor itself makes possible the engagement of a cofactor that will enable ER to manifest transcriptional activity. As proposed for tamoxifen resistance, selective pressure could result in the selection of a population of cells within a tumor that express a suitable cofactor and/or an existing cofactor made more compatible with the ER-tamoxifen complex subsequent to the activation of a signaling pathway. These observations suggested that molecules resulting in ERα destruction may be particularly useful in the treatment of breast cancer (Figure 2). Indeed, this approach was fuelled in part by the observation that high-dose estrogens, which led to a rapid down regulation of ERα expression in cells, was as effective as tamoxifen as a front-line intervention in breast cancer [61]. The first clinically approved molecule of this class, ICI182,780 (fulvestrant) was shown both in vitro and in animal models to effectively reduce ERα expression in cells and inhibit the growth of tamoxifen-resistant breast tumor xenografts [62–64]. However, the clinical results with this drug have been extremely disappointing, dampening somewhat the excitement about the potential of this class of molecule [65]. Initially, it was considered that the failure of fulvestrant indicated that the mechanism of tamoxifen resistance modeled in animals did not bear on human tumors. However, it now appears from the results of additional studies that this drug has extremely poor bioavailability and that it is difficult to get high enough levels of the drug at the tumor to effect a quantitative turnover of the receptor [66*]. Indeed, a sequential tumor biopsy study has indicated that even after long-term fulvestrant treatment at the approved dose, ER is still present at approximately 50% of the original baseline [67].Results of recent trials comparing higher doses of fulvestrant to that currently approved demonstrate increased serum steady state levels and correspondingly improved response rate, as well as increased ER turnover, although the pharmaceutical properties of fulvestrant continue to limit its use [65,68,69]. Thus, there is a tremendous amount of interest in developing SERDs that exhibit improved pharmaceutical properties. Interestingly, the compound discussed above, GW5638/DPC974, which by virtue of its effect on ERα structure can disengage cofactors, has been determined to also exhibit SERD activity [70]. Indeed, it has recently been shown that its effect on the structure of helix 12 in ERα is so dramatic that the receptor is recognized as denatured and is targeted for 26S proteasomal degradation. Whereas this molecule itself will not be developed, it has provided a chemical scaffold that can be exploited further for the development of an orally active SERD molecule [71].
Figure 2. Understanding the role of coregulators in ER action in breast tumors is instructive with respect to new drug development.
Upon binding tamoxifen ERα adopts a conformation that is distinct from apo-ERα and that which occurs upon binding estradiol. This conformational change disrupts the primary coregulator binding surface on ERα and reduces the affinity of the receptor for coregulators. Consequently, in cells where coregulators are not overexpressed or hyperactivated, tamoxifen is capable of inhibiting ER action. However, under the selective pressure of tamoxifen administration, something changes within the cell that alters the coactivator milieu such that the tamoxifen:ER complex can engage a coregulator that allows it to activate transcription. This could result from (a) the overexpression of a cofactor with which the receptor normally interacts when occupied by estradiol or (b) the expression of a cofactor that can interact in an ectopic manner with the receptor:tamoxifen complex. The ability of compounds that enable ERα to adopt a unique conformation to effectively inhibit the growth of tamoxifen resistant tumors highlights the validity of this model. It should also be apparent from this discussion why there is so much interest in developing SERDs that function by completely removing the possibility of “productive” ER-coregulator interactions.
