Abstract
The present experiment assessed whether preadolescent exposure to methamphetamine would alter adult behavioral responses to cocaine and dopamine transporter immunoreactivity in the striatum of male and female rats. Juvenile rats were injected once daily with 0 or 2 mg/kg methamphetamine from postnatal days 21 – 35 and tested in adulthood. Male rats, but not female rats, exposed to methamphetamine showed an increase in responsiveness to cocaine in the open field and an increase in dopamine transporter immunoreactivity in the striatum. These findings suggest that early exposure to methamphetamine can lead to sex-specific altered responses to psychostimulants in adulthood, which may contribute to later vulnerability to drug use.
Keywords: Methamphetamine, Rat, Juvenile, Sex Differences, Cocaine, Striatum
Stimulant exposure in young children occurs frequently in the United States and around the world [7,24]. In the US, Australia and Asia exposure to methamphetamine (MA) has been documented both prenatally and during early childhood [7,9,10,24,28]. Although this early contact with MA is known to occur, little is known about its potential impact on brain development and behavior. In humans, one risk factor for drug addiction later in life is early experience with illicit drugs [19]. Individuals exposed to MA during early developmental periods may have altered brain development, which may contribute to greater vulnerability for drug abuse as adults.
Methamphetamine causes the release and accumulation of dopamine in the cytosol and synapse via its actions at the dopamine terminal [14]. Given the pharmacological actions of MA, exposure to MA during development may lead to alterations in brain development and functioning. In humans, prenatal exposure to MA is associated with altered creatine levels in the striatum and smaller putamen and caudate [9,28]. These changes were associated with increased cognitive difficulties, including impairments in attention and visual motor integration when assessed during childhood. Likewise, long-term changes in dopaminergic markers in the striatum were reported in a rodent model of neonatal MA exposure [11]. However, it is unknown if similar changes occur when MA exposure occurs during childhood or if these changes will lead to behavioral and/or neurochemical modifications associated with drug abuse later in life.
Dopaminergic systems begin to form early in prenatal development and continue to change into adulthood [18]. Dopaminergic neurons appear during the first trimester of human fetal development [1], however, dopaminergic markers continue to modify into adulthood [17,20]. In both humans and rats, the dopamine transporter (DAT) peaks before adulthood and then decreases as the animal ages into early adulthood [17,25]. Likewise, dopamine receptors peak during adolescence in the rat and human and then decline into early adulthood [4,20]. It is unknown if early exposure to stimulant drugs that act on dopamine terminals, such as MA, will alter the development of dopaminergic systems in the brain.
There are dynamic changes in the brains of both males and females during preadolescence, [2] however the magnitude of these alterations is greater in males, especially in regions associated with reward and drug abuse. In the striatum, dopamine D1 and D2 receptors are overproduced during preadolescence in the juvenile male rat and then subsequently eliminated during early adulthood. In the female rat, however, these receptors gradually increase to adult levels during preadolescence and adolescence with no subsequent elimination [4]. Furthermore in humans, the striatum decreases in size during adolescence in males but not females [15]. Since males tend to undergo more dramatic changes in their dopaminergic systems during development, it is likely that they may be more vulnerable to the long-term effects of stimulant drugs during preadolescence compared to females.
The purpose of the current study was to assess whether early repeated exposure to MA in the rat alters their behavioral response to cocaine as adults. Since DAT in the striatum is changing during preadolescent development and cocaine works in part by blocking DAT [14,25], the current study also tested whether DAT is changed in the striatum of adult rats treated with MA during preadolescence. Sex differences exist both in the development of the dopaminergic system and in adult drug abuse [4,5,21] and may be an important consideration in examining these effects. Therefore, this study tested whether early exposure to MA leads to sex specific changes in adult responses to cocaine.
Male and female Sprague Dawley (Charles-River derived; Indianapolis, IN) rats were bred in the Psychology Department's animal colony at Northern Illinois University. Lights were maintained on a 12 hr light/dark cycle (lights on at 6:00 hr) with temperature maintained at 22±2° C. Food and water were provided ad libitum. The procedures in the current study were approved by the local Institutional Animal Care and Use Committee and followed National Institute of Health's Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1996).