Although a detailed discussion of the pharmacology of aromatase inhibitors is beyond the scope of this manuscript, there are several points that are worth mentioning in the context of SERD action. It should be apparent from the studies presented above that inhibition of aromatase and the reduction of circulating/tumor levels of estrogens should result in the inhibition of the growth of ER-positive tumors. However, it should also be clear that the inhibitory actions of these drugs could be bypassed by (a) upregulation of any of the pathways that lead to ligand-independent activation of ERα (i.e. MAPK activation) or (b) the production of, or environmental exposure to, a molecule with estrogen-like properties. Of direct relevance to the former possibility is an important paper published several years ago in which it was shown that exposing breast cancer cells to an aromatase inhibitor resulted in an adaptive change that rendered cells extremely sensitive to low levels of estrogens [72*]. With respect to the latter point is the observation that 27-hydroxycholesterol, an oxysterol produced in a stoichiometric manner from cholesterol in an aromatase-independent manner, can activate ERα and thus could contribute to resistance [73*]. Similarly, it has been shown that the androgen metabolite 3β,17β-diol can function as an estrogen in cellular models of breast cancer [74]. Clearly, in either of these potential scenarios it would be expected that SERDs would be an effective therapeutic strategy, and indeed fulvestrant was recently shown to have clinical benefit in Her2-overexpressing cancers in patients who had failed endocrine therapy [75].
Final Comments
In the post genome era, there is an abundance of potential new drug targets, a considerable number of which may be relevant to breast cancer. However, there has been a tendency to forget well-validated targets such as ERα in the belief that they have been fully exploited. This could not be further from the truth. It is clear that the more we explore the ERα signal transduction pathways in breast cancer, the more apparent it is that the existing modulators of this axis are relatively unsophisticated and can be improved. It is also clear also that we have not yet taken full advantage of the complexities in the estrogen signaling pathways in the development of new drugs. However, with a more complete understanding of ER action, as is now emerging, the field is well positioned to move from the standard empirical screening for modulators to specific mechanism-based screens of high predictive value that will likely yield useful drugs.
Acknowledgments
Some of the research discussed in this paper was supported by an NIH grant R37 DK048807.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose.
References
- 1.Clemens JA, Bennet DR, Black LJ, Jones CD. Effects of a new anti-estrogen, keoxifene ( LY156758), on growth of carcinogen-induced mammary tumors and on LH and prolactin secretion. Life Sciences. 1983;32:2869–2875. doi: 10.1016/0024-3205(83)90323-5. [DOI] [PubMed] [Google Scholar]
- 2.Jordan VC, Collins MM, Rowsby L, Prestwich G. A mono-hydroxylated metabolite of tamoxifen with potent antioestrogen activity. J Endocrinol. 1977;75:305–316. doi: 10.1677/joe.0.0750305. [DOI] [PubMed] [Google Scholar]
- 3.Harper MJK, Walpole AL. Contrasting endocrine activities of cis and trans isomers in a series of substituted triphenylethylenes. Nature. 1966;212:87–89. doi: 10.1038/212087a0. [DOI] [PubMed] [Google Scholar]
- 4.Turner CH, Sato M, Bryant HU. Raloxifene preserves bone strength and bone mass in ovariectomized rats. Endocrinology. 1994;135:2001–2005. doi: 10.1210/endo.135.5.7956922. [DOI] [PubMed] [Google Scholar]
- 5.Turner RT, Wakley GK, Hannon KS, Bell NH. Tamoxifen prevents the skeletal effects of ovarian hormone deficiency in rats. J Bone Min Res. 1987;2:449–456. doi: 10.1002/jbmr.5650020513. [DOI] [PubMed] [Google Scholar]
- 6.Gottardis MM, Robinson SP, Satyaswaroop PG, Jordan VC. Contrasting actions of tamoxifen on endometrial and breast tumor growth in the athymic mouse. Cancer Res. 1988;48:812–815. [PubMed] [Google Scholar]
- 7.McDonnell DP, Clemm DL, Hermann T, Goldman ME, Pike JW. Analysis of estrogen receptor function in vitro reveals three distinct classes of antiestrogens. Mol Endocrinol. 1995;9:659–668. doi: 10.1210/mend.9.6.8592512. [DOI] [PubMed] [Google Scholar]
- 8.Gottardis MM, Jordan VC. Development of tamoxifen-stimulated growth of MCF-7 tumors in athymic mice after long-term antiestrogen administration. Cancer Res. 1988;48:5183–5187. [PubMed] [Google Scholar]
- 9.Canney PA, Griffiths T, Latief TN, Priestman TJ. Clinical significance of tamoxifen withdrawal response. The Lancet. 1987;1:36. doi: 10.1016/s0140-6736(87)90717-3. [DOI] [PubMed] [Google Scholar]
- 10.Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, Jordan VC, Carbone PP, DeMets DL. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. New Engl J Med. 1992;326:852–856. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 11.Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, Norton L, Nickelsen T, Bjarnason NH, Morrow M, Lippman ME, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: Results from the MORE randomized trial. JAMA. 1999;281:2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 12. Safe S, Kim K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J Mol Endocrinol. 2008;41(5):263–275. doi: 10.1677/JME-08-0103.In addition to its classical mechanism of activation of transcription through direct association with DNA elements surrounding target genes, ER also modulates gene expression through a non-classical mechanism involving indirect interaction of receptor with DNA mediated through tethering interactions with other transcription factors. This review provides a current perspective of the ER action mediated through these tethering interactions.