Pregnant dams' cages were checked twice daily to determine any births (PD1). The pups were left undisturbed with their mothers until the day of weaning (PD21) and then pair housed with a same sex littermate in polycarbonate cages (46 × 25 × 21cm). Pups from a total of 17 litters were used for the experiments. The pups were pseudo-randomly assigned to a treatment group in pairs at weaning (2 pups received MA, 2 pups received saline and 2 received no injection) resulting in the following treatment groups: MA (8 males and 8 females for open field; 13 males and 11 females for DAT immunoreactivity), saline (8 males and 8 females for open field; 11 males and 8 females for DAT immunoreactivity), and not handled (9 males and 7 females for open field; 8 males and 7 females for DAT immunoreactivity). Therefore, the treatments were counter-balanced across each litter and sex.
MA-injected and saline-injected rats were weighed daily from PD21-35 and received an i.p. injection of 0.1% of their body weight of 2 mg/kg (+)-methamphetamine HCl (Sigma Laboratories, St. Louis, MO, USA) dissolved in 0.9% NaCl saline or 0.9% NaCl saline alone. The selection of dosage and developmental window was based on previous research in our laboratory [23] and on comparable dosages of other stimulants administered during similar developmental windows [2,3,8]. During PD21-35, the not handled group was left undisturbed. From PD36-80, all rats were left undisturbed except for routine cage maintenance. At 80 days old, rats were handled for 5 min per day for 5 days. The estrous cycle was not tracked in female rats because vaginal lavage has been shown to attenuate cocaine induced locomotion, which would potentially confound the behavioral measures for this study [31].
At approximately 90 days old, a subset of rats began testing in the open field. A large plywood box (75 × 75 × 29 cm) painted green was used for open field testing. For the first 4 days of testing, each rat was placed into a randomly chosen corner of the open field and allowed to explore the open field for 30 min. On the fifth day, an injection of 7.5 mg/kg (i.p.) cocaine ((-)-Cocaine HCl, RTI International, Research Triangle Park, North Carolina, USA) was given [16]. Immediately after the injection, each rat was allowed to explore the open field for 60 min. The apparatus was cleaned thoroughly between rats with a disinfectant solution. All testing sessions were recorded using a DVD-recorder attached to an overhead camera for analysis of the total horizontal distance traveled during each testing session using Noldus Ethovisions 3.0 (Noldus Information Technology, Wageningen, Netherlands).
A separate group of rats was killed via decapitation and their brains were rapidly removed at approximately 130 days old. The striata were dissected, frozen on dry ice, and stored at -80°C until Western blots for DAT was conducted [27]. Briefly, the left striatum was homogenized in 0.5mL of ice-cold 0.32M sucrose and centrifuged at low speed (800 × g, 17min, 4°C). The supernatant (S1) was removed and centrifuged at high speed (22,000 × g, 17min, 4°C) to yield pelleted (P2) synaptosomes. The pellet was resuspended in 150μL of ice-cold dH20 and an aliquot (75μL) was centrifuged at high speed (22,000 × g, 17min, 4°C). The supernatant (S3) was removed and the pellet was resuspended in ice-cold dH20 to form the membrane fraction. Protein values were determined via the Bradford method. Protein samples were then mixed with 2 × Tris-glycine- loading buffer (Invitrogen, Carlsbad, CA, USA), heated at 85°C for 5 min and stored at -80°C until assayed. Western Blot analysis was performed using Tris-glycine (10%) 10 lane gels in a Novex mini cell apparatus (Invitrogen, Carlsbad, CA, USA). Protein (5 μg) was loaded into each lane and the gel run at 150V for 90min (running buffer: 25mM TRIS; 192mM Glycine; 0.02% SDS). Gels were transferred at 120V for 2h to a polyvinylidene difluoride (PVDF) membrane (transfer buffer: 25mM TRIS; 192mM Glycine; 20% Methanol; 0.02% SDS). The membranes were then blocked for 1h at room temperature in blocking buffer (10mM TRIS; 150mM NaCl; pH 8.0; 5% non-fat dry milk and 0.05% Tween-20) and probed with DAT primary antibody (dilution 1:1250; sc 1458, Santa Cruz, CA, USA) overnight at 4°C. The following day, membranes were rinsed 3 × in TBST, probed with HRP-conjugated anti-goat secondary antibody (dilution 1:2500, sc 2768, Santa Cruz, CA, USA) for 1h at room temperature and visualized via enhanced chemiluminescence (ECL) (Amersham Biosciences, NJ, USA). The net band intensity was measured using a Kodak Gel Logic 100 imaging system (Rochester, NY, USA).
Saline and unhandled control groups did not significantly differ from each other at any time point during open field testing or in western blot analysis. Therefore, these groups were collapsed into one control group for the reported statistical analyses and presentation of results.