- 13. Stender JD, Kim K, Charn TH, Komm B, Chang KCN, Kraus WL, Benner C, Glass CK, Katzenellenbogen BS. Genome-wide analysis of estrogen receptor alpha DNA binding and tethering mechanisms identifies Runx1 as a novel tethering factor in receptor-mediated transcriptional activation. Mol Cell Biol. 2010;30(16):3943–3955. doi: 10.1128/MCB.00118-10.Using ChIP-Seq technology, the authors conduct a genome-wide analysis of ER binding comparing the wild type receptor to a DNA binding deficient mutant. Further analysis of those genes shown to be regulated by a non-classical mechanism demonstrated a role for Runx1 in mediating ER regulation of this subset of genes.
- 14.Hall JM, McDonnell DP. Coregulators in nuclear estrogen receptor action: From concept to therapeutic targeting. Mol Interventions. 2005;5:343–357. doi: 10.1124/mi.5.6.7. [DOI] [PubMed] [Google Scholar]
- 15.Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson J-A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors a and b. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 16.Hall JM, McDonnell DP. The estrogen receptor b-isoform (ERb) of the human estrogen receptor modulates ERa transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 17.Vanacker J-M, Pettersson D, Gustafsson J-Å, Laudet v. Transcriptional targets shared by estrogen receptor-related receptors (ERRs) and estrogen receptor (ER) a, but not by ERb. EMBO J. 1999;18:4270–4279. doi: 10.1093/emboj/18.15.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paech K, Webb P, Kuiper GGJM, Nilsson S, Gustafsson J-A, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERa and ERb at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 19.Barkhem T, Carlsson B, Milsson Y, Enmark E, Gustafsson J-A, Nilsson S. Differential response of estrogen receptor a and estrogen receptor b to partial estrogen agonists/antagonists. Mol Pharmacol. 1998;54:105–112. doi: 10.1124/mol.54.1.105. [DOI] [PubMed] [Google Scholar]
- 20.Peekhaus NT, Chang T, Hayes EC, Wilkinson HA, Mitra SW, Schaeffer JM, Rohrer SP. Distinct effects of the antiestrogen Faslodex on the stability of estrogen receptors-a and -b in the breast cancer cell line MCF-7. J Mol Endo. 2004;32:987–995. doi: 10.1677/jme.0.0320987. [DOI] [PubMed] [Google Scholar]
- 21.Hou YF, Yuan ST, Li HC, Wu J, Lu JSGL, Lu LJ, Shen ZZ, Ding J, Shao ZM. ERb exerts multiple stimulative effects on human breast carcinoma cells. Oncogene. 2004;23:5799–5806. doi: 10.1038/sj.onc.1207765. [DOI] [PubMed] [Google Scholar]
- 22.Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor b inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004;64:423–428. doi: 10.1158/0008-5472.can-03-2446. [DOI] [PubMed] [Google Scholar]
- 23. Gee AC, Carlson KE, Martini PGV, Katzenellenbogen BS, Katzenellenbogen JA. Coactivator peptides have a differential stabilizing effect on the binding of estrogens and antiestrogens with the estrogen receptor. Mol Endocrinol. 1999;13:1912–1923. doi: 10.1210/mend.13.11.0373.Using a fluorescent ER ligand to analyze the rate of ligand dissociation, the authors demonstrate that specific agonist-dependent interaction of ER with the nuclear receptor interaction motif of SRC-1, either as a minimal peptide or within an isolated domain of SRC-1, stabilized the ER-agonist complex. While it had been widely accepted that the nature of the bound ligand impacted the ability of the ER to recruit coactivator proteins, this study illustrated that the converse is also true: coactivator interactions can influence interaction of the receptor with its ligand.
- 24. Hall JM, McDonnell DP, Korach KS. Allosteric regulation of estrogen receptor structure, function, and coactivator recruitment by different estrogen response elements. Mol Endocrinol. 2002;16:469–486. doi: 10.1210/mend.16.3.0814.Using phage display of peptides containing the canonical LXXLL motif known to mediate interaction of several coactivators with the nuclear receptors, the authors demonstrate that the nature of the DNA element to which ER is bound influences the preference with which the receptor interacts with distinct peptide sequences. These results carry significance with respect to the level of ER transcriptional activation elicited by an ERE binding site in a given target gene and suggest that the DNA elements may dictate not only ER binding, but also the nature of the complex nucleated around the bound receptor.
- 25.Dana SL, Hoener PA, Wheeler DL, Lawrence CL, McDonnell DP. Novel estrogen response elements identified by genetic selection in yeast are differentially responsive to estrogens and antiestrogens in mammalian cells. Mol Endocrinol. 1994;8:1193–1207. doi: 10.1210/mend.8.9.7838152. [DOI] [PubMed] [Google Scholar]
- 26.Clemm DL, Macy BL, Santiso-Mere D, McDonnell DP. Definition of the critical cellular components which distinguish between hormone and antihormone activated progesterone receptor. J Steroid Biochem Molec Biol. 1995;53:487–495. doi: 10.1016/0960-0760(95)00095-h. [DOI] [PubMed] [Google Scholar]
- 27.Crabtree JS, Peano BJ, Zhang X, Komm BS, Winneker RC, Harris HA. Activity of three selective estrogen receptor modulators on hormone-dependent responses in the mouse uterus and mammary gland. Mol Cell Endocrinol. 2008;287(1–2):40–46. doi: 10.1016/j.mce.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 28.Strom A, Hartman J, Foster JS, Kietz S, Wimalasena J, Gustafsson J-Å. Estrogen receptor b inhibits 17b–estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci USA. 2004;101:1566–1571. doi: 10.1073/pnas.0308319100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher B, Dignam J, Bryant J, Wolmark N. Five versus more than five years of tamoxifen for lymph node-negative breast cancer updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst. 2001;93:684–690. doi: 10.1093/jnci/93.9.684. [DOI] [PubMed] [Google Scholar]
- 30.Smith CL, Nawaz Z, O'Malley BW. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol. 1997;11:657–666. doi: 10.1210/mend.11.6.0009. [DOI] [PubMed] [Google Scholar]
- 31.Wijayaratne AL, Nagel SC, Paige LA, Christensen DJ, Norris JD, Fowlkes DM, McDonnell DP. Comparative analyses of the mechanistic differences among antiestrogens. Endocrinology. 1999;140:5828–5840. doi: 10.1210/endo.140.12.7164. [DOI] [PubMed] [Google Scholar]
- 32.Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan X-Y, Sauter G, Kallioniemi O-P, Trent JM, Meltzer PS. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 33.Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SAW, Wong J, Allred DC, Clark GM, Schiff R. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003;95:353–361. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 34. Redmond AM, Bane FT, Stafford AT, McIlroy M, Dillon MF, Crotty TB, Hill AD, Young LS. Coassociation of estrogen receptor and p160 proteins predicts resistance to endocrine treatment: SRC-1 is an independent predictor of breast cancer recurrence. Clin Cancer Res. 2009;15:2098–2106. doi: 10.1158/1078-0432.CCR-08-1649.Using quantitative immunofluorescent microscopy of breast cancer cell lines and breast cancer samples, the authors demonstrate significantly stronger colocalization of coactivators SRC-1 and AIB1 (SRC-3) with ER in endocrine therapy resistant models and tumor samples, and that these interactions are predictive of response. This study illustrates the importance of these coactivators in determining the pharmacology of ER antagonists.