The open field habituation data were analyzed using a 2 (group—control or MA pretreatment) × 2 (sex-male or female) × 4 (day) repeated measures ANOVA. The cocaine challenge data were blocked by litter and analyzed using a 2 (group) × 2 (sex) × 3 (time- habituation day 4, first 30 min following cocaine, last 30 min following cocaine) repeated measures ANOVA. Separate 2 (group) × 3 (time) ANOVA's were conducted for each sex based on the a priori hypothesis that treatment could affect each sex differently. The western blot immunoreactivity was analyzed using a one-way ANOVA blocked by litter. Male and female rats were run on separate gels; therefore gender differences could not be calculated for DAT. Significant group differences (p<0.05) were further analyzed using Tukey's post hoc analysis. All statistical analyses were performed on SAS 9.2 software using the GLM procedure (Cary, NC, USA). Data are expressed as the mean ± SEM.
All groups habituated to the open field as indicated by a decrease in distance traveled as the number of habituation sessions increased (Main Effect of Day: F(3,132)=31.31, p<0.01; Table 1). There was a significant 3 way interaction with female rats exposed to MA during preadolescence traveling greater distances during the first day of habituation compared to control male rats (F(3,132)=3.18, p<0.05; Table 1). However, no overall group effect was found (F(1,44)=0.69, ns), nor did group interact with habituation day (F(3,132)=1.05, ns). Furthermore, no overall effect of sex was found (F(1,44)=0.46, ns), nor were sex differences seen across days of habituation (F(3,132)=1.76, ns).
Table 1.
Distance Traveled (cm) During Habituation. Means (SE) are presented for distance traveled in 30 min test. There was a decrease in distance traveled throughout the 3 days of habituation (p<.01).
| Distance Traveled (m) During Habituation Training | ||||
|---|---|---|---|---|
| Habituation Day | 1 | 2 | 3 | 4 |
| Female | ||||
| Control | 10.17 (0.46) | 8.59 (0.56) | 8.36 (0.73) | 8.09 (0.39) |
| MA | *12.12 (1.42) | 8.77 (1.20) | 7.96 (1.11) | 7.15 (0.65) |
| Male | ||||
| Control | 9.69 (0.71) | 7.79 (0.79) | 7.11 (0.61) | 7.31 (0.64) |
| MA | 10.10 (0.41) | 8.74 (0.54) | 8.18 (0.66) | 8.53 (0.73) |
p<0.05 MA Female < Control Male
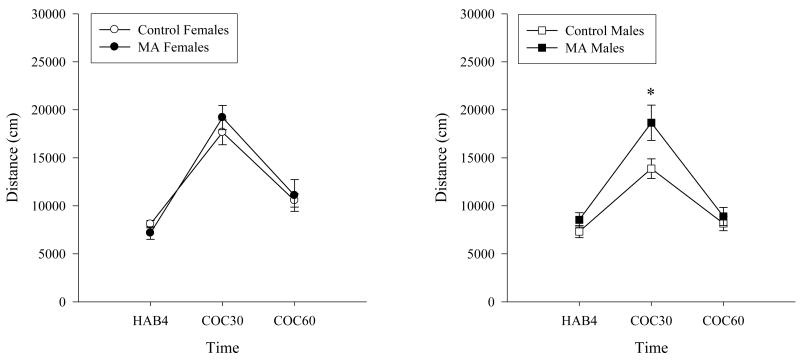
An acute injection of cocaine increased the distance traveled in all groups (Time: F(2,82)=226.84, p<0.01; Figure 1). Rats exposed to MA during preadolescence traveled a significantly greater distance in the open field during the first 30 min following the cocaine challenge compared to the control group (Time × Group Interaction: F(2,82)=5.67, p<0.05), but not at any other time point. However, no differences were found between groups in the overall distance traveled (Main Effect of Group: F(1,41)=3.34, ns).
Figure 1.
Distance traveled during the cocaine challenge in the open field. An acute injection of cocaine significantly increased the distance traveled in all groups as expected. Rats exposed to MA during preadolescence traveled significantly greater distances during the first 30 min following the cocaine challenge compared to the control group. The increased locomotion during the first 30 min following the cocaine challenge (COC30) was greater in MA exposed males compared to male controls (* p<0.05).
Female rats traveled a greater distance following the cocaine challenge than males (Time × Sex: F(2,82)=5.36, p<0.01). The overall gender by treatment interaction did not reach significance (F(2,82)=1.32, ns). When the sexes were analyzed separately based on the a priori hypothesis that drug exposure would affect each gender differently, male but not female rats exposed to MA during preadolescence had significantly increased locomotion compared to sex matched control rats during the first 30 min following the cocaine injection (Time × Group: Male F(2,46)=6.56, p<0.05; Female F(2,36)=1.18, ns).
Male rats exposed to MA from PD21-35 had a 55% increase in DAT protein immunoreactivity in the striatum compared to male control rats (F(1,19)=14.22, p<0.05; Figure 2). However, female rats exposed to MA had similar DAT immunoreactivity compared to female control rats (F(1,15)=0.12, ns).
Figure 2.
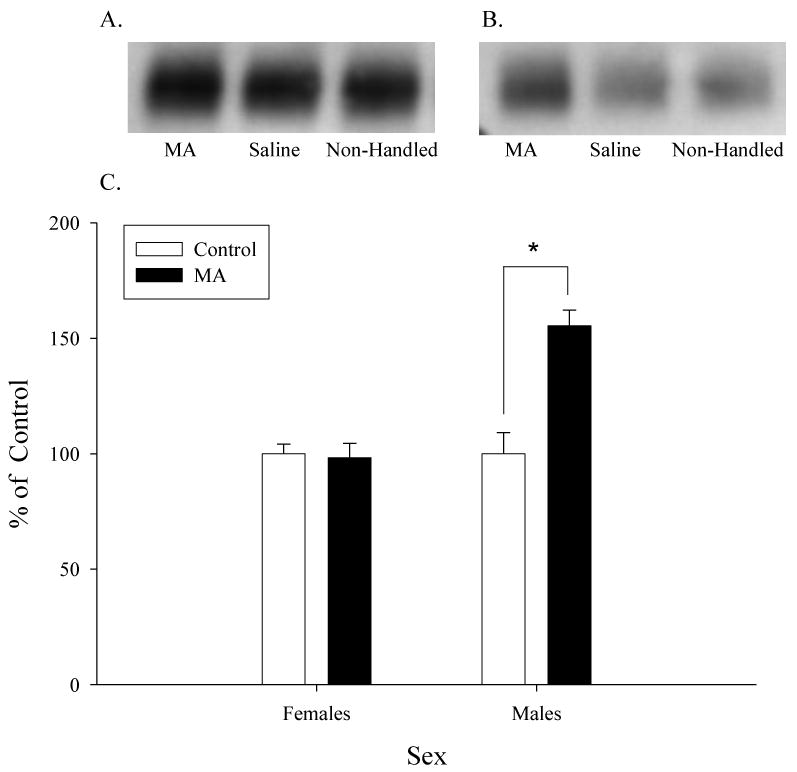
Western blot analysis of striatal DAT. Representative blots of striatal tissue (5 μg of protein/lane) taken from female (A) or male (B) rats. C. Male rats exposed to MA during preadolescence had significantly higher striatal DAT immunoreactivity compared to control male rats (* p<0.05). Female rats had no significant changes in striatal DAT immunoreactivity.
Overall, the findings of the current study suggest that early exposure to MA can alter adult responses to another stimulant, cocaine, in a sex specific manner. Male rats exposed to chronic low doses of MA had an increased locomotor response to cocaine that may be related to the observed increase in DAT immunoreactivity in the striatum. However, female rats exposed to the same MA dosing regimen from PD21-35 showed no changes in their locomotor response to cocaine as adults or in DAT levels in the striatum.
The enhanced behavioral responsiveness to cocaine in male rats treated with MA during preadolescence may be due to the action of cocaine on DAT. Cocaine blocks catecholamine transporters including the dopamine transporter, therefore the increase in DAT observed in MA exposed male rats may contribute to greater dopamine-stimulated behavior [14]. The spontaneous hypertensive rat also has been shown to have increased DAT expression in the striatum and increased responsiveness to stimulant drugs [6,32]. Furthermore, human cocaine drug abusers also show increased DAT densities in the caudate [12].
However, the increase in DAT densities should be interpreted cautiously for it may be a compensatory response due to less functional DAT. Mice lacking the orphan G protein-coupled receptor 37 have increased striatal dopamine uptake and DAT expression on the plasma membrane, but decreased overall striatal DAT protein and locomotor response to cocaine compared to wild-type mice [22]. These findings suggest that the localization and function of the transporters may play a greater role in the regulation of dopamine and stimulant-induced activity than the total amount of DAT protein. In the current study, male rats exposed to MA during preadolescence differed only in the distances traveled after the cocaine challenge but not during habituation. Our behavioral data are similar to data from DAT knockdown mice with 90% reduction of DAT who showed an increased locomotion to low doses cocaine but not to saline compared to wild-type mice [30]. Thus, the increased locomotion and the increased DAT protein found in the male rats treated with MA during preadolescence may be indicative of reduced striatal DAT function rather than changes in density.