- 35.Shou J, Mssarweh S, Osborne CK, Wakeling AE, Schiff R. Mechanisms of tamoxifen resistance: Increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 36.Kurokawa H, Lenferink AEG, Simpson JF, Pisacane PI, Sliwkowski MX, Forbes JT, Arteaga CL. Inhibition of HERs/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res. 2000;60:5887–5894. [PubMed] [Google Scholar]
- 37.Yue W, Wang J-P, Conaway M, Masamura S, Li Y, Santen RJ. Activation of the MAPK pathway enhances sensitivity of MCF-7 breast cancer cells to the mitogenic effect of estradiol. Endocrinology. 2002;143:3221–3229. doi: 10.1210/en.2002-220186. [DOI] [PubMed] [Google Scholar]
- 38.Fujimoto N, Katzenellenbogen BS. Alteration in the agonist/antagonist balance of antiestrogens by activation of protein kinase A signaling pathways in breast cancer cells: antiestrogen selectivity and promoter dependence. Mol Endo. 1994;8:296–304. doi: 10.1210/mend.8.3.7517003. [DOI] [PubMed] [Google Scholar]
- 39.Gee JM, Robertson JF, Gutteridge E, Ellis IO, Pinder SE, Rubini M, Nicholson RI. Epidermal growth factor receptor/HER2/insulin-like growth factor receptor signaling and oestrogen receptor activity in clinical breast cancer. Endocrine-Related Cancer. 2005;12:S99–S111. doi: 10.1677/erc.1.01005. [DOI] [PubMed] [Google Scholar]
- 40. Carascossa S, Dudek P, Cenni B, Briand P-A, Picard D. CARM1 mediates the ligand-independent and tamoxifen-resistant activation of the estrogen receptor alpha by cAMP. Genes & Development. 2010;24:708–719. doi: 10.1101/gad.568410.While functional interaction of activated signaling pathways with unliganded or SERM-occupied ER has long been observed, the authors demonstrate coactivator CARM1 is a specific target of cAMP induced phosphorylation and that this is necessary and sufficient for association with ER. These results suggest a mechanism other than direct ER phosphorylation by which signaling through growth factor receptors can result in agonist independent activation of ER.
- 41.Mora JFd, Brown M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol. 2000;20:5041–5047. doi: 10.1128/mcb.20.14.5041-5047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xia W, Bacus S, Hegde P, Husain I, Strum J, Liu L, Paulazzo G, Lyass L, Trusk P, Hill J, Harris J, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci USA. 2006;103:7795–7800. doi: 10.1073/pnas.0602468103.Through continuous exposure of tamoxifen resistant breast cancer cells to the EGFR and Her2 inhibitor lapatinib, the authors isolate lapatinib resistant cells and subsequently demonstrate that their mechanism involves a functional switch of the cells from dependence on Her2 to dependence on ER signaling to maintain antiapoptotic pathways. The authors further show that this switch renders the cells sensitive to antiestrogens, suggesting that these agents may have therapeutic value in treatment of Her2 inhibitor resistant breast cancers.
- 43. Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M, Jiang J, Howat WJ, Ali S, Carroll JS. Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature. 2008;456:663–667. doi: 10.1038/nature07483.The authors identify direct estrogen or tamoxifen dependent repression of ERBB2 (Her2) expression that requires receptor recruitment of PAX2. The authors further demonstrate that competition of PAX2 and SRC-3 for interaction with ER can relieve inhibition of ERBB2 expression in cells overexpressing SRC-3, providing a mechanism for SRC-3 association with ERBB2 overexpression in breast tumors.
- 44.Moi LLH, Flageng MH, Gandini S, Guerrieri-Gonzaga A, Bonanni B, Lazzeroni M, Gjerde J, Lien EA, Censi AD, Mellgren G. Effect of low-dose tamoxifen on steroid receptor coactivator 3/amplified in breast cancer 1 in normal and malignant human breast tissue. Clin Cancer Res. 2010;16:2176–2186. doi: 10.1158/1078-0432.CCR-09-1859. [DOI] [PubMed] [Google Scholar]
- 45. Goetz MP, Suman VJ, Ingle JN, Nibbe AM, Visscher DW, Reynolds CA, Lingle WL, Erlander M, Ma X-J, Sgroi DC, Perez EA, et al. A two-gene expression ratio of homeobox 13 and interleukin-17B receptor for prediction of recurrence and survival in women receiving adjuvant tamoxifen. Clin Cancer Res. 2006;12:2080–2087. doi: 10.1158/1078-0432.CCR-05-1263.The authors report increased expression of SRC-3 following short term treatment with tamoxifen in the neoadjuvent setting. Because SRC-3 expression is associated with tamoxifen resistance, these results illustrate an adaptive immediate response to tamoxifen treatment that may contribute to later development of resistance.
- 46.Ma X-J, Wang Z, Ryan PD, Isakoff SJ, Barmettler A, Fuller A, Muir B, Mohapatra g, Salunga R, Tuggle JT, Tran Y, et al. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell. 2004;5:607–616. doi: 10.1016/j.ccr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 47.Ma X-J, Hilsenbeck SG, Wang W, Ding L, Sgroi DC, Bender RA, Osborne CK, Allred DC, Erlander MG. The HOXB13:IL17BR expression index is a prognostic factor in early-stage breast cancer. J Clin Oncology. 2006;24:4611–4619. doi: 10.1200/JCO.2006.06.6944. [DOI] [PubMed] [Google Scholar]
- 48.Norris JD, Fan D, Sherk A, McDonnell DP. A negative coregulator for the human ER. Mol Endocrinol. 2002;16:459–468. doi: 10.1210/mend.16.3.0787. [DOI] [PubMed] [Google Scholar]
- 49.Yeo GW, Coufal NG, Liang TY, Peng GE, Fu X-D, Gage FH. An RNA code for the FOX2 splicing regulator revealed by mapping RNA-protein interactions in stem cells. Nature Structural & Molecular Biology. 2009;16:130–137. doi: 10.1038/nsmb.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moore TW, Mayne CG, Katzenellenbogen JA. Minireview: Not picking pockets: Nuclear Receptor Alternate-site Modulators (NRAMs) Mol Endocrinol. 2010;24:683–695. doi: 10.1210/me.2009-0362.While the ligand binding pocket has long been targeted for development of novel estrogen receptor regulators, the authors review recent advances in targeting alternative surfaces of nuclear receptors to develop novel therapeutics for treatment endocrine responsive cancers.
- 51.Parent AA, Gunther JR, Katzenellenbogen JA. Blocking estrogen signaling after the hormone: Pyrimidine-core inhibitors of estrogen receptor-coactivator binding. J Med Chem. 2008;51:6512–6530. doi: 10.1021/jm800698b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buzdar AU, Marcus C, Holmes F, Hug V, Hortobachi G. Phase II evaluation of LY156758 in metastatic breast cancer. Oncology. 1988;45:144–145. doi: 10.1159/000226637. [DOI] [PubMed] [Google Scholar]
- 53.Norris JD, Paige LA, Christensen DJ, Chang C-Y, Huacani MR, Fan D, Hamilton PT, Fowlkes DM, McDonnell DP. Peptide antagonists of the human estrogen receptor. Science. 1999;285:744–746. doi: 10.1126/science.285.5428.744. [DOI] [PubMed] [Google Scholar]
- 54.Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, Ohman L, Greene GL, Gustafsson JA, Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 55.Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95:927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 56.Vogel VG. The NSABP Study of Tamoxifen and Raloxifene (STAR) trial. Expert Rev Anticancer Ther. 2009;9(1):51–60. doi: 10.1586/14737140.9.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher LER, Pajon JJL, Wade I, Robidoux A, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes. JAMA. 2007;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 58.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon ERJL, Wade I, Robidoux A, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 trial: Preventing breast cancer. Cancer Prev Res (Phila Pa) 2010;3(6):696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wu Y-L, Yang X, Ren Z, McDonnell DP, Norris JD, Willson TM, Greene GL. Structural basis for an unexpected mode of SERM-mediated ER antagonism. Mol Cell. 2005;18:413–424. doi: 10.1016/j.molcel.2005.04.014.With as appreciation of that distinct conformations of ER that are elicited by the original serendipitously discovered SERMs, the authors devise a mechanism based screen to identify novel therapeutics that induce unique conformations of the receptor. They further demonstrate that tamoxifen resistant xenograft tumors are not cross-resistant to these novel therapeutics, validating further targeting of ER by unrelated antiestrogens in the face of tamoxifen resistance.