The sex difference that was observed following the cocaine challenge supports previous research. Kuhn and colleagues found that adult male rats have a reduced locomotor response to cocaine compared to adult female rats [21]. Interestingly, adult males showed an attenuated response to cocaine compared to juvenile rats, but adult females showed similar levels of activity regardless of the developmental period [21]. The attenuated locomotor response in the adult males may be due to the decrease in DAT densities observed in male rats as they age into adulthood [21,25]. In the current study, male rats exposed to MA during preadolescence had a similar locomotor response to cocaine as female rats but an increased locomotor response compared to the control male rats. Coupled with the increased DAT densities in the striatum, these findings may suggest that the dopaminergic systems in adult male rats exposed to MA earlier in development may be in a similar functional state as that of male juvenile rats.
In summary, the findings in the current study suggest that males may be more vulnerable to the enduring effects of early exposure to MA. These male rats had an increased locomotor response to cocaine and increased DAT immunoreactivity compared to control male rats, but female rats showed no changes in either measure. The increased DAT immunoreactivity in the striatum of MA treated male rats may be indicative of an increased potential of drug abuse. Increased DAT densities in the caudate are also seen in humans with cocaine dependence [12] and in medication naive patients with attention/deficit hyperactivity disorder who also have higher rates of substance abuse disorders [13,29]. Overall, the current study suggests that juvenile MA exposure may lead to altered responses to drugs of abuse as adults.
Acknowledgments
The authors would like to thank Laura Risley for conducting the Western blots and preliminary data analyses. Preliminary analyses of part of these data were presented at the Annual Meeting of the Society for Neuroscience in San Diego, CA (2007).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Almqvist PM, Akesson E, Wahlberg LU, Pschera H, Seiger A, Sundstrom E. First trimester development of the human nigrostriatal dopamine system. Exp Neurol. 1996;139(2):227–37. doi: 10.1006/exnr.1996.0096. [DOI] [PubMed] [Google Scholar]
- 2.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27(1-2):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 3.Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA., Jr Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002;5(1):13–4. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- 4.Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8(6):1495–8. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- 5.Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cailhol S, Mormède P. Strain and sex differences in the locomotor response and behavioral sensitization to cocaine in hyperactive rats. Brain Res. 1999;842(1):200–5. doi: 10.1016/s0006-8993(99)01742-4. [DOI] [PubMed] [Google Scholar]
- 7.Caldicott DG, Pigou PE, Beattie R, Edwards JW. Clandestine drug laboratories in Australia and the potential for harm. Aust N Z J Public Health. 2005;29(2):155–62. doi: 10.1111/j.1467-842x.2005.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 8.Carlezon WA, Jr, Mague SD, Andersen SL. Enduring behavioral effects of early exposure to methylphenidate in rats. Biol Psychiatry. 2003;54(12):1330–7. doi: 10.1016/j.biopsych.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004;132(2):95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Chomchai C, Na Manorom N, Watanarungsan P, Yossuck P, Chomchai S. Methamphetamine abuse during pregnancy and its health impact on neonates born at Siriraj Hospital, Bangkok, Thailand. Southeast Asian J Trop Med Public Health. 2004;35(1):228–31. [PubMed] [Google Scholar]
- 11.Crawford CA, Williams MT, Newman ER, McDougall SA, Vorhees CV. Methamphetamine exposure during the preweanling period causes prolonged changes in dorsal striatal protein kinase A activity, dopamine D2-like binding sites, and dopamine content. Synapse. 2003;48(3):131–7. doi: 10.1002/syn.10197. [DOI] [PubMed] [Google Scholar]
- 12.Crits-Christoph P, Newberg A, Wintering N, Ploessl K, Gibbons MB, Ring-Kurtz S, Gallop R, Present J. Dopamine transporter levels in cocaine dependent subjects. Drug Alcohol Depend. 2008;98(1-2):70–6. doi: 10.1016/j.drugalcdep.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dopheide JA, Pliszka SR. Attention-deficit-hyperactivity disorder: an update. Pharmacotherapy. 2009;29(6):656–79. doi: 10.1592/phco.29.6.656. [DOI] [PubMed] [Google Scholar]