- 60.Connor CE, Norris JD, Broadwater G, Willson TM, Gottardis MM, Dewhirst MW, McDonnell DP. Circumventing tamoxifen resistance in breast cancers using antiestrogens that induce unique conformational changes in the estrogen receptor. Cancer Res. 2001;61:2917–2922. [PubMed] [Google Scholar]
- 61.Peethambaram PP, Ingle JN, Suman VJ, Hartann LC, Loprinzi CL. Randomized trial of diethylstilbestrol vs. tamoxifen in postmenopausal women with metastatic breast cancer. An updated analysis. Breast Cancer Res Treat. 1999;54(2):117–122. doi: 10.1023/a:1006185805079. [DOI] [PubMed] [Google Scholar]
- 62.Osborne CK, Coronado-Heinson EB, Hilsenbeck SG, McCue BL, Wakeling AE, McClelland RA, Nicholson RI. Comparison of the effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast cancer. J Natl Cancer Inst. 1995;87:746–750. doi: 10.1093/jnci/87.10.746. [DOI] [PubMed] [Google Scholar]
- 63.Howell A, DeFriend D, Robertson J, Blamey R, Walton P. Response to a specific antiestrogen (ICI182,780) in tamoxifen-resistant breast cancer. Lancet. 1995;345:29–30. doi: 10.1016/s0140-6736(95)91156-1. [DOI] [PubMed] [Google Scholar]
- 64.Creighton CJ, Massarweh S, Huang S, Tsimelzon A, Hilsenbeck SG, Osborne CK, Shou J, Malomi L, Schiff R. Development of resistance to targeted therapies transforms the clinically associated molecular profile subtype of breast tumor xenografts. Cancer Res. 2008;68(18):7493–7501. doi: 10.1158/0008-5472.CAN-08-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Young OE, Renshaw L, Macaskill EJ, White S, Faratian D, Thomas JSJ, Dixon JM. Effects of fulvestrant 750 mg in premenopausal women with oestrogen-receptor-positive primary breast cancer. Eur J Cancer. 2008;44(3):391–399. doi: 10.1016/j.ejca.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 66. Robertson JFR. Fulvestrant (Faslodex)-How to make a good drug better. The Oncologist. 2007;12:774–784. doi: 10.1634/theoncologist.12-7-774.This comprehensive review details the clinical properties and patient response that has been observed following treatment with the SERD ICI 182,780 (Fulvestrant). Well discussed are the pharmacological complications of Fulvestrant as well as the clinical advantages of SERD therapy.