- 14.Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–98. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- 15.Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex. 1996;6(4):551–60. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 16.Haile CN, GrandPre T, Kosten TA. Chronic unpredictable stress, but not chronic predictable stress, enhances the sensitivity to the behavioral effects of cocaine in rats. Psychopharmacology (Berl) 2001;154(2):213–20. doi: 10.1007/s002130000650. [DOI] [PubMed] [Google Scholar]
- 17.Haycock JW, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish SJ. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. J Neurochem. 2003;87(3):574–85. doi: 10.1046/j.1471-4159.2003.02017.x. [DOI] [PubMed] [Google Scholar]
- 18.Herlenius E, Lagercrantz H. Neurotransmitters and neuromodulators during early human development. Early Hum Dev. 2001;65(1):21–37. doi: 10.1016/s0378-3782(01)00189-x. [DOI] [PubMed] [Google Scholar]
- 19.Hser YI, Longshore D, Anglin MD. The life course perspective on drug use: a conceptual framework for understanding drug use trajectories. Eval Rev. 2007;31(6):515–47. doi: 10.1177/0193841X07307316. [DOI] [PubMed] [Google Scholar]
- 20.Jucaite A, Forssberg H, Karlsson P, Halldin C, Farde L. Age-related reduction in dopamine D1 receptors in the human brain: from late childhood to adulthood, a positron emission tomography study. Neuroscience. 2010;167(1):104–10. doi: 10.1016/j.neuroscience.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn CM, Walker QD, Kaplan KA, Li ST. Sex, steroids, and stimulant sensitivity. Ann N Y Acad Sci. 2001;937:188–201. doi: 10.1111/j.1749-6632.2001.tb03565.x. [DOI] [PubMed] [Google Scholar]
- 22.Marazziti D, Mandillo S, Di Pietro C, Golini E, Matteoni R, Tocchini-Valentini GP. GPR37 associates with the dopamine transporter to modulate dopamine uptake and behavioral responses to dopaminergic drugs. Proc Natl Acad Sci U S A. 2007;104(23):9846–51. doi: 10.1073/pnas.0703368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McFadden LM, Matuszewich L. The effects of methamphetamine exposure during preadolescence on male and female rats in the water maze. Behav Brain Res. 2007;185(2):99–109. doi: 10.1016/j.bbr.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 24.Mecham N, Melini J. Unintentional victims: development of a protocol for the care of children exposed to chemicals at methamphetamine laboratories. Pediatr Emerg Care. 2002;18(4):327–32. doi: 10.1097/00006565-200208000-00024. [DOI] [PubMed] [Google Scholar]
- 25.Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Rüther E, Huether G. Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Brain Res Dev Brain Res. 2000;119(2):251–7. doi: 10.1016/s0165-3806(99)00182-0. [DOI] [PubMed] [Google Scholar]
- 26.Morissette M, Di Paolo T. Sex and estrous cycle variations of rat striatal dopamine uptake sites. Neuroendocrinology. 1993;58(1):16–22. doi: 10.1159/000126507. [DOI] [PubMed] [Google Scholar]
- 27.Quinton MS, Yamamoto BK. Neurotoxic effects of chronic restraint stress in the striatum of methamphetamine-exposed rats. Psychopharmacology. 2007;193(3):341–50. doi: 10.1007/s00213-007-0796-x. [DOI] [PubMed] [Google Scholar]
- 28.Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T. Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology. 2001;57(2):255–60. doi: 10.1212/wnl.57.2.255. [DOI] [PubMed] [Google Scholar]
- 29.Spencer TJ, Biederman J, Madras BK, Dougherty DD, Bonab AA, Livni E, Meltzer PC, Martin J, Rauch S, Fischman AJ. Further evidence of dopamine transporter dysregulation in ADHD: a controlled PET imaging study using altropane. Biol Psychiatry. 2007;62(9):1059–61. doi: 10.1016/j.biopsych.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tilley MR, Cagniard B, Zhuang X, Han DD, Tiao N, Gu HH. Cocaine reward and locomotion stimulation in mice with reduced dopamine transporter expression. BMC Neurosci. 2007;8:42. doi: 10.1186/1471-2202-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker QD, Nelson CJ, Smith D, Kuhn CM. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav. 2002;73(4):743–52. doi: 10.1016/s0091-3057(02)00883-3. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe Y, Fujita M, Ito Y, Okada T, Kusuoka H, Nishimura T. Brain dopamine transporter in spontaneously hypertensive rats. J Nucl Med. 1997;38(3):470–4. [PubMed] [Google Scholar]