- 67.Gutteridge E, Robertson JFR, Cheung KL. Effects of fulvestrant on estrogen receptor levels during long-term treatment of patients with advanced breast cancer - final results. Breast Cancer Res Treat. 2004;88 suppl 1:S177. [Google Scholar]
- 68.Robertson JF, Llombart-Cussac A, Rolski J, Feltl D, Dewar J, Macpherson E, Lindemann J, Ellis MJ. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol. 2009;27(27):4530–4535. doi: 10.1200/JCO.2008.21.1136. [DOI] [PubMed] [Google Scholar]
- 69. Leo AD, Jerusalem G, Petruzelka L, Torres R, Bondarenko I, Khasanov R, Verhoeven D, Pedrini J, Lichinitser M, Pendergrass K, Garnett S, et al. CONFIRM: A Phase III, randomized, parallel-group trial comparing fulvestrant 250 mg vs fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. Cancer Res. 2009;69(24 Suppl) Abstract nr 25.The pharmaceutical properties of fulvestrant complicate its use as a breast cancer intervention, and the low response rate to Fulvestrant originally observed in the EFECT trial is attributed to inability to achieve a therapeutic dose. The CONFIRM trial compared a high dose of fulvestrant with the established dose and observed a corresponding increase in patient response, supporting a beneficial role for SERD therapy in treatment of endocrine resistant cancer.
- 70. Wittmann BM, Sherk A, McDonnell DP. Definition of functionally important mechanistic differences among selective estrogen receptor down-regulators. Cancer Res. 2007;67:9549–9560. doi: 10.1158/0008-5472.CAN-07-1590.Through detailed analysis of ER degradation, the authors demonstrate multiple distinct mechanisms by which SERDs by which different SERDs effect a removal of ER. These results illustrate further opportunities for development of clinically useful SERDs.
- 71. Kieser KJ, Kim DW, Carlson DE, Katzenellenbogen BS, Katzenellenbogen JA. Characterization of the pharmacophore properties of novel Selective Estrogen Receptor Downregulators (SERDs) J Med Chem. 2010;53:3320–3329. doi: 10.1021/jm100047k.Through a detailed molecular analysis of a series of ligands based upon the SERD GW5638, the authors investigate the requirement of molecular aspects of this drug for SERD activity. This study may prove instructive for future SERD development.
- 72. Santen R, Jeng M-H, Wang J-P, Song R, Masamura S, McPherson R, Santner S, Yue W, Shim W-S. Adaptive hypersensitivity to estradiol: Potential mechanism for secondary hormonal responses in breast cancer patients. J Steroid Biochem Molec Biol. 2001;79:115–125. doi: 10.1016/s0960-0760(01)00151-0.Although aromatase inhibitors that reduce circulating estrogen levels have been shown to have clinical benefit in breast cancer treatment, patients frequently relapse over time as the tumors become resistant. This study was the first to illustrate that, rather than estrogen insensitivity, resistance may instead develop through hypersensitivity to estrogen.
- 73. DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. 27- hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22:65–77. doi: 10.1210/me.2007-0383.Although 27-hydroxycholesterol, a natural cholesterol metabolite, had previously been shown to antagonize ER action in the cardiovascular system, the authors report activation of ER target genes in breast cancer cells as well as partial antagonism of ER action in this model. This is the initial report of an endogenously produced SERM and broadens the scope of physiologically important interactions between estrogen action and metabolism.
- 74. Sikora MJ, Cordero KE, Larios JM, Johnson MD, Lippman ME, Rae JM. The androgen metabolite 5 alpha-androstane-3 beta, 17 beta-diol (3 alpha Adiol) induces breast cancer growth via estrogen receptor: implications for aromatase inhibitor resistance. Breast Cancer Res Treat. 2009;115:289–296. doi: 10.1007/s10549-008-0080-8.The authors report upregulation of steroidogenic enzymes by breast cancer cells to enable ER dependent growth and response to androgen metabolites. These results suggest that one mechanism of resistance to aromatase inhibitors may consist of an autocrine production of alternative ER ligands from androgens, whose levels remain unchanged despite reduction of circulating estrogen.
- 75.Robertson JF, Steger GG, Neven P, Barni S, Gieseking F, Nole F, Pritchard KI, O'Malley FP, Simon SD, Kaufman B. Activity of fulvestrant in HER2-overexpressing advanced breast cancer. Ann Oncol. 2010;21(6):1246–1253. doi: 10.1093/annonc/mdp447. [DOI] [PubMed] [Google Scholar]